INTRODUCTION
Awareness of health and environmental concerns, product safety, and biodegradable and nontoxic natural resources has increasingly attracted interest in various industrial fields. Pigments are essential components of daily life and are used in several fields including medicine, cosmetics, and food [1]. Natural pigments have attracted considerable attention compared to synthetic pigments because natural pigments are environmentally friendly, have low toxicity and carcinogenicity, and exhibit beneficial biological activities [2]. Among the natural pigments produced by microbes, melanin is structurally complex and functionally diverse [3], attracting the attention of researchers.
Melanin is a heterogeneous biopolymer with various functions in living organisms. It is produced through the process of oxidative polymerization, which involves phenolic or indolic molecules [4]. Based on its chemical structure, melanin is generally classified into eumelanin, pyomelanin, pheomelanin, neuromelanin, and allomelanin [5]. However, due to its complex polymerization, insoluble, and amorphous nature, the field of biochemistry and biophysics is currently unable to provide a definitive chemical construction of melanin. Researchers have used various techniques to provide complementary perspectives on melanin structure identification. These approaches include initial investigations focusing on physicochemical properties and pigment decolorization, examination of particle morphology using electron microscopy, and analysis of compound structures using Fourier transform infrared and UV-visible spectroscopy techniques [4].
Microbial-based melanin production is widely considered environmentally friendly, economical, and sustainable. Several reports have shown that actinomycetes produce microbial melanin. Actinomycetes are a group of bacteria characterized by their gram-positive nature and elevated guanine-cytosine (G+C) content in their DNA, exceeding 55 mol % [6]. Rare marine actinomycetes of the genera Nocardiopsis, Micromonospora, Dietzia, and Salinispora are potential sources of secondary metabolites with varied functions and distinctive molecular structures [7]. Tyrosinase mediates extracellular melanin biosynthesis in actinomycetes to produce eumelanin via the 3,4-dihydroxyphenylalanine (DOPA) pathway involving L-tyrosine transformation [8]. The structure of melanin contains numerous binding sites, primarily consisting of phenolic hydroxyl, carboxyl, and amine groups, which allow it to interact with various other molecules. Its structure is related to its biological activity, thereby playing many roles in protective functions, including absorption of free radicals and blocking of ultraviolet (UV) radiation [9].
Actinomycete melanin has been reported to have antioxidant properties by reducing free radicals that cause oxidative stress. The human body produces free radicals via various metabolic processes at the cellular level. Additionally, these molecules can be introduced from external sources such as UV radiation, environmental pollutants, and smoking. Reactive oxygen species (ROS) and reactive nitrogen species are collectively referred to as free radicals, which are characterized by their instability and high reactivity [10]. Excessive ROS production can damage lipid molecules, disrupt the structure and function of proteins, and damage DNA. Melanin has been suggested to exhibit in vitro antioxidant activity by acting as an electron acceptor or donor for free radical molecules, stabilizing them before oxidizing other cellular components [4]. Further analyses should be performed at the cellular level to understand how melanin affects various cellular processes. Therefore, a comprehensive evaluation of the antioxidant properties of melanin is important. However, its antioxidant properties remain unclear.
Research on melanin antioxidants is limited to in vitro studies, and few report melanin from rare actinomycetes. In previous studies, melanin from actinomycete species showed in vitro antioxidant activity in reducing DPPH, ABTS+•, and OH?• radicals with IC50 values ranging from 100 to 500 μg/ml [11–13]. The fission yeast, Schizosaccharomyces pombe is an important model organism for studying cellular-level melanin antioxidant mechanisms. However, the effects of melanin at the cellular level have not yet been reported. UV radiation generates free radicals, and melanin not only reduces free radicals but also has photoprotective properties as a UV absorber, as indicated by the sun protection factor (SPF) value. In this study, the marine sponge-associated actinomycete Micromonospora fulva HV6 was used as a source of melanin. The study objectives were to determine the in vitro antioxidant activity of the pigment, to examine its antioxidant effects at the cellular level, and to determine its photoprotective properties.
MATERIALS AND METHODS
Chemicals and reagents
All materials used, including L-tyrosine, glucose, NaOH, HCl, dimethyl sulfoxide (DMSO), methanol, ethanol, 1-butanol, chloroform, acetone, dichloromethane, ethyl acetate, FeCl3, CuSO4, K2S2O8, and ABTS, were of analytical grade and were obtained from Merck (Darmstadt, Germany) as were all chemicals used for microbial medium components. Rhodamine B, L-DOPA, and synthetic melanin standards (product name: Melanin—Synthetic; product number: M8631; CAS number: 8049-97-6) were sourced from Sigma-Aldrich (St. Louis, MO, USA). DPPH radicals and L-ascorbic acid were purchased from HiMedia (Mumbai, India). Zymo Research (Irvine, CA, USA) provided the Quick-DNA™ Fungal/Bacterial Miniprep Kit, while Bioline (London, UK) provided MyTaq HS Red Mix.
Isolate source and culture conditions
The marine sponge-associated actinomycete isolate coded as HV6, was isolated from the marine sponge Smenospongia sp. from Pramuka Island, Kepulauan Seribu, Indonesia (5°44′46.3 S 106°36′35.7 E), in a previous study using a pretreatment method according to Pisano et al. [14]. The HV6 isolate was used as a source of melanin pigment for the first time in this study. The isolate was regularly grown on International Streptomyces Project No. 4 (ISP-4) medium (10 g/l soluble starch, 1 g/l MgSO4.7H2O, 1 g/l NaCl, 1 g/l K2HPO4, 2 g/l (NH4)2SO4, 2 g/l CaCO3, 1 mg/l FeSO4.7H2O, 1 mg/l MnCl2.4H2O, 1 mg/l ZnSO4.7H2O, and 20 g/l agar) as per the standard cultivation procedure [15]. The yeast S. pombe strain ARC039 was used to evaluate the cellular level antioxidant activity [16]. Schizosaccharomyces pombe was cultivated in a standard yeast extract-supplemented (YES) medium (5 g/l yeast extract, 30 g/l glucose, 0.128 g/l histidine, 0.128 g/l leucine, 0.128 g/l adenine, 0.01 g/l uracil, 0.128 g/l arginine, and 20 g/l agar).
Isolate identification
Morphological characterization and melanoid pigment production
Morphological characterization of the HV6 isolate was performed after 14 d of incubation on ISP-4 medium at 30°C [13]. Macroscopic morphological characterization was performed by observing colonies, such as pigmentation of aerial mycelia and substrate and production of diffusible pigments, whereas microscopic characterization was performed by observing the shape and arrangement of spore chains. The ability of the HV6 isolate to produce extracellular black pigment was analyzed on tyrosine agar medium (5 g/l peptone, 5 g/l L-tyrosine, 3 g/l beef extract, and 20 g/l agar; pH 7–7.2) at 30°C for 14–21 d [17]. Qualitative parameters were observed in the form of brown areas of diffusible pigments produced by actinomycete.
Molecular identification
The HV6 strain was genetically identified using 16S rRNA gene sequence analysis. The strain genome was isolated using the Quick-DNA™ Fungal/Bacterial Miniprep Kit. The universal primer pair 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1429R (5′-TACGGYTACCTTGTTACGAGACTT-3′) was used to amplify the 16S rRNA gene sequence [18]. The PCR protocol involved 35 cycles of pre-denaturation (94°C, 5 minutes), denaturation (95°C, 30 seconds), annealing (55°C, 30 seconds), elongation (72°C, 1 minute 30 seconds), final elongation (72°C, 10 minutes), and post-PCR (4°C, 5 minutes). The alignment of gene sequences with data from GenBank (www.ncbi.nlm.nih.gov) was performed using the Basic Local Alignment Search Tool for Nucleotide Sequences (BLAST-N) program. MEGA software version 11.0.13 was used to construct a phylogenetic tree based on the neighbor-joining method adjusted to the p-distance model (1,000 bootstraps).
Production, extraction, and purification of melanin pigments
Confirmation of melanin production
The melanin-producing ability of M. fulva HV6 was previously confirmed by L-DOPA assay and tyrosinase enzyme activity in a tyrosine broth medium. L-DOPA was detected by spectrophotometry (Metertech SP-8001; Taiwan) at 530 nm using the L-DOPA standard curve over a concentration range of 10–100 μg/ml [19]. Tyrosinase activity was measured at 280 nm (Metertech SP-8001; Taiwan) using L-tyrosine as the substrate [17]. A single unit of tyrosinase activity causes an increase of 0.001 per minute in absorbance at pH 6.5°C and 25°C. The following equation was used to determine enzyme activity:
where df is the dilution factor, 0.001 indicates the unit change in A280 nm per minute for tyrosinase at pH 6.5°C and 25°C, and 0.1 is the volume of enzyme used (ml).
Melanin extraction and purification
Micromonospora fulva HV6 was cultured in a tyrosine broth medium for extracellular melanin production in a shaker incubator at 150 rpm and 27°C for 21 d. Melanin extraction was performed according to the acid precipitation method with slight modifications [20]. Actinomycete cultures were then centrifuged at 3,000 × g for 30 minutes at 27°C. Melanin was isolated from the resulting supernatant by precipitation in 6 M HCl, with the pH adjusted to 3.0. The mixture was maintained at 4°C for 24 hours to facilitate pigment precipitation. The precipitate was collected and washed 3–5 times with distilled water, followed by the successive addition of organic solvents namely chloroform:ethyl acetate:ethanol (ratio 1:1:1), and washed with distilled water. The pH of the solution was raised to 9.0 with the addition of 1 M NaOH. After a 30-minute incubation period, the mixture was centrifuged at 3,000 × g for 20 minutes at 27°C. Subsequently, the pH of the supernatant was lowered to 3.0 using 6 M HCl to harvest melanin. The extracted melanin was rinsed with distilled water to enhance its purity. After drying at 40°C to a constant weight, melanin was stored at 0°C. The yield was calculated based on the ratio of the final weight of melanin to the initial culture volume multiplied by 100% [21].
Characterization of melanin pigments
Physicochemical properties
The physicochemical characterization of melanin is conventionally performed as a preliminary analysis owing to its low solubility in most common organic solvents [22]. The solubility properties of melanin were determined by mixing 1 mg of melanin powder with 2 ml each of 1 M NaOH, 0.01 M NaOH, 1 M KOH, H2O, methanol, ethanol, 1-butanol, DMSO 99.0%, DMSO 10%, acetone, dichloromethane, chloroform, and ethyl acetate. After 24 hours of incubation, the mixture was centrifuged at 5,000 × g for 15 minutes. Solutions of 6 M HCl, 1% FeCl3, and 1% CuSO4 were used to test the precipitation properties of melanin by mixing 2 ml of each solution with 100 μl of a 1 mg/ml melanin solution. In addition, bleaching was evaluated by mixing 100 μl of 1 mg/ml melanin solution with 30% H2O2 and 5.25% NaOCl. This mixture was then incubated for 48 hours at 27°C to determine the effect of the oxidant.
UV-visible (Vis) spectrometry
Melanin absorption spectra were obtained via spectrophotometry [23]. The pigment was diluted in a 0.1 M NaOH solution to achieve a 0.01 mg/ml concentration. The maximum absorption spectrum of the pigment was measured using a UV-Vis spectrophotometer (Metertech SP-8001; Taiwan) in the 200–800 nm wavelength range. A blank control was prepared using a solvent. The melanin standard was used as a control to compare the obtained absorption spectra.
Scanning electron microscopy (SEM)
The surface morphology of the melanin particles was observed using SEM. Melanin powder was dropped onto the surface of the SEM plates. The SEM samples were covered with a layer of Au particles applied through sputtering (Hitachi®, Tokyo, Japan). The specimens were then examined using a scanning electron microscope (JEOL JSM-IT200; South Korea) at magnifications of 5,000 × and 15,000 ×, with a 5 µm working distance and 15 kV acceleration voltage.
Fourier transform infrared (FT-IR) spectroscopy
The functional groups and structures of melanin compounds were identified using FT-IR spectroscopy. The pigment samples were placed on an FT-IR sample plate and analyzed directly by inserting the plate into an FT-IR instrument set in the attenuated total reflection measurement mode. Measurements were performed in the wavenumber range of 4,000–500 cm-1 using an FT-IR spectrometer (BRUKER TENSOR II; Germany). The assay result was compared with those of melanin standards.
DPPH radical scavenging assay
The DPPH assay was performed using spectrophotometry with some modifications [24]. A solution containing 100 µl of 125 µM DPPH (dissolved in methanol) was combined with an equal volume of melanin at different concentrations (also in methanol). The reaction was carried out at room temperature in the dark for 30 minutes. L-Ascorbic acid was used as a positive control. The reduction of DPPH radicals by melanin was quantified at a wavelength of 517 nm using an ELISA reader (EPOC; USA). Measurements were obtained in triplicate. The percentage of inhibition was determined using the equation provided:
The 50% inhibitory concentration (IC50) was obtained by regressing the inhibition values into a linear regression equation.
ABTS+• radical scavenging assay
The ABTS radical assay was performed as previously described [25]. Briefly, a mixture of 20 μl of different melanin concentrations and 180 μl of ABTS+• was prepared and incubated for 30 minutes at room temperature in the dark. L-Ascorbic acid was used as a positive control. The reaction mixture was analyzed using an ELISA reader (EPOC; USA) with three replicates at a wavelength of 734 nm. The absorbance value was used to calculate the % inhibition, which was then regressed using a linear regression equation to obtain the IC50 value for melanin. The percentage of inhibition was determined using the equation provided:
Oxidative stress response assay
A cellular oxidative stress response assay was performed on S. pombe using the spot method [26]. Schizosaccharomyces pombe was inoculated on YES broth medium at an initial OD600 of 0.05 and then treated with different concentrations of melanin (in DMSO). YES medium with calorie restriction (CR) (reduction of glucose from 3% to 0.3% w/v) could prolong the lifespan of the yeast, whereas ascorbic acid treatment at 20 μg/ml enhanced the in vitro antioxidant properties. An inoculum inoculated on YES medium with CR and ascorbic acid was used as the positive control, and YES medium without melanin treatment and YES medium with DMSO supplementation were used as negative controls. Treated and control cultures were incubated for 24 hours at room temperature with agitation at 120 rpm. The optical density of each culture was adjusted to OD600 = 1 and serially diluted from 10-1 to 10-4. Each dilution was spot-inoculated onto YES agar supplemented with 0, 1, and 2 mM H2O2. The viability of yeast cells was observed after 3 d of incubation based on a comparison of spot density between the treatments and the control.
Detection of mitochondrial activity
A Rhodamine B fluorescent probe was used to observe the membrane potential and mitochondrial activity of yeast cells, as described by Lesmana et al. [26], with slight modifications. After 18 hours of incubation, the melanin-treated S. pombe culture with an initial optical density (OD600) of 0.05 was collected. The collection process included centrifugation at 3,000 × g for 2 minutes at room temperature. The positive controls included yeast treated with ascorbic acid and CR conditions, while the negative control was DMSO. Rhodamine B was added to the cell suspension in phosphate buffer (0.1 M, pH 7) to a final concentration of 200 nM. This process was performed without any exposure to light. Stained cells were examined using an Olympus BX51 fluorescence microscope equipped with a WU fluorescence mirror unit (#1). This unit included a DM400 dichroic mirror, BP330-385 excitation filter, and BA420 barrier filter.
Determination of photoprotective properties of melanin
The SPF value of M. fulva HV6 melanin was evaluated as described previously with some modifications [27]. Melanin solutions were prepared at concentrations of 125, 100, 50, and 25 μg/ml from an initial concentration of 1,000 μg/ml (in 0.1 M NaOH). Melanin absorbance measurements were performed on a UV-Vis spectrophotometer (Metertech SP-8001; Taiwan) in the UV spectrum, specifically from 290 to 320 nm, with readings recorded at 5 nm intervals. The SPF values were calculated using the Mansur equation:
where cf is a correction factor of 10, EE(λ) is the erythema effect spectrum, Abs(λ) is the absorbance of melanin, and I is the solar intensity spectrum. EE(λ) × I is a constant, and its value was determined according to Sayre et al. [28].
Data analysis
For the melanin antioxidant assays, DPPH and ABTS radical scavenging assays were performed in triplicate. Data were processed using Microsoft Excel version 2108 (Microsoft Inc., Redmond, WA, USA) and expressed as the mean ± standard deviation.
RESULTS AND DISCUSSION
Isolate identification
Morphological characteristics of the HV6 isolate
The HV6 isolate grew slowly on ISP-4 medium with the appearance of brown-black aerial and beige substrate mycelia, did not produce diffusible pigment, had non-sporulating colonies with a size of ±2 mm, and produced spherical spores in a monosporous-chain arrangement (Fig. 1A and 1B), similar to the general morphological characteristics of Micromonospora [29]. In addition, the isolate was able to synthesize an extracellular black pigment, presumably melanin, as indicated by the diffusion of brown color on tyrosine agar medium (Fig. 1C). These morphological characteristics are important because compared to other gram-positive bacteria, most actinomycetes show a high degree of morphological differentiation, namely, a complex mycelial structures, aerial and substrate mycelia with different pigmentation, and the ability to form spores [30]. Owing to their metabolite production capacity, marine actinomycetes can synthesize various pigments in natural and synthetic media, such as the soluble black pigment melanoid, which is commonly used as a taxonomic characterization standard [31].
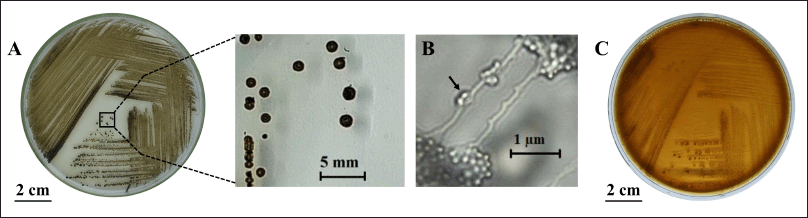 | Figure 1. Characteristics of M. fulva HV6 colonies: (A) on ISP-4 medium after 14 d of incubation at 30°C, (B) monosporous chain arrangement (arrow) at 1,000× magnification, and (C) extracellular melanin synthesis on tyrosine agar after 21 d of incubation at 30°C. [Click here to view] |
Identity of the HV6 isolate
Identification of the 16S rRNA gene sequence of the HV6 isolate showed that it was closely related to M. fulva UDF-1 (Table 1). The HV6 isolate was named M. fulva HV6. Phylogenetic tree construction showed that M. fulva HV6 was on the same branch as M. fulva UDF-1 and in a clade with M. chalcea ATCC 12452 (Fig. 2). The 16S rRNA gene sequences information of M. fulva HV6 were deposited in GenBank under the accession number PP 738051.2. A survey of microbial genomes in a review by Parra et al. [32] reported that the Micromonospora genome encodes the second largest chemical diversity in the actinomycete group and thus has the most significant potential for secondary metabolite biosynthesis, with a broad scope for pharmaceutical and industrial fields. Notably, the actinomycete group produces melanin as a primary pigment during the secondary metabolism phase with broad biological activities, such as antioxidant and radioprotective activities [33].
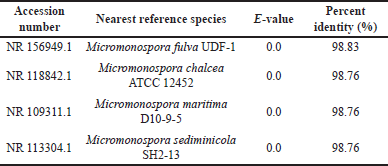 | Table 1. BLAST-N results of 16S rRNA sequences of the HV6 isolate. [Click here to view] |
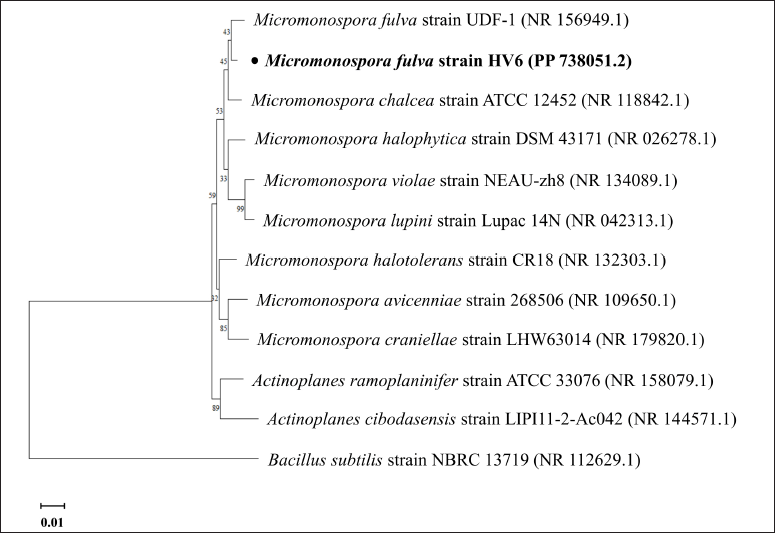 | Figure 2. Phylogenetic tree construction of M. fulva HV6 using the 1,000×neighbor-joining bootstrap method. The line scale indicates 0.01 genetic distance. [Click here to view] |
Production, extraction, and purification of melanin pigments
Confirmation of melanin production
Micromonospora fulva HV6 cultured in tyrosine broth medium underwent a color change from brown to solid black over an incubation period of 14–21 d. Based on these results, the isolate showed melanin production with positive evidence of tyrosinase production (Fig. 3A). The L-tyrosine present in the medium is a precursor used by actinomycetes to synthesize melanin. Therefore, these conditions are sufficient to explain why melanin synthesis originates from the conversion of L-tyrosine via the DOPA pathway. Melanin synthesis by microbes generally occurs via the DOPA pathway to form the eumelanin structure, which involves the transformation of L-tyrosine to L-DOPA through the action of tyrosinase, followed by conversion to dopaquinone, and autopolymerization to form melanin [34].
Melanin synthesis by M. fulva HV6 was confirmed by the detection of L-DOPA compounds and tyrosinase enzyme activity. Quantification results showed that L-DOPA production increased with increasing in tyrosinase activity in the culture. The L-DOPA concentration and tyrosinase activity reached 22 μg/ml and 4,936.67 U/ml, respectively, on Day 7 (Fig. 3B). Previous studies have reported that some melanogenic microbes, including Vibrio tyrosinaticus, Rhizobium sp., Streptomyces sp., and marine bacteria, are natural tyrosinase producers that tend to show dominant tyrosinase activity in the bioconversion process of L-tyrosine to L-DOPA [35]. The production of L-DOPA by Bacillus sp. JPJ was derived from the biotransformation of 0.5 mg/ml L-tyrosine, producing 0.461 mg/ml L-DOPA without significant optimization [36]. In addition, the Brevundimonas sp. SGJ culture under nonoptimized conditions reached a maximum L-DOPA production of 0.419 g/l when the tyrosinase activity in the culture reached a maximum of 2,015 U/mg [37].
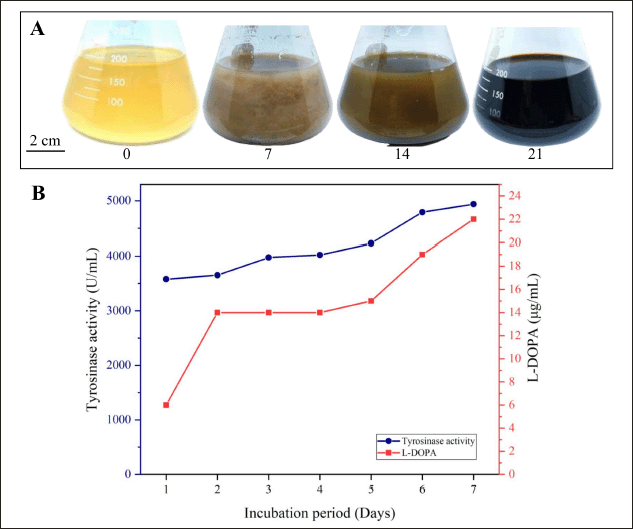 | Figure 3. Melanin production: (A) color change in the tyrosine broth medium after incubation for 14–21 d at 30°C (the numbers under the Erlenmeyer flasks indicate the incubation time in d); (B) tyrosinase activity and L-DOPA concentration quantified from the cell-free supernatant on the tyrosine broth medium. [Click here to view] |
Melanin extraction and purification
Extracellular melanin from M. fulva HV6, extracted by acid precipitation, was characterized as a coarse solid black powder with a yield of 0.03% (w/v) (Fig. 4A). In this study, melanin production in the culture medium was 310 mg/l. Melanin typically appears as an amorphous powder ranging in color from dark brown to black [4]. The physical appearance of a deep black powder typical of melanin was also observed in Streptomyces glaucescens NEAE-H melanin extracted by HCl acid precipitation with a maximum melanin yield of 350 mg/l from peptone yeast extract iron broth production medium [13] and the yeast Yarrowia lipolytica with a maximum melanin yield of 160 mg/l from production medium supplemented with a L-DOPA precursor [38]. The yield value obtained is an indicator of the effect of the extraction conditions and indicates the amount of bioactive components in melanin [21]. Acid precipitation is a commonly used technique to extract and purify extracellular melanin. In the present study, a follow-up procedure involving washing with an organic solvent was performed. Acid treatment aims to remove all protein fractions, cell debris, and residual nutrients from the production medium. Organic solvents such as absolute ethanol, ethyl acetate, and chloroform are then used to remove lipids and other residual components, thereby increasing the purity of melanin [5]. The marine actinomycete Nocardiopsis spp. produces melanin with antibacterial, antibiofilm, and anti-quorum sensing properties [7,39]. However, reports of melanin isolated from rare actinomycetes are few.
Characteristics of melanin pigments
Physicochemical properties and UV-Vis absorption spectrum
Analysis of the physicochemical properties of HV6 melanin revealed that the pigment was resistant to solubilization in all solvents tested but readily soluble in alkaline solutions and 99.0% DMSO; bleached by oxidizing agents (H2O2 and NaOCl); and precipitated by HCl, FeCl3, and CuSO4 (Fig. 5). The observed properties are similar to those found in melanin from the rare marine actinomycetes Nocardiopsis dassonvillei JN1 and Nocardiopsis sp. JN2 [39]. Conventional physicochemical characterization is an early aid in demonstrating that the extracted black pigment is melanin, as melanin has typical solubility and reactivity properties, such as low solubility in H2O and most common organic solvents, solubility in alkaline solutions, bleaching by oxidizing agents, and precipitation in acidic solutions [5]. Analysis of the UV-Vis absorption spectrum showed that M. fulva HV6 melanin exhibited a peak absorption at 213 nm in the UV region, with a gradual decrease toward the visible spectrum. This pattern was similar to that of melanin standards (Fig. 4B). According to Ghadge et al. [40], microbial melanin exhibits peak absorption within the UV spectrum, specifically between 200 and 400 nm. This characteristic was attributed to the presence of conjugated carbonyl groups, including carboxylic acids, esters, and amides. The maximum absorption of Actinoalloteichus sp. MA-32 melanin is at 300 nm [12], whereas that of N. dassonvillei JN1 and Nocardiopsis sp. JN2 is at 350 nm [39] and S. glaucescens NEAE-H is at 250 nm [13]. The pH-influenced solubility properties are related to the granule formation process and its dimensions, where lowering the pH of the melanin solution promotes aggregate formation and sedimentation, whereas increasing the pH causes the granules to break down into small oligomeric particles [22]. This is related to the properties of melanin polyelectrolytes, which are influenced by the ionization state of the phenolic, carboxyl, and amine groups [13].
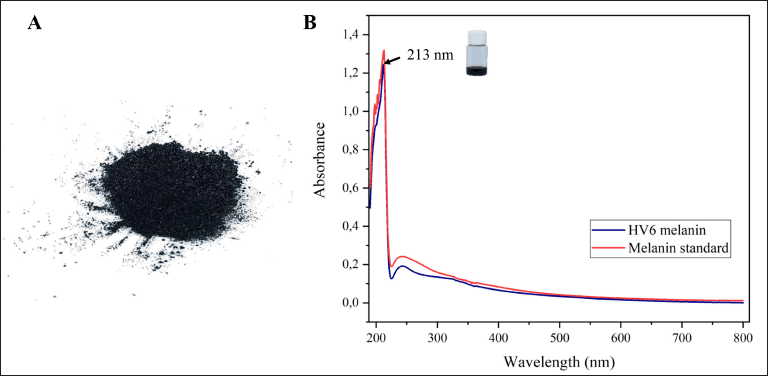 | Figure 4. Characteristics of M. fulva HV6 melanin: (A) physical characteristics; (B) UV-Vis absorption spectrum; maximum absorption is indicated by the arrow. [Click here to view] |
 | Figure 5. Physicochemical properties of M. fulva HV6 melanin: (A) solubility properties in (1) 1 M NaOH, (2) 0.01 M NaOH, (3) 1 M KOH, (4) H2O, (5) methanol, (6) ethanol, (7) 1-butanol, (8) 99.0% DMSO, (9) 10% DMSO, (10) acetone, (11) dichloromethane, (12) chloroform, and (13) ethyl acetate; (B) precipitation properties in (1) 6 M HCl, (2) 1% FeCl3, and (3) 1% CuSO4; effect of oxidants in the bleaching of (4) 1 mg/ml melanin solution by (5) 30% H2O2 and (6) 5.25% NaOCl. [Click here to view] |
Surface morphology of the pigment particles
Morphological characterization of melanin particles using SEM is an effective method for analyzing the melanin particle size [5]. The SEM images at scales of 1 and 5 μm, showed that the HV6 melanin particles had irregular shapes and surfaces (Fig. 6). Amin et al. [41] reported that depending on the source of melanin, the morphology of melanin particles varies, which is usually amorphous with an irregular surface and a size range of 30–1,000 nm. When observed under SEM, fungal melanin has particle sizes in the nanogranule range, whereas bacterial melanin has relatively small dimensions [35]. Melanin from Streptomyces sp. has black particles, rough and irregular surfaces, and porous structures [11], whereas melanin from S. glaucescens NEAE-H is observed as small spheres, similar to that of natural melanin (Sepia officinalis) [13]. In contrast, melanin of bacterial origin, Pseudomonas putida ESACB 191, appears as an amorphous population of heterogeneous fragments without distinguishable structures [42].
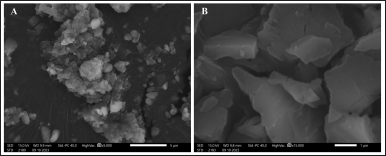 | Figure 6. Results of scanning electron microscopy imaging of melanin particles at (A) 5,000× and (B) 15,000× magnification, showing irregular shapes. [Click here to view] |
Pigment structure
The different wavenumber absorption peaks in the IR spectra of HV6 melanin revealed characteristics of the eumelanin structure. Analysis of the FT-IR spectrum of HV6 melanin (Fig. 7) revealed several characteristic peaks. A wavenumber peak at approximately 1,612.51 cm-1 was attributed to the C=O (carbonyl) functional group. The spectrum showed a moderate absorption intensity between 3,600 and 3,300 cm-1, corresponding to the stretching vibrations of the N–H group in amide compounds. An oblique spectrum with a peak at 3,271.70 cm-1 was observed due to O–H group stretching vibrations. The C–H group associated with the aromatic ring was indicated by a peak at 3,081.92 cm-1, whereas aliphatic C–H group stretching vibrations produced a peak at 2,928.93 cm-1. The aromatic ring was further confirmed by a moderate absorption intensity at 1,515.08 cm-1. CH3 bending vibrations were identified at 1,442.51 cm-1 and 1,370.26 cm-1. In addition, the C–O group of the ester compound was detected at 1,219.70 cm-1. These spectroscopic features closely matched those of melanin standards (Fig. 7). FT-IR analysis revealed several signals characteristic of the indolic/phenolic property of melanin, such as the melanin from Streptomyces nashvillensis, which shows a prominent and wide peak at 3,464 cm-1 due to stretching vibrations of –NH and –OH groups, small and weak peaks at 2,920 cm-1 and 2,851 cm-1 due to stretching vibrations of aliphatic C–H groups [43]; a strong peak between 1,650 and 1,620 cm-1 confirming the aromatic ring, a peak at 1,423.51 cm-1 derived from CH2–CH3 bending vibrations [13]; and small peaks at 1,233 cm-1 and 1,153 cm-1 due to stretching vibrations of phenolic C–OH groups [43]. These results are also supported by findings of Ribera et al. [44] as in eumelanin, the wavenumber peak marker is the presence of a broad peak with vigorous intensity between 3,500 and 3,000 cm-1 for O–H and –NH2 stretching vibrations, a signal at 2,925 cm-1 from C–H stretching vibrations, and a peak at 1,075 cm-1 from C–O groups in phenols or carboxylic acids.
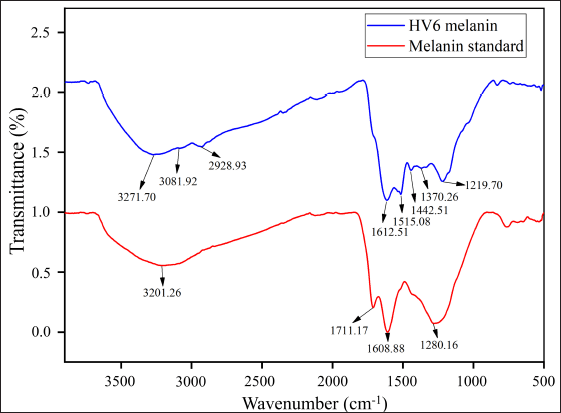 | Figure 7. Infrared (IR) spectra of M. fulva HV6 melanin compared with those of the melanin standard. [Click here to view] |
Antioxidant activity of melanin
The antioxidant activity of melanin extracted from M. fulva HV6 was evaluated using in vitro DPPH and ABTS+• radical reduction assays and was expressed as IC50 values. Astuti et al. [16] reported that compounds with the highest antioxidant activity had the lowest IC50 values. Melanin extracted from HV6 exhibited potent antioxidant activity and effectively scavenged DPPH and ABTS+• radicals. The IC50 values for these activities were determined to be 31.55 μg/ml and 63.07 μg/ml, respectively. The results of the melanin treatment were different from those of the L-ascorbic acid treatment (Table 2). Notably, melanin from M. fulva HV6 in the present study was more potent at reducing DPPH radicals than was melanin from marine Streptomyces species in a previous study with the best IC50 value at a concentration of 150 μg/ml [45] and more potent at reducing ABTS+• radicals than was melanin from S. glaucescens strain NEAE-H with an IC50 value of 100 μg/ml [13]. According to research conducted by El-Zawawy et al. [46], melanin exhibits antioxidant properties, capable of neutralizing free radicals through a sequence of single-electron transfer processes. Consequently, incorporating melanin into cosmetic formulations can help mitigate tissue damage resulting from toxic substances. However, vitamin C, also known as L-ascorbic acid, is an essential biomolecule that serves a crucial function in safeguarding cellular components from oxidative stress [47].
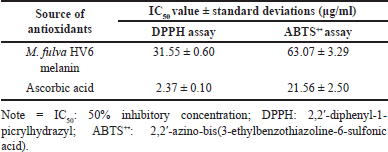 | Table 2. Comparison of IC50 values of antioxidant activity. [Click here to view] |
Oxidative stress response
The effect of melanin treatment on the antioxidant potential was further analyzed at the cellular level based on S. pombe cell viability. The fission yeast S. pombe is an ideal system for analyzing cellular processes common to higher eukaryotic cells, including response pathways to cellular stress induced by cellular redox imbalances. Schizosaccharomyces pombe shows remarkable conservation in multicellular eukaryotes, where fluctuations in intracellular H2O2 exposure trigger the MAP kinase pathways Sty1/Spc1 and Pap1 to induce adaptive responses to H2O2 levels [48]. Notably, HV6 melanin at a concentration of 240 μg/ml was able to provide resistance of S. pombe cells under severe oxidative stress (2 mM H2O2) better than the positive control ascorbic acid and solvent control, as evidenced by viability and growth that reached 10-3 dilutions (Fig. 8). The growth of yeast cells in the negative control (DMSO and YES medium) was viable only up to 10-2 dilutions and showed growth defects at lower dilutions. To the best of our knowledge, this research is the first to demonstrate the antioxidant potential of melanin pigments from Micromonospora in a rare actinomycete group. Previous research reported that the active fraction of Bacillus haikouensis AGS112 yellow–red pigment, when applied at 35 μg/ml, enhanced S. pombe survival under 2 mM H2O2-induced oxidative stress, achieving results comparable to the positive control used in the experiment [49]. In addition, melanin-treated cells showed an oxidative stress-resistant phenotype comparable to that under CR conditions despite being cultured in a high-glucose medium. López-Lluch and Navas [50] reported that one of the features of CR is the modulation of mitochondrial activity and the reduction of oxidative damage, thus ensuring cell longevity. These results suggest that HV6 melanin can induce the intracellular stress response in S. pombe against oxidative stress.
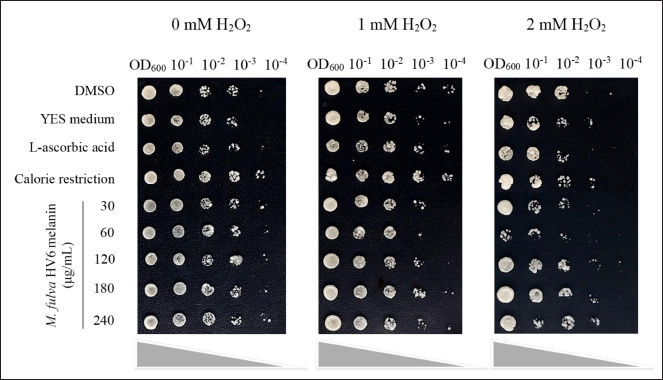 | Figure 8. Effect of treatment with different concentrations of M. fulva HV6 melanin on S. pombe cell viability under H2O2 stress conditions in comparison with both negative controls (DMSO and YES medium) and positive controls (20 μg/ml ascorbic acid and CR condition with 0.3% glucose). [Click here to view] |
Mitochondrial activity
In this study, the effect of melanin on the cellular aspects of S. pombe related to oxidative stress was investigated by monitoring mitochondrial activity. As speculated, the application of HV6 melanin at its maximum concentration (240 μg/ml) significantly increased mitochondrial activity. The cells treated with melanin, ascorbic acid, and CR fluoresced brightly in contrast to those treated with DMSO, which did not fluoresce (Fig. 9). Evidence of mitochondrial activation also occurred following CR treatment, as reported in other studies [16,51]. Under these conditions, target of rapamycin signaling is downregulated and responds efficiently to mitochondrial electron transport chain activity [52]. The mitochondrial membrane potential then increases, accompanied by ROS production, which provides adaptive signaling during growth, induces stress tolerance, and increases cell longevity. Research has shown that yeast cells treated with HV6 melanin can survive the severe oxidative stress caused by H2O2 exposure (Fig. 8). Our data suggest that HV6 melanin-induced oxidative stress tolerance likely occurs through mitochondrial adaptive ROS signaling, as observed under CR conditions. Melanin in cells is thought to act as a pro-oxidant that triggers the activation of hormesis; that is, the induction of mild stress can trigger adaptive responses in cells to prevent further damage from severe stress [50]. Therefore, HV6 melanin can induce a defense system against stress and increase the tolerance to oxidative stress.
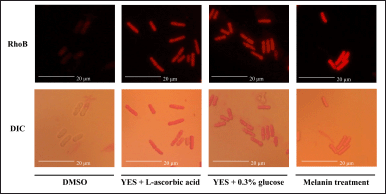 | Figure 9. Effect of HV6 melanin on mitochondrial activity in the fission yeast S. pombe based on Rhodamine B (RhoB) staining compared to its contrast visualization in differential interference contrast brightfield microscopy. The orange-reddish fluorescence signals indicate the presence of active mitochondria. Yeast in the YES + DMSO medium was used as a negative control (solvent control), whereas YES + ascorbic acid 20 μg/ml medium and the CR condition (0.3% glucose) were used as positive controls. [Click here to view] |
Photoprotective properties of melanin
The photoprotective properties of M. fulva HV6 melanin were indicated by the SPF value, which is an index of the protective potential of a compound against UVB rays. The SPF value for HV6 melanin indicated a moderate level of protection (20.78). This index is consistent with Commission Recommendation 2006/647/EC [53] that categorizes SPF values into four qualitative classes of low (6–10), moderate (15–25), high (30–40), and very high (50+) protection. Sufficient evidence shows that HV6 melanin, when used as a sunscreen, protects against UVB skin damage. Sunscreen effectiveness is measured using the SPF value. According to Dutra et al. [54], the United States Food and Drug Administration recommends sunscreens with an SPF of 15 to guard against sunburn, premature skin aging, and skin cancer. To our knowledge, this research represents the first investigation into the sun-protective qualities of melanin generated by the actinomycete Micromonospora. The SPF values of Pseudomonas koreensis UIS 19 and Bacillus safensis melanin showed very high levels of protection with values of 61.55 and 53.36, respectively [27,55]. In contrast, commercial synthetic melanin has an SPF value of 59.34 with very high protection [55]. However, HV6 melanin in this study is promising for future sunscreen formulations as a natural compound with fewer side effects than those of synthetic sunscreen components [46]. Melanin has physiological and photoprotective properties, absorbs the UV-visible spectrum, and is reported to be a stronger UV absorber than mycosporine-like amino acids [56]. Currently, the primary commercial uses of melanin are as a coloring agent in sunglasses (https://espeyewear.com/) and as an antioxidant component in sun protection products (https://chicet.com/product/melanin-sunscreen-for-dry-skin/).
CONCLUSION
The melanin extracted from M. fulva HV6 exhibited potent in vitro antioxidant properties, effectively neutralizing DPPH and ABTS+• radicals. The IC50 values for these scavenging activities were measured at 31.55 ± 0.60 μg/ml and 63.07 ± 3.29 μg/ml, respectively. Higher concentrations of melanin maintained the viability of S. pombe cells under severe H2O2 oxidative stress conditions comparable to that under CR conditions, indicating that melanin could induce the intracellular stress response system of yeast cells against oxidative stress. Melanin also stimulated the oxidative stress defense system of S. pombe, as occurs under CR, by enhancing mitochondrial function and promoting oxidative stress tolerance via mitochondrial adaptive ROS signaling. In addition, melanin exhibited protective properties against UVB rays, providing a moderate level of protection when used as sunscreen. The results of this study have particular relevance for pharmaceutical and industrial applications as well as for the search for natural antioxidant candidates owing to increasing health and environmental concerns. However, the analysis of S. pombe antioxidative gene expression is required to fully understand the antioxidant potential of HV6 melanin.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took Fart in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All generated and evaluated data are presented in the research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Kiki MJ. Biopigments of microbial origin and their application in the cosmetic industry. Cosmetics. 2023;10(2):1–17. CrossRef
2. Barreto JV de O, Casanova LM, Junior AN, Reis-Mansur MCPP, Vermelho AB. Microbial pigments: Major groups and industrial applications. Microorganisms. 2023;11(12):1–38. CrossRef
3. Gosset G. Biotechnological production of melanins with microorganisms. In: Singh OV, editor. Bio-pigmentation and biotechnological implementations, New York, NY: John Wiley & Sons, Inc; 2017. pp. 161–71. CrossRef
4. El-Naggar NEA, Saber WIA. Natural melanin: current trends, and future approaches, with especial reference to microbial source. Polymers. 2022;14(7):1339. CrossRef
5. Pralea IE, Moldovan RC, Petrache AM, Ilie? M, Heghe? SC, Ielciu I, et al. From extraction to advanced analytical methods: the challenges of melanin analysis. Int J Mol Sci. 2019;20(16):3943. CrossRef
6. Udhyakumar K, Ramalingam S, Saravanan R, Dheeba B. Extraction of actinomycetes (Streptomyces sp.) pigment and evaluation of its anticancer property on HeLa cell line. Der Pharma Chem. 2017;9(24):106–13.
7. Sundar R, Sivaperumal P. Melanin pigments from sediment-associated Nocardiopsis sp. marine actinobacterium and antibacterial potential. J Adv Pharm Technol Res. 2022;13(5):88–92. CrossRef
8. Guo J, Rao Z, Yang T, Man Z, Xu M, Zhang X, et al. Cloning and identification of a novel tyrosinase and its overexpression in Streptomyces kathirae SC-1 for enhancing melanin production. FEMS Microbiol Lett. 2015;362(8):1–7. CrossRef
9. Guo J, Rao Z, Yang T, Man Z, Xu M, Zhang X. High-level production of melanin by a novel isolate of Streptomyces kathirae. FEMS Microbiol Lett. 2014;357(1):85–91. CrossRef
10. Phaniendra A, Jestadi DB, Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem. 2015;30(1):11–26. CrossRef
11. Li C, Ji C, Tang B. Purification, characterisation and biological activity of melanin from Streptomyces sp. FEMS Microbiol Lett. 2018;365(19):1–8. CrossRef
12. Manivasagan P, Venkatesan J, Senthilkumar K, Sivakumar K, Kim SK. Isolation and characterization of biologically active melanin from Actinoalloteichus sp. MA-32. Int J Biol Macromol. 2013;58:263–74. CrossRef
13. El-Naggar NEA, El-Ewasy SM. Bioproduction, characterization, anticancer and antioxidant activities of extracellular melanin pigment produced by newly isolated microbial cell factories Streptomyces glaucescens NEAE-H. Sci Rep. 2017;7(1):1–19. CrossRef
14. Pisano MA, Sommer MJ, Lopez MM. Application of pretreatments for the isolation of bioactive actinomycetes from marine sediments. Appl Microbiol Biotechnol. 1986;25(3):285–8. CrossRef
15. Shirling E, Gottlieb D. Methods for characterization of streptomyces species. Int J Syst Bacteriol. 1966;16(3):313–40. CrossRef
16. Astuti RI, Prastya ME, Batubara I, Budiarti E, Ilmiyawati A. Antiaging and antioxidant bioactivities of Asteraceae plant fractions on the cellular functions of the yeast Schizosaccharomyces pombe. Adv Pharmacol Pharm Sci. 2021;2021(9):1–12. CrossRef
17. Raval KM, Vaswani PS, Majumder DR. Biotransformation of a single amino-acid L-tyrosine into a bioactive molecule L-DOPA. Int J Sci Res Publ. 2012;2(5):1–9.
18. Benget VV, Retnaningrum E. Activities and molecular characterization of petroleum hydrocarbons degrading rhizobacteria from mangrove plants (Rhizophora sp.) in Kulon Progo, Yogyakarta, Indonesia. Biodiversitas. 2020;21(1):21–7. CrossRef
19. Arnow LE. Colorimetric determination of the components of 3,4-dihydroxyphenylalanine-tyrosine mixtures. J Biol Chem. 1937;118(2):531–7. CrossRef
20. El-Batal AI, El-Sayyad GS, El-Ghamery A, Gobara M. Response surface methodology optimization of melanin production by Streptomyces cyaneus and synthesis of copper oxide nanoparticles using gamma radiation. J Clust Sci. 2017;28(3):1083–112. CrossRef
21. Adusei S, Otchere JK, Oteng P, Mensah RQ, Tei-Mensah E. Phytochemical analysis, antioxidant and metal chelating capacity of Tetrapleura tetraptera. Heliyon. 2019;5(11):1–15. CrossRef
22. Suwannarach N, Kumla J, Watanabe B, Matsui K, Lumyong S. Characterization of melanin and optimal conditions for pigment production by an endophytic fungus, Spissiomyces endophytica SDBR-CMU319. PLoS One. 2019;14(9):1–15. CrossRef
23. Fu X, Xie M, Lu M, Shi L, Shi T, Yu M. Characterization of the physicochemical properties, antioxidant activity, and antiproliferative activity of natural melanin from S. reiliana. Sci Rep. 2022;12(1):1–10. CrossRef
24. Batubara I, Mitsunaga T, Ohashi H. Screening antiacne potency of Indonesian medicinal plants: antibacterial, lipase inhibition, and antioxidant activities. J Wood Sci. 2009;55(3):230–5. CrossRef
25. Lee KJ, Oh YC, Cho WK, Ma JY. Antioxidant and anti-inflammatory activity determination of one hundred kinds of pure chemical compounds using offline and online screening HPLC assay. Evid Based Complement Alternat Med. 2015;2015:1–13. CrossRef
26. Lesmana D, Andrianto D, Astuti RI. Antiaging properties of the ethanol fractions of clove (Syzygium aromaticum l.) bud and leaf at the cellular levels: study in yeast Schizosaccharomyces pombe. Sci Pharm. 2021;89(4):1–13. CrossRef
27. Eskandari S, Etemadifar Z. Melanin biopolymers from newly isolated Pseudomonas koreensis strain UIS 19 with potential for cosmetics application, and optimization on molasses waste medium. J Appl Microbiol. 2021;131(3):1331–43. CrossRef
28. Sayre RM, Agin PP, LeVee GJ, Marlowe E. A comparison of in vivo and in vitro testing of sunscreening formulas. Photochem Photobiol. 1979;29(3):559–66. CrossRef
29. Barka EA, Vatsa P, Sanchez L, Gaveau-Vaillant N, Jacquard C, Meier-Kolthoff JP et al.. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol Mol Biol Rev. 2016;80(4):1–43. CrossRef
30. Jose PA, Maharshi A, Jha B. Actinobacteria in natural products research: progress and prospects. Microbiol Res. 2021;246(11):1–14. CrossRef
31. Dastager S, Wen-Jun LA, Shu-Kun T, Xin-Peng T, Xiao-Yang Z, Li- Hua X, et al. Seperation, identification and analysis of pigment (Melanin) production in Streptomyces. African J Biotechnol. 2006;5(11):1131–4.
32. Parra J, Beaton A, Seipke RF, Wilkinson B, Hutchings MI, Duncan KR. Antibiotics from rare actinomycetes, beyond the genus Streptomyces. Curr Opin Microbiol. 2023;76(10):1–13. CrossRef
33. Kraseasintra O, Sensupa S, Mahanil K, Yoosathaporn S, Pekkoh J, Srinuanpan S, et al. Optimization of melanin production by Streptomyces antibioticus NRRL B-1701 using Arthrospira (Spirulina) platensis residues hydrolysates as low-cost L-tyrosine supplement. BioTech. 2023;12(1):1–17. CrossRef
34. Tran-Ly AN, Reyes C, Schwarze FWMR, Ribera J. Microbial production of melanin and its various applications. World J Microbiol Biotechnol. 2020;36(11):1–9. CrossRef
35. Singh S, Nimse SB, Mathew DE, Dhimmar A, Sahastrabudhe H, Gajjar A, et al. Microbial melanin: recent advances in biosynthesis, extraction, characterization, and applications. Biotechnol Adv. 2021;53(5):1–22. CrossRef
36. Surwase SN, Jadhav JP. Bioconversion of L-tyrosine to L-DOPA by a novel bacterium Bacillus sp. JPJ. Amino Acids. 2011;41(2):495–506. CrossRef
37. Surwase SN, Patil SA, Apine OA, Jadhav JP. Efficient microbial conversion of L-tyrosine to L-DOPA by Brevundimonas sp. SGJ. Appl Biochem Biotechnol. 2012;167(5):1015–28. CrossRef
38. Apte M, Girme G, Bankar A, RaviKumar A, Zinjarde S. 3, 4-dihydroxy-L-phenylalanine-derived melanin from Yarrowia lipolytica mediates the synthesis of silver and gold nanostructures. J Nanobiotechnol. 2013;11(1):3–11. CrossRef
39. Kamarudheen N, Naushad T, Rao KVB. Biosynthesis, characterization and antagonistic applications of extracellular melanin pigment from marine Nocardiopsis sps. Indian J Pharm Educ Res. 2019;53(2):S112–20. CrossRef
40. Ghadge V, Kumar P, Singh S, Mathew DE, Bhattacharya S, Nimse SB, et al. Natural melanin produced by the endophytic Bacillus subtilis 4NP-BL associated with the halophyte Salicornia brachiata. J Agric Food Chem. 2020;68(25):6854–63. CrossRef
41. Amin S, Rastogi RP, Sonani RR, Ray A, Sharma R, Madamwar D. Bioproduction and characterization of extracellular melanin-like pigment from industrially polluted metagenomic library equipped Escherichia coli. Sci Total Environ. 2018;635(4):323–32. CrossRef
42. Ferraz AR, Pacheco R, Vaz PD, Pintado CS, Ascensão L, Serralheiro ML. Melanin: production from cheese bacteria, chemical characterization, and biological activities. Int J Environ Res Public Health. 2021;18(20):1–18. CrossRef
43. Restaino OF, Manini P, Kordjazi T, Alfieri ML, Rippa M, Mariniello L, et al. Biotechnological production and characterization of extracellular melanin by Streptomyces nashvillensis. Microorganisms. 2024;12(2):1–17. CrossRef
44. Ribera J, Panzarasa G, Stobbe A, Osypova A, Rupper P, Klose D, et al. Scalable biosynthesis of melanin by the basidiomycete Armillaria cepistipes. J Agric Food Chem. 2018;67:132–139. CrossRef
45. Sheefaa MI, Sivaperumal P. Antioxidant activities from melanin pigment produced by marine actinobacterium of Streptomyces species. J Adv Pharm Technol Res. 2022;13(5):S84–S87. CrossRef
46. El-Zawawy NA, Kenawy ER, Ahmed S, El-Sapagh S. Bioproduction and optimization of newly characterized melanin pigment from Streptomyces djakartensis NSS-3 with its anticancer, antimicrobial, and radioprotective properties. Microb Cell Fact. 2024;23(1):1–26. CrossRef
47. Du J, Cullen JJ, Buettner GR. Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim Biophys Acta—Rev Cancer. 2012;1826(2):443–57. CrossRef
48. Vivancos AP, Jara M, Zuin A, Sansó M, Hidalgo E. Oxidative stress in Schizosaccharomyces pombe: different H2O2 levels, different response pathways. Mol Genet Genomics. 2006;276(6):495–502. CrossRef
49. Cahlia U, Astuti RI, Nomura J, Wahyudi AT. Antioxidant properties of active fraction extract derived from yellow-red pigment produced by the marine sponge-associated bacterium Bacillus haikouensis AGS112 and identification of related compounds. HAYATI J Biosci. 2023;30(5):874–84. CrossRef
50. López-Lluch G, Navas P. Calorie restriction as an intervention in ageing. J Physiol. 2016;594(8):2043–60. CrossRef
51. Fauzya AF, Astuti RI, Mubarik NR. Effect of ethanol-derived clove leaf extract on the oxidative stress response in yeast Schizosaccharomyces pombe. Int J Microbiol. 2019;2019(7):1–7. CrossRef
52. Pan Y, Schroeder EA, Ocampo A, Barrientos A, Shadel GS. Regulation of yeast chronological life span by TORC1 via adaptive mitochondrial ROS signaling. Cell Metab. 2011;13(6):668–78. CrossRef
53. European Commission. Commission Recommendation of 22 September 2006 on the efficacy of sunscreen products and the claims made relating thereto. Off J Eur Union. 2006;265:39–43.
54. Dutra EA, Da Costa E Oliveira DAG, Kedor-Hackmann ERM, Miritello Santoro MIR. Determination of sun protection factor (SPF) of sunscreens by ultraviolet spectrophotometry. Rev Bras Ciencias Farm J Pharm Sci. 2004;40(3):381–5. CrossRef
55. Tarangini K, Mishra S. Production of melanin by soil microbial isolate on fruit waste extract: two step optimization of key parameters. Biotechnol Rep. 2014;4(1):139–46. CrossRef
56. Solano F. Photoprotection and skin pigmentation: melanin-related molecules and some other new agents obtained from natural sources. Molecules. 2020;25(7):1–18. CrossRef