INTRODUCTION
Rheumatoid arthritis (RA) is a chronic disease with a prevalence of 0.5%–1% of the industrialized world’s population. Smoking, gender (women are three times more susceptible to RA than men), obesity, age, and genetics are risk factors for RA [1,2]. RA is a multisystem autoimmune disease characterized by inflammation of the synovial membrane, swelling, and the production of anti-citrullinated protein antibodies, which is associated with excessive production of proinflammatory cytokines [3–5]. These cytokines are directly involved in many immune processes that correlate with the pathogenesis of RA [6]. Increased transcription factor Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB, nuclear factor of activated T cells, activator protein-1 (AP-1), and other such as signal transducer and activator of transcription family of proteins, interferon regulatory factors (IRFs), Forkhead (Fox) family pro-teins, T-box transcription factor 21/T-boxexpressed in T cells (T-bet), the the CCAAT/enhancer-binding proteins (C/EBPs) family and the Ets transcription factor family) [7] and cytokine expression are part of the mechanism that causes joint degeneration in RA. Interleukins (ILs) and tumor necrosis factor (TNF)-α have been associated with the etiology of arthritis. TNF-α is the main cytokine that regulates the formation of other inflammatory mediators in synovial tissue and also destroys bone and cartilage by activating chondrocytes apoptosis and osteoclasts. IL-6 and IL-1β are other critical cytokines involved in the pathogenesis of RA. IL-6 involves various physiological processes, such as immune response, inflammation, and bone metabolism. IL-6 also regulates osteoclastogenesis in combination with TNF. Meanwhile, IL-1β plays an essential role in the development of RA, especially in production by monocytes and macrophage cells [3]. IL-1β involved in a variety of immunological functions such as proliferation, activation, and differentiation as well as in the recruitment of additional inflammatory cells [8]. Macrophages, which are the primary source of the proinflammatory cytokines, express HLA class II molecules. By virtue of their proximity to T cells, they may also function as antigen-presenting cells, thereby perpetuating immunological responses within the joint [9]. Natural killer cells, T cells, B cells, endothelial cells, synovial cells, and neutrophils also produce IL-1β. IL-1β activates monocytes/macrophages, thereby causing increased inflammation. In addition, IL-1β activates chondrocytes, causing cartilage damage, and activates osteoclasts that cause bone resorption [3]. These proinflammatory cytokines are also responsible for the formation of matrix-metalloproteases, inducible nitric oxide synthase, osteoclast differentiation, and expression of cell adhesion molecules [2]. As a result of the imbalance between proinflammatory and anti-inflammatory states, synovial membrane inflammation and joint injury occur [1]. If left untreated or not appropriately controlled, this disease can cause damage to cartilage and bones and reduce the sufferer’s quality of life or even cause disability [5].
In RA therapy, pharmacological therapy consists of biological and non-biological disease-modifying anti-rheumatic drugs and anti-inflammatory therapy with non-steroidal anti-inflammatory drugs or glucocorticoids [10]. Anti-rheumatic drugs have a variety of significant benefits. However, its clinical use is limited for several reasons, including high cost and side effects. Hormonal irregularities, decreased immunity, digestive tract disorders, infections, osteoporosis, and cyclical vomiting syndrome difficulties have all been reported as side effects of RA drug use [1,11]. Glucocorticoid drugs have been reported to increase the risk of cardiovascular disease in RA patients due to their potential adverse effects on lipids, glucose tolerance, and the development of hypertension and obesity [6]. Based on this, many anti-RA products have been developed which are derived from natural ingredients. One of them was Kasumba Turate, more known as Safflower.
Carthamus tinctorius Linn., commonly known as Safflower, is widely used as traditional medicine in Indonesia. More than 200 compounds have been isolated from C. tinctorius: flavonoids, phenylethanol glycosides, coumarins, fatty acids, steroids, polysaccharides, and quinochalcones. Quinochalcones comprise almost all the red and yellow pigments in Safflower. The main component of yellow pigment is Hydroxysafflor yellow A (HSYA). Modern pharmacological studies show that Safflower has many beneficial bioactivities, such as anti-inflammatory, modulating the immune system, antioxidant, and antitumor effects [12–15]. Safflower has been a very effective treatment for RA [16]. The flavone luteolin and its glucopyranosides, such as luteolin 7-O-beta-Dglucopyranoside and luteolin-7-O-(6’’-O acetyl)- beta-D-glucopyranoside have been reported to provide anti-inflammatory effects in vitro and in vitro. In vivo studies and several studies show that Safflower inhibits NF-κB activity at concentrations in the low micromolar range [16]. In another study, it was shown that ethanol extract from safflower leaves protects the LPS-lipopolysaccharide HaCaT cells by inhibiting the expression of iNOS, IL-6, and IL-1β and suppressing the phosphorylation of the p38, p65, phosphoactivated Jun N-terminal kinase via inactivation of mitogen-activated protein kinases/NF-κB signaling pathway [17].
Therefore, this study aims to determine the anti-RA activity of the ethanol extract of Safflower (C. tintorius Linn.) on reducing edema in animal models of RA mice and to determine the anti-RA activity of the ethanol extract of Safflower (C. tinctorius). Linn.) against rheumatoid factor (RF) in RA mice.
MATERIALS AND METHODS
Extraction
1 kg of dried Safflower (C. tinctorius Linn.) was obtained from the Safflower plantation in Waemppubu Village, Amali District, Bone Regency, South Sulawesi. Then, it was powdered and continued by macerating with ethanol for 3 × 24 hours. The filtrate collected was then evaporated to obtain a concentrated extract for further experiments.
Cell viability (CV) assay
HEK293 (ATCC, USA) cell lines were maintained in Roswell Park Memorial Institute Medium 1640 medium (Gibco®, USA) and supplemented with 10% fetal bovine serum (Gibco®, New York, NY) and 1% penicllin/streptomycin (Gibco®, New York, NY). The cells were then incubated in an incubator at 37°C with 5% of CO2. After a confluence of 80%, the HEK293 cells (4 × 105) were seeded in 96 well plates in the incubator. After 24 hours, the old medium was discarded, washed twice with phosphate buffer saline (PBS) pH 7.4 (Gibco®, USA), replaced with a new medium containing samples at various concentrations, and incubated for 24 hours. Then, the medium containing samples was discarded, washed with PBS pH 7.4 (2x), and incubated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide solution for 4, followed by dissolving the formazan crystal by dimethyl sulfoxide. Then, the absorbance was measured at λ570 nm. The %CV was calculated as follows: [18].
RA modelling in experimental animals
The study protocol was approved by the Institute of Research, Ethic Committee and Community Service, Universitas Halu Oleo with approval number (160/UN29.20.1.2/ PG/2023). Briefly, 30 Male mice (Mus musculus, BALB/c strain from Gold Mice Farm Mandai Maros, Makassar, Indonesia) weighing 20–30 g were used as experimenta l animals. The mice were acclimatized for seven days in a standard environment and accessed to food and water ad libitum in the Laboratory of the Faculty of Pharmacy, Halu Oleo University. After acclimatization, the mice were induced with 0.1 ml of complete Freund’s adjuvant (CFA) intraplantarly, except the normal group. Then, they measured their paws’ thickness (T0) and day 17 (Tt) using a gauge meter. The RF was also evaluated on day 17. In addition, the RA index was scored to determine whether the animals met the requirement as an RA mice model with a score > 1 was considered as experiencing RA [19,20]. RA index was scored in Table 1 below.
Anti RA activity assay
The RA mice model was then treated accordingly daily with one dose for 15 days of 0.5% of sodium carboxymethyl cellulose (Na-CMC) C(-) or negative control group, 2.5 mg/kg body weight (BW) of methotrexate for the positive group (C(+)), and with Safflower ethanolic extract at dose 100, 200, and 400 mg/kg BW, respectively (CTE100, CTE200, and CTE400) for 14 days. At day 31, their paws’ thickness were re-measured (Tt). In addition, the normal group was a group which not receive CFA or any treatment. Then, the edema percentage (%edema) for each group was calculated as follows:
Then, the inhibition percentage (% RA inhibition) was calculated as follows:
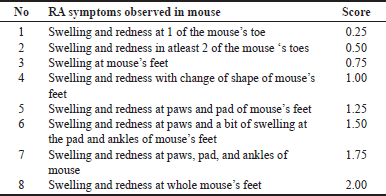 | Table 1. The RA parameters. [Click here to view] |
After that, the mice were sacrificed, and blood was collected intracardially. The collected blood was put in an EDTA-containing sterile tube and centrifuged for 30 minutes at 3,000 rpm at 2°C. The plasma was then analyzed with the RF direct latex test to assay the agglutination of antigen, as well as anti-inflammatory cytokines by measuring its TNF-α, IL-1β, and IL-6 levels as per kit protocol’s TNF-α, IL-1β, and IL-6 ELISA kit.
Molecular docking studies
The study utilized the molecular targets, namely IL 1β protein data bank (PDB code 5R8Q), IL-6 (PDB code 1ALU), and TNF-α (PDB code 7JRA). These targets were obtained from the RCSB website (https://www.rcsb.org/). After obtaining the targets, they were prepared by removing the bound ligands, solvent, and water molecules from the structures using BIOVIA Discovery Studio 2020.
Several compounds in C. tinctorius L. (Carthamine, HSYA, Anhydrosafflor yellow B, Safflor yellow A, and Safflor yellow B) were drawn, and their 3D structures were built using ChemDraw Ultra Professional 15.0 [21]. The geometrical structures of compounds were minimized and optimized using the semi-empirical AM1 method [22]. AutoDockTools 1.5.6 was used to prepare the targets and compounds before the docking process. Firstly, the proteins were protonated, and Kollman and Gasteiger charges were assigned to the target and compound structures [23].
Protein-ligand docking simulations were performed between several compounds in C. tinctorius L. and IL-1β, IL-6, and TNF-α with the assistance of AutoDock Vina [24]. The docking coordinates were set to the center positions of 1-methyl-N-{[(2S)-oxolan-2-yl]methyl}-1H-pyrazole-3-carboxamide (JGY) in IL-1β, Tartaric acid; VGY = 2-[5-(3-chloro-4-{[(1R)-1-(2-fluorophenyl)ethyl]amino}quinolin-6-yl)pyrimidin-2-yl]propan-2-ol (TLA) in IL-6, and VGY in TNF-α, with a grid area of 40 × 40 × 40 and 0.375 Å point spacing. The validated procedures identified a root mean square deviation of JGY, TLA, and VGY below 2 Å (Fig. 1). Other docking procedures were set to default. Finally, the simulation results were analyzed through visualization using BIOVIA Discovery Studio 2020.
Statistical analysis
Data collected was presented as mean ± SD and analyzed statistically using one-way analysis of variance with a significance of 0.05.
RESULTS AND DISCUSSION
CV asssay
Evaluation of C. tinctorius extract for its potential cytotoxicity is considered an essential step in evaluating its suitability for further applications. The in vitro cytotoxicity test of C. tinctorius extract was performed in human embryonic kidney 293 (HEK293) cell lines to determine the CV whether it is safe or not to normal cells. The CV measure the percentage of number of living cell post treatment of samples [25,26].
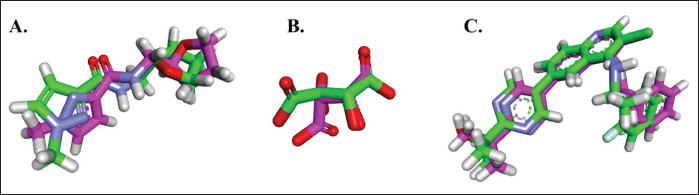 | Figure 1. Overlaying the X-ray crystallography pose (in green) and the docked pose (in pink) of JGY with IL-1β (1.834 Å), TLA with IL-6 (1.813 Å), and VGY with TNF-α (1.179 Å) [Click here to view] |
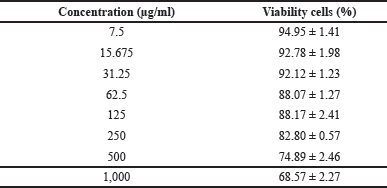 | Table 2. The HEK293 CV. [Click here to view] |
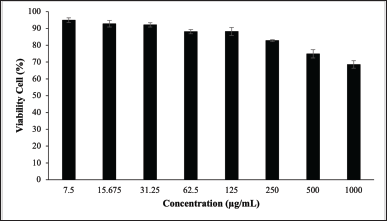 | Figure 2. The CV (%) of HEK293 post treated with C. tinctorius at various concentrations. [Click here to view] |
It was found that C. tinctorius extract at various concentrations from 7.5 to 250 μg/ml were safe to HEK293 cells with %CV ≥ 80% (Table 2 and Fig. 2), while concentration 500 and 1,000 μg/ml were considered as weak toxicity. Hence, it can be concluded that C. tinctorius extract is safe against HEK293 cell lines [26].
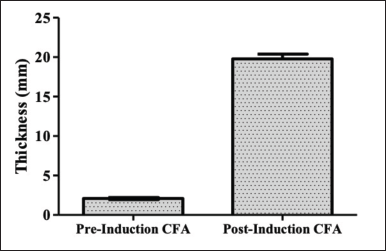 | Figure 3. The effect of CFA injection on mice paw thickness. Data is presented as mean±SD. [Click here to view] |
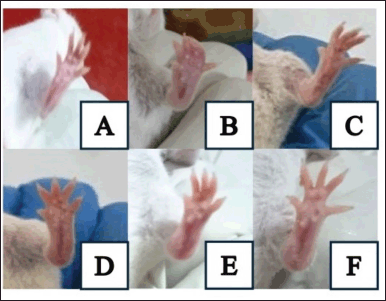 | Figure 4. Representative pictures of RA model in mice, examined on day 30 after induced with CFA. (A) normal group. (B) C(-), which was induced with CFA and received no treatment. (C) C(+), which was induced with CFA and received 2.5 mg/kg BW of methotrexate. (D–F) treatment groups, which were induced with CFA and received C. tinctorius extract at concentration 100; 200; and 400 mg/kgbw (CTE100, CTE200, and CTE400). [Click here to view] |
RA modelling in mice
CFA-induced RA in mice is a test animal model commonly used for preclinical studies of arthritis due to its short test duration and easy measurements [27]. After administration of CFA, inflammation occurs caused by fluid exudation, neutrophil infiltration, and mast cell activity, including stimulating the phagocytosis and cytokine release such as IL-1β, IL-6, and TNF-α. The role of inflammatory mediators and serological and pathological changes in the CFA-induced arthritis model is similar to RA in humans [19,28,29].
Our result found that on day 17, Figure 3 exhibited the increased edema volume of mice’s paws by measuring the thickness (in mm) of paws. The thickness of the mice’s paw indicated the edema occurred post-inducing with CFA, causing RA. They significantly differed before and after inducing with CFA (p<0.05). In addition, the arthritis index scoring demonstrated that the index obtained was more than 1. It means that the mice were successfully modelled for the RA model. RF also proved that the administration of CFA was causing agglutination as a response in latex tests [19,30].
Anti-RA activity
The RA is shown in Figure 4. Inflammatory signs were characterized by swelling and redness (Fig. 4(B)). Figure 4(C–F) exhibit the improvement, indicated with decreased edema in mice’s paw.
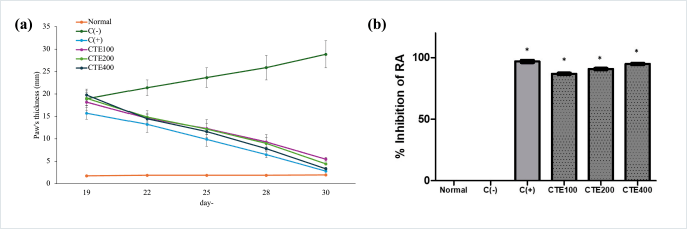
The anti-RA effect can be seen from the paw’s thickness (Fig. 5(a)) and percentage of RA inhibition. The paw’s thickness of mice were reduced after treatment with methotrexate, CTE100, CTE200, and CTE400. This measurement indicated the reduction of edema. The highest reduction was found in day 30, which ranking as follows: methotrexate > CTE400 > CTE200 > CTE100. After measuring the paw’s thickness of mice, the RA inhibition was calculated. Based on the data in Figure 5(b), RA inhibition was calculated. The calculation of the percentage of RA inhibition starts from day 17 to day 30. The percentage of inhibition was calculated using the thickness of the mice paw edema at time t (Tt) subtracted by the thickness of the initial mice paw edema (T0). The percentage of inhibition of the paw edema of the normal mice group, CTE100. CTE200, and CTE400 were calculated and compared with the C(-). The RA inhibition according to treatment groups can be seen in Figure 5.
According to the result obtained, C. tinctorius provided an effect in a concentration-dependent manner, with the highest concentration used which was CTE400, followed by CTE200 and Group CTE100, with concentrations of 200 and 100 mg/Kg BW, respectively. The highest percentage of RA inhibition was showed with C(+) as a positive group, which was 98.88%, followed by CTE400, CTE200, and CTE100, which were 94.94%, 90.86%, and 86.99%, respectively. While C(-) did not provide anti-RA activity, as shown with %RA inhibition as 0%. Moreover, the normal group did not show any RA signs. CTE400 showed a good anti-RA result as a positive control, which was 2.5 mg/KgbBW of methotrexate (p > 0.05).
CTEs decreased the TNF-α levels in a dependent-concentration manner, with CTE200 having higher activity compared to CTE100 and CTE50, respectively (p < 0.05) compared to the RA model and C(-), yet not significantly different to C(+) used (p > 0.05). It is illustrated at Figure 6. It means that CTE at a concentration of 200 mg/kg BW has similar potency in lowering TNF-α levels with control positive used, 2.5 mg/kg BW of methotrexate.
CTEs were lowering IL-1β in a dependent-concentration manner with higher concentration provided higher activity, starting from CTE200, CTE100, and CTE50, respectively (p < 0.05), compared to the RA model, as illustrated at Figure 7.
On the other hand, CTEs also lowered IL-6 levels in a dependent concentration manner. CTE200 had a higher ability to lower IL-6 levels, followed by CTE100 and CTE50, respectively (p < 0.05), compared to the RA model. It is presented in Figure 8.
In addition, C. tinctorius at a concentration of 100, 200, and 400 mg/kgbW showed no agglutination formation on the RF assay. RF is an immunoglobulin that can be detected in RA patients. RF potentiated antigen presentation to T cells through dendritic cell uptake of immune complexes with exogenous antigens and via B cells. RF plays a helpful role in diagnosis, providing information about prognosis, predicting patient subgroups, and predicting the onset of RA. In several studies, immunosuppressive treatment reduced RF serum levels by inhibiting the pro-inflammatory cytokines [31,32].
 | Figure 5. The % RA inhibition. Data is presented as mean ± SD (* indicates the significance difference to group I (p<0.05)). [Click here to view] |
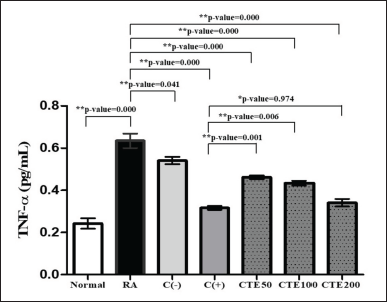 | Figure 6. The effect of C. tinctorius extract on lowering TNF-α levels. Data is presented as mean ± SD. [Click here to view] |
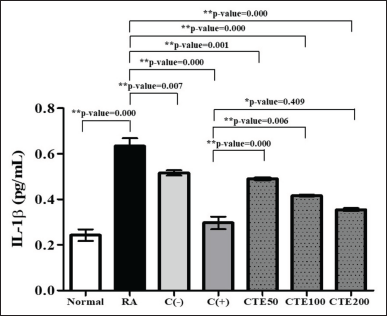 | Figure 7. The effect of C. tinctorius extract on lowering IL-1β levels. Data is presented as mean ± SD. [Click here to view] |
The anti-RA activity of the ethanol extract of C. tinctorius flowers might be affected by the presence of alkaloids, saponins, terpenoids, flavonoids, tannins and anthraquinones and has been proven to have antipyretic, analgesic, antioxidant, anti-inflammatory [14], immunosuppressive activity [13].
Molecular docking
In the IL-1β target, 5 compounds were identified to have had a satisfying affinity with a binding energy range of −6.9 to −7.9 kcal/mol (Table 2). Carthamine and Safflor Yellow B exhibited the most negative binding energy among these compounds. Interestingly, these two compounds showed different interactions when bound to the active site of IL-1β (Fig. 9; Table 4). Specifically, Carthamine was observed to have formed four hydrogen bonds with the residues Val41, Lys65, Asn66, and Pro23, as well as two hydrophobic interactions with Val19 and Leu67. On the other hand, Safflor Yellow B had formed four hydrogen bonds with the residues Tyr24, Leu80, Leu82, and Val132, and one hydrophobic interaction with Lys77. A summary of these interactions can be seen in Table 4.
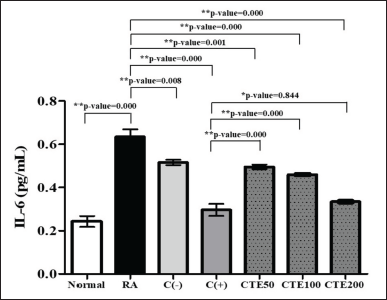 | Figure 8. The effect of C. tinctorius extract on lowering IL-6 levels. Data is presented as mean ± SD. [Click here to view] |
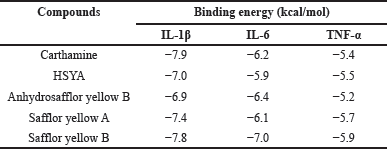 | Table 3. Summary of binding energies for several compounds in C. tinctorius L. against IL-1β, IL-6, and TNF-α. [Click here to view] |
IL-1β interacts with the IL-1 receptor (IL-1R) to trigger an inflammatory response. The residues Lys65, Asn66, Leu80, and Leu82 are amino acids in the binding domain crucial for the activity of IL-1β [32]. The binding of IL-1β by Carthamine and Safflor Yellow B can disrupt or inhibit the interaction between IL-1β and IL-1R, potentially altering or halting the inflammatory response initiated by IL-1β [33].
In the IL-6 target, the identified compounds from C. tinctorius L. exhibited a binding energy range of −5.9 to −7.0 kcal/mol (Table 3). Safflor yellow B and Anhydrosafflor yellow B displayed better affinity than other compounds in this target. Safflor Yellow B was observed to have interacted with the residues Glu37, Pro47, Met49, Glu154, Ser151, Phe155, and Arg161 of IL-6 (Table 4), forming hydrogen bonds. Meanwhile, Anhydrosafflor Yellow B had only formed two hydrogen bonds with the residues Lys48 and Gln57 (Fig. 10; Table 4). Interestingly, all compounds exhibited the same hydrophobic interactions with Phe56 of IL-6. In IL-6, one of the domains involved in interactions with the receptor is the binding site loop domain, located in the amino acid sequence 30–60 in its three-dimensional structure [34]. The compounds Safflor Yellow B and Anhydrosafflor Yellow B are capable of interacting with this domain, and it is believed that they can disrupt the interaction of IL-6 with the IL-6 receptor to form homodimerization with gp130, thereby inhibiting the activation of the inflammatory signaling system [35].
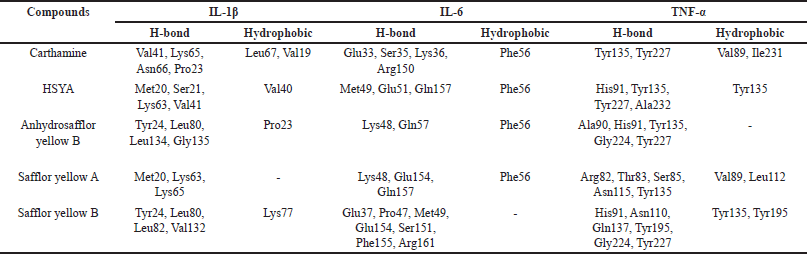 | Table 4. Summary of molecular interactions for several compounds in C. tinctorius L. against IL-1β, IL-6, and TNF-α. [Click here to view] |
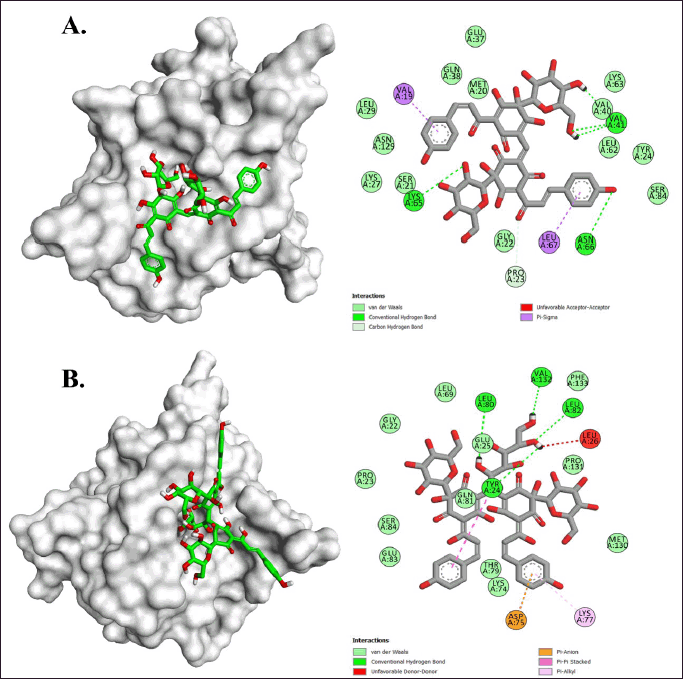 | Figure 9. Molecular interactions of (A) Carthamine and (B) Safflor yellow B against IL-1β. [Click here to view] |
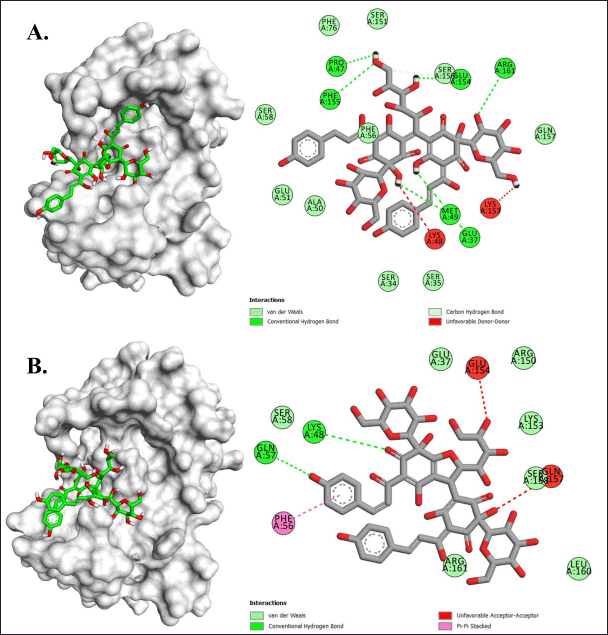 | Figure 10. Molecular interactions of (A) Safflor yellow B and (B) Anhydrosafflor yellow B against IL-6. [Click here to view] |
In the TNF-α target, all compounds showed binding energies ranging from approximately −5.2 to −5.9 kcal/mol (Table 3). In this target, Safflor yellow A and B were estimated to have better affinity for TNF-α than other compounds. Safflor yellow A formed hydrogen bonds with residues Arg82, Thr83, Ser85, Asn115, and Tyr135, as well as hydrophobic interactions with Val89 and Leu112. Meanwhile, Safflor yellow B formed hydrogen bonds with His91, Asn110, Gln137, Tyr195, Gly224, and Tyr227, as well as hydrophobic interactions with Tyr135 and Tyr195 (Fig. 11; Table 4). Interestingly, the interactions with Tyr135, Tyr227, and Tyr195 resembled the interactions of the inhibitor VGY with TNF-α [36]. This study revealed the ability of Safflor yellow B to form hydrogen bonds and hydrophobic interactions with TNF-α, leading to a significant disruption of the TNF receptor binding site. Based on these results, it was reasonable to assume that the binding of Safflor Yellow B to TNF-α monomers prevented trimer formation, which was necessary for the signaling process [37,38].
This computational study highlights the potential of Safflor yellow B, a compound derived from C. tinctorius L., in playing a crucial role in anti-inflammatory effects, especially in RA. RA is a complex autoimmune disease characterized by chronic inflammation. Understanding interactions at the molecular level is highly beneficial in developing effective RA therapy. The ability of Safflor yellow B to tightly bind to IL-1β, IL-6, and TNF-α, which play a crucial role in the pathogenesis of RA, indicates that this compound could be a promising candidate for the development of anti-inflammatory drugs related to RA.
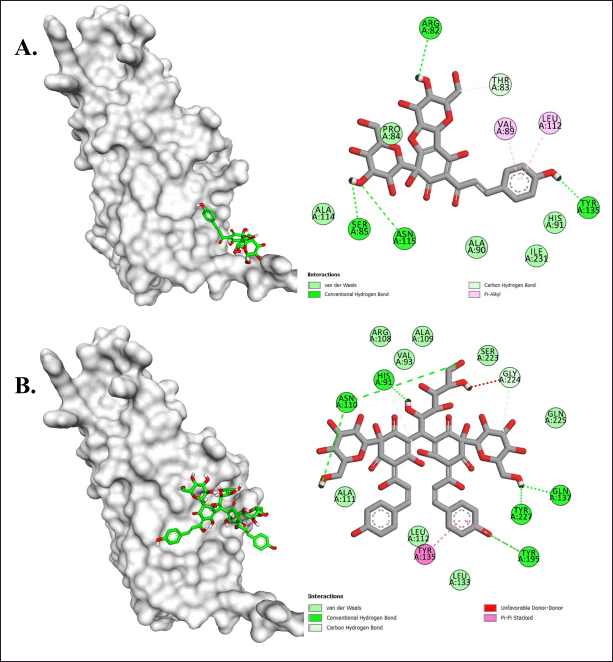 | Figure 11. Molecular interactions of (A) Safflor yellow A and (B) Safflor yellow B against TNF-α. [Click here to view] |
CONCLUSION
Safflower (C. tinctorius Linn.) ethanolic extract is safe to HEK293 and has anti-RA activity by reducing paw edema in RA mice-model by inhibiting RF by not forming the agglutination of immunoglobulin, as markers for rheumatoid post treatment with C. tinctorius extract. It also can be concluded that by inhibiting the RF, the levels of pro-inflammatory cytokines serum such as TNF-α, IL-1β, and IL-6 will also be supressed . Moreover, In addition, 200 and 400 mg/kgBW were the most effective (p < 0.05) They reduced the paws edema and reducing the inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, as well the rheumatoid factor by not forming the agglutination of immunoglobulin, as markers for rheumatoid post treatment with C. tinctorius extract.
ACKNOWLEDGMENTS
The World Class Professor 2023 Program by Directorate of Resources Affairs, Directorate General of Higher Education, Research, and Technology, Ministry of Education, Culture, Research and Technology, Republic of Indonesia (to A.F and T.M).
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
Publication has been funded by The World Class Professor program 2023 by Directorate of Resources Affairs, Directorate General of Higher Education, Research, and Technology, Ministry of Education, Culture, Research and Technology, Republic of Indonesia.
CONFLICTS OF INTEREST
The authors declare no conflict of interest and no competing financial interest.
ETHICAL APPROVALS
The study protocol was approved by the Institute of Research, Ethic Committee and Community Service of Faculty of Pharmacy, Halu Oleo of University, Kendari, Indonesia (Approval no.: 160/UN29.20.1.2/ PG/2023).
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Alzarea SI, Alasmari AF, Alanazi AS, Alzarea AI, Alharbi M, Alshammari A, et al. Butin attenuates arthritis in complete freund’s adjuvant-treated arthritic rats?: possibly mediated by its antioxidant and anti-inflammatory actions. Front Pharmacol. 2022;13:810052. CrossRef
2. Mateen SS, Moin S, Shahzad S, Khan AQ. Level of inflammatory cytokines in rheumatoid arthritis patients: correlation with 25-hydroxy vitamin D and reactive oxygen species. PLoS One. 2017;12(6):1–11. CrossRef
3. Kondo N, Kuroda T, Kobayashi D. Cytokine Networks in the pathogenesis of rheumatoid arthritis. Int J Mol Sci. 2021;22(20):10922. CrossRef
4. Zhang X, Dong Y, Dong H, Zhang W, Li F. Investigation of the effect of phlomisoside F on complete Freund’s adjuvant-induced arthritis. Exp Ther Med. 2017;13(2):710–6. CrossRef
5. Li Y, Guo R, Oduro PK, Sun T, Chen H, Yi Y, et al. The Relationship between porphyromonas gingivalis and rheumatoid arthritis?: a meta-analysis. front cell infect microbiol. 2022;12(956417):1–10. CrossRef
6. Ahmed OAMEG, Abo-youssef AM, Abo-saif AA. Effect of losartan in complete freund’ s adjuvant—induced arthritis in rats. IJPR. 2018; 17(4):1420–30.
7. Okamoto H, Cujec TP, Yamanaka H, Kamatani N. Molecular aspects of rheumatoid arthritis: role of transcription factors. FEBS J. 2008;275(18):4463–70.
8. Chung YH, Kim DH, Lee WW. Monosodium urate crystal-induced pro-interleukin-1β production is post-transcriptionally regulated via the p38 signaling pathway in human monocytes. Sci Rep. 2016;6(1):34533.
9. Kay J, Calabrese L. The role of interleukin-1 in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford, England). 2004;43(3):iii2–iii9. CrossRef
10. Schönenberger KA, Schüpfer A, Gloy VL, Hasler P, Stanga Z, Kaegi-braun N, et al. Effect of anti-inflammatory diets on pain in rheumatoid arthritis?: a systematic review and meta-analysis. Nutrients. 2021;13(2):4221. CrossRef
11. Minozzi S, Bonovas S, Lytras T, Pecoraro V, González-lorenzo M, Bastiampillai AJ, et al. Risk of infections using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis?: a systematic review. Expert Opin Drug Saf. 2016;15(1):11–34. CrossRef
12. Malik F, Malaka MH, Fristiohady A, Wahyuni W, Hamsidi R, Sahidin S, et al. Cytotoxic activity of kasumba flower ethanol extract turate (Carthamus tinctorius Linn.) against the line of cancer cells T47D breasts. JFSP. 2021;7(3):189–98. CrossRef
13. Asgarpanah J, Kazemivash N. Phytochemistry, pharmacology and medicinal properties of Carthamus tinctorius L. Chin J Integr Med. 2013;19(2):153–9. CrossRef
14. Hamsidi R, Wahyuni W, Fristiohady A, Malaka MH, Sahidin I, Ekasari W, et al. Steroid compounds isolation from Carthamus tinctorius linn as antimalarial. RJPT. 2021;14(10):5297–304. doi: 10.52711/0974-360X.2021.00924
15. Fristiohady A, Wirhamsah AR, Rathapon A, La Ode MJP. Phytochemistry, pharmacology and medicinal uses of Carthamus tinctorius linn: an updated review. Biointerface Res Appl Chem. 2023;13(5):1–26. CrossRef
16. Sanskriti GSB, Sameer SN. Detailed study on therapeutic properties, uses and pharmacological applications of safflower (Carthamus tinctorius L.). Int J Ayurveda Pharma Res. 2014;2(3):5–16. CrossRef
17. Kim S, Hong M, Deepa P, Sowndhararajan K, Park SJ, Park S, Kim S. Carthamus tinctorius suppresses responses by inhibiting the MAPKs / NF-κB signaling pathway in HaCaT cells. Sci Pharm. 2023;91:1–14. CrossRef
18. Asasutjarit R, Sooksai N, Fristiohady A, Lairungruang KNgSF, Fuongfuchat A. Optimization of production parameters for andrographolide-loaded nanoemulsion preparation by microfluidization and evaluations of its bioactivities in skin cancer cells and UVB radiation-exposed skin. Pharmaceutics. 2021;13(8):1290. CrossRef
19. Nasuti C, Fedeli D, Bordoni L, Piangerelli M, Servili M, Selvaggini R, et al. Anti-inflammatory, anti-arthritic and anti-nociceptive activities of Nigella sativa oil in a rat model of arthritis. Antioxidants. 2019;8(9):342. CrossRef
20. Siharis FS, Saranani S, Nurlansi N. Activity of tokulo (Kleinhovia hospita L.) as anti rheumatoid arthritis and anti-inflammatory in white rats induced by complete freud adjuvant (CFA). J Trop Pharm Chem. 2021;5(3):p-ISSN:2087–7099; e-ISSN:2407–6090. CrossRef
21. Cousins KR. ChemDraw ultra 9.0. CambridgeSoft, 100 CambridgePark drive, cambridge, MA 02140. www. cambridgesoft.com. See web site for pricing options. J Am Chem Soc. 2005;127(11):4115–6. CrossRef
22. Gupta VP. 4—Approximate molecular orbital theories. In Principles and applications of quantum chemistry. Boston, MA: Academic Press; 2016. pp. 127–53.
23. Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–91.
24. Adan A, Kiraz Y, Baran Y. Cell proliferation and cytotoxicity assays. Curr Pharm Biotechnol. 2016;17(14):1213–21.
25. Somaida A, Tariq I, Ambreen G, Abdelsalam AM, Ayoub AM, Wojcik M, et al. Potent cytotoxicity of four cameroonian plant extracts on different cancer cell lines. Pharmaceuticals. 2020;13(11):357. CrossRef
26. López-García J, Lehocký M, Humpolí?ek P, Sáha P. HaCaT keratinocytes response on antimicrobial atelocollagen substrates: extent of cytotoxicity, cell viability and proliferation. J Funct Biomater. 2014;5(2):43–57. CrossRef
27. Oleg T, Arthur JO. AutoDock vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–61. CrossRef
28. Gou KJ, Zeng R, Ren XD, Dou QL, Yang QB, Dong Y, et al. Anti-rheumatoid arthritis effects in adjuvant-induced arthritis in rats and molecular docking studies of Polygonum orientale L. extracts. Immunol Lett. 2018; 201:59–69. CrossRef
29. Naz R, Ahmed Z, Shahzad M, Shabbir A, Kamal F. Amelioration of rheumatoid arthritis by anacardium occidentale via inhibition of collagenase and lysosomal enzymes. eCAM. 2020;2020:11. CrossRef
30. Singh VS, Dhawale SC, Shakeel F, Faiyazuddin M, Alshehri S. Antiarthritic potential of calotropis procera leaf fractions in FCA-induced arthritic rats: involvement of cellular inflammatory mediators and other biomarkers. Agriculture. 2021;11:68. CrossRef
31. Smit F. Picrorhiza scrophulariiflora. Tesis. Universiteit Utrecht, The Netherlands; 2000.
32. Ingegnoli F, Castelli R, Gualtierotti R, RFs: clinical applications. Dis Mark. 2013;35(6):727–34. CrossRef
33. Nichols C, Keshu JNgA, Kelly G, Conte MR, Marber MS, Fraternali F, et al. Mining the PDB for tractable cases where X-Ray crystallography combined with fragment screens can be used to systematically design protein–protein inhibitors: two test cases illustrated by IL1β-IL1R and P38α–TAB1 complexes. J Med Chem. 2020;63(14):7559–68. doi: 7559–68. CrossRef
34. Ruscitti P, Cipriani P, Carubbi F, Liakouli V, Zazzeroni F, Di Benedetto P, et al. The role of IL-1β in the bone loss during rheumatic diseases. Mediat Inflamm. 2015;2015:782382. CrossRef
35. Somers W, Stahl M, Seehra JS. 1.9 Å crystal structure of interleukin 6: implications for a novel mode of receptor dimerization and signaling. EMBO J. 1997;16:989–97. CrossRef
36. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):1–16. CrossRef
37. Xiao HY, Li N, Duan JJW, Jiang BZ, Lu KNJ, Tino LM, et al. Biologic-like in vivo efficacy with small molecule inhibitors of TNFα identified using scaffold hopping and structure-based drug design approaches. J Med Chem. 2020;63(23):15050–71. CrossRef
38. Niu J, Cederstrand AJ, Eddinger GA, Yin B, Checco JW, Bingman CA, et al. Trimer-to-monomer disruption mechanism for a potent, protease-resistant antagonist of tumor necrosis factor-α signaling. J Am Chem Soc. 2022;144(22):9610–7. CrossRef