INTRODUCTION
Based on data from the World Health Organisation, more than 80% of the world’s population depends on traditional herbal medicine as their main means of getting fundamental healthcare [1]. Due to the emergence of negative impact and the development of intolerance to chemically based medications, individuals have shifted their attention to ethno-pharmacognosy. Some individuals often assert the benefits of particular natural or herbal products. The bioactive compounds found in medicinal plants, such as alkaloids, phenolics, and terpenoids, are important in their biological effects. These effects include their ability to reduce inflammation, prevent the incidence of diabetes, and act as antioxidants [2]. Due to the diverse groups of chemical compounds in plants, the extraction process to obtain bioactive compounds with desired health benefits remains an important issue [3]. Prior research has indicated that the effectiveness of extracting compounds is mainly influenced by factors such as pH, temperature, the ratio of sample to solvent, and the polarity of the solvent [3].
Some extraction procedures, such as conventional and advanced methods, can be used to extract the plants. Conventional methods, i.e., maceration, are generally applied to extract medicinal plants. The advantage of this method is that it is cheap, but the maceration method needs a high extraction time, extra solvent requirements, and the loss of volatile compounds [4]. Meanwhile, microwave-assisted extraction (MAE) and ultrasonic-assisted extraction (UAE) methods, which are modern, have the advantages of less time, lesser solvent requirements, and lower capital investment in the extraction process. The advantage of UAE compared to MAE is that the UAE method requires less power or energy and higher heat-sensitive compound retention [4,5]. Therefore, the extraction method used in this study was UAE.
Plant-derived medications have been globally employed in traditional medical methods to treat various illnesses. Plants utilized in traditional medicine possess a diverse array of chemical compounds that have the potential to cure both chronic and infectious disorders [6] effectively. Duraipandiyan et al. [6] stated that numerous phytochemicals obtained from plants can function as potent and safe alternative options for medical purposes with minimal adverse effects. Numerous beneficial biological activities have been documented from natural materials, which include the ability to inhibit the growth of cancer cells, inhibit the growth of microbes, scavenge free radicals, inhibit the occurrence of diarrhea, provide analgesics, and accelerate wound healing. Populations of people worldwide have long employed plants possessing medicinal uses as raw materials for traditional remedies and nutritional supplements [7]. Herbal medicines in Asia have a rich history that reflects the deep connection between humans and the environment. It is now widely recognized that the medicinal properties of plants are derived from the bioactive compounds that induce specific physiological responses in the human body.
Peronema canescens Jack, often known as Sungkai or Jati Sabrang, is an indigenous plant species in Indonesia that originates in the Lamiaceae family, specifically in ethnobotany. This plant is commonly found in the Sumatra and Kalimantan islands, and its stems, leaves, flowers, and seeds have been used as folk medicines in the local community [8]. Fransisca et al. [9] stated that the tribe of Dayak on Kalimantan Island traditionally employs P. canescens as a medication to treat flu, fever, stomach ache, and worms (ringworms), as well as as a mouthwash to prevent toothache. Empirically, the Lembak people in the Bengkulu, Sumatra island, have used P. canescens plants as traditional remedies for fever, worms, antiseptics, diarrhea, malaria, and toothaches [10]. By brief bibliometric analysis, we have noted around 33 P. canescens or Sungkai articles found in the Scopus database. The development of this research was started in 2014 (1 article) by exploring the wood quality of the sungkai tree. The works on this plant have been continued in 2020 until mid-2024 which focus on the uses of P. canescens leaves for health. This lack number of articles on the database showed that this plant has not been optimally explored. To date, Indonesia has published the most works about P. canescens leaves, followed by Malaysia in second place. Since 2022, people started to pay attention to the antioxidant activity of P. canescens plant as well as their activities as anti inflammatory and immunomodulator. However, the ability of P. canescens to treat and maintain health through various bioactivity has not been much investigated. Moreover, the research related to the isolation and identification of active compounds that play a role in P. canescens leaves bioactivity is still very limited.
Various secondary metabolites are known present in the leaves of P. canescens, including phenolic compounds, flavonoids, alkaloids, terpenoids, steroids, tannins, and saponins. The compounds in question have been identified as exerting specific pharmacological actions [9,11]. The extract of P. canescens leaf in the ethanol solvent shows the strongest antioxidant activity, along with high levels of flavonoids and phenolic compounds [12–14]. In their research finding, Sutomo et al. [15] reported that P. canescens extracted with methanol exhibits significant antioxidant activity, demonstrated by an IC50 value of 63.977 ppm. The extract has been qualitatively confirmed to contain phenolic, flavonoid, steroid, alkaloid, tannin, and saponin compounds. The characterization of secondary metabolites derived from the analysis results utilizing GC-MS indicated that P. canescens leaf extract comprises 17 chemical compounds categorized within the fatty acid, steroid, terpenoid, phenolic, and carbohydrate groups. Phenolic compounds, including phenol, 2-methoxy-4-vinylphenol, caryophyllene, and phytol, have been identified for their significant antioxidant activity [16].
Previous research by Muharni et al. [17] showed that P. canescens extract has compounds called betulinic acid and stigmasterol that are very good at lowering cholesterol levels in vitro assays. In their research results, Latief et al. [18] also stated that P. canescens extract contains β-sitosterol and 5,7-dihydroxy isoflavone compounds. Betulinic acid is a terpenoid compound, while stigmasterol and β-sitosterol are steroid compounds (phytosterols) that play an important role as anticholesterol, antihyperlipidemic, and pancreatic lipase inhibitors [19–21]. In vivo, Pratiwi et al. [22] have proven that P. canescens extract has significant antihyperlipidemic activity in experimental animals. Therefore, the pancreatic lipase inhibition assays are important to be carried out in this study to obtain information on the pancreatic lipase inhibitory capacity of the extract and all fractions of P. canescens and to determine the bioactive compounds that play a role in this activity.
As far as we studied, the pancreatic lipase inhibitor activity of P. canescens leaves has not been investigated. Furthermore, the determination of P. canescens antioxidant activity yielded inconsistent results. This study focused on comprehensively characterizing the phytochemicals in P. canescens extract and fraction samples, followed by determining antioxidant activity and the ability to inhibit pancreatic lipase activity. The metabolite profile of P. canescens was also found using ultra-high-performance liquid chromatography-high resolution mass spectrometry (UHPLC-HRMS) and principal component analysis (PCA). This approach allowed for the classification of chemicals in P. canescens extracts and fractions. Identifying active compounds by metabolite profile and determining their biological activity will improve our knowledge of ethnomedicine based on herbal preparations of P. canescens.
MATERIALS AND METHODS
Materials
Peronema canescens leaves have been acquired from Bengkulu, located on the island of Sumatera in Indonesia. It has been identified (voucher specimen BO-1618223) by the Botanical Characterization Laboratories, a division of the National Research and Innovation Agency of Indonesia. The analytical chemicals and standards utilized in this investigation, including chloroform, ethyl acetate, methanol, Folin-Ciocalteu, gallic acid, ascorbic acid, perchloric acid, and glacial acetic acid, were procured from Merck, Germany. Quercetin, trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2’-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), dimethyl sulfoxide, potassium persulfate, p-nitrophenylbutyrate, and standard ursolic acid have been obtained from Sigma Aldrich (St. Louis, MO, USA). Orlistat (Xenical) was obtained from a local distributor.
Extraction process
Unexposed to direct sunlight, the leaves of P. canescens were dry in the air for 7 days. The moisture content of P. canescens leaf simplicia has been measured, and the results obtained are 5.49% ± 1.45%. Dried leaves of P. canescens were then powdered and subjected to ultrasound-assisted extraction (UAE, 220-240 V, 50/60 Hz, and 550 W), employing 96% ethanol as the solvent for 1.5 hours, formulated with a solid-to-solvent ratio of 1:6 (w/v). The process of extraction was carried out three times at ambient temperatures. The extracted sample underwent filtration, collection, and concentration utilizing a BUCHI Rotavapor® R-300 rotary evaporator at a temperature of 50°C to get a crude extract. Finally, the resultant extract was dehydrated by freeze-drying (BUCHI Lyovapor™ L-200), and the percentage of extract obtained was determined using the following formula [23]:
Fractionation
An 80-g ethanol extract (EE) sample was mixed in 300 ml of water. The resulting mixture was then separated using the liquid–liquid partitioning method, with the solvents n-hexane, chloroform, ethyl acetate, and n-butanol in equal quantities. This process was repeated thrice, each partitioning step lasting 30 minutes. This partitioning aimed to obtain fractions based on increasing solvent polarity. Finally, the resulting fractions from the solvents n-hexane, chloroform, ethyl acetate, n-butanol, and aqueous (FH, FC, FEA, FB, and FA, respectively) were evaporated and lyophilized for further assays. Each series of extraction methods with ethanol and fractionation with various solvents was conducted thrice, so three sets of yield data (%) were obtained.
Total phenolic content
The Folin-Ciocalteu reaction method was used to measure the amount of phenolic group in the extract and fractions in this study, with minor adjustments [24]. A 250 μl aliquot of a 1 mg/ml sample was blended with 3.75 ml of water that was distilled and 250 ml of Folin-Ciocalteu solution, then vortexed until homogeneous. The resulting solution was then incubated for 8 minutes at 25°C–28°C. After the incubation, 750 ml of a Na2CO3 solution (20% w/v in water) was put in, vortexed, and then subjected to a further 2-hour incubation while protected from light. Afterward, 200 μl of every mixture was placed into a 96-well microplate. The absorbance was measured by a microplate reader at 765 nm wavelength using the instrument Multiskan Go from Thermo ScientificTM. Values of absorbances were compared with a standard gallic acid within the concentration range of 60–300 µg/ml. All experiments were conducted three times, and the resulting data have been presented as milligrams of gallic acid equivalent (GAE) per gram of extract or fractions.
Total flavonoid content
Total flavonoids were quantified by the modified aluminum chloride colorimetric method [25] with slight modification. A 10 μl sample (0.5 mg/ml) and standard quercetin solution with various concentrations (25–600 μg/ml) were put into a 96-well microplate. Furthermore, 60 μl methanol, 10 ml of potassium acetate, 10 ml of aluminum chloride, and 120 ml of water that had been distilled were administered. After incubating the resulting mixture for 30 minutes, the absorbance was measured at a wavelength of 415 nm. A reference standard was established using quercetin, and the total amount of flavonoids was quantified as milligrams of quercetin equivalent (QE) per gram of extract or fractions.
Total terpenoid content
Terpenoid quantity was conducted using a colorimetric technique [26] with slight modifications. Extract and fractions samples were prepared with 800 µg/ml concentration in methanol p.a. Subsequently, a solution of ursolic acid was made at various concentrations ranging from 10 to 500 µg/ml. A volume of 0.1 ml of sample solution and standard ursolic acid with various concentrations were put into a test tube with a dark screw. Then, 0.2 ml of 5% vanillin and 1 ml of concentrated perchloric acid (60%) were added. Then, the mixture that had been prepared was incubated in a water immersion maintained at a temperature of 60°C for 45 minutes. After 45 minutes of incubation, the resultant mixture remained at ambient temperature for 15 minutes. Next, 2.5 ml of pure acetic acid (glacial) was added and vortexed until homogeneous. After homogenization, 200 μl of the resulting mixture was added to the 96-well microplate, and the absorbance value was measured at 548 nm using a microplate reader (Multiskan Go, Thermo ScientificTM). This study expressed the quantitative measure of terpenoid content as milligrams of UAE per gram of extract or fraction, using ursolic acid as the reference standard.
DPPH radical scavenging assay
The free radical scavenging capacity of the extract samples and all fractions of P. canescens was determined in vitro by the 2,2-DPPH assay, following the methodology published by Mahmood et al. [27] with a few modifications. The details of this method can be seen in Supplementary Material 1.
ABTS radical scavenging assay
Assessment of free radical scavenging activity using the ABTS method was carried out using the procedure previously described by Re et al. [28] with slight modification. Details of this method are available in the Supplementary Material 1.
Ferric-reducing antioxidant power (FRAP) assay
The ferric-reducing capacity of the plant extracts and fractions was investigated using the Benzie and Strain [29] method with slight modification. A description of this method can be viewed in Supplementary Material 1.
Assay of inhibiting pancreatic lipase
The effectiveness of the extract and fractions in suppressing pancreatic lipase activity was evaluated. The experiment was accomplished by utilizing spectrophotometry to measure the rate at which nitrophenol was formed due to pancreatin hydrolyzing p-nitrophenylbutyrate [30]. First, the extract and fractions were diluted in dimethyl sulfoxide to create solutions with 10 and 100 mg/ml concentrations, respectively. As a consequence, the final concentration of the mixture was 0.38 and 3.8 mg/ml, respectively. Ten ml of completely dissolved extract and fraction were mixed with an equivalent volume of pancreatin solution (made from 1 mg of pancreatic enzyme dissolved in 1 ml of saline phosphate buffer solution, pH 6.8). Next, the mixture was incubated for 5 minutes at 37°C. After incubation, 240 ml of substrate (A solution of 0.165 mM p-nitrophenylbutyrate in PBS) was added to the solution mixture. Then, the absorbance value of the mixture was measured at a specific wavelength of 415 nm with two observations, namely at intervals of 0 and 35 minutes. The lipase inhibitory action was quantified as the amount of μM nitrophenol produced per minute. As a positive control, 120 μg/ml orlistat was employed. To establish a nitrophenol standard curve, nitrophenol was diluted into solutions with concentrations of 0, 1, 2, 5, 10, and 20 μg/ml [31].
Identification of phytochemical constituents using UHPLC-HRMS
Liquid chromatography (Thermo Scientific™ Vanquish™ UHPLC Binary Pump) and Orbitrap high-resolution mass spectrometry (Thermo Scientific™ Q Exactive™ Hybrid Quadrupole-Orbitrap™ High-Resolution Mass Spectrometer) were employed to assess the phytochemical composition of the extract and fractions. The extract was dissolved in 1 ml MS grade methanol, vortexed for 30 seconds, sonicated for 30 minutes, and filtered through a 0.22 μm filter. MS-grade water (A) and MS-grade methanol (B) were the mobile phases employed in the experiment. Each mobile phase contained 0.1% formic acid. The gradient method was implemented, and the discharge rate was set at 0.3 ml/minute. Initially, the concentration of mobile phase B was modified to 5%, progressively increasing to 90% within 16 minutes. After that, maintain a 90% concentration for 4 minutes before reverting to the initial state of 5% of mobile phase B for 25 minutes. The injection volume was 3 μl, and the column temperature was maintained at 40°C. A comprehensive MS/dd-MS2 collection technique was used for the untargeted screening, incorporating either positive or negative ionization polarities/states. The voltage of spraying was adjusted to 3.30 kilovolts (kV), the capillary temperature was set at 320°C, and the auxiliary gas heater was kept at a temperature of 30°C. In both positive and negative ionization modes, the scan range was conducted from 66.7 to 1,000 m/z, with a resolution of 70,000 for full MS and 17,500 for dd-MS2. Furthermore, the Compound Discoverer 3.2 software from Thermo ScientificTM was employed to evaluate the raw chromatography results. The analysis included the utilization of the two locally stored and online databases, such as ChemSpider (www.chemspider.com) and mzCloud (www.mzcloud.org) [32]. The validation method run in this work related to linearity, limit of detection (LOD), and limit of quantification (LOQ) was explained by Wenzi et al. [33] method, which involved ten repetitions to obtain precise data. On the other hand, Aydo?an [34] stated that the quantification value of the acteoside compound was higher than the LOQ and LOD, which were 58 ng/ml and 64 ng/ml, respectively. Meanwhile, other polyphenol compounds had lower quantification values than the LOD and LOQ.
Chemometrics study based on metabolomic analysis
In phytochemical investigations, PCA was employed to distinguish and classify the metabolite compound variable. The chemical compounds in P. canescens extracts and fractions can be separated into some principal components with PCA. The chemometrics analysis was conducted using the publicly accessible MetaboAnalyst 6.0 software, which may be accessed at https://www.metaboanalyst.ca. The compound areas derived from untargeted metabolomic analysis were utilized as variables in PCA, and then 32 variables were selected for chemometric analysis. Chemometrics analysis was conducted using six samples, which included EE, FH, FC, FEA, FB, and FA. Thirty-two variables were chosen from the metabolomics analysis to be included in the chemometrics investigation. The Compound Discoverer software applied a filtering process that considered the compound names, preferred the most precise match finds using mzCloud, and considered the DDA fragments for the specified ion. The purpose of the filter was to decrease the number of variables from the initial magnitude. Before chemometric analysis, the data underwent Pareto scaling to ensure optimal variation. PCA was analyzed using a PCA score plot, PCA Biplot, and variable importance of projections value [32].
RESULTS
Yield, total phenolic content (TPC), total flavonoid content (TFC), and total terpenoid content (TTeC) of P. canescens leaf extract and fraction
The percentage yield information from P. canescens that was extracted with 96% ethanol and subsequently fractionated with n-hexane, chloroform, ethyl acetate, n-butanol, and aqueous as solvents is presented in Table 1. According to Table 1, the fraction using chloroform as the solvent had the highest extraction yield (28.11% ± 0.43%). Instead, the aqueous fractions demonstrated the lowest extraction yield (4.56% ± 0.44%).
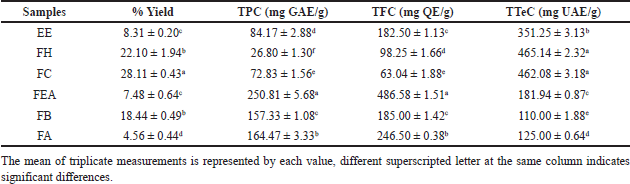 | Table 1. Yield (%), TPC, TFC, and TTEC of P. canescens leaf extract and fraction. [Click here to view] |
In addition, the results of the determination of the total phenolic, flavonoid, and terpenoid compounds are also shown in Table 1. The result of TPC and TFC from extract and fraction of P. canescens in this study showed linear results. The highest TPC was shown in the FEA with a value of 250.81 ± 5.68 mg GAE/g, and the highest TFC was also demonstrated in the FEA with a value of 486.58 ± 1.51 mg QE/g. Phytochemicals from the terpenoid group were also determined in this study (Table 1). The TTeC obtained in this study ranged from 110.00 ± 1.88 to 465.14 ± 2.32 mg UAE/g, with the highest total terpenoid content values found in FH and FC (465.14 ± 2.32 and 462.08 ± 3.18, respectively).
Antioxidant activity of P. canescens leaf extract and fraction
The results of the antioxidant activity assays of the extract and fractions samples of P. canescens in this study are presented in Table 2. The results obtained are shown in IC50 values. According to the DPPH free radical scavenging activity analysis, the FEA showed the highest antioxidant activity, with an IC50 value of 39.09 ± 0.46 μg/ml. Furthermore, FEA also has the highest ABTS free radical scavenging ability, with an IC50 value of 33.85 ± 0.41 μg/ml. The samples of extract and fraction were also evaluated for their ability to reduce power in the redox reaction using the FRAP assays. The results revealed that the FEA had the highest level of reducing activity, measuring 171.22 ± 0.57 mg TE/g.
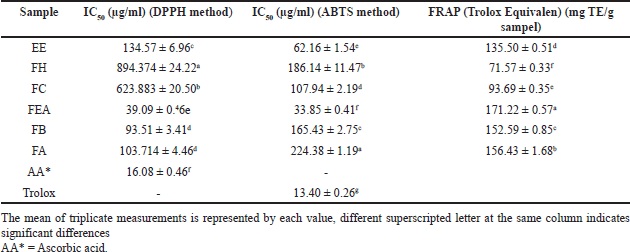 | Table 2. The result of IC50 antioxidant activity of P. canescens extract and fraction. [Click here to view] |
Pancreatic lipase inhibition assay
The results of this investigation demonstrated that the n-hexane fraction exhibited the highest level of inhibition of pancreatic lipase, with a percentage of 42.94%. Furthermore, the EE exhibited moderate inhibition, with a percentage of 24.73%. In contrast, the pancreatic lipase enzyme was not inhibited by the fractions derived from aqueous solvents, n-butanol, ethyl acetate, or chloroform, as demonstrated in Table 3.
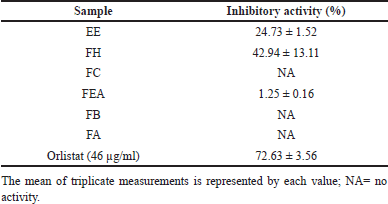 | Table 3. Pancreatic lipase inhibitory activity of P. canescens extract and fraction. [Click here to view] |
Principle component analysis of phytochemical constituent
The PCA performed in this study effectively classified the variables that indicate the region of each chemical compound in the extract and fractions (Table 4). The bioactivity of plant extracts and fractions can be affected by chemical compounds. The PCA was used to illustrate the correlation between the amounts of phytochemicals and the bioactivity of extracts and fractions. Figure 1 illustrates the score plot of PCA for evaluating 32 chemical compounds in extract and fraction samples with various solvents. Based on the data set obtained, the first principal component identified 31.6% of the total variation in the data set, while the second principal component represented 15.0%. Together, these two components represented a considerable variable region, 46.60% of the data set.
The investigation results, as illustrated by the Biplot and Score Plot PCA (Figs. 1 and 2), indicate that FH has a significant amount of chemical components classified as terpenoid and fatty acid compounds. In the interim, flavonoid compounds dominate in fractions that contain semi-polar to polar solvents, specifically the FEA and FB groups. Furthermore, the Hierarchical Clustering Heatmaps software application by MetaboAnalyst 6.0 was employed to visualize the clustering of multivariate data. The highest or lowest value in the data set is represented by each colored cell on the map, as illustrated in Figure 3.
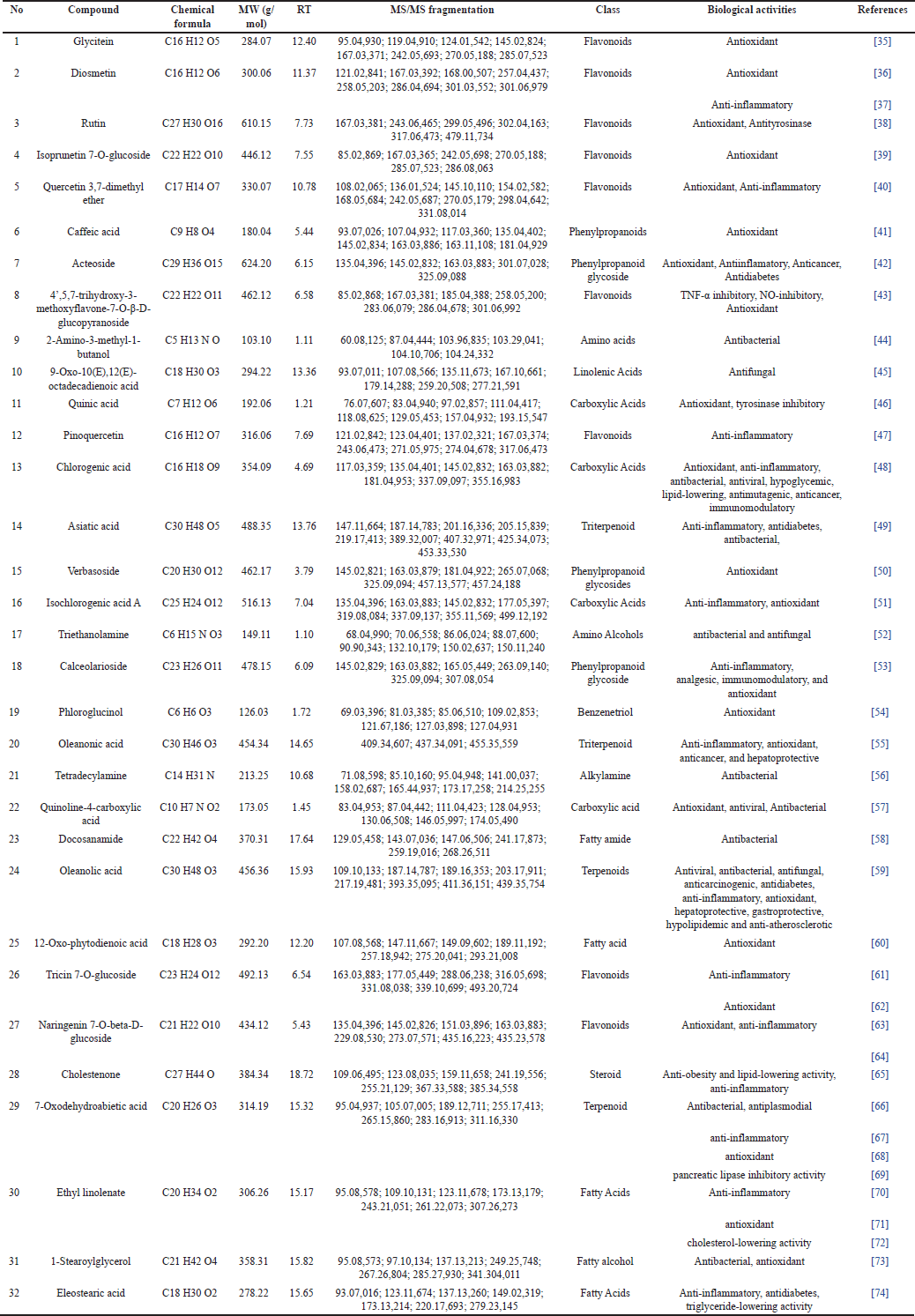 | Table 4. The chemical compound of P. canescens extract and fractions. [Click here to view] |
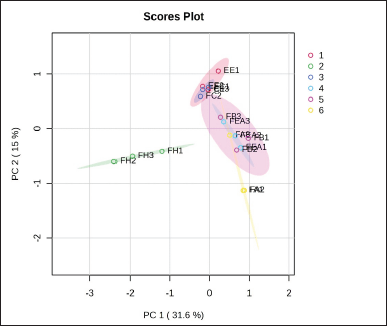 | Figure 1. Score plot of PCA based on different extract and fractions of P. canescens. [Click here to view] |
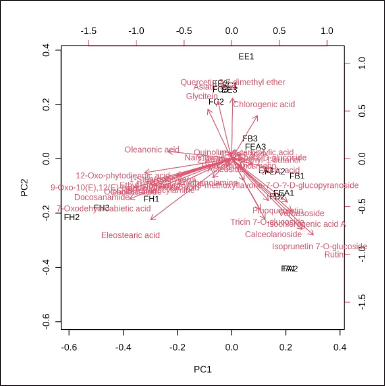 | Figure 2. Biplot of PCA of the phytochemical compound of different extract and fractions of P. canescens. [Click here to view] |
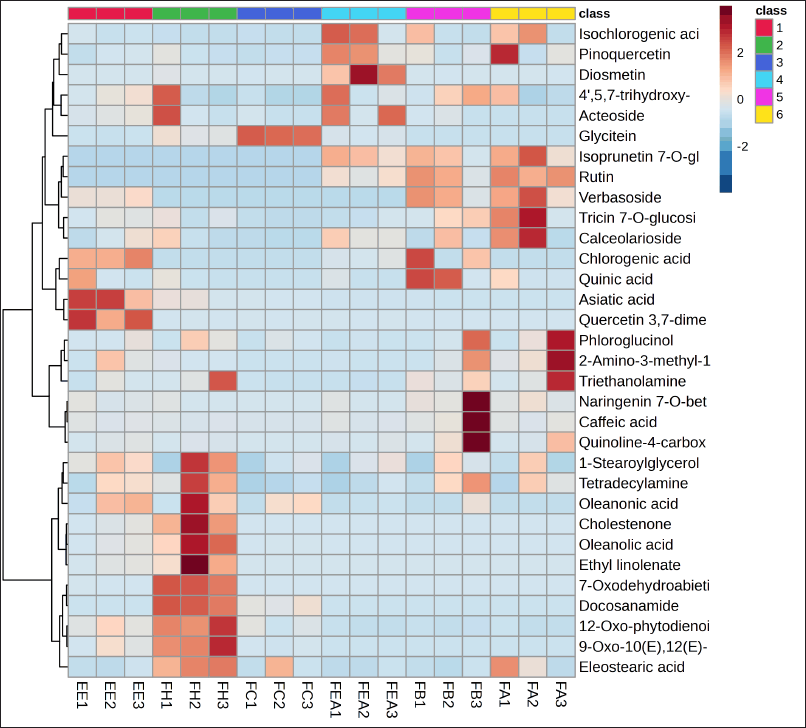 | Figure 3. Heat map of top 32 phytochemicals compound correlation with different extract and fraction of P. canescens (class 1=Ethanol extract; 2= n-hexane fraction; 3=chloroform fraction; 4= ethyl acetate fraction; 5=n-butanol fraction; 6= aqueous fraction). [Click here to view] |
DISCUSSION
Yield, TPC, TFC, and TTeC of P. canescens Jack. leaf extract and fraction
The phytochemicals are extracted from plants through one series of processes, such as milling, grinding, homogenizing, and extraction. The primary technique employed to extract and separate phytochemicals from plant sources is extraction [75]. The effectiveness of an extraction is influenced by the chemical composition of the plant, which specifies the selection of extraction techniques, sample particle size, type of solvent, and the presence of interfering compounds. The extraction yield can be affected by various factors such as solvent polarity, pH, temperature, extraction time, and sample composition [76]. In this study, the extraction method used was UAE. UAE is an extraction method that uses ultrasonic waves. With the help of the kinetic energy of the ultrasonic wave motion, the cell wall membrane will break, and chemical compounds will be mass-diffused into the solvent [77]. Ultrasonic frequencies range from 18 to 40 kHz, effectively extracting bioactive compounds from plant materials, including compounds with antioxidant potential [78]. Therefore, this study used an ultrasonic frequency of 20 kHz to extract bioactive compounds from P. canescens leaves. The optimal time required for extraction is an important factor in determining each plant extract antioxidant capacity, TPC, and TFC. Several research results state that with the UAE method, the optimal extraction time to obtain the best antioxidant activity, TPC, and TFC is 10 minutes [79], 20–120 minutes [80], 0–30 minutes [81], and 60 minutes [82]. Yim et al.[83] stated that an extraction time longer than the maximum limit required will cause a decrease in the antioxidant potential and polyphenol content of plant extracts. Due to the interaction of phenol with other plant components, the extraction process of phenol compounds is hampered. The extraction method of P. canescens leaves can be optimized for further research by shortening the extraction time to 30–60 minutes and using a combination of ultrasound and microwave-assisted extraction methods. This method combines microwaves and ultrasounds so that when high momentum and energy are applied, the ruptured plant cells will release more metabolite compounds in a shorter time.
This study used various solvents to fractionate the ethanolic crude extract of P. canescens. The different polarities of the solvent were applied during extraction to get the desired metabolites. Solvents with different polarities are expected to obtain different metabolites in each fraction. Polar solvents such as methanol, ethanol, and water form hydrogen bonds with other molecules. Higher phenolic compounds were extracted using a more polar solvent [84]. Some plant metabolites have hydrophobic properties, and these compounds can only be extracted by non-polar solvents [85]. For example, in a previous study, sesquiterpene artemisinin, which has highly active antimalarial activity, was extracted using hexane [86].
The results obtained in this study showed that the percentage yield of the 96% EE was 8.31%. Compared to the results obtained by Fadlilaturrahmah et al. [87] (yield of 7.28%), which also used 96% ethanol as a solvent, the percentage yield in this study was higher. It may be due to differences in extraction methods and the location of origin of P. canescens leaves. Fadlilaturrahmah et al. [87] obtained extracts from the maceration method, and the location of origin of P. canescens leaves were from the province of South Kalimantan. Differences in the geographical location of the plant origin determine the concentration and type of metabolites contained in the plant. Plants growing in areas with geographical conditions with high ecological pressure will produce more metabolite compounds than those growing in nutrient-rich areas [31,88]. Benhssain et al. [89] in their research stated that the results of extract yield, polyphenol, flavonoid, and tannin content of a plant are highly dependent on the geographical, bioclimatic, and edaphic conditions at each location where the plant was sampled.
Next, the raw EE (80 g) performed liquid-liquid partitioning using a series of solvents with progressively higher polarities. Thus, chemical compounds are separated based on their affinity for the solvent [90]. In this study, the fraction using a semi-polar solvent, ethyl acetate, showed linear results for total phenolic and flavonoid compounds. Meanwhile, the fraction with non-polar solvents, specifically n-hexane and chloroform, produced the highest quantity of terpenoid compound. The linear correlation between the total phenolic and total flavonoid contents of P. canescens suggests that the predominant phenolic compounds present are semi-polar to polar flavonoids.
Furthermore, the ethyl acetate solvent used in the fractionation technique significantly attracts the flavonoid compounds in the crude EE [91]. Similar to the findings of Muharni et al. [92], the present investigation confirms that the ethyl acetate fraction of P. canescens exhibited the most significant levels of total phenolic and flavonoid content compared to the hexane and methanol fractions. However, the total content of phenolic and flavonoids obtained by the current study exceeded the results of Muharni et al. [92]. It could be due to differences in extraction and fractionation methods and the source of P. canescens used.
Among all of the extracts and fractions of P. canescens investigated in the current study, FH and FC showed the highest content of chemical compounds in the terpenoid group. The results obtained from the present study are consistent with those of Juswardi et al. [16]. Some main chemicals are found in young, mature, and old P. canescens are carbohydrates, fatty acids, steroids, and terpenoids. The chemical substances mentioned are methyl stearate, resibufogenin, hexadecanoic acid, pregnan-20-one, 3-(acetyloxy)-5,6:16, 17-diepoxy-, and butyl 4,7,10,13,16,19-docosahexaenoate.
Antioxidant activity of P. canescens leaf extract and fraction
The IC50 value of a substance is inversely proportional to its antioxidant activity. This value demonstrated the quantity of antioxidants necessary to reduce the concentration of DPPH by 50%. The resultant value is calculated by interpolating using linear regression analysis. Chemical compounds with lower IC50 values demonstrate stronger antioxidant activity [93]. The antioxidant activity of the crude extract and fractions of P. canescens is examined in this research using a variety of assays, including DPPH, ABTS, and FRAP. Several in vitro assays were developed to quantify the effectiveness of antioxidants in plant and food samples. It has been established that various testing methods are required to comprehensively evaluate the antioxidant capacity of a sample against various sources of free radicals. Various assays differ in their fundamental principles and experimental conditions [94].
Among all the assessment methods, the FEA had the highest antioxidant activity. The antioxidant activity of the fraction is attributed to its high concentration of phenolic and flavonoid compounds. The results align with prior research showing a linear association between the quantity of polyphenols and their ability to act as antioxidants [95]. Following Ait Chaouche et al. [96], the presence of phenolic compounds is helpful as a key indicator of the antioxidant capability of plants. The plant predominantly comprises phenolic and flavonoid compounds, renowned for their potent antioxidant properties [97]. The chemical structure of phenolic and flavonoid compounds, particularly the benzene ring, and the quantity and placement of -OH functional groups are accountable for their antioxidant activity. The mechanism of phenolics and flavonoids as antioxidants can be through radical scavenging, metal chelation, or enzyme inhibition [98,99].
Antioxidant free radicals and a non-radical substrate were obtained by donating the H-atom from the phenolic compound to the free radical substrate. For example, gallic acid. Its benzene ring stabilizes the radicals due to the resonance effects, while the -OH functional group on the phenolic compound contributes to forming antioxidant free radicals [99]. Another mechanism of phenolic and flavonoid compounds as antioxidants is metal chelation. The phenolics and flavonoids chelate with the metal ions, such as copper or iron. The flavonoid or phenolic compounds can inhibit the reduction of metal ions, consequently preventing reactive oxygen species (ROS) formation [98,99]. Xanthine Oxidase is an example of an enzyme in the body that needs to be inhibited because it can directly or indirectly produce ROS in the body. This enzyme has been reported to be inhibited by flavonoids [100].
The research results of this study accord with the findings stated by Muharni et al. [92], which suggests that the fraction with ethyl acetate as a solvent exhibits the strongest antioxidant activity among the other fractions examined, with an IC50 value of 320 ug/ml. Many more studies have performed antioxidant examinations on extracts derived from P. canescens. Pindan et al. [8] investigated the capacity of antioxidants of P. canescens by employing different solvents for the fractionation process. The values of the IC50 for the crude extract of ethanol were determined to be 25,549 ppm; for the n-hexane fraction, it was 607,475 ppm; for the ethyl acetate fraction, 12,986 ppm; and for the remains of the ethanol fraction, it was 15,766 ppm [8]. The differences in the findings of this investigation compared to other studies may be due to many factors, such as variations in secondary metabolite content, solvents used for extraction, and extract composition [101]. The extraction procedures and conditions also affect the antioxidant activity, such as temperature and time [102].
In this study, as positive controls, the antioxidants used were Ascorbic acid and Trolox. The IC50 values produced by Ascorbic acid and Trolox were 16.08 ± 0.46 and 13.40 ± 0.26, respectively. This IC50 value is higher than the IC50 value of ascorbic acid produced in the study by Irnameria and Okfrianti [103], which was 5,440 ppm. However, the results of these two positive control assays are still within the range of very strong antioxidant activity (IC50 <50 ppm) [104]. The difference in IC50 values from the ascorbic acid standard in this study can be caused by several environmental factors during testing, including temperature, humidity, and light exposure that affect the stability of the ascorbic acid molecular structure [105].
Pancreatic lipase inhibition assay
Pancreatic lipase is an enzyme that has an essential function in absorbing triacylglycerols from the diet. This enzyme causes the breakdown of triacylglycerol into 2-monoacylglycerol and fatty acids. It has been established that the intestinal tract cannot directly absorb fat from the diet before interacting with pancreatic lipase [106]. Several natural compounds, such as terpenoids, tannins, and saponins, can inhibit intestinal lipase, preventing dietary fat breakdown. Consequently, inhibiting pancreatic lipase activity by natural compounds would limit the decomposition of dietary lipids, reducing the incidence of hypercholesterolaemia [107].
The measurement results accord with the elevated total terpenoid level obtained in the n-hexane fraction. The n-hexane fraction, known for its abundant terpenoid content, is likely to contain specific chemicals that exhibit an inhibitory effect against pancreatic lipase. Multiple research findings have indicated that the substances oleanolic acids (triterpenes) and 7-Oxodehydroabietic acid (diterpenes) had a potent ability to hinder the activity of the enzyme pancreatic lipase [108].
Principle component analysis of phytochemical constituent
Identifying active compounds in herbal plant extracts and fractions is essential to determining a suitable application technique [109]. This research employed the UHPLC-HRMS combined with chemometric analysis to perform untargeted metabolomics profiling and classify the chemical compounds of P. canescens extract and fractions. Chemometrics is an effective statistical technique widely applied to analyze secondary metabolites from plants. Identifying bioactive compounds derived from untargeted metabolomic analysis helps obtain specific compounds in each treatment group [110].
Based on the score plot PCA, the n-hexane fraction of P. canescens is separated from other fractions by PC1 (Fig. 1). Meanwhile, the chloroform fraction can be separated from the FEA, FB, and FA by PC2 but not separated from EE. Fractions with medium polarity (FEA and FB) to high polarity (FA) do not separate. This indicates that some of the same compounds are present in the three fractions. The classification results with this score plot PCA are linear with the results of determining the content of total phenolic and total flavonoid compounds (Table 1), which shows that FEA, FB, and FA contain significantly higher amounts of phenolic and flavonoid compounds compared to FH and FC. Likewise, the antioxidant activity of FEA, FB, and FA produces strong to very strong antioxidant activity. This antioxidant activity results from the high phenolic and flavonoid compounds in the three fractions. Pindan et al.[8] in their study also stated that FEA from P. canescens has potent antioxidant activity and contains alkane, alkene, alcohol, and fatty acid compounds based on identification using GC-MS analysis. In addition, the results score plot PCA also shows that fraction with low polarity (FH) has linearity when determining the content of terpenoid compounds. This is in line with its activity, namely pancreatic lipase inhibition activity. Therefore, the chemical compounds separated and contained in FH may be the compounds responsible for pancreatic lipase inhibition activity. This clearly shows that the active compounds made from an extract or fraction are greatly affected by the type of plant and the polarity of the solvent [2]. This study, based on the PCA Biplot (Fig. 2) shows that FH contains several specific compounds that are well separated from other fractions, including compounds 7-Oxodehydroabietic acid, oleanonic acid, oleanolic acid, docosanamide, 9-Oxo-10(E), 12(E)-octadecadienoic acid, eleostearic acid, 1-stearoylglycerol, ethyl linolenate, and 12-Oxo-phytodienoic acid. While FEA, FB, and FA contain several of the same compounds and are not clearly separated, these compounds are grouped in one quadrant near the coordinate point 0.0. The chemical compound in FH was a compound from the terpenoid and fatty acid groups. Several research results confirm that terpenoid compounds have lipase-inhibitory activity [107].
Sosnowska et al. [111] reported in their study that strawberry and raspberry fruit extracts, which have high fatty acid concentrations, strongly inhibit the effect on pancreatic lipase enzymes. Fatty acid components that bind to bile acids can inhibit pancreatic lipase enzymes. The binding of fatty acids to bile acids will affect the surface properties of the substrate emulsion so that lipase cannot bind to the substrate and will be absorbed in the oil-water interface [112].
Furthermore, analysis using PCA also showed the clustering results of compounds by the heatmap of the top 32 phytochemical compounds (Fig. 3); it shows that diosmetin (trihydroxyflavone group) and pinoquercetin (pentahydroxyflavone group) compounds are found in high amounts in FEA. These findings are consistent with the investigation conducted by Latief et al. [18], which states that flavonoid compounds (5.7 dihydroxy isoflavones) can be isolated from the ethyl acetate extract of P. canescens. Flavonoid compounds are a class renowned for their decisive antioxidant action. Various mechanisms, such as the direct scavenging of ROS, the stimulation of antioxidant enzymes, the enhancement of metal chelation activity, the elevation of α-tocopherol radicals, the suppression of NADPH oxidase, the alleviation of oxidative stress induced by NO, the augmentation of uric acid levels, and the reinforcement of the antioxidant capacity of small-molecule antioxidants, are used by flavonoids to exhibit antioxidant activity in vitro [113]. Flavonoids with trihydroxyflavone and pentahydroxyflavone functional groups work as antioxidants by directly capturing reactive oxygen by structural flavonoid molecules [114].
CONCLUSION
The study presented that the ethyl acetate fractions of P. canescens had the highest amounts of phenolic and flavonoid compounds and produced very strong antioxidant activity. The compounds diosmetin and pinoquercetin were identified as compounds that may contribute to the antioxidant activity of the ethyl acetate fraction of P. canescens. In addition, the n-hexane fraction showed significant terpenoid content and a strong inhibitory effect on the lipase enzyme. The compounds 7-Oxodehydroabietic acid, oleanonic acid, oleanolic acid, docosanamide, 9-Oxo-10 (E), 12 (E) -octadecadienoic acid, eleostearic acid, 1-stearoylglycerol, ethyl linolenate, and 12-oxo-phytodienoic acid have been identified from the n-hexane fraction as compounds that play an important role in pancreatic lipase inhibitory activity. Further research to determine the correlation between the amount of active compound content and the bioactivity of the extract and fraction of P. canescens leaves can be done to complement this study’s limitations. Enzyme kinetics analysis and molecular mechanisms of bioactive compounds can be used to validate the data obtained in this study. In addition, further research using experimental animals and optimization of safer solvents will provide more comprehensive bioactivity information and increase our knowledge of ethnomedicine based on P. canescens herbal preparations. The alternative solvents to replace n-hexane and chloroform that are less toxic suggested the use of bio-based solvents, such as sunflower oil, as a substitute for n-hexane and dimethyl carbonate as an alternative to chloroform replacement.
ACKNOWLEDGMENT
The authors thank the Research Center for Food Technology and Processing -National Research and Innovation Agency (PRTPP-BRIN) for providing facilities and collaboration among the research group division.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research is financially supported by the Program House-New Agricultural and Food Product Prototypes-Agricultural and Food Research Organization—National Research and Innovation Agency, Fiscal Year 2024.
CONFLICT OF INTEREST
We officially affirm that no conflicts of interest arise from any financial, personal, or other affiliations with individuals or organizations related to the subject matter addressed in the journal.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Verma S, Singh SP. Current and future status of herbal medicines. Vet World. 2008;1(11):347–50.
2. Ng ZX, Samsuri SN, Yong PH. The antioxidant index and chemometric analysis of tannin, flavonoid, and total phenolic extracted from medicinal plant foods with the solvents of different polarities. J Food Process Preserv. 2020;44:1–11.
3. Chew SY, Teoh SY, Sim YY, Nyam KL. Optimization of ultrasonic extraction condition for maximal antioxidant, antimicrobial, and antityrosinase activity from Hibiscus cannabinus L. leaves by using the single factor experiment. J Appl Res Med Aromat Plants. 2021;25:100321.
4. Rao MV, Sengar AS, Sunil CK, Rawson A. Ultrasonication—A green technology extraction technique for spices: a review. Trends Food Sci Technol. 2021;116:975–91.
5. El Maaiden E, Bouzroud S, Nasser B, Moustaid K, El Mouttaqi A, Ibourki M, et al. A comparative study between conventional and advanced extraction techniques: pharmaceutical and cosmetic properties of plant extracts. Molecules. 2022;27:2074.
6. Duraipandiyan V, Ayyanar M, Ignacimuthu S. Antimicrobial activity of some ethnomedicinal plants used by Paliyar tribe from Tamil Nadu, India. BMC Complement Altern Med. 2006;6:1–17.
7. Ochwang’i DO, Kimwele CN, Oduma JA, Gathumbi PK, Mbaria JM, Kiama SG. Medicinal plants used in treatment and management of cancer in Kakamega County, Kenya. J Ethnopharmacol. 2014;151:1040–55.
8. Pindan NP, Daniel, Saleh C, Magdaleni AR. Phytochemical test and antioxidant activity test of n-hexane fraction extract, ethyl acetate and remained ethanol from leaf of sungkai (Peronema canescens Jack.) using DPPH method. J At. 2021;06:22–7.
9. Fransisca D, Kahanjak DN, Frethernety A. Uji aktivitas antibakteri ekstrak etanol daun sungkai (Peronema canescens Jack) terhadap pertumbuhan Escherichia coli dengan metode difusi cakram Kirby-Bauer. J Pengelolaan Lingkung Berkelanjutan. 2020;4:460–70.
10. Ahmad I, Ibrahim A. Bioaktivitas ekstrak metanol dan fraksi n-heksana daun sungkai (Peronema canescens jack) terhadap larva udang (Artemia salina Leach). J Sains dan Kesehat. 2015;1:114–9.
11. Ramadenti F, Sundaryono A, Handayani D. Uji fraksi etil asetat daun Peronema canescens terhadap Plasmodium berghei pada Mus musculus. Alotrop J Pendidik dan Ilmu Kim. 2017;2:89–92.
12. Kusriani RH, Nawawi A, Turahman T. Uji aktivitas antibakteri ekstrak dan fraksi kulit batang dan daun sungkai (Peronema canescens Jack) terhadap Staphylococcus aureus ATCC 25923 dan Escherichia coli ATCC 25922. J Farm Galen Vol. 2015;2:8–14. [Internet] Available from: https://www.jfg.stfb.ac.id/index.php/jfg/article/view/24
13. Maigoda T, Judiono J, Purkon DB, Haerussana ANEM, Mulyo GPE. Evaluation of Peronema canescens leaves extract: fourier transform infrared analysis, total phenolic and flavonoid content, antioxidant capacity, and radical scavenger activity. Open Access Maced J Med Sci. 2022;10:117–24.
14. Okfrianti Y. Aktivitas antioksidan ekstrak etanol daun sungkai (Peronema canescens Jack). J Kesehat. 2022;13:333–9. [Internet] Available from: http://ejurnal.poltekkes-tjk.ac.id/index.php/JK
15. Sutomo S, Arnida A, Yulistati FR, Normaidah N, Pratama MRF. Pharmacognostic study and antioxidant activity of sungkai (Peronema canescens Jack.) methanol extract from Indonesia. Bull Pharm Sci Assiut. 2022;45:655–65.
16. Juswardi J, Delsy Amalia I, Sriwijaya U. Metabolite profile of false elder leaves (Peronema canescens Jack.) based on development levels. Int J Life Sci. 2023;11:143–50.
17. Muharni M, Ferlinahayati F, Yohandini H, Riyanti F, Pakpahan Nap. The anticholesterol activity of betulinic acid and stigmasterol isolated from the leaves of sungkai (Peronema canescens Jack). Int J Appl Pharm. 2021;13:198–203.
18. Latief M, Sutrisno, Dasrinal E, Safitri W, Tarigan IL. Immunomodulator activity of 5,7-dihydroxy isoflavones and β-Sitosterol from Peronema canescens Jack leaves methanol and ethyl acetate extract. Proceedings of the 4th Green Development International Conference (GDIC 2022) 2023 [Internet]; Amsterdam, The Netherlands: Atlantis Press; 2023. pp 558–72. Available from: http://dx.doi.org/10.2991/978-2-38476-110-4_57
19. Yao P, Liu Y. Terpenoids: natural compounds for non-alcoholic fatty liver disease (NAFLD) therapy. Molecules. 2023;28(1):272.
20. Ramabulana T, Ndlovu M, Mosa RA, Sonopo MS, Selepe MA. Phytochemical profiling and isolation of bioactive compounds from Leucosidea sericea (Rosaceae). ACS Omega. 2022;7:11964–72.
21. De Melo CL, Queiroz MGR, Arruda Filho AC, Rodrigues AM, De Sousa DF, Almeida JGL, et al. Betulinic acid, a natural pentacyclic triterpenoid, prevents abdominal fat accumulation in mice fed a high-fat diet. J Agric Food Chem. 2009;57:8776–81.
22. Pratiwi U, Muharni M, Ferlinahayati F, Yohandini H, Suheryanto S. Antihyperlipidemia activity of sungkai (Peronema canescens) leaves extract in albino rats Rattus noverticus (Wistar strain) with propylthiouracil-induced hyperlipidemia. Med Plants. 2022;14:589–96.
23. Nguyen HC, Lin KH, Huang MY, Yang CM, Shih TH, Hsiung TC, et al. Antioxidant activities of the methanol extracts of various parts of Phalaenopsis orchids with white, yellow, and purple flowers. Not Bot Horti Agrobot Cluj-Napoca. 2018;46:457–65.
24. Nowak A, Czyzowska A, Efenberger M, Krala L. Polyphenolic extracts of cherry (Prunus cerasus L.) and blackcurrant (Ribes nigrum L.) leaves as natural preservatives in meat products. Food Microbiol. 2016;59:142–9. [Internet] Available from: https://doi.org/10.1016/j.fm.2016.06.004
25. Chang C, Yang M, Wen H, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–82.
26. Lin MS, Yu ZR, Wang BJ, Wang CC, Weng YM, Koo M. Bioactive constituent characterization and antioxidant activity of Ganoderma lucidum extract fractionated by supercritical carbon dioxide. Sains Malaysiana. 2015;44:1685–91.
27. Mahmood A, Ngah N, Omar MN. Phytochemicals constituent and antioxidant activities in Musa x paradisiaca flower. Eur J Sci Res. 2011;66:311–8.
28. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7.
29. Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: the FRAP assay. Anal Biochem. 1996;239:70–6.
30. Lévuok Mena KP, Patiño Ladino OJ, Prieto Rodríguez JA. In vitro inhibitory activities against α-glucosidase, α-amylase, and pancreatic lipase of medicinal plants commonly used in Chocó (Colombia) for type 2 diabetes and obesity treatment. Sci Pharm. 2023;91:49.
31. Wiyono T, Frediansyah A, Sholikhah EN, Pratiwi WR. UHPLC-ESI-MS analysis of Javanese Tamarindus indica leaves from various tropical zones and their beneficial properties in relation to antiobesity. J Appl Pharm Sci. 2022;12:137–47.
32. Windarsih A, Suratno, Warmiko HD, Indrianingsih AW, Rohman A, Ulumuddin YI. Untargeted metabolomics and proteomics approach using liquid chromatography-Orbitrap high resolution mass spectrometry to detect pork adulteration in Pangasius hypopthalmus meat. Food Chem. 2022;386:132856. [Internet] Available from: https://doi.org/10.1016/j.foodchem.2022.132856
33. Wenzl T, Haedrich J, Schaechtele A, Robouch P, Stroka J. Guidance document on the estimation of LOD and LOQ for measurements in the field of contaminants in feed and food. EUR 28099. Luxembourg: Publications Office of the European Union; 2016. doi: https://doi.org/10.2787/8931
34. Aydo?an C. Recent advances and applications in LC-HRMS for food and plant natural products: a critical review. Anal Bioanal Chem. 2020;412:1973–91.
35. Park JS, Park HY, Kim DH, Kim DH, Kim HK. Ortho-dihydroxyisoflavone derivatives from aged Doenjang (Korean fermented soypaste) and its radical scavenging activity. Bioorganic Med Chem Lett. 2008;18(18):5006–9.
36. Liao W, Chen L, Ma X, Jiao R, Li X, Wang Y. Protective effects of kaempferol against reactive oxygen species-induced hemolysis and its antiproliferative activity on human cancer cells. Eur J Med Chem. 2016;114:24–32.
37. Seo YH, Kang SY, Shin JS, Ryu SM, Lee AY, Choi G, et al. Chemical constituents from the aerial parts of Agastache rugosa and their inhibitory activities on prostaglandin E2 production in lipopolysaccharide-treated RAW 264.7 Macrophages. J Nat Prod. 2019;82(12):3379–85.
38. Kishore N, Twilley D, Blom Van Staden A, Verma P, Singh B, Cardinali G, et al. Isolation of flavonoids and flavonoid glycosides from Myrsine africana and their inhibitory activities against mushroom tyrosinase. J Nat Prod. 2018;81(1):49–56.
39. Boukaabache R, Kerkatou W, Bensouici C, Benayache F, Boumaza O. A new isoflavonoid derivative, evaluation of the antioxidant capacity and phenolic content of Genista lobelii DC. Nat Prod Res. 2024;38:3842–7.
40. Cuong TD, Hung TM, Kim JC, Kim EH, Woo MH, Choi JS, et al. Phenolic compounds from Caesalpinia sappan heartwood and their anti-inflammatory activity. J Nat Prod. 2012;75(12):2069–75.
41. Losada Barreiro S, Bravo Díaz C. Free radicals and polyphenols: the redox chemistry of neurodegenerative diseases. Eur J Med Chem. 2017;133:379–402.
42. Xiao Y, Ren Q, Wu L. The pharmacokinetic property and pharmacological activity of acteoside: a review. Biomed Pharmacother. 2022;153:113296.
43. Yang JH, Kondratyuk TP, Jermihov KC, Marler LE, Qiu X, Choi Y, et al. Bioactive compounds from the fern Lepisorus contortus. J Nat Prod. 2011;74(2):129–36.
44. Yang X, Huang E, Yuan C, Zhang L, Yousef AE. Isolation and structural elucidation of brevibacillin, an antimicrobial lipopeptide from Brevibacillus laterosporus that combats drug-resistant Gram-positive bacteria. Appl Environ Microbiol. 2016;82(9):2763–72.
45. Cantrell CL, Case BP, Mena EE, Kniffin TM, Duke SO, Wedge DE. Isolation and identification of antifungal fatty acids from the basidiomycete Gomphus floccosus. J Agric Food Chem. 2008;56(13):5062–8.
46. Choi JY, Lee JW, Jang H, Kim JG, Lee MK, Hong JT, et al. Quinic acid esters from Erycibe obtusifolia with antioxidant and tyrosinase inhibitory activities. Nat Prod Res. 2021;35(18):3026–32.
47. Swaminathan P, Kalva S, Saleena L. E-Pharmacophore and molecular dynamics study of flavonols and dihydroflavonols as inhibitors against dihydroorotate dehydrogenase. Comb Chem High Throughput Screen. 2014;17(8):663–73.
48. Miao M, Xiang L. Pharmacological action and potential targets of chlorogenic acid. Adv Pharmacol. 2020;87:71–88.
49. Lv J, Sharma A, Zhang T, Wu Y, Ding X. Pharmacological review on asiatic acid and its derivatives: a potential compound. SLAS Technol. 2018;23(2):111–27.
50. Peixoto JAB, Álvarez-Rivera G, Costa ASG, Machado S, Cifuentes A, Ibáñez E, et al. Contribution of phenolics and free amino acids on the antioxidant profile of commercial lemon verbena infusions. Antioxidants. 2023;12(2):251.
51. Huang J, Xie M, He L, Song X, Cao T. Chlorogenic acid: a review on its mechanisms of anti-inflammation, disease treatment, and related delivery systems. Front Pharmacol. 2023;14:1218015.
52. Zardini HZ, Davarpanah M, Shanbedi M, Amiri A, Maghrebi M, Ebrahimi L. Microbial toxicity of ethanolamines—multiwalled carbon nanotubes. J Biomed Mater Res—Part A. 2014;102(6):1774–81.
53. Pieretti S, Saviano A, Mollica A, Stefanucci A, Aloisi AM, Nicoletti M. Calceolarioside a, a phenylpropanoid glycoside from calceolaria spp., displays antinociceptive and anti-inflammatory properties. Molecules. 2022;27(7):2183.
54. Goswami SK, Gangadarappa SK, Vishwanath M, Razdan R, Jamwal R, Bhadri N, et al. Antioxidant potential and ability of phloroglucinol to decrease formation of advanced glycation end products increase efficacy of sildenafil in diabetes-induced sexual dysfunction of rats. Sex Med. 2016;4(2):106–14.
55. Jesus JA, Lago JHG, Laurenti MD, Yamamoto ES, Passero LFD. Antimicrobial activity of oleanolic and ursolic acids: an update. Evidence-based Complement Altern Med. 2015;(1):620472.
56. Xue J, Zhu L, Zhu X, Li H, Ma C, Yu S, et al. Tetradecylamine-MXene functionalized melamine sponge for effective oil/water separation and selective oil adsorption. Sep Purif Technol. 2021;259:118106.
57. Fikriya SH, Cahyana AH. Study of antioxidant activity of the derivatives of quinoline-4-carboxylic acids by the modification of isatin via pfitzinger reaction. Makara J Sci. 2023;27(2):9.
58. El Sayed ZI, Hassan WHB, Abdel-Aal MM, Al-Massarani SM, Abdel-Mageed WM, Basudan OA, et al. Chemical and biological characterization of the ethyl acetate fraction from the red sea marine sponge Hymedesmia sp. Pharmaceuticals. 2024;17(6):724.
59. Castellano JM, Ramos-Romero S, Perona JS. Oleanolic acid: extraction, characterization and biological activity. Nutrients. 2022;14(3):623.
60. Chen L, Tang Y, Tao Y, Seerat A, Zhu P, He T, et al. Accumulation of salicylic acid and 12-oxophytodienoic acid acting on the antioxidant pathway to keep stability of striped leaves of variegated temple bamboo. J Am Soc Hortic Sci. 2024;149(1):27–36.
61. Zhu JX, Guo MX, Zhou L, Yi LT, Huang HL, Wang HL, et al. Evaluation of the anti-inflammatory material basis of Lagotis brachystachya in HepG2 and THP-1 cells. J Ethnopharmacol. 2024;318:117055.
62. C T S, Balachandran I. LC/MS characterization of antioxidant flavonoids from Tragia involucrata L. Beni-Suef Univ J Basic Appl Sci. 2016;5(3):231–5.
63. Céliz G, Alfaro FF, Cappellini C, Daz M, Verstraeten S V. Prunin- and hesperetin glucoside-alkyl (C4-C18) esters interaction with Jurkat cells plasma membrane: Consequences on membrane physical properties and antioxidant capacity. Food Chem Toxicol. 2013;55:411–23.
64. Cai J, Wen H, Zhou H, Zhang D, Lan D, Liu S, et al. Naringenin: a flavanone with anti-inflammatory and anti-infective properties. Biomed Pharmacother. 2023;164:114990.
65. Nagao K, Inoue N, Suzuki K, Shimizu T, Yanagita T. The cholesterol metabolite cholest-5-en-3-one alleviates hyperglycemia and hyperinsulinemia in obese (db/db) Mice. Metabolites. 2022;12(1):26.
66. Ribeiro VP, Arruda C, Aldana-Mejia JA, Bastos JK, Tripathi SK, Khan SI, et al. Phytochemical, antiplasmodial, cytotoxic and antimicrobial evaluation of a Southeast brazilian brown propolis produced by Apis mellifera Bees. Chem Biodivers. 2021;18(9):2100288.
67. Yang XW, Feng L, Li SM, Liu XH, Li YL, Wu L, et al. Isolation, structure, and bioactivities of abiesadines A-Y, 25 new diterpenes from Abies georgei Orr. Bioorganic Med Chem. 2010;18(2):744–54.
68. Suárez-Rebaza LA, de Albuquerque RDDG, Zavala E, Alva-Plasencia PM, Ganoza-Suárez MM, Ganoza-Yupanqui ML, et al. Chemical composition and antioxidant capacity of purified extracts of Prosopis pallida (Humb. & Bonpl. ex Willd.) Kunth (Fabaceae) fruits from Northern Peru. Bol Latinoam y del Caribe Plantas Med y Aromat. 2023;22(5):594–606.
69. Yoshioka Y, Yoshimura N, Matsumura S, Wada H, Hoshino M, Makino S, et al. α-glucosidase and pancreatic lipase inhibitory activities of diterpenes from Indian mango ginger (Curcuma amada roxb.) and its derivatives. Molecules. 2019;24(22):4071.
70. Park SY, Seetharaman R, Ko MJ, Kim DY, Kim TH, Yoon MK, et al. Ethyl linoleate from garlic attenuates lipopolysaccharide-induced pro-inflammatory cytokine production by inducing heme oxygenase-1 in RAW264.7 cells. Int Immunopharmacol. 2014;19(2):253–61.
71. Warinthip N, Liawruangrath B, Natakankitkul S, Pojanakaroon T, Rannurags N, Pyne SG, et al. Chemical constituents from leaves of Gardenia sootepensis and Pseudomussaenda flava biological activity and antioxidant activity. Chiang Mai Univ J Nat Sci. 2022;21(1):2022004.
72. Jin Z, Li L, Zheng Y, An P. Inhibition of Bacillus cereus by garlic (Allium sativum) essential oil during manufacture of white sufu, a traditional Chinese fermented soybean curd. LWT. 2020;130:109634.
73. Hussein K, Eswaramoorthy R, Melaku Y, Endale M. Antibacterial and antioxidant activity of isoflavans from the roots of Rhynchosia ferruginea and in silico study on dna gyrase and human peroxiredoxin. Int J Second Metab. 2021;8(4):321–36.
74. Kobori M, Ohnishi-Kameyama M, Akimoto Y, Yukizaki C, Yoshida M. α-Eleostearic acid and its dihydroxy derivative are major apoptosis-inducing components of bitter gourd. J Agric Food Chem. 2008;56(2):10515–20.
75. El Houda Lezoul N, Belkadi M, Habibi F, Guillén F. Extraction processes with several solvents on total bioactive compounds in different organs of three medicinal plants. Molecules. 2020;25(20):4672
76. Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ismadji S, et al. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal. 2014;22:296–302. [Internet] Available from: http://dx.doi.org/10.1016/j.jfda.2013.11.001
77. Jha AK, Sit N. Extraction of bioactive compounds from plant materials using combination of various novel methods: a review. Trends Food Sci Technol. 2022;119:579–91.
78. González-Centeno MR, Knoerzer K, Sabarez H, Simal S, Rosselló C, Femenia A. Effect of acoustic frequency and power density on the aqueous ultrasonic-assisted extraction of grape pomace (Vitis vinifera L.)—a response surface approach. Ultrason Sonochem. 2014;21(6):2176–84.
79. Muzykiewicz-Szyma?ska A, Kucharska E, Pe?ech R, Nowak A, Jakubczyk K, Kucharski ?. The optimisation of ultrasound-assisted extraction for the polyphenols content and antioxidant activity on Sanguisorba officinalis l. Aerial parts using response surface methodology. Appl Sci. 2024;14(20):9579.
80. Thoo YY, Ho SK, Liang JY, Ho CW, Tan CP. Effects of binary solvent extraction system, extraction time and extraction temperature on phenolic antioxidants and antioxidant capacity from mengkudu (Morinda citrifolia). Food Chem. 2010;120(1):290–5.
81. Xu DP, Zhou Y, Zheng J, Li S, Li AN, Li H Bin. Optimization of ultrasound-assisted extraction of natural antioxidants from the flower of Jatropha integerrima by response surface methodology. Molecules. 2016;21(1):18.
82. Indrianingsih AW, Styaningrum P, Suratno, Windarsih A, Suryani R, Noviana E, et al. The effect of extraction method on biological activity and phytochemical content of Artocarpus heterophyllus (jackfruit) leaves extract concurrent with its principal component analysis. Process Biochem. 2024;143:135–47.
83. Yim HS, Chye FY, Koo SM, Matanjun P, How SE, Ho CW. Optimization of extraction time and temperature for antioxidant activity of edible wild mushroom, Pleurotus porrigens. Food Bioprod Process. 2012;90(2):235–42.
84. González Montelongo R, Gloria Lobo M, González M. Antioxidant activity in banana peel extracts: testing extraction conditions and related bioactive compounds. Food Chem. 2010;119:1030–9.
85. Kaczorová D, Karalija E, Dahija S, Bešta-Gajevi? R, Pari? A, ?avar Zeljkovi? S. Influence of extraction solvent on the phenolic profile and bioactivity of two achillea species. Molecules. 2021;26:1601–16.
86. Lapkin AA, Plucinski PK, Cutler M. Comparative assessment of technologies for extraction of artemisinin. J Nat Prod. 2006;1653–64.
87. Fadlilaturrahmah F, Khairunnisa A, MP Putra A, Sinta I. Uji aktivitas tabir surya dan antioksidan ekstrak etanol daun sungkai (Perenema canescens Jack). J Ilm Ibnu Sina Ilmu Farm dan Kesehat. 2021;6:322–30.
88. Mundim FM, Pringle EG. Whole-plant metabolic allocation under water stress. Front Plant Sci. 2018;9:852–63.
89. Benhssain K, Aabdousse J, Salim N, Oussif I, Ramchoun M, Elhabty M, et al. Effect of geographical origin on yield and secondary metabolite content of extracts of Moroccan Juniperus thurifera. Aust J Crop Sci. 2023;17:591–9.
90. Saada M, Falleh H, Catarino MD, Cardoso SM, Ksouri R. Plant growth modulates metabolites and biological activities in retama raetam (forssk.) webb. Molecules. 2018;23:1–17.
91. Singh A, Kumar V. Phyto-chemical and bioactive compounds of pumpkin seed oil as affected by different extraction methods. Food Chem Adv. 2023;2:1–9. [Internet] Available from: https://doi.org/10.1016/j.focha.2023.100211
92. Muharni M, Ferlinahayati F, Yohandini H. Antioxidant, antibacterial, total phenolic and flavonoid contents of sungkai leaves (Peronema canescens). Trop J Nat Prod Res. 2021;5:528–33.
93. Gulcin ?. Antioxidants and antioxidant methods: an updated overview. Arch Toxicol. 2020;94(3):651–715.
94. Faraone I, Rai DK, Chiummiento L, Fernandez E, Choudhary A, Prinzo F, et al. Antioxidant activity and phytochemical characterization of Senecio clivicolus wedd. Molecules. 2018;23:1–17.
95. Lremizi I, Ait A, Chawki O, Marie B, Fauconnier L. Chemical composition, antioxidant, anticholinesterase, and alpha?glucosidase activity of Stevia rebaudiana Bertoni extracts cultivated in Algeria. J Food Meas Charact. 2023;17:2639–50.
96. Ait Chaouche FS, Mouhouche F, Hazzit M. Antioxidant capacity and total phenol and flavonoid contents of Teucrium polium L. grown in Algeria. Med J Nutrition Metab. 2018;11:135–44.
97. Elufioye TO, Chinaka CG, Oyedeji AO. Antioxidant and anticholinesterase activities of Macrosphyra longistyla (DC) hiern relevant in the management of Alzheimer’s disease. Antioxidants. 2019;8:400.
98. Nimse SB, Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015;5:27986–8006.
99. Zeb A. Concept, mechanism, and applications of phenolic antioxidants in foods. J Food Biochem. 2020;44(9):13394.
100. Mohamed Isa SSP, Ablat A, Mohamad J. The antioxidant and xanthine oxidase inhibitory activity of Plumeria rubra flowers. Molecules. 2018;23:400–18.
101. Olszowy-Tomczyk M. Synergistic, antagonistic and additive antioxidant effects in the binary mixtures. Phytochem Rev. 2020;19(5):63–103 [Internet]. doi: https://doi.org/10.1007/s11101-019-09658-4
102. Alara OR, Abdurahman NH, Ukaegbu CI. Extraction of phenolic compounds: a review. Curr Res Food Sci. 2021;4:200–14. [Internet] Available from: https://doi.org/10.1016/j.crfs.2021.03.011
103. Irnameria D, Okfrianti Y. Perbedaan aktivitas antioksidan ekstrak daun sungkai dengan menggunakan pelarut metanol dan aquades. Agritepa. 2023;10:219–28.
104. Molyneux P. The use of the stable free radical Diphenylpicryl-hydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol. 2004;26:211–9.
105. Liu Y, Liu C, Li J. Comparison of vitamin C and its derivative antioxidant activity: evaluated by using density functional theory. ACS Omega. 2020;5:25467–75.
106. Han LK, Kimura Y, Kawashima M, Takaku T, Taniyama T, Hayashi T, et al. Anti-obesity effects in rodents of dietary teasaponin, a lipase inhibitor. Int J Obes. 2001;25(10):1459–64.
107. Patil M, Patil R, Bhadane B, Mohammad S, Maheshwari V. Pancreatic lipase inhibitory activity of phenolic inhibitor from endophytic Diaporthe arengae. Biocatal Agric Biotechnol. 2017;10:234–8.
108. Birari RB, Bhutani KK. Pancreatic lipase inhibitors from natural sources: unexplored potential. Drug Discov Today. 2007;12:879–89.
109. Ahda M, Jaswir I, Khatib A, Ahmed QU, Mahfudh N, Ardini YD, et al. Phytochemical analysis, antioxidant, α-glucosidase inhibitory activity, and toxicity evaluation of Orthosiphon stamineus leaf extract. Sci Rep. 2023;13:1–11.
110. Kang C, Zhang Y, Zhang M, Qi J, Zhao W, Gu J, et al. Screening of specific quantitative peptides of beef by LC-MS/MS coupled with OPLS-DA. Food Chem. 2022;387:132932. [Internet] Available from: https://doi.org/10.1016/j.foodchem.2022.132932
111. Sosnowska D, Pods?dek A, Redzynia M, ?y?elewicz D. Effects of fruit extracts on pancreatic lipase activity in lipid emulsions. Plant Foods Hum Nutr. 2015;70:344–50.
112. Kahlon TS, Smith GE. In vitro binding of bile acids by blueberries (Vaccinium spp.), plums (Prunus spp.), prunes (Prunus spp.), strawberries (Fragaria X ananassa), cherries (Malpighia punicifolia), cranberries (Vaccinium macrocarpon) and apples (Malus sylvestris). Food Chem. 2007;100:1182–7.
113. Shen N, Wang T, Gan Q, Liu S, Wang L, Jin B. Plant flavonoids: classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022;383:132531.
114. Zuo AR, Dong HH, Yu YY, Shu QL, Zheng LX, Yu XY, et al. The antityrosinase and antioxidant activities of flavonoids dominated by the number and location of phenolic hydroxyl groups. Chinese Med (United Kingdom). 2018;13:1–12. [Internet] Available from: https://doi.org/10.1186/s13020-018-0206-9
SUPPLEMENTARY MATERIAL
The supplementary material can be accessed at the link here: [https://japsonline.com/admin/php/uploadss/4515_pdf.pdf]