INTRODUCTION
Citrus species, part of the Rutaceae family, are globally renowned for their popular fruits and diverse health benefits. Among them, sweet oranges (Citrus sinensis) play a significant role, constituting approximately 70% of the world’s citrus production and consumption. Alongside sweet oranges, other widely cultivated and consumed Citrus species include tangerines or mandarins (Citrus reticulata), grapefruits (Citrus vitis), limes (Citrus aurantifolia), and lemons (Citrus limon) [1].
Indonesia has a substantial Citrus production of approximately 2.6 million tons per year, encompassing 255 different varieties, including sweet (C. sinensis), mandarin (C. reticulata), sour (Citrus aurantium), pomelo oranges (Citrus maxima), tangerines (Citrus nobilis), lemons, limes, and so on [2,3]. Citrus, beyond its application as a condiment, is utilized in sweet delicacies in European nations, enhancing dishes such as pan-seared chicken with orange-brandy sauce or pork tenderloin with blood oranges [4]. Notably, C. limon and Citrus hystrix are extensively used as spices in Asian countries due to their distinctive scents and natural oils [5,6].
Citrus essential oils (EOs), obtained through hydro distillation of citrus peels, are rich sources of bioactive compounds with applications in the pharmaceutical and food industries [7]. Comprising a complex mixture of aldehydes, esters, alcohols, ketones, acids, monoterpenes, and sesquiterpenes [8], these EOs exhibit diverse biological activities, including antibacterial, antiviral, fungicidal, and antioxidant properties [9,10]. Beyond their antimicrobial effects, Citrus EOs also serve a crucial role as antioxidative agents, protecting organisms and tissues from damage caused by reactive oxygen species (ROS) [11].
Previous studies have unveiled the phytochemical makeup of EOs derived from Citrus species. Among these components, the pivotal active compound found in Citrus EOs is D-limonene. However, its concentration significantly fluctuates across Citrus species, spanning from 34.2% to 81.9% of the total compounds. Lemon EO exhibits stronger antioxidant activity, as assessed by (2,2- azinobis-3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and (2,2-diphenyl-1-picrylhydrazyl) (DPPH) free radicals, compared to other Citrus EOs [12]. The major components of this EO are Limonene (67.1%), α-terpinene (8.0%), and α-pinene (11.0%) [13]. In another study, C. limon EO also demonstrated significant inhibition in the growth of Solanum lycopersicum and Lepidium sativum germinating seeds at a level of 100 µg/mL in comparison with C. myrtifolia and C. bergamia EOs [14]. Additionally, this EO was reported to exhibit moderate toxicity in animal gavage with 500 and 100 mg/kg doses at the sub-chronic stage [15].
Nevertheless, the precise phytochemical constituents responsible for the biological properties of Citrus EOs, especially across diverse Citrus species in Indonesia, remain unknown. This study aims to determine these associations by examining the chemical composition of EOs and their corresponding antioxidant, allelopathic, and cytotoxic characteristics, utilizing principal component analysis (PCA) and hierarchical cluster analysis (HCA). The significance of this study extends beyond uncovering the therapeutic potential inherent in Citrus EOs; it also delves into exploring their promising applications across agricultural, medicinal, and interdisciplinary domains.
MATERIALS AND METHODS
Plant materials and EO extraction
The fruits of C. sinensis, C. limon, and C. hystrix were purchased commercially (Jogjakarta market). A total of 5 kg of each Citrus species was peeled manually and the peels were air dried for 12 hours before being extracted by a Clevenger hydro distillation system. Lactuca sativa and Raphanus sativus seeds were obtained commercially (PT. Panah Merah, Purwakarta, Indonesia). These seeds were tested for germination and more than 90% were alive before being used in a plant inhibitory assay.
DPPH radical scavenging activity
The method described by Andriana et al. [16] was employed to assess the DPPH free radical scavenging activity of Citrus EOs. The absorbances of samples were measured at 517 nm and expressed as radical scavenging activity percentage.
Plant inhibitory potentials
Plant inhibitory assay was conducted using moist filter paper placed in a 12 well-plate (22.1 mm in diameter and 35 mm in depth) following the method reported by Minh et al. [17]. The seeds of the indicator plants were lettuce (L. sativa) and radish (R. sativus). Plant inhibitory potentials on L. sativa and R. sativus were expressed in inhibitory percentage of germination, shoot, and root size over control.
Evaluation of cytotoxicity by the brine shrimp lethality assay
Cytotoxic activity of Citrus EOs was assessed using the brine shrimp (Artemia salina) lethality bioassay, with 5 mg of each extract dissolved in DMSO and serial dilutions in simulated seawater. After 24 hours, surviving brine shrimp nauplii were counted, and mortality was determined based on the absence of regulated forward movement within 30 seconds [18,19].
Total phenolic contents
Total phenolic contents in Citrus EOs were determined using the Folin Ciocalteu (FC) reagent as reported previously [16] and expressed as milligrams of gallic acid equivalent (GAE) per gram of the sample.
Total flavonoid contents
Total flavonoid contents were evaluated based on the method reported previously and expressed as quantified and reported in milligrams of quercetin (QE) per gram of the sample [20].
Identification of functional groups by Fourier-transform infrared spectroscopy (FTIR)
An FTIR analysis was performed to find the chemical functional groups in EO samples following the procedure described previously by Indrianingsih et al. [21]. The spectra were measured by the attenuated total reflection method and displayed as the percentage of transmittance.
Identification of chemical constituents by gas chromatography-mass spectrometry (GC-MS)
The phytochemical constituents of Citrus EOs were identified by a GC-MS system (Agilent 7890B/MSD 5977 A, Agilent Technology, Inc., Santa Clara, CA) following the previous method [22]. Data peak processing was managed by the Agilent Chem Station software, incorporating the National Institute of Standards and Technology mass spectral library (Agilent Technology, USA).
Statistical analysis
The data analysis was conducted using MetaboAnalyst 5.0 software (https://metaboanalyst.ca/) with the one-way analysis of variance method and Tukey’s test with a 95% confidence interval (p < 0.05) to determine the significance level among samples. A multivariate analysis was employed to reduce dimensional parameters, utilizing a correlation matrix consisting of 9 samples and their replicates as well as 103 variables (GC/MS peak area %) and antioxidant activities. HCA was applied to illustrate clusters and interrelationships between samples, forming the basis for the hierarchical clustering algorithms [23].
RESULTS
Assessment of DPPH scavenging activity, total phenolic and flavonoid contents
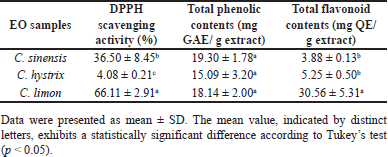
The antioxidant activities, total flavonoid, and phenolic contents of EOs from three different types of citruses are shown in Table 1. Citrus sinensis EO demonstrated the strongest antioxidant activity compared to the other samples. Conversely, the total flavonoid contents of C. limon EO exhibited the highest value among all samples. There is a relationship between the total phenolic contents and the antioxidant activities of the samples.
 | Table 1. DPPH scavenging activity, total flavonoid, and phenolic contents. [Click here to view] |
 | Table 2. Brine shrimp lethality property of EOs from three species of Citrus. [Click here to view] |
Brine shrimp lethality assay
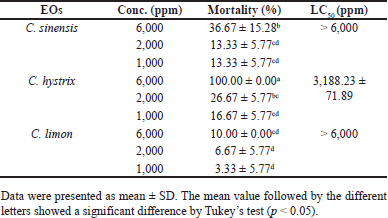
Table 2 shows the brine shrimp lethality test of EO samples. The C. hystrix EO demonstrated the highest death rate, with an LC50 value of 3188.23 ± 71.89 ppm. On the contrary, C. sinensis and C. limon EOs exhibit a requirement exceeding 6,000 ppm to induce mortality in brine shrimp nauplii.
Plant inhibitory activity
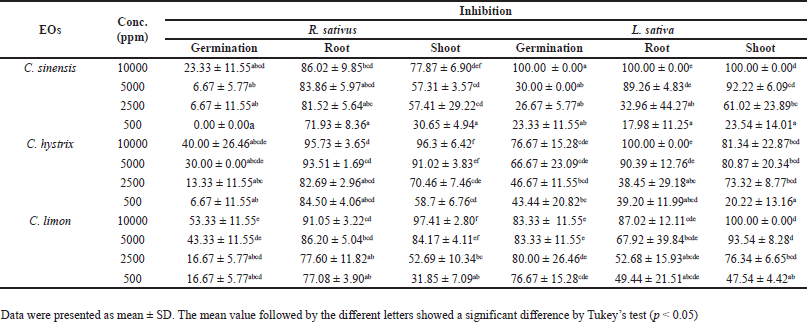
Table 3 illustrates the inhibitory activity of Citrus EOs on the germination and growth of R. sativus and L. sativa. The C. limon EO exhibited a significant inhibitory effect on R. sativus germination and shoot growth. At a concentration of 10,000 ppm, the observed inhibition rates on R. sativus germination and shoot length were the highest, measuring at 53.33% ± 11.55% and 97.41% ± 2.80%, respectively. On the other hand, C. hystrix EO demonstrated a strong inhibitory activity on the root length of R. sativus (95.73% ± 3.65%). The C. sinensis EO completely inhibited the germination and growth of L. sativa at a concentration of 10,000 ppm followed by the C. limon and C. hystrix EOs, respectively.
Identification of the functional group of Citrus EOs
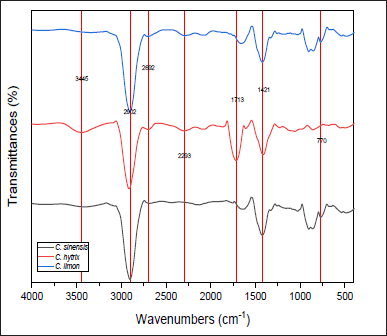
Figure 1 shows the FTIR spectra of EOs from three species of Citrus. According to this figure, there are transmittances in wave numbers 3445, 2902, 2692, 2293, 1713, 1421, and 770 cm-1. The wavenumber range of 3650–3250 cm-1 indicated the presence of hydrogen bond (OH) presence in EO samples. C. hystrix EO showed a higher intensity in hydrogen bonds. Furthermore, all samples have a very low transmittance in wavenumber of 2,902 cm−1, indicating the presence of aliphatic compounds in all samples. A slight transmittance intensity was also shown at a wavenumber of 2,293 cm−1, indicating the presence of triple bond carbon because it was followed with spectra between 1,600 and 1,300 cm−1. Moreover, wavenumber 1,713 cm−1 indicated the double bond carbon (C=C), and all samples seem to possess a high-intensity transmittance at 1,421 cm−1 that indicates the presence of Vinyl C–H in-plane bend. The final transmittance detected at 770 cm−1 showed the content of the phenyl group in the EO samples [24].
 | Table 3. Plant inhibitory potentials of EOs from three species of Citrus species. [Click here to view] |
 | Figure 1. FTIR spectra of EOs from three species of Citrus. [Click here to view] |
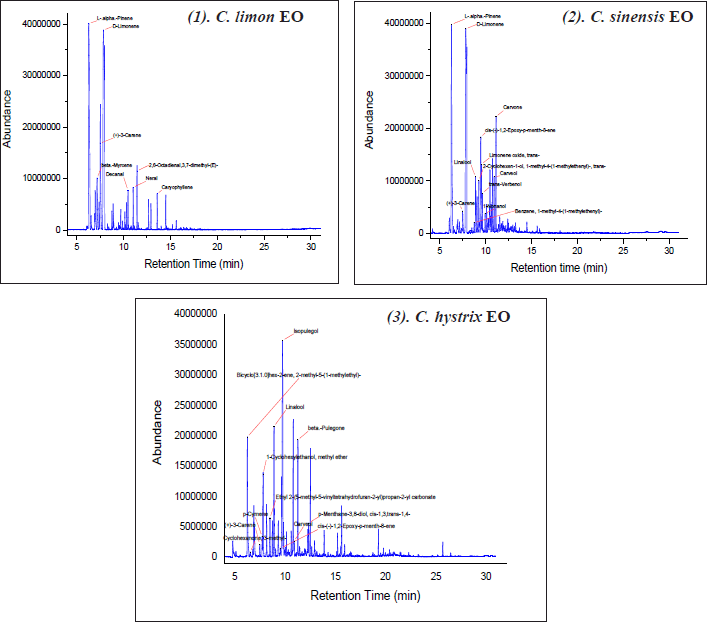 | Figure 2. GC-MS chromatograms of EO samples. [Click here to view] |
Identification of phytochemical constituent of essential oils of Citrus by GC-MS
Supplementary Material 1 shows the phytochemical constituents of Citrus EOs, while Figure 2 illustrates their GC-MS chromatograms. A total of 103 compounds from various chemical classes were identified in EO samples by a GC-MS system. Of which, D-limonene (27.86%), L-α-Phinene (16.64%), cis-(-)-1,2-Epoxy-p-menth-8-ene (5.24%), and Carvone (4.65 %) were accounted as major components of C. sinensis EO. On the other hand, p-Cymene (5.96%), Ethyl 2-(5-methyl-5-vinyltetrahydrofuran-2-yl) propan-2-yl carbonate (7.02%), Isopulegol (15.95%), trans-Carveol (6.79), and β-Pulegone (6.91) were the dominant compounds containing in C. hystrix EO. While for C. limon EO, L-α-Pinene (27.43%), D-limonene (36.41%), and p-Menthatriene (9.26%) were significant contents. These various chemical components might affect the biological activity of Citrus EO. However, which components contribute to their antioxidant, plant inhibitory, and cytotoxic properties require more investigation.
Chemometric analysis based on GC-MS chemical profiles concerning antioxidant activity
Figure 3 shows PCA score plots of GC-MS chemical profiles and antioxidant activities as the target cell of classification. PCA successfully reduced variables measured from all samples into two principal components, PC1 of 58.8% and PC2 of 34.7%, which explained about 93.1% of the variation in the dataset. Notably, C. limon and C. sinensis EOs exhibited similar antioxidant activity, so they are placed in the same negative ordinate (quadrants II and III).
Figure 4 displays the PCA loading plot based on the chemical profiles and antioxidant activities of EO samples determined by the GC-MS system. The Citrus EO samples were clearly dispersed along the PCs in the PCA plot. D-limonene and α-terpineol were among the most discriminating chemicals on negative PC1, while for negative PC2, they were caryophyllene and (+)-3-carene. Conversely, β-pinene and (-)-spathulenol exhibited high selectivity for positive PC1; however, trans-p-mentha-1(7),8-dien-2-ol and trans-verbenol affected the positive PC2.
Figure 5 illustrates the HCA dendrogram, which reflects the relationship between EOs’ chemical components and their antioxidant activities. This dendrogram categorized the EOs from Citrus species into two primary clusters. Cluster II grouped EOs from C. sinensis and C. limon, indicating their closeness in phytochemical components and antioxidant activities. In contrast, C. hystrix EO was situated in Cluster I, suggesting distinct properties compared to the other samples.
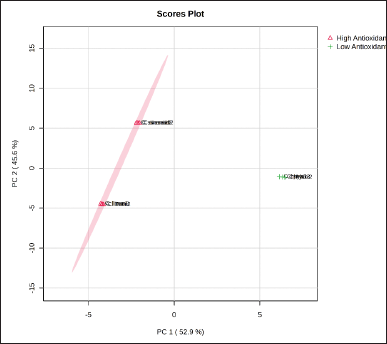 | Figure 3. Score plot from PCA analysis on antioxidant activity citrus EOs. [Click here to view] |
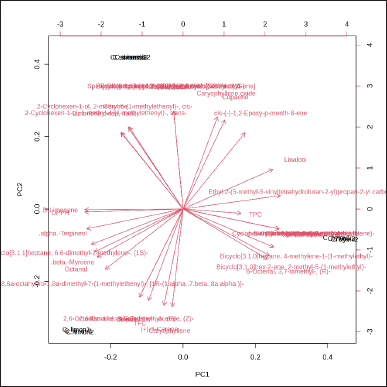 | Figure 4. PCA loading plot based on GC-MS chemical profiles and biological activities of EO. [Click here to view] |
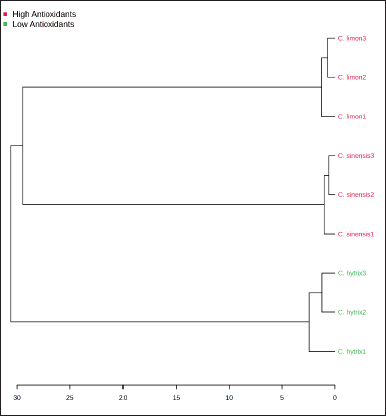 | Figure 5. Dendrogram of HCA to determine the closeness of EO samples. [Click here to view] |
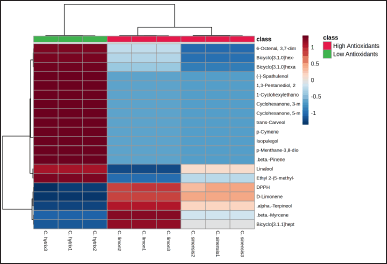 | Figure 6. Heatmap of top 20 phytochemical components of EOs from three Citrus species in correlation with antioxidant activity. [Click here to view] |
A heatmap analysis was conducted to assess the relationship between the chemical compounds of EO samples and their biological properties. This visualization, presented in Figure 6, showcased the top 20 compounds present in the EO samples derived from the percentage of peak area in GC-MS analysis, utilizing color intensity to represent their abundance.
In the assessment of antioxidant activity, the EO derived from C. limon exhibited notably high potential as an antioxidative agent, as evidenced by the percentage of DPPH scavenging activity. This observation indicated a dominant presence of antioxidant compounds in C. limon EO compared to the other EOs studied. Among the 20 identified compounds, D-limonene, Linalool, α-Terpineol, and β-Myrcene were the most abundant in both C. limon and C. sinensis EOs. These compounds likely contribute significantly to the antioxidant activity observed in these EOs. Nonetheless, further investigations are essential to precisely determine the specific roles of these compounds.
DISCUSSION
In the current study, we evaluated the antioxidant, plant inhibitory, and cytotoxic properties of three distinct Citrus EOs originating from Indonesia as well as their phytochemical components. Utilizing chemometric techniques, HCA, and PCA, we discriminated between EO samples to elucidate their relationship in terms of antioxidant activity and phytochemical compositions.
In terms of antioxidant properties, the EO from C. limon exhibited the highest potency at 66.11% DPPH scavenging activity, surpassing the activity levels of C. sinensis and C. hytrix EOs, which recorded values of 36.50% and 4.08%, respectively. This result was similarly reflected in the total flavonoid contents of the EO samples, with C. limon EO showing the highest concentration at 30.56 mg QE/g extract. These observations might be attributed to the notably elevated concentration of antioxidative agents in C. limon EO such as D-limonene compared to the other samples. This result aligned with prior studies that identified D-limonene as the predominant constituent in C. limon peel [12,25] and leaf EO [26]. Notably, D-Limonene stands out as the primary volatile compound in lemon EO, at levels usually ranging between 70% and 48% [27]. Subsequent investigations have elucidated the diverse antioxidant properties of D-limonene through in vitro assays, including DPPH, ABTS, FRAP, iron chelating, hydroxyl radical scavenging, and superoxide radical scavenging assays, showcasing its efficacy in reducing ROSs through varied mechanisms [28]. Additionally, in vivo assessments have revealed that D-limonene enhances antioxidant levels and augments the protein expression of inducible cyclooxygenase-2 (COX-2) and in nitric oxide synthase (iNOS) UC rats [29].
In terms of plant growth inhibition, the C. hystrix EO demonstrated the strongest inhibitory activity. Prior research has shown that a methanol extract derived from C. hystrix, at a concentration of 10 mg/ml, effectively inhibited the germination of lettuce seeds and impeded the growth of their roots. This finding suggests the potential use of the extract as a bioherbicide in the future for weed management [30]. In this study, the main phytochemical compounds C. hystrix EO were Ethyl 2-(5-methyl-5-vinyltetrahydrofuran-2-yl) propan-2-yl carbonate (7.02%) and Isopulegol (15.95%). The scientific literature documents the phytotoxic activity of EOs, which manifests in varying degrees of inhibition on seed germination and radical elongation. This activity appears to be caused by monoterpenes, specifically oxygenated compounds, especially ketones, alcohols, aldehydes, and phenols [31].
Furthermore, in the field of cytotoxicity assays, which evaluates the capacity of cytotoxic substances to induce cellular harm or cell mortality, the EO derived from C. hystrix exhibited a greater mortality rate in comparison to other citrus oils. This higher cytotoxicity aligns with prior studies that showed C. hystrix EO had cytotoxic activity on A. salina and demonstrated a greater cytotoxicity effect on different cell lines including human cervix carcinoma, murine melanoma, and human lung fibroblast (MRC-5) [32]. Moreover, the current investigation demonstrates that the EO of C. hystrix possessed a notably elevated level of Isopulegol (15.95%). Isopulegol is a monoterpene found in different plant species that have been scientifically proven to possess pharmacological properties [33].
In the realm of biological activity, citrus EOs have demonstrated inhibitory effects against several microorganisms. For instance, the EO from C. limon has been shown to inhibit bacteria including Lactobacillus plantarum, Lactobacillus mesenteroides, and Escherichia coli [34]. Similarly, C. sinensis EO exhibits inhibitory activity against gram-positive bacteria such as Staphylococcus aureus, Lactobacillus monocytogenes, and Enterococcus faecium along with some gram-negative bacteria including Salmonella enteritidis and Pseudomonas aeruginosa [35]. Furthermore, C. hystrix EO has demonstrated an inhibitory effect against various bacteria such as S. aureus, Enterococcus faecalis, E. coli, Staphylococcus epidermidis, and Proteus vulgaris [36]. The observed inhibitory effects of Citrus EOs against these bacteria are attributed to specific volatile components such as D-limonene and isopulegol, which are believed to contribute significantly to their antimicrobial properties [37,38].
The present study found the isopulegol and ethyl 2-(5-methyl-5-vinyltetrahydrofuran-2-yl) propan-2-yl carbonate as the major component present in C. hystrix EO. In contrast, a previous study reported different major compounds in the Citrus peel EO are sabinene, β-pinene, citronellal, limonene, terpinen-4-ol, and α-pinene [39]. Limonene was identified as the predominant constituent in nearly all Citrus peel EO samples, except C. hystrix and C. micrantha EOs, which were dominated by β-pinene. The most abundant compounds were monoterpenes, followed by sesquiterpenes and ester. According to another study, the kaffir lime peel EO has three primary chemical components: D-limonene (17.10%), 3-carene (13.77%), and γ-terpinene (12.56%). All three chemicals belong to the monoterpene hydrocarbon group and are characterized by the presence of double bonds, which are known as alkenes [40].
The variations in the primary chemical constituents of EOs between the current findings and previous studies can be attributed to disparities in the geographical regions where the plants are cultivated, resulting in distinct chemical compositions. The chemical composition of EOs within a plant is determined by various factors including the plant species, climate, geographical location, season, soil composition, extraction technique, and the specific plant portion used for oil extraction. These variations highlight the importance of considering multiple factors when studying and comparing EO compositions from different sources [41,42].
To understand the relation of antioxidant and phytochemical components of Citrus EOs as well as closeness among samples, PCA and hierarchy cluster analysis were employed. PCA and HCA analyses successfully discriminated EO samples into two groups, namely EO with high and low antioxidant activity. EOs of C. limon and C. sinensis were clustered in the same place showing the closeness of both samples. However, C. hystrix EO was put in a different cluster meaning the different properties of this sample to C. limon and C. sinensis EOs. PCA and HCA were widely used to discriminate many samples based on their similarity, e.g., to cluster EO of Juniperus rigida [43] and Foeniculum vulgare [44]. In line with the previous study, the present study highlights the power of chemometric analysis to classify and cluster as well as to determine the relationship among observed variables.
CONCLUSION
In the present study, EOs extracted from the peels of three distinct Citrus species from Indonesia, namely C. sinensis, C. limon, and C. hystrix, were evaluated for their antioxidant potential, plant inhibitory effects, and lethality properties against brine shrimp. We employed HCA and PCA to gauge the proximity of these samples concerning their antioxidant activity. Among the EOs tested, C. limon EO exhibited the most robust antioxidant activity against the DPPH free radical, while C. sinensis EO demonstrated the highest inhibitory effect on the growth of L. sativa, a vegetable plant. GC-MS analysis unveiled D-limonene as the predominant component in C. limon and C. sinensis EOs, constituting 36.41% and 27.86%, respectively. Meanwhile, C. hystrix EO featured Isopulegol as the major volatile compound, accounting for 15.95% of its composition. PCA and HCA analyses effectively classified the Citrus EO samples into two distinct categories. Specifically, C. limon and C. sinensis were grouped together as samples with high antioxidant activity, whereas C. hystrix EO exhibited lower activity. The clustering in HCA highlighted the proximity between C. limon and C. sinensis EOs. This study underscores the potent antioxidant, plant inhibitory, and brine shrimp cytotoxic effects exhibited by Citrus species EOs, suggesting their potential as sources for natural antioxidative, herbicidal agents, and pharmaceutical substances. However, further elucidation is necessary to ascertain the specific volatile components responsible for these observed biological activities.
ACKNOWLEDGEMENTS
The authors extend their gratitude to LPPM Universitas Terbuka (UT) for their support through the applied research scheme project, under contract number B/551/UN31.LPPM/PT.01.03/2023. Furthermore, the authors express gratitude to the Advanced Characterization Laboratories Serpong, National Research and Innovation Agency, for providing facilities and valuable scientific and technical assistance through E-Layanan Sains (ELSA) and Badan Riset dan Inovasi Nasional (BRIN).
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study did not involve experiments on animals or human subjects.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Sharma K, Mahato N, Cho MH, Lee YR. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition. 2017;34:29–46. CrossRef
2. BPS-Statistics Indonesia. Statistics of Horticulture. Jakarta: BPS-Statistics Indonesia; 2022.
3. Yulianti F, Adiredjo AL, Soetopo L, Ashari S. Short communication: morphology and genetic characteristics of potential citrus rootstock in Indonesia. Biodiversitas. 2020;21(11):5514–20.
4. Duarte A, Fernandes MJ, Bernardes JP, Miguel MG. Citrus as a component of the mediterranean diet. JSOD. 2016;4(4):289–304.
5. Al Othman HI, Alkatib HH, Zaid A, Sasidharan S, Rahiman SSF, Lee TP, et al. Phytochemical composition, antioxidant and antiproliferative activities of Citrus hystrix, Citrus limon, Citrus pyriformis, and Citrus microcarpa leaf essential oils against human cervical cancer cell line. Plants. 2022;12(134):1–15. CrossRef
6. Kaskoos RA. Essential oil analysis by GC-MS and analgesic activity of Lippia citriodora and Citrus limon. J Essent Oil Bearing Plants 2019;22(1):273–81. CrossRef
7. Hsouna, AB, Ben Halima N, Smaoui S, Hamdi N. Citrus lemon essential oil: chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017;16(146):1–11.
8. Agarwal P, Sebghatollahi Z, Kamal M, Dhyani A, Shrivastava A, Singh KK, et al. Citrus essential oils in aromatherapy: therapeutic effects and mechanisms. Antioxidants. 2022;11(12):1–45.
9. Moosavy MH, Hassanzadeh P, Mohammadzadeh E, Mahmoudi R, Khatibi SA, Mardani K. Antioxidant and antimicrobial activities of essential oil of lemon (Citrus limon) peel in vitro and in a food model. J Food Qual Hazards Control. 2017;4(2):42–8.
10. Mitropoulou G, Fitsiou E, Spyridopoulou K, Tiptiri-Kourpeti A, Bardouki H, Vamvakias M, et al. Citrus medica essential oil exhibits significant antimicrobial and antiproliferative activity. LWT. 2017;84:344–52. CrossRef
11. Manzur M, Luciardi MC, Blázquez MA, Alberto MR, Cartagena E, Arena ME. Citrus sinensis essential oils an innovative antioxidant and antipathogenic dual strategy in food preservation against spoliage bacteria. Antioxidants. 2023;12(246):1–18. CrossRef
12. Li Y, Liu S, Zhao C, Zhang Z, Nie D, Tang W, Li Y. The chemical composition and antibacterial and antioxidant activities of five citrus essential oils. Molecules. 2022;27(20):1–14. CrossRef
13. Himed L, Merniz S, Monteagudo-Olivan R, Barkat M, Coronas J. Antioxidant activity of the essential oil of citrus limon before and after its encapsulation in amorphous SiO2. Sci Afr. 2019;6:1–9. CrossRef
14. Caputo L, Cornara L, Bazzicalupo M, De Francesco C, De Feo V, Trombetta D, et al. Chemical composition and biological activities of essential oils from peels of three citrus species. Molecules. 2020;25(8):1890.
15. Adokoh CK, Asante DB, Acheampong DO, Kotsuchibashi Y, Armah, FA, Sirikyi IH, et al. Chemical profile and in vivo toxicity evaluation of unripe Citrus aurantifolia essential oil. Toxicol Rep. 2019;6:692–702.
16. Andriana Y, Xuan TD, Quy TN, Tran H-D, Le Q-T. Biological activities and chemical constituents of essential oils from Piper cubeba Bojer and Piper nigrum L. Molecules. 2019;24(10):1–16. CrossRef
17. Minh TN, Xuan TD, Van TM, Andriana Y, Viet TD, Khanh TD, et al. Phytochemical analysis and potential biological activities of essential oil from rice leaf. Molecules. 2019;24(546):1–14.
18. Niksic H, Becic F, Koric E, Gusic I, Omeragic E, Muratovic S, et al. Cytotoxicity screening of Thymus vulgaris L. essential oil in brine shrimp nauplii and cancer cell lines. Sci Rep. 2021;11:1–9. CrossRef
19. Syahmi ARM, Vijayaratna S, Sasidharan S, Latha LY, Kwan YP, Lau YL, et al. Acute oral toxicity and brine shrimp lethality of Elaeis guineensis Jacq.,(oil palm leaf) methanol extract. Molecules. 2010;15(11):8111–21.
20. Tuyen PT, Xuan TD, Khang DT, Ahmad A, Quan, NV, Tu Anh TT, et al. Phenolic compositions and antioxidant properties in bark, flower, inner skin, kernel and leaf extracts of Castanea crenata Sieb. et Zucc. Antioxidants. 2017;6(2):31. CrossRef
21. Indrianingsih AW, Windarsih A, Noviana E, Asari SM, Pratiwi SI. In vitro evaluation of antioxidant, α-glucosidase inhibitor, and antibacterial activities of Frangipani flower and the principal component analysis of its constituents. Process Biochem. 2023;130:347–57. CrossRef
22. Andriana Y, Fajriani NA, Iwansyah AC, Xuan TD. Phytochemical constituents of Indonesian adlay (Coix lacrima-jobi L.) and their potential as antioxidants and crop protection agents. Agrochemicals. 2023;2(1):135–49. CrossRef
23. Sebastiani P, Peris TT. Detection of significant groups in hierarchical clustering by resampling. Methods. 2016;7(144):1–10.
24. Nandiyanto ABD, Ragadhita R, Fiandini M. Interpretation of Fourier transform infrared spectra (FTIR): a practical approach in the polymer /plastic thermal decomposition. Indonesian J Sci Technol. 2023;8(1):113–26. CrossRef
25. Kehal F, Chemache L, Himed L, Barkat M. Antimicrobial and antioxidant activities of citrus limon peel essential oils and their application as a natural preservative in fresh cream: effects on oxidative and sensory properties. Acta Universitatis Cibiniensis Ser E Food Tech. 2023;27(1):1–14.
26. Petretto GL, Vacca G, Addis R, Pintore G, Nieddu M, Piras F, et al. Waste Citrus limon leaves as source of essential oil rich in limonene and citral: chemical characterization, antimicrobial and antioxidant properties, and effects on cancer cell viability. Antioxidants. 2023;12(1238):1–21. CrossRef
27. Klimek-Szczykutowicz M, Szopa A, Ekiert H. Citrus limon (lemon) phenomenon—a review of the chemistry, pharmacological properties, applications in the modern pharmaceutical, food, and cosmetics industries, and biotechnological studies. Plants. 2020;9(119):1–24.
28. González-Mas MC, Rambla JL, López-Gresa MP, Blázquez MA, Granell A. Volatile compounds in Citrus essential oils: a comprehensive review. Review. 2019;10(12):1–18.
29. Shah BB, Mehta AA. In vitro evaluation of antioxidant activity of d–limonene. Asian J Pharm and Pharmacol. 2018;4(6):883–7. CrossRef
30. Yu L, Yan J, Sun Z. D-limonene exhibits antiinflammatory and antioxidant properties in an ulcerative colitis rat model via regulation of iNOS, COX-2, PGE2 and ERK signaling pathways. Mole Med Rep. 2017;15:2339–46. CrossRef
31. Phuagphong P, Nawanopparatsakul S, Kitcharoen N. Effects of Citrus aurantifolia (Christm.), Citrus hystrix and Alpinia galanga on plant growth inhibition. The International Conference on Herbal Traditional Medicine. 2015, pp 43–52.
32. Smeriglio A, Trombetta D, Cornara L, Valussi M, De Veo V, Caputo L. Characterization and phytotoxicity assessment of essential oils from plant byproducts. Molecules. 2019;24(2941):1–16.
33. Aumeeruddy-Elalfi Z, Ismaël IS, Hosenally M, Zengin G, Mahomoodally MF. Essential oils from tropical medicinal herbs and food plants inhibit biofilm formation in vitro and are non-cytotoxic to human cells. 3 Biotech. 2018;8(9):1–11.
34. Ramos AGB, de Menezes IRA, da Silva MSA, Pessoa RT, de Lacerda Neto LJ, Passos FRS, et al. Antiedematogenic and anti-inflammatory activity of the monoterpene isopulegol and its β-cyclodextrin (β-CD) inclusion complex in animal inflammation models. Foods. 2020;9(5):1–16.
35. Raspo MA, Vignola MB, Andreatta AE, Juliani HR. Antioxidant and antimicrobial activities of citrus essential oils from Argentina and the United States. Food Biosci. 2020;36:100651.
36. Espina L, Somolinos M, Lorán S, Conchello, P, García D, Pagán R. Chemical composition of commercial citrus fruit essential oils and evaluation of their antimicrobial activity acting alone or in combined processes. Food Control. 2011;22(6):896–902.
37. Sreepian A, Sreepian PM, Chanthong C, Mingkhwancheep T, Prathit P. Antibacterial activity of essential oil extracted from Citrus hystrix (Kaffir Lime) peels: an in vitro study. Tropical Biomed. 2019;36(2):531–41.
38. Zhang Z, Vriesekoop F, Yuan Q, Liang, H. Effects of nisin on the antimicrobial activity of D-limonene and its nanoemulsion. Food Chem. 2014;150:307–12.
39. Ürgeová E, Uvá?ková ?, Vaneková M, Maliar T. Antibacterial potential of microwave-assisted extraction prepared hydrolates from different salvia species. Plants 2023; 12(6): 1325.
40. Baccati C, Gibernau M, Paoli M, Ollitrault P, Tomi F, Luro F. Chemical variability of peel and leaf essential oils in the Citrus subgenus Papeda (Swingle) and few relatives. Plants. 2021;10(1117):1–15. CrossRef
41. Husni E, Putri US, Dachriyanus. Chemical content profile of essential oil from kaffir lime (Citrus hystrix DC.) in Tanah Datar Regency and antibacterial activity. Adv Health Sci Res. 2021;40:174–81.
42. Lawal OA, Ogunwande IA, Owolabi MS, Giwa-Ajeniya AO, Kasali AA, Abudu FA, et al. Comparative analysis of essential oils of Citrus aurantifolia Swingle and Citrus reticulata Blanco, from two different localities of Lagos State, Nigeria. Am J Essent Oils Nat Prod. 2014;2(2):08–12.
43. Feng X, Zhang W, Wu W, Bai R, Kuang S, Shi B, et al. Chemical composition and diversity of the essential oils of Juniperus rigida along the elevations in Helan and Changbai Mountains and correlation with the soil characteristics. Ind Crops Prod. 2021;159:1–12. CrossRef
44. Kalleli F, Rebey IB, Wannes WA, Boughalleb F, Hammami M, Tounsi MS, et al. Chemical composition and antioxidant potential of essential oil and methanol extract from Tunisian and French fennel (Foeniculum vulgare Mill.) seeds. J Food Biochem. 2019;43(8):e12935. CrossRef