INTRODUCTION
Exposure to ultraviolet (UV) radiation is known to have harmful effects on the skin, such as photoaging and photocarcinogenesis [1]. To minimize damage from UV exposure, topical preparations can be applied to the skin. Sunscreen is a topical preparation that protects against UV rays and is characterized by its sun protection factor. Many sunscreen formulas incorporate active substances from plants that are effective skin protection agents against sun exposure. The active compounds include flavonoids, saponins, antioxidants, and quercetin [2,3]. Centella asiatica (L.) is a herbal plant containing active substances that has skincare benefits and protects the skin from the sun [4].
Centella asiatica (CA) fraction can be formulated into nanoemulsion due to its poor lipophilic properties and limited solubility. Asiaticoside is a compound found in the CA fraction that exerts a therapeutic effect on UV-induced photoaging. Huang et al. [5] demonstrated that asiaticoside can prevent photoaging of the skin through the inhibition of human dermal fibroblast proliferation induced by UV-A exposure [5]. Asiaticoside has a molecular weight of 959.12 g/mol, a water solubility of 307.347 μg/mL, and a partition coefficient of 2.24 [6]. The low solubility and the high molecular weight inhibit their skin absorption, causing limitations for its use in topical formulations [7,8]. To overcome this, nanoemulsion technology offers a promising approach for the development of CA fraction products. Nanoemulsions, as an encapsulation process in nanoparticle technology, provide a method for developing drug delivery system formulations [7].
Nanoemulsion is the dispersion of two unmixed liquids, oil in water (O/W) or water in oil (W/O), stabilized using a suitable surfactant. The selection of nanoemulsion preparations can increase the solubility of the active substance, provide good stability against phase separation, appear transparent, and reduce particle size, thus, offering a high surface area for better absorption of the active ingredients [9]. The selection of oil, surfactant, and cosurfactant phases is an important factor in the manufacture of stable nanoemulsion formulations [10]. The oil phase in the nanoemulsion formula affects the size of the droplets and the stability of the formed nanoemulsions. As a carrier, the oil phase can dissolve active lipophilic substances. Olive oil was used as the oil phase in this study because it contains saturated, monounsaturated, and polyunsaturated fatty acids, as well as palmitic, stearic, and oleic acids.
Centella asiatica (L.) Urb. is an herbal plant that is widely used in the treatment of skin diseases and skincare. Active compounds in the CA fraction that exert pharmacological effects on the skin include asiaticosides, madecasosides, asiatic acid, and madecassic acid. Asiaticoside is the primary active constituent in CA. In the CA fraction, saponins and their derivatives, namely pentacyclic triterpenoids, were the most abundant, with an asiaticoside content of approximately 8%. Asiaticoside has a therapeutic effect on UV-induced photoaging by lowering the ROS content. Topical treatment using asiaticoside improved skin that had been subjected to hyperpigmentation photoaging due to UV radiation exposure from the sun [11,12].
Empirical approaches to developing formulations have several limitations. These include time restrictions in conducting experiments, potential errors in formulation, and financial constraints. These problems can be resolved using molecular modeling approaches that enable one to accurately predict and describe the atomic interactions between the emulsion excipient and active ingredient during the preparation process. Moreover, these studies could minimize trial and error during the experimental process as presented in previous studies [13,14]. The results of the molecular modeling approach were compared with empirical observations. This study aimed to comprehend in silico modeling using molecular dynamics (MD) simulations and empirically observe the formulation of a nanoemulsion using asiaticoside from the CA fraction and olive oil as a penetration enhancer. The in silico modeling of nanoemulsion formation, supported by a computational approach, can be applied in empirical studies to predict the optimum conditions and composition of the olive oil lipid phase and asiaticoside required to achieve a good nanoemulsion drug delivery system.
MATERIALS AND METHODS
Materials
The materials used in this study were CA fraction, ethanol PA (Emsure®), n-hexane PA (Technical Grade), ethyl acetate PA (Technical Grade), diethylene amine PA (Technical Grade), asiaticoside comparator (Cosmetic Grade), TLC silica gel GF254 plate, olive oil (Technical Grade), Tween 80 (Technical Grade), propylene glycol (Technical Grade), ethanol 70% (Merck Germany), white tip, aluminum foil, filter paper (Whatman no, 1) and aquadest.
Molecular dynamics simulations of nanoemulsion formation
The formation of nanoemulsion was studied using an in silico approach; that is, MD simulations were performed using GROMACS version 2023.2. Prior to the MD simulations, three-dimensional molecular models of the studied compounds, that is, asiaticoside, Tween-80, propylene glycol, and palmitic, linoleic, and oleic acids, were generated using Avogadro and energy-minimized using the MMFF94 force field with 1,000 steps and the steepest descent algorithm. In the MD simulations, palmitic, linoleic, and oleic acids represented the olive oil composition.
The topology files of each compound (itp files) were generated using the online software (http://www. swissparam. ch/) and the optimized potentials for liquid simulations in all-atom version (OPLS-AA) force field explaining the atomic interactions were selected. An asiaticoside model was chosen as the initial coordinate, followed by the addition of other asiaticoside, Tween-80, propylene glycol, and palmitic, linoleic, and oleic acids, with the number of molecules to describe the experimental stoichiometric ratio (Table 1). These molecular models were placed randomly in a cubic box with dimensions of 7.487 × 7.487 × 7.487 Å3 filled with 7,961 water molecules as the solvent. Subsequently, energy minimization was performed with a maximum number of steps of 5,000 to perform the steepest descent minimization followed by the 1-ns NVT and NPT equilibration phases. These two-stage equilibration phases were configured to reach the desired temperature of 37°C and pressure of 1 bar and were controlled using V-rescale and Parrinello-Rahman coupling, respectively. NVT equilibration was carried out to show that the nanoemulsion system achieved stability at 37°C. The NPT equilibration process aimed to stabilize the pressure in the nanoemulsion system. Considering that NPT equilibration may require a longer time for the nanoemulsion system to stabilize its potential energy, this indicated that the nanoemulsion system achieved isothermal isobaric equilibrium.
 | Table 1. Number of molecules nanoemulsion. [Click here to view] |
The 250-ns MD simulation production phases were subjected to short-range van der Waals (vdW) and long-range electrostatic cutoff (1.2 nm) while applying the particle mesh Ewald method. Finally, 250-ns MD simulations were conducted, and simulation snapshots were saved every 100 ps. The MD simulation results were then processed to compute the system energies, that is, vdW and Coulomb short-range interactions, using gmx energy commands. The dynamic changes in nanoemulsion formation were visualized using PyMOL.
Preparation of plant extracts
The CA fraction was extracted via maceration in 70% ethanol at a 1:5 (w/v) ratio in an Erlenmeyer flask. A 50 g sample of CA simplicia powder (mesh size of 60) was weighed and transferred to an Erlenmeyer flask containing 250 ml (1:5) of 70% alcohol (v/v). The powder was macerated for 24 hours at 150 rpm. Following this, the samples were filtered through a buchner funnel. The sample was subjected to three rounds of maceration, each lasting for 24 hours with 250 ml of the total solvent. The extract was evaporated during the entire maceration process, and the extract was stored in a weighed porcelain dish. The thick CA extract was then weighed periodically until a stable weight was obtained [15].
Nanoemulsion formulation of CA fraction
The nanoemulsion were prepared according to the formulas listed in Table 2. Olive oil was used as the oil phase in beaker glasses, along with Tween 80 and the CA fraction. The mixture was stirred using a magnetic stirrer for 10 minutes at 1,000 rpm. After 10 minutes, the aquadest at a temperature of 70°C was gradually added, and the stirring speed was increased to 1,250 rpm. Sonication was performed for 50 minutes to remove bubbles [16].
 | Table 2. Nanoemulsion formula of CA fraction. [Click here to view] |
Particle size
The particle size of the nanoemulsion preparations was measured using dynamic light scattering with a particle size analyzer. Ten milliliters of the sample were dissolved in aquadest at a 1:1 ratio. The particle size were < 100 nm [17].
Transmission electron microscopy
Transmission electron microscopy (TEM; JEOL JEM 1400) was used to visualize the morphology and form of the nanoemulsion. A drop of the sample was applied to a copper grid using an autocarbon-coated tool. The samples were allowed to dry stand at room temperature 25°C for 24 hours. Following the drying period, the sample was positioned in a holder and examined under a 100–200 kV microscope to observe its morphology using a TEM apparatus [18,19].
RESULTS AND DISCUSSION
Molecular dynamics simulations
Before performing the MD simulations to depict nanoemulsion formation, the process began with solvation, followed by energy minimization. The solvation systems before and after energy minimization are shown in Figure 1. As shown in Figure 1a, the systems appear to be more disordered, with an extremely high-energy system, of 252,772 kJ/mol. During the energy minimization step, the system energy was exponentially lowered until it reached -433,601 kJ/mol, (Fig. 1b). Additionally, the molecular conformations were adjusted to yield a lower energy. The process of energy minimization eliminates unfavorable interactions among all the molecules in the system. Simultaneously, it ensured favorable conditions for non-bonding interactions to occur between asiaticoside, Tween-80, representative olive oil molecules, and the surrounding solvent molecules [20]. After the two-stage equilibrium phases, all molecules appeared relatively clustered, starting to form a nanoemulsion system, as shown in Figure 1c. The system employed periodic boundary conditions (PBC) in the two-stage equilibration phase, followed by 250-ns MD simulations, allowing the use of tiny computational units to model the characteristics of large-scale systems. Notably, the application of PBC in MD simulations helps prevent problems related to sudden changes in interatomic forces at the edges of the simulation cell. However, at the edges of the simulation cell, the modeled molecules and their assembled structures appeared disjointed or incomplete, indicating a core problem of PBC [21]. Hence, we employed the supercell feature of PyMOL, which displays multiple copies of the simulation cell, to visualize nanoemulsion formation.
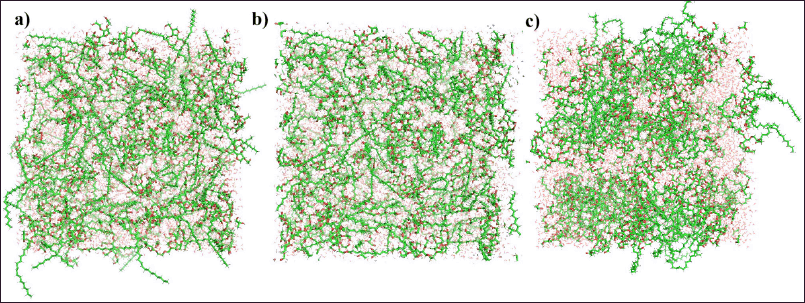 | Figure 1. The solvated simulation system at initial coordinate (a), after energy minimization (b), and after NPT equilibration (c). [Click here to view] |
Figure 2 shows the formation of the nanoemulsion from the first snapshot (0 ns) to the final snapshot (250 ns) of the MD production phase, and the stimulation was visualized by capturing snapshots every 50 ns during the entire simulation. Figure 2a clearly shows that the system is attempting to form a nanoemulsion system, which is consistent with a study conducted by Koshiyama and Wada [22]. Thereafter, the nanoemulsion likely adopted a nearly spherical shape at a simulation time of 50 ns. This observation could be energetically explained by the decrease in van der Waals energies, which might be attributed to nanoemulsion formation. Notably, after 50 ns, the nanoemulsion remained relatively stable throughout the simulation. These results were confirmed by the energy calculation results, which showed no significant energy changes in the van der Waals and Coulomb energies (Figs. 3 and 4). Thus, the 1-ns two-stage equilibration period may be crucial in initiating the process of nanoemulsion formation. Furthermore, these results correlated well with those of a previous study, indicating the importance of the equilibration phase for the generation of consistent structural ensembles of charged glycolipids [23]. Hence, additional studies validating the initial atomic coordinates before and after the time-dependent equilibration phase, prior to the production phase, are required.
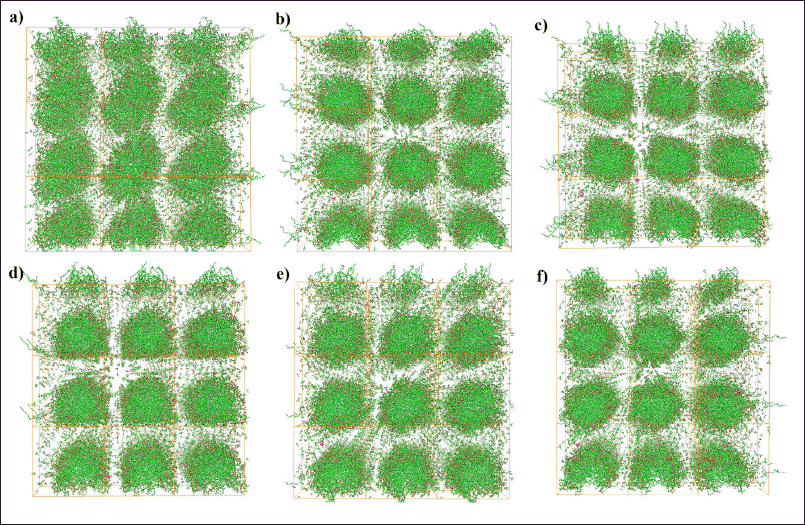 | Figure 2. The snapshots of nanoemulsion formations figured as copies of the simulation cell at 0 (a), 50 (b), 100 (c), 150 (d), 200 (e), and 250 (f) ns. [Click here to view] |
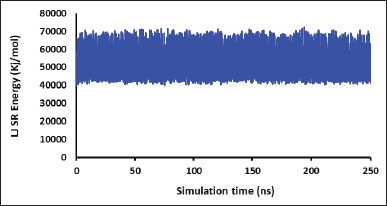 | Figure 3. The changes of short-range van der Waals energy throughout the simulations taken every 100 ps. [Click here to view] |
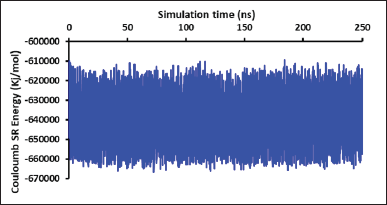 | Figure 4. The changes of short-range coulomb energy throughout the simulations taken every 100 ps. [Click here to view] |
As we examined the snapshots during the simulations, Tween-80 and the representative olive oil components interacted in the outer and inner nanoemulsion systems, forming nonhydrophobic contacts in the outer system, whereas hydrophobic contacts were observed in the inner nanoemulsion system. Asiaticoside molecules formed hydrogen bonds with Tween-80 and propylene glycol molecules in the outer nanoemulsion system (Fig. 5). Our research showed that asiaticoside molecules interacted with the nanoemulsion surface, specifically creating non-hydrophobic contacts with the outer part of the nanoemulsion system. Additionally, these results could potentially explain the experimentally observed formation of nanoemulsion in this study, consistent with the computational modeling approaches described in a previous study [13]. Hence, MD simulations may serve as a valuable tool for further investigation, for predicting and elucidating the formation and behavior of nanoemulsions.
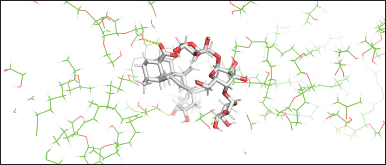 | Figure 5. Asiaticoside molecule (red–white-colored model) interacted on the nanoemulsion surface displaying its non-hydrophobic contacts (yellow dashed line) with Tween-80 and propylene glycol molecules [Click here to view] |
Preparations of plant extract
Centella asiatica (L.) is an Indonesian herbal plant with effective antiphotoaging, anti-inflammatory, and wound healing agents [24,25]. The maceration extraction method for CA aims to separate the active compounds of the plant while preserving those that are heat-sensitive [26]. Maceration of CA extracts several compounds such as triterpenoids, flavonoids, saponins, alkaloids, phenolics, tannins, and carotenoids depending on the type of solvent and extraction time used [27]. In this study, the yield of the CA fraction reached 30.6%, exceeding the minimum requirement of 7.3%. Asiaticoside is known to have activity as an antiphotoaging agent, anti-inflammatory, and wound healing activities [28]. However, the use of asiaticoside in topical formulations is often hampered by their low solubility in water, which results in low permeability and bioavailability through biological membranes [6]. Nanoemulsion was selected to increase the solubility of asiaticoside and its absorption during its topical use as a sunscreen. The small particle size of nanoemulsions minimizes the coagulation of emulsion droplets, suppresses precipitation, and assists in the delivery of active substances [29].
Particle size
Particle size testing aims to determine the average particle size and ensure the quality of preparation [30,31]. Particle size testing was performed using dynamic light scattering method [17]. The smaller the particle size, the greater the surface area of the substance, which accelerates its solubility. The increased surface area allows for greater interaction between the solute and solvent. A particle size ≤100 nm makes nanoemulsions more soluble or easily absorbed by the skin. The particle sizes of the CA fraction nanoemulsion were 15.2 consecutively, 14.8, 13.2, and 14.4, all which meets the criterion of < 100 nm [32,33].
Because the particle size test of the nanoemulsion preparation of the CA fraction was very small, the sunscreen was classified as a chemical sunscreen. The nanoemulsion particle size distribution curves in triplicate measurements showed values of 15.40 (a), 15.00 (b), and 15.20 nm (c), having a polydispersity index of 0.301, 0.368, and 0.380, respectively. The small particle size of the preparation could enable the nanoemulsion to be easily absorbed into the inner layer of the skin. Chemical sunscreen works by absorbing UV radiation and converting it into energy radiation that is harmless to humans [34]. The particle size of the nanoemulsion and the corresponding transmittance measurements are presented in Table 3.
 | Table 3. Particle size and transmittance test results. [Click here to view] |
Particle morphology
TEM was used to observe the morphology of the nanoemulsion particles (Fig. 6). Bright-field enhancement in conjunction with a diffraction model facilitated the production of a higher-resolution nanoemulsion structure. Morphological measurements utilizing TEM were used to ascertain the size and morphology of the droplet surface [35]. After dissolving the nanoemulsion and placing it in a holder, TEM was used to observe the nanoemulsion particle morphology. TEM analysis was performed on the nanoemulsion samples to view the sample grain shape under a 100–200 kV microscope [18]. TEM observation revealed that the shape or morphology of the nanoemulsion particles carrying the active ingredient asiaticoside formed a monodisperse system.
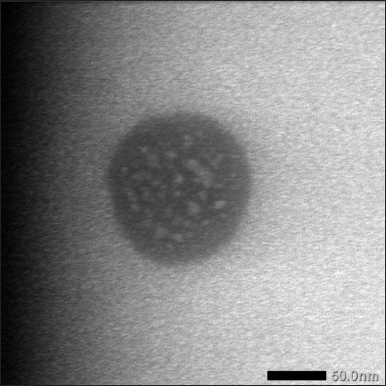 | Figure 6. TEM morphology of nanoemulsion. [Click here to view] |
CONCLUSION
The empirical experimental results showed that the particle size of the nanoemulsion reached the nanometer scale. Nanoemulsion particles containing the active substance asiaticoside were spherical in shape, with a smooth surface, showing no signs of aggregation or separation. This indicated that the inner core, composed of an oil phase, particularly olive oil, was surrounded by a thin outer layer of surfactants, Tween-80, and PG, encapsulating the nanoemulsion droplets. These findings were consistent with the in silico modeling studies, demonstrating that the nanoemulsion developed during the initial production run of the 250-ns MD simulations remained stable throughout the simulations. These findings can contribute substantially to the better understanding of computational and experimental studies complementing each other.
ACKNOWLEDGMENTS
This research was funded by the Directorate of Research, Technology, and Community Services, the Directorate General of Higher Education, Research, and Technology, the Indonesian Ministry of Education, Culture, Research, and Technology (No. 107/E5/PG.02.00.PL/2024) awarded to Dr. apt. Rini Dwiastuti.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Puglia C, Damiani E, Offerta A, Rizza L, Tirendi GG, Tarico MS, et al. Evaluation of nanostructured lipid carriers (NLC) and nanoemulsions as carriers for UV-filters: characterization, in vitro penetration and photostability studies. Eur J Pharm Sci [Internet]. 2014;51(1):211–7. Available from: http://dx.doi.org/10.1016/j.ejps.2013.09.023
2. Salsabila S, Rahmiyani I, Sri Zustika DS. Nilai Sun Protection Factor (SPF) pada Sediaan Lotion Ekstrak Etanol Daun Jambu Air (Syzygium aqueum). Majalah Farmasetika. 2021;6(Suppl 1):123–32.
3. Zainuddin S. Saifullah TN. Pamudji G. Pegagan dan minyak zaitun. Formulasi Krim Kombinasi Herbapegagan (Centella asiatica L.) dan Minyak Zaitun sebagai Tabir Surya secara In Vitro. 2019;2(1):27–38.
4. Fernenda L, Ramadhani AP, Syukri Y. Aktivitas pegagan (Centella asiatica) pada dermatologi. J Sains Farm Klin. 2023;9(3):237.
5. Huang J, Gong Y, Liu K, Chen J, Zhou X. Anti-photoaging properties of asiaticoside in ultraviolet A-irradiated human dermal fibroblasts by activating the PI3K-AKT pathway and inhibiting the NF-κB pathway. Explor Res Hypothesis Med. 2023;8(4):319–37.
6. Zhang CZ, Niu J, Chong YS, Huang YF, Chu Y, Xie SY, et al. Porous microspheres as promising vehicles for the topical delivery of poorly soluble asiaticoside accelerate wound healing and inhibit scar formation in vitro & in vivo. Eur J Pharm Biopharm [Internet]. 2016;109:1–13. Available from: http://dx.doi.org/10.1016/j.ejpb.2016.09.005
7. Rigano L, Lionetti N. Nanobiomaterials in galenic formulations and cosmetics. Amsterdam, The Netherlands: Elsevier Inc.; 2016. 121–48 pp.
8. Redhita LA, Beandrade MU, Putri IK, Anindita R. Formulasi Dan Evaluasi Nanoemulsi Ekstrak Daun Kemangi (Ocimum basilicum L.) Dengan Variasi Konsentrasi Tween 80. J Mitra Kesehat. 2022;4(2):80–91.
9. Çinar K. A review on nanoemulsions: preparation methods and stability. Trak Univ J Eng Sci [Internet]. 2017;18(1):73–83. Available from: http://dergipark.gov.tr/tujes
10. Nurfauziah R, Rusdiana T. Review: Formulasi Nanoemulsi Untuk Meningkatkan Kelarutan Obat Lipofilik. Farmaka Suplemen. 2018;16(1):352–60.
11. Muthaiyah B, Ramadoss SK, Gouri YS, Bandyopadhyay B. Proteomic alterations in human dermal fibroblasts under photo-induced pollution caused by excessive solar irradiations such as Infra-Red, Blue Light, UVA and UVB. J Cosmet Dermatological Sci Appl. 2023;13(01):16–32.
12. Jiang H, Zhou X, Chen L. Asiaticoside delays senescence and attenuate generation of ROS in UV?exposure cells through regulates TGF?β1/Smad pathway. Exp Ther Med. 2022;24(5):1–13.
13. Dwiastuti R, Radifar M, Putri DCA, Riswanto FDO, Hariono M. In silico modeling and empirical study of 4-n-Butylresorcinol nanoliposome formulation. J Biomol Struct Dyn [Internet]. 2022;40(21):10603–13. Available from: https://doi.org/10.1080/07391102.2021.1946430
14. Dwiastuti R, Radifar M, Marchaban, Noegrohati S, Istyastono EP. Molecular dynamics simulations and empirical observations on soy lecithin liposome preparation. Indones J Chem. 2016;16(2):222–8.
15. Setiawati A, Maharani BA, Sari PAP, Wyantara KA, Saputra BW, Febriansyah R, et al. Deciphering the molecular pathway of an asiaticoside-rich fraction of Centella asiatica as an anti-melanogenesis agent. J HerbMed Pharmacol. 2024;13(2):269–79.
16. Yuliani SH, Hartini M, Pudyastuti B, Istyastono EP. Comparison of physical stability properties of pomegranate seed oil nanoemulsion dosage forms with long-chain triglyceride and medium-chain triglyceride as the oil phase. Tradit Med J. 2016;21(2):93–8.
17. Chrismaurin F, Dwiastuti R, Chabib L, Yuliani H. The effect of olive oil, tween 60 and span 20 on physical characteristics of Quercetin nanoemulgel. Int J Appl Pharm. 2023;15(1):212–7.
18. Hanifah M, Jufri M. Formulation and stability testing of nanoemulsion lotion containing Centella asiatica extract. J Young Pharm. 2018;10(4):404–8.
19. Kristiani M, Ramayani SL, Yunita K, Saputri M. Formulasi dan Uji Aktivitas Nanoemulsi Minyak Atsiri Daun Kemangi ( Ocimum basilicum L .) Terhadap Salmonella typhii nanoemulsion formulation and activity test of essential oil basil leaves ( Ocimum basilicum L .) against Salmonella typhii thypoid fever. J Farm Indones. 2019;16(1):14–23.
20. Parchekani J, Allahverdi A, Taghdir M, Naderi-Manesh H. Design and simulation of the liposomal model by using a coarse-grained molecular dynamics approach towards drug delivery goals. Sci Rep [Internet]. 2022;12(1):1–15. Available from: https://doi.org/10.1038/s41598-022-06380-8
21. Bruininks BMH, Wassenaar TA, Vattulainen I. Unbreaking assemblies in molecular simulations with periodic boundaries. J Chem Inf Model. 2023;63(11):3448–52.
22. Koshiyama K, Wada S. Collapse of a lipid-coated nanobubble and subsequent liposome formation. Sci Rep. 2016;6:1–8.
23. Messias A, Santos DES, Pontes FJS, Lima FS, Soares TA. Out of sight, out of mind: the effect of the equilibration protocol on the structural ensembles of charged glycolipid bilayers. Molecules. 2020;25(21):1–16.
24. Prakash V, Jaiswal N, Srivastava M. A review on medicinal properties of Centella asiatica. Asian J Pharm Clin Res. 2017;10(10):69–74.
25. Arribas-López E, Zand N, Ojo O, Snowden MJ, Kochhar T. A systematic review of the effect of Centella asiatica on wound healing. Int J Environ Res Public Health. 2022;19(6):3266.
26. Zulkarnaen, Alifia PF, Oktavia EP, Penetapan Kadar Asiatikosida Ekstrak Etanol 70% Pegagan (Centella asiatica) menggunakan Metode LC-MS. Majalah Kesehatan. 2016;2(2):99–107.
27. Chowdhury S, Yusof F, Faruck MO, Sulaiman N. Process optimization of silver nanoparticle synthesis using response surface methodology. Procedia Eng. 2016;148:992–9.
28. Bandopadhyay S, Mandal S, Ghorai M, Jha NK, Kumar M, Ghosh, RA, et al. Therapeutic properties and pharmacological activities of asiaticoside and madecassoside: a review. J Cell Mol Med. 2023;27(5):593–608.
29. Arianto A, Cindy C. Preparation and evaluation of sunflower oil nanoemulsion as a sunscreen. Open Access Maced J Med Sci. 2019;7(22):3757–61.
30. Dhamoon RK, Popli H, Aggarwal G, Gupta M. Particle size characterization-techniques, factors and quality-by-design approach. Int J Drug Deliv. 2018;10(1):1–11.
31. Husnawiyah N, Wibisono N, Widyaningrum I. Pengaruh Jenis Lipid Padat Terhadap Sifat Fisika Dan Kimia Pada Sistem Penghantar Obat Nanostructired Lipid Carriers (Nlc). HYPERLINK “https://jim.unisma.ac.id/index.php/jbm/index” J Bio Komplementer Med. 2023;10(2):1–7.
32. Kurniasari D, Atun S. Pembuatan Dan Karakterisasi Nanopartikel Ekstrak Etanol Temu Kunci (Boesenbergia pandurata) Pada Berbagai Variasi Komposisi Kitosan. J Sains Dasar. 2017;6(1):31–5.
33. Maha HL, Sinaga KR, Masfria. Formulation and evaluation of miconazole nitrate nanoemulsion and cream. Asian J Pharm Clin Res. 2018;11(3):319–21.
34. Santos Caetano JP, Abarca AP, Guerato M, Guerra L, Schalka S, Perez Simão DC, et al. SPF and UVA-PF sunscreen evaluation: are there good correlations among results obtained in vivo, in vitro and in a theoretical sunscreen simulator? a real-life exercise. Int J Cosmet Sci. 2016;38(6):576–80.
35. Faria-Silva AC, Costa AM, Ascenso A, Ribeiro HM, Marto J, Gonçalves LM, et al. Nanoemulsions for cosmetic products. Nanocosmetics Fundam Appl Toxic. 2020;(1):59–77.