INTRODUCTION
Diabetes mellitus (DM) is a non-infectious chronic metabolic disease characterized by hyperglycemia. In 2021, it was estimated that 536.6 million people live with DM globally. Their age ranged from 20 to 70 years old. The global number of people with DM was predicted to increase by 46% in 2045 which was equivalent to 783.2 million people. The prevalence of DM in Southeast Asia in 2015 was recorded at 415 million cases. This number was predicted to rise to 642 million in 2040 [1]. Research of Basic Health (Riskesdas) by Indonesia’s Health Ministry in 2018 recorded an increase in the prevalence of people with DM in Indonesia’s reproductive age to 8.5% from previously 6.9% in 2013 [2].
Hyperglycemia-induced diabetes was the main cause of oxidative stress. The increasing reactive oxygen species (ROS) caused by hyperglycemia in people with DM created an oxidative imbalance that induce apoptosis [3]. Oxidative stress was reported to affect the physiological function of the male reproductive organ [4]. Apoptosis in testicle tissue caused a reduction in Leydig cells, Sertoli cells, and spermatogenic cells. It also increased the lumen diameter of the seminiferous tubule but decreased its epithelial thickness which caused hypo-spermatogenesis [5] and degraded sperm quality and quantity [6]. Current diabetes treatments have limitations and side effects and pose cardiovascular risks. Insulin has side effects of hypoglycemia and weight gain, sulfonylureas have direct effects on cardiovascular disorders, and pioglitazone increases the risk of heart failure. Increased cardiovascular risk can lead to various complications [7]. Therefore, an exploration of natural constituent alternatives is needed. A previous study stated that flavonoid content from active fraction I of Physalis angulata (Pa) extract has anti-hyperglycaemia activity [8].
Apoptosis occurs in testicular tissue due to oxidative injury characterized by increased proapoptotic biomarkers such as Bax, Bad, and c-jun N-terminal kinase and decreased antiapoptotic biomarkers such as Bcl-2 and Bcl-xl. The mechanism of apoptosis occurs due to an increase in the Bax/Bcl-2 ratio. Bax and Bcl-2 are essential in maintaining the integrity of the mitochondrial membrane pore. An increase in the Bax/Bcl-2 expression ratio indicates the occurrence of mitochondrial stress-induced apoptosis [9].
Testicular tissue is one of the tissues most susceptible to oxidative stress caused by increased ROS production. DM damages cell structure and function in testicular tissue, which can further interfere with spermatogenesis [10]. Increased ROS production in diabetic conditions decreases mitochondrial function in the testes, causing damage [11]. This can cause a decrease in Adenosine Triphosphate production due to dysregulation of the electron transport chain in mitochondria [12].
Antioxidants are reported to be able to repair hyperglycemia-induced oxidative damage to male reproductive organs [13]. Superoxide dismutase (SOD1) is a type of antioxidant that can protect cellular damage caused by oxidative stress through the conversion of free radicals to oxygen. Cellular damage due to oxidative stress through the conversion of free radicals into oxygen and hydrogen peroxide [14]. One herbal candidate that contains antioxidants and has an antidiabetic activity that has the potential to repair testicular damage due to hyperglycemia is Pa [15]. Pa is a plant that has anti-inflammatory, antiapoptotic, antioxidant [16], antidiabetic [17], and anti-infertility [18] activities. Pa contains alkaloids, saponins, flavonoids, and tannins with antidiabetic activity. Research on the administration of Pa extract at a dose of 150 mg/kgBW was reported to have an antihyperglycemic effect shown by drastically lowering the blood sugar levels of DM model rats [19].
Research on the active fraction of Pa extract in hyperglycemia conditions has yet to be widely conducted. Wahyuningsih et al. [8] reported that the fraction I of four fractions from Pa contained flavonoids that had the best antihyperglycemic activity on myoblast cells. To date, no studies have investigated the effects of the active fraction of Pa on the testes of diabetic rats. This study utilized specimen materials in the form of fractions, which contain fewer bioactive compounds and mixtures compared to specimens in extract form. This study can serve as a fundamental source of information on the utilization of natural materials to control blood glucose levels in hyperglycemic conditions and as an effort to provide protection against testicular damage caused by hyperglycemia, which holds potential for further development as an alternative supplement aimed at protecting male reproductive organs under diabetic conditions, so this research has become a new study in herbal medicine and male reproductive health.
MATERIALS AND METHODS
This research has been approved by the Medical and Health Research Ethics Committee Faculty of Medicine, Public Health, and Nursing (FMPHN) Universitas Gadjah Mada, Indonesia, through letter number EC: KE/FK/1703/EC/2023. This research employed a quasi-experimental design: post-test-only controlled group. There were 5 groups: control, DM group (DM), DM treated with Pa 8.5 (Pa 1), 34 (Pa 2), and 136 mg/kgBW (Pa 3), respectively, each group consisted of 5 male adult rats weight 200–250 g giving a total of 25 rats used in this research. The dosage determination in this study was based on the ability of myoblast cells to reduce blood glucose levels at a concentration of 100 µg/ml. The dose was then converted for rats, resulting in a minimum dose of 8.5 mg/ml [8].
The making of a model animal with DM I
Adult male rats were conditioned with no food for 12 hours and then induced with a single dose of streptozotocin (STZ) 60 mg/kgBW via the intraperitoneal route. Blood glucose level was measured 72 hours after induction of STZ. Model animal with DM I was successfully made if blood glucose level > 300 mg/dl.
Administering a fraction of Pa
The fraction I of Pa was sourced from The Center for Herbal Medicine FMPHN, Universitas Gadjah Mada, Indonesia. The fraction was dissolved in CMC-Na 0.5% solution and aquadest. Administration of the fraction to animal in groups Pa 1, Pa 2, and Pa 3 was done once daily using a nasogastric tube. Control and DM groups were treated with a placebo containing CMC-Na 0.5% and aquadest.
Measuring blood glucose level
Blood glucose level was measured once every 2 weeks using a blood glucose strip from Easy Touch® code 2886 by Bioptik Technology. The blood sample was taken from the Wistar rat’s vena caudalis.
Extracting testicles
The testes of rats were extracted after 2 months of treatment. Before the procedure, the rats were anesthetized with ketamine 50 mg/kgBW, xylazine 2 mg/kgBW, and acepromazine 0.5 mg/kgBW. Perfusion of NaCl 0.9% through the left ventricle. The pair of testes were separated for different purposes: one was kept in RNA preservation solution for examination of Bax mRNA expression, while the other was kept in neutral buffered formalin solution to be used in histological examination using hematoxylin-eosin dye.
Histological examination
Testicle tissue was cut longitudinally, turned into a paraffin block then dyed with hematoxylin-eosin. The examination of testicle histomorphology was performed under a light microscope with 100× magnification. The measurement of seminiferous tubule was performed with the software Image J on randomly selected 25 observation fields for each sample.
Molecular examination
The isolation of testicle tissue’s RNA was performed following a protocol from FAVORGEN Tri RNA Reagent catalog number FATRR-001. cDNA was created following a protocol from SMOBIO ExcelRTTM Reverse Transcription Kit II cat. No: RP1400. Polymerase chain reaction mix was made based on protocol SensiFAST SYBR Lo-ROX Kit catalog number: BIO-94005 with an annealing temperature is 60°C. The primer specifications were designed using the primer designing tool website (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) (Table 1).
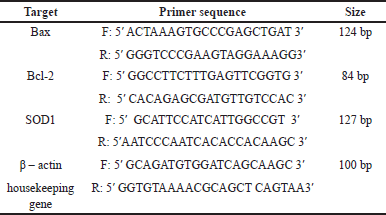 | Table 1. The primer specifications using NCBI Primer Blast software. [Click here to view] |
Analysis of the results
All statistical analysis was performed using SPSS 27. ANOVA was used to analyze the mean differences of data among groups. A significant difference was determined by p < 0.05 value and followed by a post hoc test using least significant difference and Mann–Whitney test.
RESULTS AND DISCUSSION
Blood glucose concentration
The blood glucose level in both DM and treated groups was significantly higher (p < 0.05) than the control group. Administration of Pa fraction insignificantly reduced blood glucose levels in the treated groups (p > 0.05) and the Pa 3 group showed the lowest level (377.06 ± 19.47 mg/dl) compared to the DM group (459.79 ± 18.90 mg/dl). However, Pa did not reduce blood glucose levels to the normal range. The mean blood glucose levels in diabetic rats treated with Pa fractions are presented in Table 2.
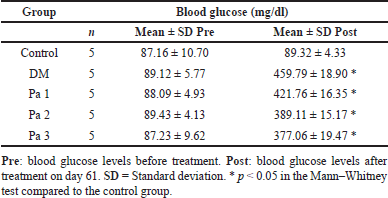 | Table 2. The mean blood glucose level in rats before and after treatment. [Click here to view] |
Body weight
The mean difference in body weight in the control group showed an increase of 16.6% by the end of the maintenance period, from 256.6 ± 18.89 to 299.17 ± 17.01 g. In contrast, the mean body weight difference in the DM group, Pa 1, Pa 2, and Pa 3 showed reductions of 18.27%, 13.9%, 18.65%, and 13.7%, respectively, following treatment. The body weight in both the DM and treated groups reduced after treatment, and the weight loss in the treated groups was lower than DM group (Table 3). However, there was no significant difference in weight loss between the DM and treatment groups (p > 0.05).
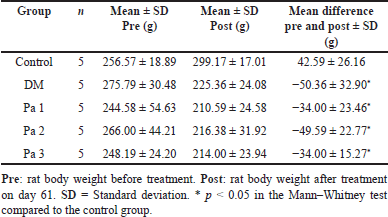 | Table 3. The mean body weight in rats before and after treatment. [Click here to view] |
Seminiferous tubule’s lumen diameter
The lumen of the seminiferous tubules was filled with few spermatogenic cells and the lumen diameter looked larger in the DM group compared to the control group. The groups treated with a fraction of Pa (Pa 1, Pa 2, and Pa 3) showed that the lumen diameter of the seminiferous tubules in group Pa 3 was narrower than Pa 1, Pa 2, and DM groups but it is wider than the control (Fig. 1). The statistical test revealed that the lumen diameter in treated groups [Pa 1 (39.34 ± 2.88 µm), Pa 2 (37.23 ± 2.77 µm) and Pa 3 (35.88 ± 2.37 µm)] were significantly narrower (p < 0.05) compared to DM (59.41 ± 3.93 µm).
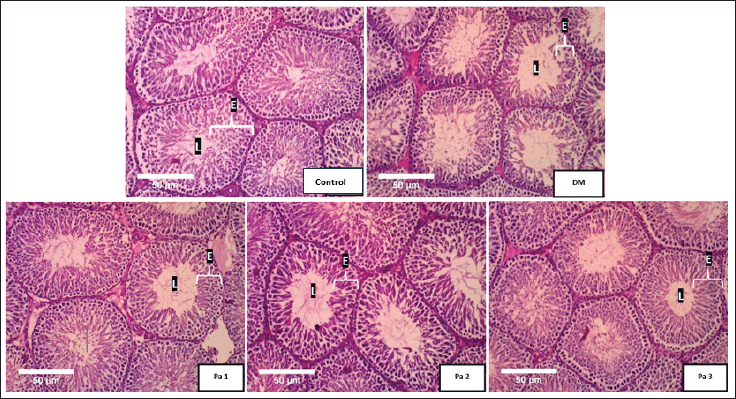 | Figure 1. Histology of lumen diameter and epithelial thickness of seminiferous tubules. Histological image of Wistar rat testicles with HE staining at 100× magnification in the control group, DM group (DM), DM group + 8.5 mg/kgBW fraction of Pa (Pa 1), DM group + 34 mg/kg BW active fraction of Pa (Pa 2), and DM group + 136 mg/kgBW fraction of Pa (Pa 3). L = lumen, E = epithelial cells. [Click here to view] |
Seminiferous tubule’s epithelial thickness
Seminiferous tubule epithelium in the DM group was thinner than the control group. Administration of the Pa fraction showed an increase in epithelial thickness compared to the DM group. The increase in seminiferous tubule epithelium thickness was found in the Pa 3 group. The statistical test showed that the epithelial layer of the seminiferous tubule was significantly thicker (p < 0.05) in the treated groups [Pa 1 (5.90 ± 1.50 mm), Pa 2 (6.30 ± 0.60 mm) and Pa 3 (6.50 ± 0.78 mm)] compared to DM (3.70 ± 0.73 mm) and epithelial layer in the Pa 3 group was thickest (Fig. 1).
Bax mRNA expression
Diabetes caused increased Bax mRNA in the DM group (1.97 ± 0.28) when compared to the control group (1.05 ± 0.16). Administration of the fraction of Pa showed lower Bax mRNA expression in the treatment group compared to the DM group. Administration of the fraction of Pa at a dose of 136 mg/kgBW (Pa 3) showed the lowest decrease in expression compared to other doses. Bax mRNA expression in the group given the fraction of Pa extract showed a decrease in expression along with the increase in the dose given. Bax mRNA expression showed significant differences between treated groups [Pa 1 (1.50 ± 0.2), Pa 2 (1.34 ± 0.1), and Pa 3 (1.19 ± 0.08)] and DM group (p < 0.001). Bax mRNA expression in the Pa 3 group exhibited a statistically insignificant difference to the control group (p > 0.05) (Fig. 2).
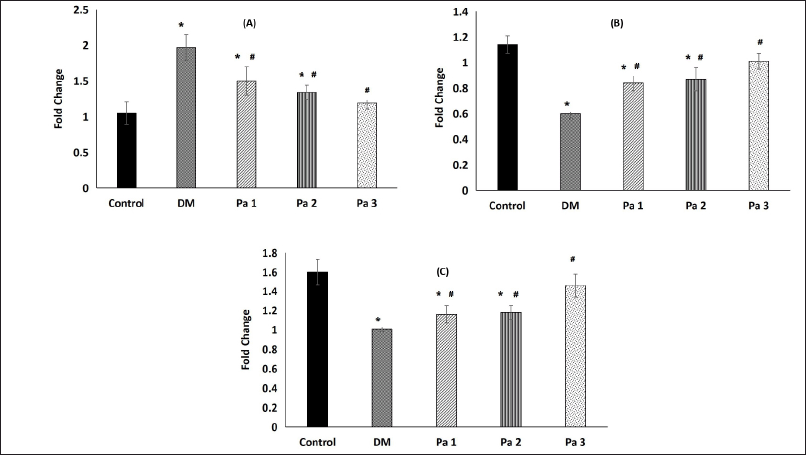 | Figure 2. Bax mRNA, Bcl-2 mRNA, and SOD1 mRNA expression in the testicular tissue of diabetic rat’s model. * show statistical differences p <0.05 compared to control and # show statistical differences p < 0.05 compared to DM group. The results are presented as mean ± SD. (A) Expression of Bax mRNA. (B) Expression of Bcl-2 mRNA. (C) Expression of SOD1 mRNA. [Click here to view] |
Bcl-2 mRNA expression
The expression of Bcl-2 mRNA was significantly increased in Pa 1 (0.84 ± 0.06), Pa 2 (0.87 ± 0.09), and Pa 3 (1.01 ± 0.05) compared to DM group (0.6 ± 0.04) with p < 0.001. Bcl-2 mRNA expression in the Pa 3 group exhibited a statistically insignificant difference to the control group (p > 0.05) (Fig. 2).
SOD1 mRNA expression
There was a significant decrease in the expression of SOD1 in the DM (1.1 ± 0.14) group compared to the control group (1.6 ± 0.2) (p-value = 0.01). The mean of SOD1 mRNA expression in the treated groups [Pa 1 (1.16 ± 0.11), Pa 2 (1.18 ± 0.17), and Pa 3 (1.46 ± 0.19)] were higher than DM group (1.1 ± 0.14) but only Pa 3 group was significantly higher (p < 0.05). The expression of SOD1 in the Pa 3 group exhibited a statistically insignificant difference to the control group (p > 0.05) (Fig. 2).
The administration of the fraction of Pa in this study showed the improvement of testicular damage in STZ-induced DM model rats. The fraction of Pa increased the size of epithelial thickness, mRNA expression of Bcl-2, and SOD1; however, it reduced the luminal diameter of the seminiferous tubules and the expression of Bax mRNA in a testicular diabetic rat model. Induction of STZ increased blood glucose levels in the DM, Pa 1, Pa 2, and Pa 3 groups with an average measurement of > 300 mg/dl. Previous research states that the administration of a single dose of STZ 60 mg/kgBW induces the occurrence of type 1 DM, which damages various organs, including the testes, with its diabetogenic properties [20].
A significant reduction in body weight was observed in the STZ-induced diabetic rat model group on day 12, compared to the control [21]. Another study conducted by Dzydzan et al. [22] reported a 9% decrease in body weight in diabetic rats after 14 days of treatment, whereas the control group exhibited a 33% increase. Weight loss in diabetic animal models is closely associated with enhanced gluconeogenic activity. This increase in gluconeogenesis results from impaired glucose uptake from carbohydrate sources due to STZ-induced pancreatic β-cell destruction [23]. STZ has been reported to damage pancreatic β-cells and reduce their cellular mass. The cytotoxicity of STZ toward β-cells is further aggravated by elevated oxidative stress, which contributes to mitochondrial dysfunction and exacerbates cellular damage [20].
The histological of the testis showed seminiferous tubule structures with tight inter-tubule spaces. The epithelial were thick with tightly arranged spermatogenic cells on the lining, and the lumens were filled with spermatozoa. Histological image from the DM control group (DM) showed relatively smaller seminiferous tubule structures with slightly loose inter-tubule spaces compared to the specimen from the control group. Among the groups treated with an active fraction of Pa, seminiferous tubules of Pa 3 had smaller lumen diameter and thicker epithelial compared to Pa 1 and Pa 2.
The results showed that there was a difference in lumen diameter and epithelial thickness between DM, Pa 1, Pa 2, Pa 3, and control groups. This may be due to the content of quercetin which is a derivative of flavonoids in Pa. Quercetin plays a role in stimulating the production of testosterone hormones that can stimulate the development of primary and secondary spermatocytes. This causes an increase in the thickness of the tubule epithelium and a decrease in the diameter of the seminiferous tubules [24]. Another study reported a successful effort in improving the seminiferous tubule’s diameter and epithelial thickness by administering pomegranate extract, which contains flavonoids and has antioxidant properties [25]. Flavonoids presumably could modulate the activity of intracellular antioxidant enzymes. It was proven by increasing the activity of SOD after treated with flavonoid [26]. DM reportedly made the apoptosis rate in spermatogenic cells relatively higher compared to the control [27]. Thinning of the seminiferous tubule epithelium indicates cell degeneration of spermatogenic cells and germ cells. The decrease in epithelial diameter and seminiferous tubule diameter due to diabetes can interfere with the male reproductive system which causes a decrease in fertility [28].
The results of this study showed that the expression of Bax and Bcl-2 mRNA is in accordance with previous research which found that there was a decrease in Bax mRNA expression and an increase in Bcl-2 expression in treatment groups compared to STZ-induced DM model animals. DM rats reportedly experienced a significant reduction in Bcl-2 gene expression and an increase in Bax gene expression compared to healthy controls. Bcl-2 contributes to the regulation of antioxidant pathway [29]. A significant increase in the Bax/Bcl-2 ratio occurred in DM rats, causing apoptosis in testicle tissue which would activate p38 mitogen-activated protein kinase and activation of p53 signaling a cascade associated with the cell death due to mitochondrial stress [30]. The fraction of Pa revealed a significant increase in SOD1 mRNA expression (Fig. 2). SOD1 enzyme deficiency may cause testicular shrinkage and increase susceptibility to heat stress [31]. SODs are the first line of defense against reactive oxygen species (mediated damage). These proteins catalyze the dismutation of superoxide anion-free radical into molecular oxygen and hydrogen peroxide and lower the O2 level, which destroys the cells at high concentrations [32].
The fraction of Pa in this research has been tested through bioassay-guided fractionation tested contains flavonoids. Flavonoids reduce the occurrence of oxidative stress through several mechanisms including acted as antioxidant, provided protection from free radicals, could help with inflammation, and activated insulin receptors [33]. Flavonoid act as antioxidants by eliminating free radicals, chelating metals, suppressing the activation of enzymes involved in the creation of free radicals, and stimulating the activation of endogenic-antioxidant-related enzymes [34]. Flavonoids could restrict the activity of free radical-producing enzymes such as xanthine oxidase, lipoxygenase, Protein Kinase C, cyclooxygenase, microsomal monooxygenase, and mitochondrial succinoxydase [35].
Administering hesperidin and morin, two flavonoids from different subclasses, to Sprague–Dawley rats could significantly prevent programmed cell death in testicle tissue [36,37]. Most anti-apoptosis proteins such as Bcl-2 and less pro-apoptosis proteins such as Bax and Caspase 3 were present. Flavonoids could improve mitochondrial membrane potential which explains their anti-apoptosis potential and the protection they provide at sub-cellular level [38]. This research showed that a fraction of Pa extract was effective in protecting and mitigating any damage caused by oxidative stress to the testicle in DM condition. The therapeutic effect of Pa makes it a potential alternative in the treatment of reproductive dysfunction in males especially in case of infertility caused by oxidative stress. Further research is needed to see the safety level of the active fraction of Pa extract by conducting toxicity tests. This study has a limitation in that it does not include an examination of apoptotic marker proteins in testicular tissue. As a result, the researchers were unable to determine which specific areas of the testicular tissue underwent apoptosis and which did not.
CONCLUSION
The administration of Pa fraction in STZ-induced diabetic rats revealed the testes tissue improvements of lumen and epithelial seminiferous tubules, Bcl-2, and SOD1 mRNA expression and decreased the Bax mRNA expression.
ACKNOWLEDGMENTS
The authors are thankful to the Faculty of Medicine, Public Health and Nursing, Gadjah Mada University Research community fund grant (DAMAS).
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICT OF INTEREST
The author reports no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Ethical approvals details is given in the ‘Materials and Methods’ section.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. International Diabetes Federation (IDF). Diabetes atlas. 7th ed. Brussels, Belgium: International Diabetes Federation; 2015.
2. Ministry of Health Republic of Indonesia. National basic health research finding report (RISKESDAS) 2018. Jakarta, Indonesia: The National Institute of Health Research and Development; 2019. 123–43 pp.
3. Prattichizzo F, De Nigris V, Mancuso E, Spiga R, Giuliani A, Matacchione G, et al. Short-term sustained hyperglycaemia fosters an archetypal senescence-associated secretory phenotype in endothelial cells and macrophages. Redox Biol. 2018;15:170–81. CrossRef
4. Ding GL, Liu Y, Liu ME, Pan JX, Guo MX, Sheng JZ, et al. The effects of diabetes on male fertility and epigenetic regulation during spermatogenesis. J Androl. 2015;17(6):948–53. CrossRef
5. Turner TT, Jeffrey JL. Stress oxidative: a common factor in testicular dysfuction. J Androl. 2008;29(5):488–98. CrossRef
6. Aitken RJ, Gibb Z, Baker MA, Drevet J, Gharagozloo P. Causes and consequences of oxidative stress in spermatozoa. Reprod Fertil Dev. 2008;28(1-2):1–10. CrossRef
7. Dalama B, Mesa J. New oral hypoglycemic agents and cardiovascular risk. Crossing the metabolic border. Rev Esp Cardiol. 2016;69(11):1088–97. CrossRef
8. Wahyuningsih MSH, Wiwekananda KSS, Putri APR, Nugrahaningsih DAA, Yunianti MM. Bioassay guided fractionation of ciplukan (Physalis angulata L.) monitored by glucose consumption assay and thin layer chromatography on myoblast cells. Trad Med J. 2023;28(1):22–30.
9. Aubrey BJ, Kelly GL, Janic A, Herold MJ, Strasser A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression?. Cell Death Differ. 2018;25(1):104–13. CrossRef
10. Asadi N, Bahmani M, Kheradmand A, Kopaei MR. The impact of oxidative stress on testicular function and the role of antioxidants in improving it: a review. J Clin Diagn Res. 2017;11(5):1–5. CrossRef
11. Amaral S, Oliveira PJ, Ramalho-Santos J. Diabetes and the impairment of reproductive function: possible role of mitochondria and reactive oxygen species. Curr Diabetes Rev. 2008;4(1):46–54.
12. Davila MP, Munoz PM, Tapia JA, Ferrusola CO, da Silva CCB, Pena FJ. Inhibition of mitochondrial complex I leads to decreased motility and membrane integrity related to increased hydrogen peroxide and reduced ATP production, while the inhibition of glycolysis has less impact on sperm motility. PLoS One. 2015;10(9):1–21. CrossRef
13. Minas A, Talebi H, Ray MT, Eisalou MGA, Razi M. Insulin treatment to type 1 male diabetic rats protects fertility by avoiding testicular apoptosis and cell cycle arrest. Gene. 2021;799(145847):1–10.
14. Eleutherio ECA, Magalhães RSS, de Araújo Brasil A, Neto JRM, Holanda Paranhos L. SOD1, more than just an antioxidant. Arch Biochem Biophys. 2021;697(108701):1–16. CrossRef
15. Sutjiatmo AB, Sukandar EY, Ratnawati Y, Kusmaningati S, Wulandari A, Narvikasari S. Antidiabetic effect of ciplukan herb (Physalis angulata Linn.) in alloxan-induced diabetic mice. J Farm Indones. 2021;5(4):166–71.
16. Dewi S, Isbagio H, Purwaningsih EH, Kertia N, Setiabudy R, Setiati S. A double-blind, randomized controlled trial of ciplukan (Physalis angulata Linn.) extract on skin fibrosis, inflammatory, immunology, and fibrosis biomarkers in scleroderma patients. Acta Med Indones Indones J Intern Med. 2019;51(4):303–10.
17. Iwansyah AC, Luthfiyanti R, Ardiansyah RCE, Rahman N, Andriana Y, Hamid HA. Antidiabetic activity of Physalis angulata L. fruit juice on streptozotocin-induced diabetic rats. S Afr J Bot. 2022;145:313–9. CrossRef
18. Ukwubile CA, Bingari MS, Angyu AE, Galba LC. Physalis angulata Linn. (Solanaceae) leaf extract boosts fertility, sperm production and haematological parameters in swiss male albino rats. Int J Med Plants Nat Prod. 2018;4(3):1–10. CrossRef
19. Maliangkay HP, Rumondor R, Kantohe M. Phytochemical screening and antidiabetic potential of ethanol extract of ciplukan herb (Physalis angulata l.) in alloxan-induced white rats (Rattus norvegicus). Bio-Edu. 2019;4(3):98–107.
20. Wu J, Yan LJ. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. Diabetes Metab Syndr Obes. 2015;8:181–8. CrossRef
21. Jiang S, Xu L, Xu Y, Guo Y, Wei L, Li X, et al. Antidiabetic effect of Momordica charantia saponins in rats induced by high-fat diet combined with STZ. Electron J Biotechnol. 2020;43:41–7. CrossRef
22. Dzydzan O, Bila, I, Kucharska AZ, Brodyak I, Sybirna N. Antidiabetic effects of extracts of red and yellow fruits of cornelian cherries (Cornus mas L.) on rats with streptozotocin-induced diabetes mellitus. Food Funct. 2019;10(10):6459–72. CrossRef
23. Furman BL, Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol. 2015;70:5.47.1–20.
24. Kadhim ZH. Effect of flavonoids on thickness and diameter of testicular seminiferous tubules in adult males wistar rates exposed to oxidative stress by lead acetate. MINAR Int J Appl Sci Technol. 2022;4(1):291–308. CrossRef
25. Utomo B, Daningtia NR, Yuliani GA, Yuniarti WM. Effects of a standardized 40% ellagic acid pomegranate (Punica granatum L.) extract on seminiferous tubule histopathology, diameter, and epithelium thickness in albino Wistar rats after heat exposure. Vet World. 2019;12(8):1261–65. CrossRef
26. Zhang Q, Yang W, Liu J, Liu H, Lv Z, Zhang C, et al. Identification of six flavonoids as novel cellular antioxidants and their structure-activity relationship. Oxidative Med Cell Longev. 2020;2020(4150897):1–12. CrossRef
27. Chen Y, Jiao N, Jiang M, Liu L, Zhu Y, Wu H, et al. Loganin alleviates testicular damage and germ cell apoptosis induced by AGEs upon diabetes mellitus by suppressing the RAGE/p38MAPK/NF-κB pathway. J Cell Mol Med. 2020;24(11):6083–95.
28. Sisman AR, Kiray M, Camsari UM, Evren M, Ates M, Baykara B, et al. Potential novel biomarkers for diabetic testicular damage in streptozotocin-induced diabetic rats: nerve growth factor Beta and vascular endothelial factor. Dis Markers. 2014;2014(108106):1–7. CrossRef
29. Mohamed AAR, Khater SI, Arisha AH, Metwally MMM, Hedeab GM, El-shetry ES. Chitosan-stabilized selenium nanoparticles alleviate cardio-hepatic damage in type 2 diabetes mellitus model via regulation of caspase, Bax/Bcl-2, and Fas/FasL-pathway. Gene. 2024:768(145288):1–13.
30. Solgi T, Amiri I, Asl SS, Saidijam M, Seresht BM, Artimani T. Antiapoptotic and antioxidative effects of cerium oxide nanoparticles on the testicular tissues of streptozotocin-induced diabetic rats: an experimental study. Int J Reprod Biomed. 2021;19(7):589–98.
31. Hussain T, Kandeel M, Metwally E, Murtaza G, Kalhoro DH, Yin Y, et al. Unraveling the harmful effect of oxidative stress on male fertility: a mechanistic insight. Front Endocrinol. 2023;14(:1070692):1–13.
32. Kangralkar VA, Patil SD, Bandivadekar RM. Oxidative stress and diabetes: a review. Int J Pharm Appl. 2010;1(1):38–45.
33. Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016;5(e47):1–15. CrossRef
34. Banjarnahor SD, Artanti N. Antioxidant properties of flavonoids. Med J Indones. 2014;23(4):239–44. CrossRef
35. Nile SH, Keum YS, Nile AS, Jalde SS, Patel RV. Antioxidant, anti-inflammatory, and enzyme inhibitory activity of natural plant flavonoids and their synthesized derivatives. J Biochem Mol Toxicol. 2018;32(1):1–8. CrossRef
36. Arisha AH, Ahmed MM, Kamel MA, Attia YA, Hussein MMA. Morin ameliorates the testicular apoptosis, oxidative stress, and impact on blood-testis barrier induced by photo-extracellularly synthesized silver nanoparticles. Environ Sci Pollut Res Int. 2019;26(28):28749–62. CrossRef
37. Shokoohi M, Khaki A, Shoorei H, Khaki AA, Moghimian M, Abtahi-Eivary SH. Hesperidin attenuated apoptotic-related genes in testicle of a male rat model of varicocoele. Andrology. 2020;1:249–58.
38. Samie A, Edaghat R, Baluchnejadmojarad T, Roghani M. Hesperetin, a citrus flavonoid, attenuates testicular damage in diabetic rats via inhibition of oxidative stress, inflammation, and apoptosis. Life Sci. 2018;210:132–9. CrossRef