INTRODUCTION
The 2022 Global Burden of Cancer Study indicates that breast cancer is the most often diagnosed cancer worldwide, with 2,296,840 new cases (11.7% of all cancer cases), and is the second most significant cause of cancer-related mortality, resulting in 684,996 deaths [1]. Breast cancer is the most common cancer diagnosis in Indonesia [2]. The cancer prevalence rate is roughly 1.4 per 100 individuals, amounting to 347,000 cases, with cervical and breast cancer demonstrating the most significant incidence rates [3,4]. Research conducted by the American Society of Clinical Oncology analyzed data and samples from 2,062 patients, revealing that the significant subtype of breast cancer was hormone-dependent estrogen receptor (ER+/progesterone receptor+/human epidermal growth factor receptor2−) [5,6].
Prior studies have shown that increased molecular expression of Michigan Cancer Foundation (MCF)-7 is essential in breast cancer, primarily through mechanisms associated with ERs [7,8]. MCF-7 cells exhibit active ERs that, when over-expressed, can enhance cellular responses to estrogen as a growth factor. It promotes cellular proliferation and cancer by activating signaling pathways such as PI3K/Akt and mitogen-activated protein kinase/extracellular signal-regulated kinase, which regulate the cell cycle and their transition from the G1 phase to the S phase [9,10]. Furthermore, increased expression of transcription factors such as c-Myc and Cyclin D1 proximities aberrant cell division [11].
Current research indicates that of the 250,000 plant species in the Plantae kingdom, only 10% have been studied for their anti-cancer potential [12,13]. Many plants are yet unexamined and insufficiently researched for their potential as anti-cancer agents despite their ethnos-pharmacological users as anticancer medications, menstruation stimulants, and birthing facilitators. This is a substantial possibility for identifying anti-cancer chemicals from herbal sources [14]. Amaranthaceae is a medicinal plant with several pharmacological properties, including pro-apoptotic, antioxidant, and anti-inflammatory [15]. Breast cancer is a multifactorial disease with complex molecular mechanisms involving ER [16]. Aerva Sanguinolenta (AS), a member of their Amaranthaceae family, widely known as Sambang Colok (SC), comprises polyphenolic compounds, flavonoids, and tannins acknowledged for their natural antioxidant properties [17]. Researchers can assess these anti-proliferative effects and mechanisms of action or plant-extracted substances in breast cancer cells using vitriol methods [18].
The AS plant is empirically utilized as a cancer preventative, to aid menstruation, and as a therapy for fever. AS exhibits pharmacological activity such as anti-inflammatory, anti-cancer, analgesic, anti-diabetic, and antipyretic effects [17–20]. The high antioxidant activity of AS can aid in preserving homeostasis against free radicals and oxidative stress, which can harm DNA, proteins, and cell lipids, critical elements in cancer progression [21]. Numerous studies suggest that antioxidant chemicals can impede the development of cancer cells and trigger apoptosis [22]. Antioxidant compounds such as flavonoids and polyphenols can regulate apoptosis signaling pathways, including the intrinsic pathway involving mitochondria and the extrinsic pathway involving death receptors [23]. Compounds found in red wine can increase the expression of pro-apoptotic genes, such as Bax, and reduce the expression of anti-apoptotic genes, such as Bcl-2, which contribute to the death of cancer cells [24]. Vitamin C can induce excessive oxidative stress in cancer cells, triggering the apoptosis pathway through caspase activation and producing reactive oxygen species [25].
This study will determine SC efficacy (AS) as an anticancer drug via cytotoxicity assays on MCF-7 breast cancer cell lines. AS was extracted utilizing many solvents, including methanol, ethanol, ethyl acetate, hexane, and butanol. We also fractionated their methanol extract using ethyl acetate, n-hexane, and butanol. All extracts and fractions were subjected to assays. The most active extract/fraction was further tested via Liquid Chromatography High-Resolution Mass Spectrophotometry analysis (LC-HRMS) to determine their bioactive components. We also examine the antioxidant activity, total phenolic content (TPC), and total flavonoid content (TFC). MCF-7 is a cell line originating from human breast carcinoma, exhibiting threats representative of prevalent breast cancer subtypes, including sensitivity to estrogen hormones [26]. This enables researchers to assess the impact of anticancer agents in hormone receptor-positive cells, which is crucial for advancing hormonal therapy. Furthermore, MCF-7 offers replication and laboratory upkeep benefits, facilitating repeated study and results validation [27]. Previous research has shown that MCF-7 is highly responsive to various anticancer compounds from natural and synthetic sources, making it an effective tool for early screening in discovering new drugs [28]. Through this cell model, researchers can gain insights into the mechanisms of actions of compounds and the potential side effects that may occur, which is critical for developing safer and more effective therapies [6].
MATERIAL AND METHODS
Sample preparation, extraction, and fractionation of AS
AS, known as SC (AS), was harvested from Tawangmangu, Central Java, Indonesia. As was recognized at their School of Life Science and Technology, ITB Indonesia, under certificate 7881/IT1.C11.2/TA.00/2023. One kilogram of AS plants is cleaned of dirt and dust, dried in an oven (Memmert, Germany) at 40°C–50°C for 24 hours, then ground using a blender (Moulinex, France) until it becomes a fine powder. Next, the extraction is carried out using the maceration method. As much as 500 g of plant powder was placed into the maceration device, and 1 l of technical ethanol (Merck, Germany) was added. The mixture from the maceration is stirred using a magnetic stirrer. After soaking, the mixture is filtered using filter paper to separate the residue from the resulting extract. The filtered extract is then placed into a rotary vacuum evaporator (Büchi, Switzerland) to evaporate the solvent at 40°C and a pressure of 300 mBar, resulting in a thick extract. After the extraction process, the next step is fractionating the obtained methanol extract. As much as 500 g of methanol extract was placed into the fractionation separator (Perekan, Germany). To separate the compounds based on polarity, 200 ml of n-hexane (Merck, Germany) was added to the methanol extract. The mixture was stirred for 10 minutes and allowed to sit for 30 minutes to ensure the fraction separation occurred. After the separation phase, the n-hexane fraction in the upper layer is taken and stored in a storage bottle. The process is done by adding 200 ml of ethyl acetate (Merck, Germany) to the remaining methanol extract, stirring for 10 minutes, and letting it sit for 30 minutes before collecting the ethyl acetate fraction. Next, 200 ml of butanol (Merck, Germany) will be added, and the same process will be performed to obtain the butanol fraction. Finally, the remaining extract is processed by adding 200 ml of water, stirring, and separating it into the aqueous fraction. All the resulting fractions were then evaporated using a rotary vacuum evaporator at the same temperature and pressure to remove the solvent, yielding n-hexane, ethyl acetate, butanol, and water fractions. These fractions are stored in amber glass bottles to maintain stability and quality.
Antioxidant activity: 2,2-dipheinyl-1-picrylhydrazyl free radical scavenging assay
The antioxidant activity was assessed by evaluating the free radical scavenging vcapacity of their AS extract and fraction against their stable 2,2-dipheinyl-1-picrylhydrazyl (DPPH) free radical, following the method established by [16,29] Sarkar et al. [29] and Gulcin and Alwasel [30] with minor changes. 500 mg of their samples and 5 ml of their methanol reaction mixture were combined, vortexed, and sonicated to create a stock solution. Sixteen microliters of each sample were mixed with 1,584 μl of methanol, followed by the addition of 400 μl of DPPH, and their mixture was vortexed. The test samples were generated in triplicate at five concentrations ranging from 62.5 to 1,000 ppm, with their maximum concentration being 1,000 ppm (80 μl + 79,20 μl of methanol). The solution was incubated at an ambient temperature for 30 minutes before measuring their absorbance at a wavelength of 517 nm with a microplate reader (FLUOstar omega). The quantities of several extracts were quantified, and their IC50 value (their concentration necessary to block 50% of DPPH radicals) was established. All assays were conducted in triplicate. Aquadeis served as controls. Meithanoil, combined with DPPH, served as their control. L-(+)-ascorbic acid (Vitamin C) with a final concentration of 0–5 μg/ml was used as the standard antioxidant, measured absorbance value was transformed into the percentage of radical scavenging activity utilizing their subsequent equation [31,32]
The percentage of radical trapping activity can be determined using their subsequent equation: AB denotes their sample absorption, while AB signifies their absorption of the Blank.
Total flavonoid content
The AlCl3 approach, predicated on the complexation of AlCl3 with flavonoids exhibiting a peak absorbance at 415 nm, was employed to quantify the TFC [33,34]. In summary, 60 μl of ethanol, 5 μl of 10% AlCl3, and 20 μl of each extract (0.5 mg/ml) were mixed separately. After adding 110 μl of distilled water and 5 μl of 1 M potassium acetate each week, their reaction mixture was incubated for 25 minutes. To guarantee experimental repeatability, each measurement was conducted in triplicate. A quercetin calibration curve was employed to determine their TFC; the absorbance was measured at 510 nm. The TFC results are expressed as milligrams of quercetin equivalent (QE) per gram of dry extract weight (mg QE/g) via generating a standard curve with a series of concentrations of 0–100 μg/ml of rutin (x-axis) against absorbance (y-axis).
Total phenolic content
The Folin-Coicalteu method, with slight modifications, was employed to quantify their TPC as described by Cacique et al. [35]. In a 96-well microtiter plate, 20 μl of their sample (0.5 mg/ml) of plant extracts, our standard, and 100 μl of Folin-Coicalteu reagent (1:10; v/v) were added, and their initial absorbance was recorded. The combination was subsequently combined with 80 μl of 1 M Na2CO3 solution to achieve a final volume of 200 μl, followed by a 25-minute incubation period. The Synergy LX Multi-Moidei Reader with Gein5 3.08.01 software recorded absorbance at 765 nm. A gallic acid calibration curve was employed to determine their TPC; a standard solution of gallic acid at 1,000 ppm was prepared by dissolving 10 mg of gallic acid in 10 ml of methanol, and a series of standard solutions with concentrations of 300, 250, 200, 150, and 100 ppm were made, results expressed as milligrams of gallic acid equivalent (GAE) per gram of dry extract weight (mg GAE/g). Each measurement was conducted in triplicate for validation purposes.
Cell culture
The MCF-7 cell line, derived from human breast cancer, was procured from the Laboratory for Agro and Industrial Biomedical Technology Development at the National Research and Innovation Agency (BRIN) in Serpong, Tangerang Selatan, Indonesia. It is utilized in the research. MCF-7 cells cultured in Roswell Park Memorial Institute (RPMI) 1640 (Gibco Life Technologies) media contain 10% fetal bovine serum (FBS), red phenol, 100 U/ml penicillin, 0.1 mg/ml streptomycin, 2 mM glutamine, and 1 mM sodium pyruvate. The cells are maintained in a 75 cm² container at 37°C, with 5% CO2 and 95% humidity.
Cytotoxic assay in vitro
The cytotoxicity experiment of MCF-7 breast cancer cells was conducted using 3-(4,-5-dimethylthiazo-2-yl)-2,5-diphenyltetrazolium bromide) (MTT) techniques. MCF-7 breast cancer cells were cultured in 96-well plates for 24 hours. A 96-well plate has 1 × 104 cells pair well, utilizing RPMI media with phenol Red, supplemented with 10% FBS, 0.1 mg/ml streptomycin, 100 U/ml penicillin, and one mM sodium pyruvate. The cells were incubated for 24 hours at 37°C, 5% CO2, and in a humidified atmosphere at 95%. Subsequently, their media was altered with samples in their growth medium at six varying concentrations and incubated for 24 hours under the same conditions. The salt wash is performed utilizing a phosphate saline buffer. The incorporation of MTT solution (3-(4.5-Dimethylthiazol-2-yl)-2.5-Diphenyltetrazolium) into their medium was succeeded by a 4-hour incubation at 37°C, 5% CO2, and 95% humidity to ascertain viable cells. The color intensity is quantified using an ELISA reader at 570 nm, using an EnSpire™ 2,300 multilabel plate reader manufactured by Perkin Elmer in the United States. The formula used to calculate the percentage of inhibition was (Atreatment/Acontrol cell) × 100, where Atreatment represents the absorbance of the treatment and Acontrol cell represents the absorbance of the control cell. Data are evaluated by probit analysis to determine IC50 [36,37].
Liquid chromatography high-resolution mass spectrophotometry (HRMS) analysis
This analysis utilized liquid chromatography (Thermo ScientificTM VanquishTM UHPLC Binary Pump) in conjunction with Orbitrap HRMS (Thermo ScieintificTM Q ExactiveiTM Hybrid Quadrupoilei-OrbitrapTM HRMS). The liquid chromatography employed a Thermo ScieintificTM AccucoreTM Phenyl-Hexyl analytical column measuring 100 × 2.1 mm ID × 2.6 μm. The methodology employed was derived from [38] utilizing the mobile phase of MS-grades water with 0.1% formic acid (A) and mass spectrometry (MS)-grades methanol with 0.1% formic acid (B), implemented via a gradient approach at a flow rate of 0.3 ml/min. The gradient commenced at 5% mobile phase B, progressively escalated to 90% over 16 minutes, maintained at 90% for 4 minutes, and thereafter reverted to their initial condition of 5% B by their 25-minute mark. The column temperature was sustained at 40°C, with an injection volume of 3 μl. The non-target screening was performed in a complete MS/dd-MS2 acquisition model with either positive or negative polarity or ionization state. The arbitrary units for nitrogen were 32, 8, and 4, and it served as a sheath, auxiliary, and purge gas. The auxiliary gas heater temperature was set to 30°C, the capillary temperature to 320°C, and the spray voltage to 3.30 kV. The resolution was 70,000 for full MS and 17,500 for dd-MS2 in both positive and negative ionization models, with a scanning range of 66.7 to 1000 m/z. The device was controlled using XCalibur 4.4 software from Thermo Scientific, Bremen, Germany. To ensure optimal instrument performance and robustness in mass accuracy investigations, their device was calibrated weekly in positive and negative ESI models utilizing Thermo Scientific Perce ESI join calibration solution (Waltham, MA).
Data analysis
All experiments were conducted in triplicate. Data are presented as mean values with a ± SD. Using the GraphPad prism and one-way analysis of variance (ANOVA), We identified significant results using a p-value of less than 0.05 using IBMSPSS Statistics 22 (ANOVA) software. All values are represented by (*).
RESULTS AND DISCUSSIONS
Antioxidant activity, TFC, and TPC against AS
The antioxidant efficacy of their extract and fraction was assessed utilizing the DPPH assay. Figure 1 illustrates the effectiveness of AS extract, produced with different solvents, in neutralizing DPPH free radicals compared to gallic acid across multiple concentrations ranging from 31.25 to 500 μg/ml. The DPPH scavenging activity of these different extracts is contingent upon concentration, augmenting with higher extract concentrations. The AS N-Hexane extract and fraction from each tested plant had the best antioxidant activity among the extracts analyzed.
| Figure 1. Concentration-dependent DPPH inhibition activity of AS A. Extract of AS, B. Fraction of AS. [Click here to view] |
Table 1 shows that the N-Hexane extract and fraction exhibit the best test parameter value compared to their antioxidant extracts and fractions, with the extract value (Antioxidant: 35.53 μg/ml, TFC: 14.96 mg QE/g, TPC: 25.61 mg GAE/g) and fraction values. (Antioxidant: 42.4 μg/ml, TFC: 15.51 mg QE/g, TPC: 25.31 mg GAE/g).
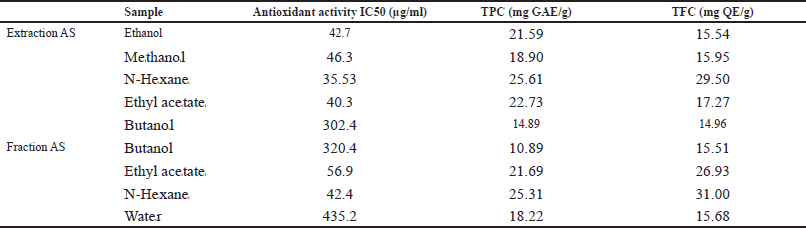 | Table 1. The levels of antioxidant activity, TFC, and TPC against AS extract and fractions. [Click here to view] |
Furthermore, we established a Pearson correlation among TPC, TFC, and the antioxidant activity of their test plants to ascertain the relationship between these biocompositions and their plants’ antioxidant activity (Table 2). A very positive association was identified between TFC and the antioxidant activity of the AS plant. The positive correlation suggests that the elevated antioxidant activity of the AS plant extract is attributable to a greater concentration of TPC. The AS fraction exhibits a favorable association and a robust link between antioxidant activity and TFC. Our findings corroborate earlier research indicating a strong correlation between TFC activity and antioxidant activity, as TFC is a prominent antioxidant agent in numerous medicinal plants.
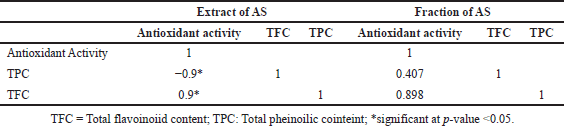 | Table 2. Pearson correlation between antioxidant activity, with fenolic content and flavonoid content. [Click here to view] |
The research results show that the antioxidant test, TPC, and TFC in the n-hexane and ethyl acetate extracts of AS have good levels, with a very high Pearson correlation value between TFC and antioxidant activity, namely 0.900 for the extract and 0.898 for the fraction. This correlation indicates that flavonoid compounds significantly contribute to the antioxidant potential of AS, which is in line with various recent studies affirming the role of flavonoids as cellular protective agents with the ability to capture free radicals and reduce oxidative stress [39]. According to the latest journal, flavonoids not only function as antioxidants but also possess anti-inflammatory activity and can contribute to the prevention of degenerative diseases, such as cancer and heart disease. TPC, which includes other phenolic compounds, also enhances antioxidant activity, indicating synergy between flavonoids and phenolic compounds in the extract [39]. With its high levels of TFC and TPC, AS has the potential to be an effective natural source in traditional and modern medicine, supporting disease prevention strategies through increased intake of bioactive compounds [40]. Table 3 shows that AS extracts have potential as anti-cancer based on the IC50 values produced by N-Hexane and Ethyl acetate extracts have potential, with strong values shown by ethyl acetate extracts and followed by medium N hexane, and for methanol and ethanol extracts have no potential at all.
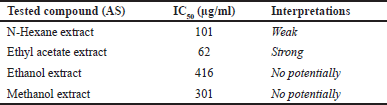 | Table 3. IC50 value of AS extract with several different solvents. [Click here to view] |
Cytotoxic activity based on MCF-7 cell viability
Cytotoxic assay results indicate a decline in their viability curves if MCF-7 cells beginning at five concentrations (31.25, 62.5, 125, 250, 500, and 1,000 μg/ml), which visibly diminished their growth curve. This interpretation of viability, based on three repetitions, is illustrated in Figure 3. N-hexane and ethyl acetate extracts demonstrated dases that inhibited 50% of MCF7 cell growth, ranging from 62.5 to 1,000 μg/ml. The IC50 values for each extract are calculated to ascertain the specific amounts that can inhibit the proliferation of MCF-7 cells, as illustrated in Table 4.
 | Table 4. IC50 value of AS fraction with several different fractions. [Click here to view] |
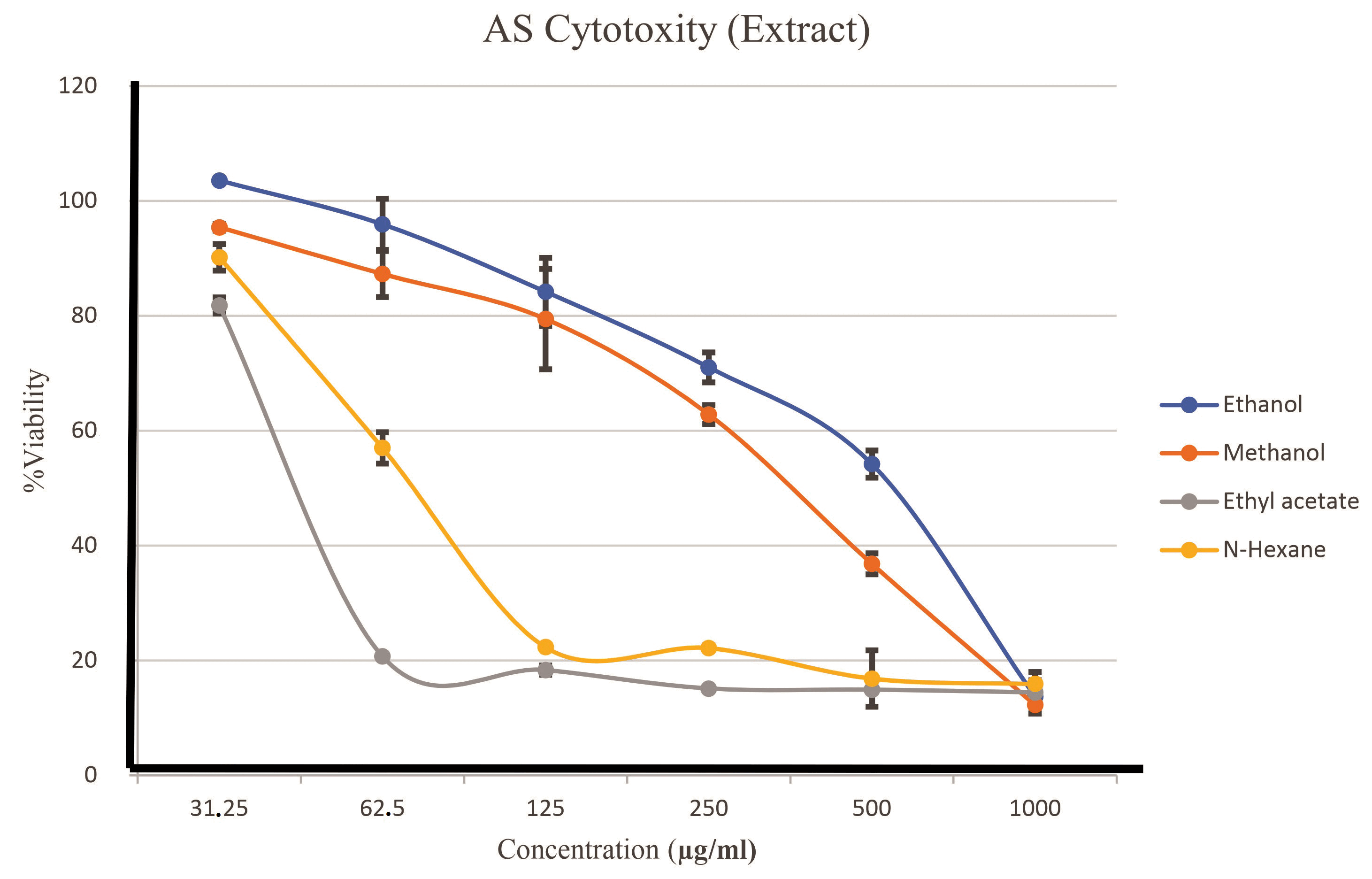 | Figure 2. Comparison of growth curves of all extracts of AS. [Click here to view] |
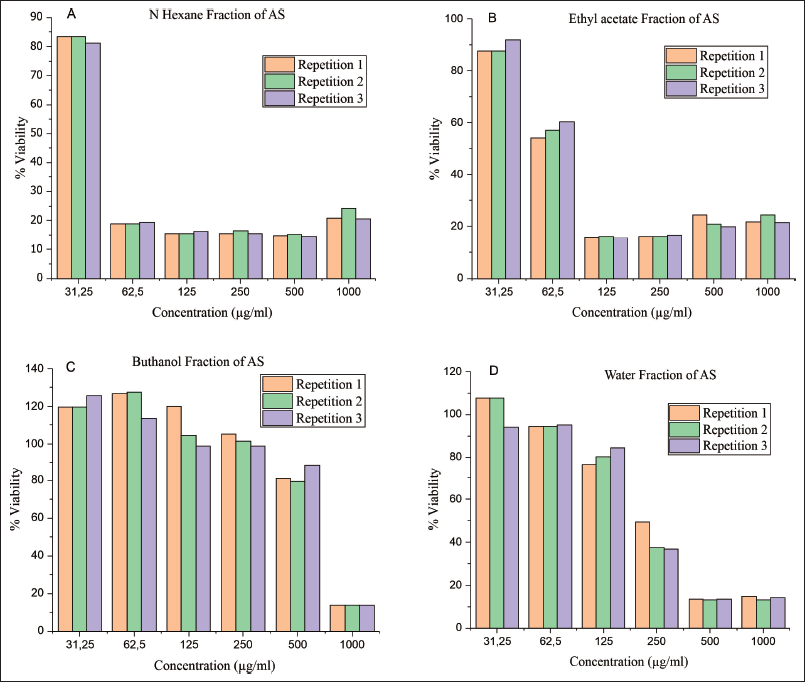 | Figure 3. The MCF-7 cell growth curve is compared to several AS fractions: (A) N-Hexane fraction of AS, (B) Ethyl acetate fraction of AS, (C) Butanol fraction of AS, and (D) Water fraction of AS. [Click here to view] |
The growth curve in Figure 2 shows a variation in the growth curve between the solvents used. However, the AS growth chart with their ethyl acetate solvent decreased growth at a concentration close to their growth point of concentration of 62 μg/ml. Table 1 provides evidence of a 50% growth barrier concentration for their MCF-7 cell population. The IC50 calculation yielded a score of 62 μg/ml, indicating a patient inhibition of the growth of MCF-7 breast cancer cells.
The AS continued fractional testing with three repetitions. Inter-fraction variation results indicated a reduction in cell proliferation of their MCF-7 line corresponding to an increase in the concentration of the sample utilized (31.25, 62.5, 125, 250, 500, 1,000 μg/ml) (Figure 3). The N-hexane and ethyl acetate fractions exhibited potential anticancer IC50 values of 49 μg/ml (strong) and 89 μg/ml (strong), respectively (Table 2); the comparison of growth curves among AS fractions indicates a decline in growth at varying concentrations. Figure 4 shows the growth curve with variations between the fractions obtained. However, the growth graph of AS with N Hexane fraction decreased growth at a concentration close to the growth point concentration of 49 μg/ml. Table 4 provides evidence of a growth barrier concentration of 50% for the MCF-7 cell population. The IC50 calculation resulted in a score of 49 μg/ml, which indicates the inhibition of breast cancer cell growth against the MCF-7 cell line.
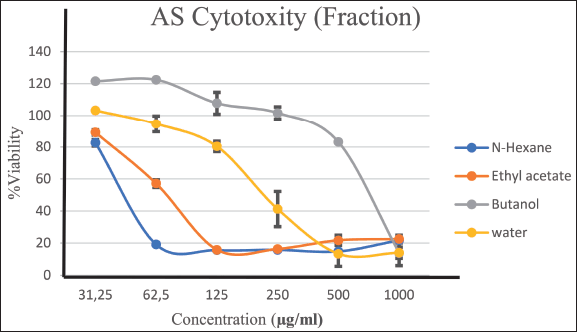 | Figure 4. Comparison of growth curves of all fractions of AS. [Click here to view] |
Table 4 shows the calculation of the IC50 value for each fraction, which is used to determine precisely what concentrations can inhibit the growth of MCF-7 cells.
A reduction in cell viability is noted at doses beginning at 5 μg/ml, with data indicating a diminishing growth curve if MCF-7 cells as their extract concentration escalates. The n-hexane and ethyl acetate extracts inhibit 50% MCF-7 cell proliferation at 62.5–1000 μg/ml. This indicates that these chemicals possess cytotoxic properties. The reduction in viability signifies that the active chemicals in their extract can compromise cell integrity and hinder the multiplication of cancer cells. These findings correspond with prior studies indicating that plant extracts can be anticancer agents, influencing apoptotic pathways and obstructing their cancer cell cycle [41]. Consequently, their subsequent stage involves further screening to isolate the bioactive compounds accountable for this anticancer activity and executing docking analysis to investigate the connections between these compounds and molecular targets. This discovery establishes a robust basis for advancing plant-derived cancer therapeutics, which may offer an alternative to traditional treatments that frequently include detrimental side effects.
Data variation among fractions, conducted with three repetitions, indicates a notable reduction in the proliferation of MCF-7 cells as the concentration of their sample escalates, particularly from 31.25 to 1,000 μg/ml. The reduction in cell viability signifies that their active chemicals in the n-hexane and ethyl acetate fractions effectively impede cancer cell proliferation. The IC50 value for their n-hexane fraction is 49 μg/ml; their ethyl acetate fraction is 89 μg/ml, demonstrating significant anticancer activity in both fractions. These findings align with prior studies indicating that extracts from specific plants exhibit cytotoxic effects against various cancer cell types, including MCF-7, via mechanisms such as apoptosis induction and cell cycle inhibition [42,43]. This discovery underscores the necessity of further investigating the bioactive chemicals in that fraction, which may be developed as novel therapeutic agents for cancer treatment.
Hexane extract and fractions tend to extract lipophilic compounds such as specific terpenoids and flavonoids known to have anticancer activity. These compounds can interact with the cancer cell membrane, disrupt the cell proliferation process, and trigger apoptosis (programmed cell death). On the other hand, although polar and capable of extracting polar compounds, ethanol, and methanol extracts may not contain high concentrations of bioactive compounds that effectively inhibit the growth of MCF-7 cells. Previous research by Zhang et al. [44] showed that lipophilic compounds have a better affinity for targeting signaling pathways involved in cancer growth [45]. This may explain why ethanol and methanol-based extracts do not exhibit the same potential. Butanol solvent on extract and fraction with medium polarity may also not contain the optimal active compounds to inhibit cancer cell growth. According to Chehelgerdi [46] bioactive compounds isolated from medicinal plants often have varying efficiencies depending on the type of solvent used. In this case, the components extracted with butanol may not be sufficiently effective in targeting the active oncogenic pathways in MCF-7 cells [47].
Analysis of AS N-Hexane fraction compounds with LC-HRMS
This study uses an LC-HRMS analysis to identify the sensitivities present in the AS N-Hexane fraction. LC-HRMS enables comprehensive molecular fingerprinting in a sample, following up on the procedure of Windarsih et al. [38]. The analysis's results indicate the predicted relationship between the identified subject and the m/z cloud base data. A high-resolution spectrometer analyses various molecules using the MS and MS/MS spectra presented in the mzCloud basis data. This spectrum can be used as a reference when looking up uncommon words. Identification of molecules is based on high-resolution data from single molecules and all fragments of molecules. The Compound Discover tool compares the fragmentation patterns and molecular ions with those found in the m/zCloud database to determine the best match. In the case of a compound match, the software will show ranges from 0 to 100, with a higher score indicating a more significant number of the best matches of the number match of ions [38].
A total of 4 compounds were successfully filtered by considering their best value to be from cloud best match confidence, with a minimum value ranging from 95% to 100%, to proceed with silicon analysis and obtain screening results in the form of potential compounds that can reduce cancer cell proliferation. Screening Results are shown in Table 5.
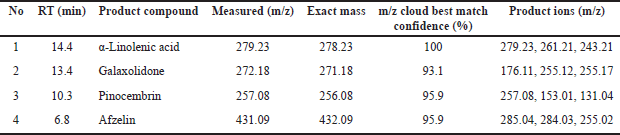 | Table 5. Prediction of phytochemical compounds Fraction of ethyl acetate AS. [Click here to view] |
Information regarding the retention time (RT) on the total ion current (TIC) graph in phytochemical screening results using LC-HRMS is essential for identifying and analyzing compounds. Each compound has a characteristic RT, so the peaks that appear on the TIC graph can be used to determine when a compound elutes from the chromatography column, as shown in Figure 5.
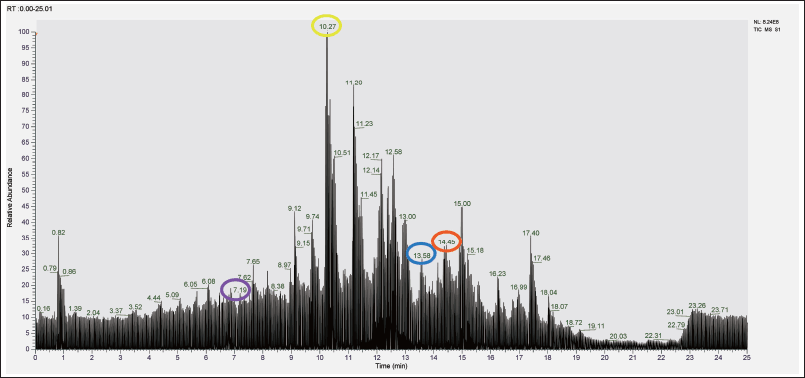 | Figure 5. TIC graph in phytochemical screening results (Red circle: α-Linolenic acid; Blue Circle: Galaxolidone; Yellow Circle: Pinocembrin; Purple Circle: Afzelin). [Click here to view] |
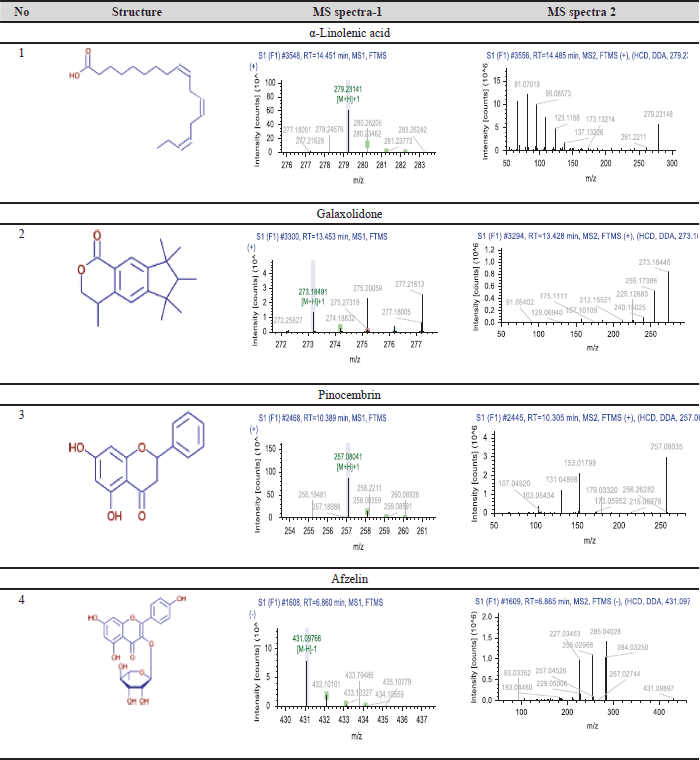 | Table 6. Chemical formula and MS-MS spectra. [Click here to view] |
The LC HRMS testing results indicated that four substances were accurately identified and selected based on optimal mzCloud match values, with scores between 95% and 100%. This filtering procedure is essential for their methods, such as silicon analysis, to identify possible chemicals limiting cancer cell proliferation. These characteristics derived from existing, including Measured (m/z), Exact mass m/z, Cloud Best Match confidence (%), and Product coins (m/z), are essential for their identification of pertinent substances. The measured m/z offers insights into the mass-to-charge ratio, whereas the exact mass m/z facilitates precise compound identification [48]. The Cloud Best Match confidence (%) signifies the degree of assurance concerning the data match, guaranteeing that the chosen compounds are representative and pertinent. Production (m/z) data elucidate the fragmentation of compounds, adding to the comprehension of their chemical structure and potential biological action. By synthesizing the outcomes from LC HRMS and in silico analysis, researchers may effectively forecast interactions between drugs and molecular targets, thereby expediting the development of anticancer medicines. Our research findings align with prior studies demonstrating that a multidisciplinary approach in drug development, incorporating LC HRMS technology and in silico analysis, can improve the efficacy and safety of cancer therapies derived from natural compounds [49,50]
The LC-HRMS test found a group of phytochemicals that are very interesting. These are Galaxolidone, α-linolenic acid, pinocembrin, and afzelin. The m/z values indicate their easy identification. Galaxolidone (279.23 m/z) and α-linolenic acid (272.18 m/z) were detected with perfect Cloud Best Match. Confidence values, 100% and 93.1%, respectively, indicate that these compounds have strong potential to provide biological effects. Also, pinocembrin (257.08 m/z) and afzelin (431.09 m/z) have high confidence values (95.9%), which means that these compounds may help the extract work as expected in the pharmaceutical field.
Galaxolidone is a phytochemical compound synonymous with estradiol and is an aromatic compound that can reduce inflammation and protect cells from damage. It can stop cancer cells from multiplying by controlling signaling pathways in the cell cycle and induced apoptosis mechanism [51]. Galaxolidone or Galaxolide is a sesquiterpene lactone, a chemical frequently occurring in flora. It is recognized for its possible anti-inflammatory and antibacterial characteristics [52]. Galaxolidone has been extracted from multiple plant species, including those within their Asteraceae family. Flavonoids are recognized for their antioxidant characteristics and possible health advantages [53]. Pinocembrin has been investigated for its prospective anti-inflammatory, neuroprotective, and anticancer properties [54]. α-Linolenic acid, an omega-3 fatty acid, is also known to help fight cancer. Studies have shown that this compound can cause apoptosis in MCF-7 cells and stop cancer cells from spreading and migrating. Various plants contain pinocembrin, a flavonoid that exhibits cytotoxic effects against MCF-7 cells and can inhibit signaling pathways in cancer cell growth [55].
Afzelin, a flavonoid glycoside, possesses significant potential in inhibiting the growth of MCF-7 cells. Previous research has shown that afzelin can induce cell death through apoptosis and disrupt the proliferation process of cancer cells [56,57]. Overall, these compounds show potential as promising anticancer agents, and further research is needed to understand their mechanisms of action and effectiveness in cancer therapy. Because there is much evidence that these compounds can fight cancer, AS can be seen as a valuable natural source for making cancer treatments that work better and are safer.
CONCLUSION
With a value of 35.53 μg/ml, total phenol is 25.61mg GAE/g, and total flavonoid is 29.50 mg QE/g, this study shows that N-hexane extract may be a better antioxidant than other test materials. This is true for both the extract and fraction of AS. These data are also supported by the inhibition of MCF-7 cell growth with an IC50 value of 42 μg/ml in the n-hexane extract, which is the best IC50 value. This is a further indicator for evaluating the LC-HRMS phytochemical profile of N-Hexane AS extract, and four phytochemical compounds are obtained. Galaxolidone, linolenic acid, pinocembrin, and afzelin.
ACKNOWLEDGMENTS
This work was funded by Research Grant 2024 from the Health Research Organization, National Research and Innovation Agency (BRIN), Indonesia. We also thank KS Gunung Kidul (Umar Anggara Jenie) Kawasan Sains Umar Anggara Jenie BRIN Yogyakarta for the LC-HRMS analyses.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63. doi: CrossRef
2. Kemenkes RI, Kementerian Kesehatan RI. Panduan Nasional Penanganan Kanker Kanker Payudara (PNPKKP). Jakarta, Indonesia, 2021.
3. Makar S, Saha T, Swetha R, Gutti G, Kumar A, Singh SK. Rational approaches of drug design for the development of selective estrogen receptor modulators (SERMs), implicated in breast cancer. Bioorg Chem. 2020;94:103380. doi: CrossRef
4. Singh D, Vignat J, Lorenzoni V, Eslahi M, Ginsburg O, Lauby-Secretan B, et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob Health. 2023;11(2):e197–206. doi: CrossRef
5. Pandit P, Patil R, Palwe V, Gandhe S, Patil R, Nagarkar R. Prevalence of molecular subtypes of breast cancer: a single institutional experience of 2062 patients. Eur J Breast Health. 2020;16(1):39–43. doi: CrossRef
6. Chen G, Liu W, Yan B. Breast cancer MCF-7 cell spheroid culture for drug discovery and development. J Cancer Ther. 2022;13(3):117–30. doi: CrossRef
7. Al-Shami K, Awadi S, Khamees A, Alsheikh AM, Al-Sharif S, Ala’ Bereshy R, et al. Estrogens and the risk of breast cancer: a narrative review of literature. Heliyon. 2023;9(9):e20224. doi: CrossRef
8. Miziak P, Baran M, B?aszczak E, Przybyszewska-Podstawka A, Ka?afut J, Smok-Kalwat J, et al. Estrogen receptor signaling in breast cancer. Cancers (Basel). 2023;15(19):4689. doi: CrossRef
9. Linares RL, Benítez JG, Reynoso MO, Romero CG, Sandoval-Cabrera A. Modulation of the leptin receptors expression in breast cancer cell lines exposed to leptin and tamoxifen. Sci Rep. 2019;9(1):19189. doi: CrossRef
10. You M, Xie Z, Zhang N, Zhang Y, Xiao D, Liu S, et al. Signaling pathways in cancer metabolism: mechanisms and therapeutic targets. Signal Transduct Target Ther. 2023;8(1):196. doi: CrossRef
11. Pawlonka J, Rak B, Ambroziak U. The regulation of cyclin D promoters – review. Cancer Treat Res Commun. 2021;27:100338. doi: CrossRef
12. Usman M, Khan WR, Yousaf N, Akram S, Murtaza G, Kudus KA, et al. Exploring the phytochemicals and anti-cancer potential of the members of Fabaceae family: a comprehensive review. Molecules. 2022;27(12):3863. doi: CrossRef
13. Masyudi M, Hanafiah M, Rinidar R, Usman S, Marlina M. Phytochemical screening and GC-MS analysis of bioactive compounds of Blumea balsamifera leaf extracts from South Aceh, Indonesia. Biodiversitas. 2022;23(3):1346–54. doi: CrossRef
14. Sak K. Anticancer action of plant products: changing stereotyped attitudes. Explor Target Antitumor Ther. 2022;3(4):423–7. doi: CrossRef
15. Rao GV, Kavitha K, Gopalakrishnan M, Mukhopadhyay T. Isolation and characterisation of a potent antimicrobial compound from Aerva sanguinolenta Blume.: an alternative source of Bakuchiol. J Pharm Res. 2012;5(1):174–6. Available from: http://jprsolutions.info
16. Adegbola PI, Adetutu A, Olaniyi TD. Antioxidant activity of Amaranthus species from the Amaranthaceae family – a review. S Afr J Bot. 2022;133:111–7. doi: CrossRef
17. Lalee A, Pal P, Bhattacharaya B, Samanta A. Evaluation of anticancer activity of Aerva Sanguinolenta (L.) (Amaranthaceae) on Ehrlich’s Ascites cell induced Swiss Mice. Int J Drug Dev Res. 2012;4(1):203–9. Available from: http://www.ijddr.in
18. Patel HK, Bihani T. Selective estrogen receptor modulators (SERMs) and selective estrogen receptor degraders (SERDs) in cancer treatment. Pharmacol Ther. 2018:186:1–24. doi: CrossRef
19. Anurup Mandal AM, Durbadal Ojha DO, Asif Lalee AL, Sudipta Kaity SK, Mousumi Das MD, Debprasad Chattopadhyay DC, et al. Bioassay directed isolation of a novel anti-inflammatory cerebroside from the leaves of Aerva sanguinolenta. Med Chem Res. 2015;24(5):1952–63. doi: CrossRef
20. Yanti Y, Setiawan T, Lay BW. Antibacterial, antibiofilm and quorum sensing inhibitory activities of Clitoria ternatea anthocyanin against Streptococcus mutans. Int J Infect Dis. 2018;73:143–4. doi: CrossRef
21. Lestari U, Mufti N, Lutfiyah DA, Fitriyah U, Annisa Y. UV irradiation enhanced in-vitro cytotoxic effects of ZnO nanoparticle on human breast cancer. J Phys: Conf Ser. 2018;1093:012046. doi: CrossRef
22. Safrina D. Pengaruh ketinggian tempat tumbuh dan pengeringan terhadap flavonoid total sambang colok (Iresine Herbstii). Indones J Agric Postharvest Res. 2018;15(3):147–54.
23. Abotaleb M, Samuel SM, Varghese E, Varghese S, Kubatka P, Liskova A, et al. Flavonoids in cancer and apoptosis. Cancer. 2018;11(1):28 doi: CrossRef
24. Ka?mierczak-Bara?ska J, Boguszewska K, Adamus-Grabicka A, Karwowski BT. Two faces of vitamin c—antioxidative and pro-oxidative agent. Nutrients. 2020;12(5):1501. doi: CrossRef
25. Özsoy S, Soykut G, Becer E. Quercetin: a phytochemical with pro-apoptotic effects in colon cancer cells. Cyprus J Med Sci. 2022;7(5):587–92. doi: CrossRef
26. Tran V, Kim R, Maertens M, Hartung T, Maertens A. Similarities and differences in gene expression networks between the breast cancer cell line michigan cancer foundation-7 and invasive human breast cancer tissues. Front Artif Intell. 2021;4:674370. doi: CrossRef
27. Dehelean CA, Marcovici I, Soica C, Mioc M, Coricovac D, Iurciuc S, et al. Plant-derived anticancer compounds as new perspectives in drug discovery and alternative therapy. Molecules. 2021;26(4):1109. doi: CrossRef
28. Shrihastini V, Muthuramalingam P, Adarshan S, Sujitha M, Chen JT, Shin H, et al. Plant derived bioactive compounds, their anti-cancer effects and in silico approaches as an alternative target treatment strategy for breast cancer: an updated overview. Cancers. 2021;13(24):6222. doi: CrossRef
29. Sarkar N, Farheen S, Chakraborty M, Mukherjee S, Haldar PK. Exploration of phytochemical and in-vitro antioxidant and antidiabetic properties of Aerva sanguinolenta (L.) Blume. Res J Pharm Technol. 2022;15(11):5267–72. doi: CrossRef
30. Gulcin, I and Alwasel SH. DPPH Radical Scavenging Assay. Processes 2023 ;11- 2248. 8. https:// doi.org/10.3390/pr11082248
31. Baliyan S, Mukherjee R, Priyadarshini A, Vibhuti A, Gupta A, Pandey RP, et al. Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of Ficus religiosa. Molecules. 2022;27(4):1326. doi: CrossRef
32. Windarto S, Hsu JL, Lee MC. First report of antioxidant potential of peptide fraction derived from Colaconema formosanum (Rhodophyta) protein hydrolysates. Biocatal Agric Biotechnol. 2024;58:103232. doi: CrossRef
33. Wang X, Ha D, Yoshitake R, Chan YS, Sadava D, Chen S. Exploring the biological activity and mechanism of xenoestrogens and phytoestrogens in cancers: emerging methods and concepts. Int J Mol Sci. 2021;22(16):8798. doi: CrossRef
34. Hedvat M, Emdad LK, Das S, Kim K, Dasgupta S, Thomas S, et al. Selected approaches for rational drug design and high throughput screening to identify anti-cancer molecules. Anticancer Agents Med Chem. 2012;12(9):1143–55. doi: CrossRef
35. Cacique AP, Barbosa ÉS, Pinho GP, Silvério FO. Maceration extraction conditions for determining the phenolic compounds and the antioxidant activity of catharanthus roseus (L.) g. don. Ciencia e Agrotecnologia. 2020;44:1–12. doi: CrossRef
36. Agustini K, Sangande F, Nuralih N, Harahap AM, Ningsih S, Bahtiar A. Molecular mechanism elucidation of Ocimum basilicum as anticancer using system bioinformatic approach supported by in vitro assay. Pharmacia. 2024;71:1–12. doi: CrossRef
37. Agustini K, Kusumaningrum S, Sulfianti A, Wink M. Estrogenic activity of Bryonia dioica Jacq. through in silico and in vitro studies on pS2 gene expression in the breast cancer cell line MCF-7. Pharmacia. 2023;70(4):951–8. doi: CrossRef
38. Windarsih A, Suratno, Warmiko HD, Indrianingsih AW, Rohman A, Ulumuddin YI. Untargeted metabolomics and proteomics approach using liquid chromatography-Orbitrap high resolution mass spectrometry to detect pork adulteration in Pangasius hypopthalmus meat. Food Chem. 2022;386:132856. doi: CrossRef
39. Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016;5:e47. doi: CrossRef
40. Arias A, Feijoo G, Moreira MT. Exploring the potential of antioxidants from fruits and vegetables and strategies for their recovery. Innov Food Sci Emerg Technol. 2022;77:102974. doi: CrossRef
41. Zulkipli NN, Rahman SA, Taib WR, Razali RM, Ismail I, Ahmad WA, et al. The cytotoxicity effect and identification of bioactive compounds of Prismatomeris glabra crude leaf extracts against breast cancer cells. Beni Suef Univ J Basic Appl Sci. 2024;13(1):33. doi: CrossRef
42. Rahmawati J, Yuliani R. Cytotoxic and antiproliferation activity of n-hexane fraction of avocado seed (Persea americana Mill.) on MCF7 cell. Pharmacon: Jurnal Farmasi Indonesia. 2022;19(1):35–44. Available from: http://journals.ums.ac.id/index.php/pharmacon
43. Rahmawati N, Ismail NH, Wahyuni FS, Hamidi D. Cytotoxic activity, cell migration and apoptosis effects of Uncaria nervosa Elmer leaf fractions on MCF-7 HER 2 cells. Trop J Nat Prod Res. 2024;8(6):7494–8. doi: CrossRef
44. Zhang Z, Zhou L, Xie N, Nice EC, Zhang T, Cui Y, et al. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct Targeted Ther. 2020;5:113. doi: CrossRef
45. Khan NG, Correia J, Adiga D, Rai PS, Dsouza HS, Chakrabarty S, et al. A comprehensive review on the carcinogenic potential of bisphenol A: clues and evidence. Environ Sci Pollut Res Int. 2021;28(16):19643–63. doi: CrossRef
46. Chehelgerdi M, Chehelgerdi M, Allela OQB, Pecho RDC, Jayasankar N, Rao DP, et al. Progressing nanotechnology to improve targeted cancer treatment: overcoming hurdles in its clinical implementation. Mol Cancer. 2023;22(1):169. doi: CrossRef
47. Lee JE, Jayakody JT, Kim JI, Jeong JW, Choi KM, Kim TS, et al. The influence of solvent choice on the extraction of bioactive compounds from asteraceae: a comparative review. Foods. 2024;13(19):3151. doi: CrossRef
48. Nijssen R, Blokland MH, Wegh RS, de Lange E, van Leeuwen SP, Berendsen BJ, et al. Comparison of compound identification tools using data dependent and data independent high-resolution mass spectrometry spectra. Metabolites. 2023;13(7):777. doi: CrossRef
49. Aryal B, Adhikari B, Aryal N, Bhattarai BR, Khadayat K, Parajuli N. LC-HRMS profiling and antidiabetic, antioxidant, and antibacterial activities of acacia catechu (L.f.) Willd. Biomed Res Int. 2021;2021:7588711. doi: CrossRef
50. Teoh WY, Yong YS, Razali FN, Stephenie S, Dawood Shah M, Tan JK, et al. LC-MS/MS and GC-MS analysis for the identification of bioactive metabolites responsible for the antioxidant and antibacterial activities of Lygodium microphyllum (Cav.) R. Br., Separations. 2023;10(3):215. doi: CrossRef
51. Emond R, Griffiths JI, Grolmusz VK, Nath A, Chen J, Medina EF, et al. Cell facilitation promotes growth and survival under drug pressure in breast cancer. Nat Commun. 2023;14(1):3851. doi: CrossRef
52. Rorije E, Aldenberg T, Peijnenburg W. Read-across estimates of aquatic toxicity for selected fragrances. Altern Lab Anim. 2013;41(1):77–90. doi: CrossRef
53. Arenas M, Martín J, Santos JL, Aparicio I, Alonso E. An overview of analytical methods for enantiomeric determination of chiral pollutants in environmental samples and biota. TrAC Trends Anal Chem. 2021;143:116370. doi: CrossRef
54. Rasul A, Millimouno FM, Ali Eltayb W, Ali M, Li J, Li X. Pinocembrin: a novel natural compound with versatile pharmacological and biological activities. Biomed Res Int. 2013;2013:379850. doi: CrossRef
55. Montecillo-Aguado M, Tirado-Rodriguez B, Huerta-Yepez S. The involvement of polyunsaturated fatty acids in apoptosis mechanisms and their implications in cancer. Int J Mol Sci. 2023;24(14):11691. doi: CrossRef
56. Mediani A, Hamezah HS, Rohani ER, Kamal N, Perumal V, Salim F, et al. Afzelin: advances on resources, biosynthesis pathway, bioavailability, bioactivity, and pharmacology. In: Xiao J, editor. Handbook of dietary flavonoids, Cham: Springer International Publishing, 2023. pp. 1–45. doi: CrossRef
57. Yang LJ, Han T, Liu RN, Shi SM, Luan SY, Meng SN. Plant-derived natural compounds: a new frontier in inducing immunogenic cell death for cancer treatment. Biomed Pharmacother. 2024;177:117099. doi: CrossRef