INTRODUCTION
Green nanotechnology offers a superior alternative to conventional nanoparticle synthesis methods, mainly due to its focus on environmental sustainability and biocompatibility. Traditional chemical and physical methods were effective. Nevertheless, they require toxic reagents, high energy consumption, and substantial waste, leading to environmental degradation and potential health risks associated with the resultant Nanoparticles (NPs) [1]. On the contrary, green nanotechnology employs natural biological agents, i.e., extracts and supernatants from plants, bacteria, and fungi, as reducing and stabilizing agents. This significantly minimizes the release of hazardous by-products, reduces energy usage, and fosters an environmentally safer production process [2]. Furthermore, green nanotechnology operates at ambient temperatures and pressures, making it more convenient than traditional nanofabrication methods [3].
In the biomedical and pharmaceutical sectors, green nanotechnology has proven invaluable. Its biocompatibility and reduced toxicity make it a suitable platform for numerous applications. It has been widely reported that NPs exhibit noteworthy antimicrobial, antioxidant, antitumor, antidiabetic, antibiofilm, antiparasitic, wound healing, and anti-inflammatory properties [4–6]. Also, one of the most advantageous applications in the current scenario is drug delivery systems, wherein biologically fabricated NPs provide a targeted approach to delivering the drug of our interest. Recent studies also opined that a nanoparticle-based biosensor supports researchers in diagnosing diseases rapidly. Some were designed to accurately determine the disease biomarkers for cancer and diabetes [3]. Thus, green or biologically synthesized NPs offer numerous benefits. In particular, its environmental compatibility, combined with its potential for antimicrobial and cancer treatments, biofilm hindrance in medical devices, and diagnostics, highlights its notorious application in the field of biomedicine and pharmaceuticals [1,7].
The rapid rise of antimicrobial resistance (AMR) has become a significant global health issue, mainly driven by the overuse and misuse of antibiotics in both healthcare. In recent times, the development of multidrug-resistant (MDR) bacteria has elevated rapidly and become very difficult to combat, leading to long-term illness, incurring substantial healthcare costs, and increased mortality rates. Furthermore, conventional antibiotics often fail to encounter these resistant strains, thereby making clinicians move to use more toxic, higher end antibiotic treatments, which are reported to cause severe side effects, including organ damage [8]. Likewise, reactive oxygen species (ROS) are the by-products of cellular metabolism that, when overproduced, lead to oxidative stress, ultimately resulting in cancer development and progression. It has been reported that elevated ROS levels result in the oxidation of biochemical constituents such as lipids, proteins, and DNA, leading to mutations that contribute to carcinogenesis [9]. This situation has significantly pressed for alternative solutions to address this growing issue [10].
Green nanotechnology has emerged as the most promising alternative to combat MDR bacterial strains, ROS, and cancer development in recent years. The nanosized (1–100 nm) nature of NPs and their distinct characteristics allow researchers to utilize them extensively in healthcare. NPs such as Ag, Zn, Mg, Cu, Se, Pd, and several nanocomposites produced through biological routes using plant extracts were claimed to show excellent antioxidant, antibacterial, and antitumor properties by inducing ROS-mediated apoptosis in tumor cells [11,12]. Among the various metal NPs, Titanium dioxide nanoparticles (NPs) <100 nm were found to be effectively used in cosmetics, pharmaceuticals, biomedicine, biosensors, implants, and drug delivery systems.
Seaweeds, called marine macroalgae, are rich in phytoconstituents such as polysaccharides, flavonoids, and phenolic compounds, which play a significant role in green nanoparticle synthesis. Marine algae are utilized as folklore medicine and functional food by coastal peoples due to enriched minerals and vitamins. Also, previous studies have shown that seaweed-bioactive compounds exert antiviral, anti-inflammatory, antifouling, anticoagulant, and cardiovascular protection [6]. Literature has also evidenced that NPs derived from seaweeds present an environmentally sustainable alternative to traditional methods. For instance, Ag and Au NPs synthesized using seaweeds viz. Sargassum sp., Enteromorpha sp., Gracillaria sp., Hypnea sp., Codium sp., and Ulva sp. with noteworthy bioactivities [13]. Ulva lactuca (UL), widely recognized as sea lettuce, is rich in phytochemical constituents. It exhibits wide-spectral antibacterial, antifungal, antiviral, antioxidant, immunomodulatory, anticancer, anti-obesity, and anti-inflammatory properties [14]. Furthermore, UL is enriched with essential nutrients and other bioactive compounds such as vitamins, minerals, fatty acids, and polysaccharides that enhance its health-promoting attributes, including antidiabetic activity. These characteristics emphasize the biopotential of UL as a therapeutic agent within the realms of food, pharmaceutical, and nutraceutical applications [15–17]. The biological synthesis of NPs is eco-friendly, scalable, energy-efficient, and suitable for large-scale industrial production. Viewing environmental sustainability and notorious biomedical potentials, researchers focus on fabricating seaweed-based NPs. The present study explores the biological synthesis of titanium dioxide NPs using macroalgae UL and further in vitro bioassays authenticate its antibacterial, anti-inflammatory, and anticancer properties.
MATERIAL AND METHODS
Preparation of seaweed UL extract
For the present study, the green seaweed UL was collected from Kanyakumari Coast, Tamilnadu, India. The collected seaweed was washed, dried, and powdered. The dried seaweed powder was added to distilled water, boiled (60°C), and filtered to obtain an aqueous extract, which was subsequently used for the green synthesis of TiO2NPs.
Biosynthesis of UL-TiO2NPs
Around 90 ml of titanium tetra isopropoxide (1 mM) solution was added to 10 ml of aqueous extract of UL and stirred mechanically for 2 hours in a magnetic stirrer at 70°C. Then, the content was filtered, centrifuged, and washed with water, followed by ethanol. The UL-TiO2NPs were subjected to calcination in a muffle furnace at 300°C and then used for further study.
Characterization of UL-TiO2NPs
The green synthesized UL-TiO2NPs were subjected to spectroscopic analysis to determine their characteristics. The UV-Vis spectrophotometer was used to find out the optical properties of the UL-TiO2NPs, and X-ray diffraction (XRD) analysis was performed to check out the crystallinity of the NPs. Morphological properties of the green synthesized UL-TiO2NPs were determined using scanning electron microscopy (SEM). Fourier transform-infrared spectroscopy (FT-IR) spectroscopy was determined to find the active functional groups in the range of 400–4,000 cm–1.
Antibacterial activity
The antibacterial properties of UL-TiO2NPs were studied against the selected bacterial pathogens using an agar diffusion assay. In brief, overnight culture of (Clinical isolates—Enterobacter cloaceae, Enterococcus avium, Proteus mirabilis, Pseudomonas aeruginosa; microbial type culture collection isolates – Streptococcus pyogenes, Salmonella typhi, and Enterococcus faecalis were obtained from Tamilnadu Dr. J. Jayalalithaa Fisheries University, Chennai, India) with the cell density of 1 × 108 cells/ml was swabbed over Muller Hinton Agar plates. Then, 100 µl containing 10 µg of UL-TiO2NPs was loaded onto the wells and incubated for 24 hours at 37°C. The halos (mm) formed around the well were recorded [18].
Anti-inflammatory activity
Albumin denaturation method
The anti-inflammatory efficacy of the biosynthesized titanium dioxide NPs was examined using the albumin denaturation method. Briefly, different concentrations of TiO2NPs (25–100 µg/ml) were added to 0.45 ml of 1% aqueous bovine serum albumin solution, with the pH adjusted to 6.3 using 1N HCl. The components were incubated for 20 minutes and then heated at 55°C for 30 minutes. Thereafter, the samples were read at 660 nm. Diclofenac was used as the positive control (25–100 µg/ml). Then, the protein denaturation (%) was calculated by following Missier et al. [19].
Cytotoxicity effect of UL-TiO2NPs
The (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) (MTT) assay was carried out to evaluate the cytotoxic effect of TiO2NPs against oral cancer oral carcinoma cell line (KB cells) in 96 multi-titer plates. DMEM medium (0.1ml) was prepared with KB cells (1.2 × 104 cells/ml) poured into each well of 96 multi-well plates, and to the mixture, varying concentrations (1–100 µg/ml) of TiO2NPs were added and kept incubated at 37°C for 24 hours. Then, well-plates were added with 0.1 ml of MTT (Sigma Aldrich) solution and continued for 4 hours in a COD incubator at 37°C. After incubation, the resulting formazan crystals were dissolved using 0.1 ml of DMSO, and the cell viability (%) was determined using an ELISA reader at 570 nm. The concentration of UL-TiO2NPs (IC50) with a 50% decrease in the KB cells was calculated.
Statistical analysis
The results obtained from experiments were shown as the Mean ± SD of triplicates. Additionally, using SPSS 26.0, ANOVA with post hoc multiple comparisons of the Dunnett and SNK test with different significant levels was mentioned.
RESULT AND DISCUSSION
UV-Vis spectroscopy is the most preliminary step in confirming the synthesized NPs, providing insights into their optical properties. Herein, the result of the color change from light green to brown and a sharp absorption peak at 370 nm in the UV-Vis spectrum confirmed the biogenic synthesis of UL-TiO2NPs (Fig. 1). The high absorption intensity at this wavelength affirms intense Surface Plasmon Resonance activity, which is typical for TiO2 NPs. Characteristics of distinct absorption peaks ranging between 300 and 400 have also been reported in previous studies [20,21].
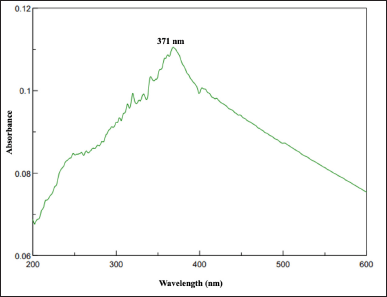 | Figure 1. UV-Vis spectra of UL-TiO2NPs biosynthesized by U. lactuca. [Click here to view] |
The FTIR spectrum shows the key functional groups associated with UL-TiO2NPs due to the phytoconstituents within the UL extract (Fig. 2). Herein, a broad bending vibration found at 3,382.80 cm−1 suggested O-H stretching, indicating hydroxyl groups, likely from alcohol or phenols. Similarly, the stretching vibration recorded at 2,338.66 and 2,084.01 cm−1 inferred the presence of aliphatic hydrocarbons. The band at 1,445.25 cm−1 implied C-H bending, linked to methylene or methyl groups, while the peak at 1,250.16 cm−1 corresponds to C-N stretching, indicating the presence of amines. Likewise, a sharp peak at 1,100.87 cm−1 signifies C-O stretching, commonly observed in alcohols and esters. A distinct peak at 859.87 and 646.94 cm−1 indicated the stretching vibration of Ti-O-Ti NPs and thereby affirmed the formation of TiO2 nanoparticles. These findings suggest that UL-TiO2NPs clearly showed the occurrence of functional groups, including hydroxyls, carbonyls, methylene, aliphatic and aromatic hydrocarbons, amines, and ethers that could strongly bind with the metal ions and aid in nanoparticle formation. Furthermore, these functional groups suggested the presence of phytochemicals such as flavonoids, alkaloids, terpenoids, and phenolic compounds on the surface, which were believed to play a pivotal role in the capping and stabilization of NPs. The functional groups observed in the present study agree with the findings of previous studies exploring TiO2 NPs from marine plants [20,22,23].
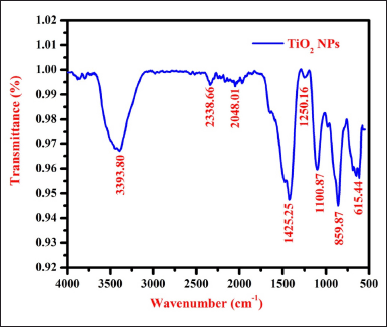 | Figure 2. FT-IR spectrum of UL-TiO2NPs biosynthesized by U. lactuca. [Click here to view] |
The XRD pattern of UL-TiO2NPs showed distinct peaks, describing the crystalline structure of titanium (Fig. 3). The predominant 2θ peaks recorded at 2θ = 25.3° correspond to the (011) plane, confirming the presence of the anatase phase as identified by JCPDS No. 98-000-5224. Subsequently, peaks at 2θ values of 37.8°, 48.1°, 53.9°, 55.0°, and 62.7° correspond to the Bragg’s reflection planes of (004), (020), (121), (024), and (025) planes, indicating the anatase crystalline structure of TiO2 NPs. Furthermore, the average crystalline size of the UL-TiO2NPs based on the Debye-Scherrer equation was between 20 and 50 nm nanoscale range. These results agree with Narayanan et al. [20], who made similar observations while investigating the titanium dioxide NPs using Cymodaceae serrulata.
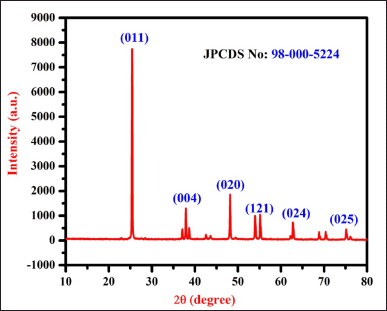 | Figure 3. XRD spectrum of UL-TiO2NPs biosynthesized by U. lactuca. [Click here to view] |
In the present investigation, the morphology of UL-TiO2NPs determined through SEM mainly showed spherical particles with minimal clustering, likely due to weak inter-particle interactions (Fig. 4). The UL-TiO2NPs are uniform in size with smooth surfaces, indicating good crystallinity. Despite slight aggregation, distinct particle boundaries are visible, suggesting a dense packing indicating a high surface area. In line with the results of the present study, Sundar et al. [22] and Balaraman et al. [23] observed the formation of spherical-shaped titanium dioxide NPs while utilizing S. isoetifolium and S. myriocystum as a reductant for the biosynthesis of TiO2NPs.
 | Figure 4. FE-SEM of UL-TiO2NPs biosynthesized by U. lactuca. [Click here to view] |
In recent years, NPs have been believed to be the best alternative to antibiotics to combat bacterial-related infections. This is mainly due to the rise in AMR bacterial pathogens. For instance, as per WHO [24], the Bacterial Priority Pathogen List was reported to enlist 24 pathogens belonging to fifteen antibiotic-resistant families. The listed AMR strains were recorded globally with specific issues such as ease of transmission, lesser incidence of treatability, and control. In this context, NPs, due to their nanosized structure, can easily intrude on the bacterial cell membrane and exert antibacterial properties. In the present study, the results on the antibacterial activity of UL-TiO2NPs inferred that it had significantly (p < 0.05) suppressed the growth of bacterial pathogens with varying degrees of inhibitory zones (Fig. 5). Interestingly, in the current study, all three clinical isolates (E. cloacae – 15 mm, E. avium – 12 mm, and P. mirabilis – 19 mm) were found to be more highly susceptible than the other three bacterial pathogens (S. pyogenes, S. typhi, and E. faecalis) tested, wherein it recorded moderate inhibitory zones ranged between 9 and 14 mm. Consistent with the findings of the present study, numerous studies on green-synthesized TiO2NPs have evidenced a significant reduction in growth inhibition of both gram-positive and gram-negative bacterial strains. These studies further advocated TiO2NPs as a viable alternative to conventional antibiotics, especially against MDR pathogens [25,26]. The remarkable antagonistic activity exhibited by UL-TiO2NPs could be attributed to the generation of ROS that significantly damages the bacterial cellular components like cell membrane, protein leakage, mitochondrial disruption, hindering exopolysaccharide production, DNA, and protein interaction and thus leading to cell death. In consonance with the present study titanium dioxide NPs derived from seaweed Sargassum myriocystum (50–90 nm), Caulerpa racemosa, Codium fragile, and Cystoseira Myrica – 125–131 nm were found to show striking antibacterial activity bacterial pathogens such as MRSA, V. cholerae, S. typhi, E. ludwigii, Morganella morganii, Klebsiella pneumoniae, and E. faecium [20,27].
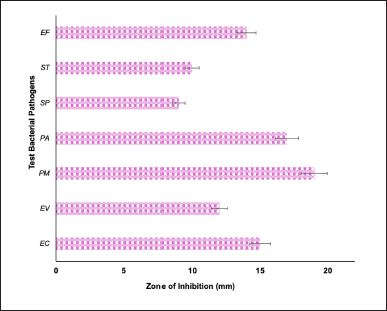 | Figure 5. Antibacterial activity of UL-TiO2NPs biosynthesized by U. lactuca. EC: E. cloaceae, ST: S. typhi, SP: S. pyogenes, PA: P. aeruginosa, PM: P. mirabilis, EA: E. avium, and EF: E. faecalis. Each bar is the mean ± SD of triplicates. [Click here to view] |
The host-mediated inflammatory response generally triggers the immune functions after bacterial infection and impedes microbial growth. However, this process may also occasionally lead to hypervirulent and MDR biofilm and may subsequently lead to inflammation in the surrounding tissues [28]. Herein, the results of the anti-inflammatory activity of UL mediated-TiO2NPs significantly (p < 0.05) inhibited the denaturation (22% to 78%) of egg albumin in a dose-dependent manner (25 to 100 µg/ml) (Fig. 6). The observed results strongly validate the UL-TiO2NP’s ability to impede protein denaturation involved in the inflammatory process. Coinciding with the results of the present study, Venkatappa et al. [29] and Bharathi et al. [30] depicted that TiO2 NPs derived from Terena asiatica and Gymnema sylvestre showed a 73% and 91.52% inhibitory effect on protein denaturation, respectively. The remarkable anti-inflammatory activity of UL-TiO2NPs could be ascribed to their ability to stabilize the tertiary and quaternary structure of proteins [31]. NPs have been claimed to have excellent anti-inflammatory activity than their bulky counterparts. Several authors investigated the anti-inflammatory mechanism of TiO2NPs and evidenced that it significantly blocks the essential signaling pathways such as MAPK and NF-kβ. These pathways are the key to the transcription of pro-inflammatory cytokines such as IL-6 and tumor necrosis factor-alpha. Furthermore, TiO2NPs were reported to reduce the expression of phosphorylated forms of proteins that are involved in these signaling pathways, thereby impeding the synthesis of inflammatory cytokines and mediators, including nitric oxide and TNF-∝ [32,33]. Diclofenac, used as standard, displayed comparatively higher anti-inflammatory activity (51%–97%) than the NPs.
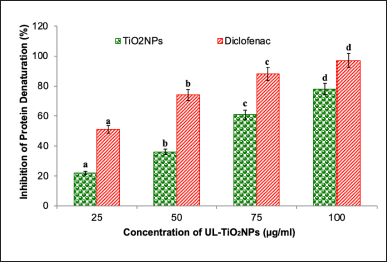 | Figure 6. Anti-inflammatory activity of UL-TiO2NPs. Each bar is the mean ± SD of triplicates. Each set of bars implies different concentrations of UL-TiO2NPs and Diclofenac (a–d) with different superscript letters are statistically significant (One-Way ANOVA test; p < 0.05, further, the post hoc multiple comparisons with SNK test). [Click here to view] |
The cytotoxicity of UL-TiO2NPs against oral KB carcinoma cells was determined through an MTT assay. After 24 hours, a dose-dependent relationship between UL-TiO2NPs and oral KB cell viability (%) was observed (Fig. 7A–C). At the lowest concentration (2.5 μg/ml), the viability of oral KB cells was relatively high, at 94.31%. But, as the concentrations of UL-TiO2NPs increased, a consistent reduction in cell viability was noticed with 82.29%, 68.57%, 47.81%, and 38.22% with respect to a progressive increase in concentration from 5, 10, 25, and 50 μg/ml. However, at 75 and 100 μg/ml, the UL-TiO2NPs exhibited 25.61% and 17.4% cell viability, indicating >75% death of cells. The observed reduction in cell viability signifies the cancer cell growth inhibition ability of the NPs. The calculated IC50 value for 50% growth inhibition of oral KB carcinoma cells was 28.74 μg/ml. The promising cytotoxic activity exerted by UL-TiO2NPs could be ascribed to a smaller size, which makes them permeate the cancer cells and elicit ROS, interfere with metabolic processes, disrupt the cellular processes, and cause DNA damage. Supporting the results of the present study, Garcia-Contreras et al. [34] evidenced that TiO2NPs could significantly impede the growth of human oral squamous cell carcinoma in a dose-dependent manner and pointed out that it could be due to mitochondrial dysfunction, raise in ROS levels, and apoptotic induction by the NPs. The results are also in consonance with the previous findings [35]. Additionally, it was also reported that titanium dioxide NPs improve the proficiency of chemotherapeutic drugs such as doxorubicin by enhancing intracellular drug retention and enabling drug-incited cytotoxicity.
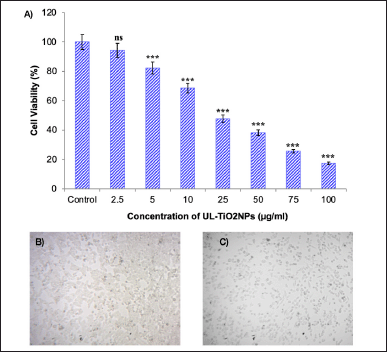 | Figure 7. A) Anticancer activity of UL-TiO2NPs against oral KB carcinoma cells. Mean ± SD of triplicates indicates a significant reduction in cell viability (%) of KB cells at different concentrations in comparison with control (one-way ANOVA, Dunnett test, *** p < 000.1; ns: non-significant), B) Control KB cells, and C) KB cells treated with UL-TiO2NPs. (Scale bar: 20 µm). [Click here to view] |
CONCLUSION
In conclusion, the results of the present research explored the biogenic synthesis of titanium dioxide NPs from the aqueous extract of UL, thereby emphasizing an environmentally sustainable method of fabricating NPs with no organic solvent and no harmful chemical usage. The TiO2NPs were spherical shaped and exhibited prodigious bioactivity against a panel of medically important bacterial pathogens. Inhibition of protein denaturation by titanium dioxide NPs validated a robust anti-inflammatory activity. The cytotoxic property of the TiO2NPs emphasized its promising anticancer activity against oral cancer cells. However, further extensive in vivo and toxicity studies are essential to effectively use this nanoparticle in biomedical instruments to prevent medical complications.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Patwardhan SV, Manning JR, Chiacchia M. Bioinspired synthesis as a potential green method for the preparation of nanomaterials: opportunities and challenges. Curr Opin Green Sustain Chem. 2018;12:110–6. CrossRef
2. Aravind M, Amalanathan M, Mary MSM. Synthesis of TiO2 nanoparticles by chemical and green synthesis methods and their multifaceted properties. SN Appl Sci. 2021;3:1–10. CrossRef
3. Kalyanasundaram S, Prakash MJ. Biosynthesis and characterization of titanium dioxide nanoparticles using Pithecellobium dulce and Lagenaria siceraria aqueous leaf extract and screening their free radical scavenging and antibacterial properties. Int Lett Chem Phys Astron. 2015;50:80–95.
4. Mathesh A, Mohanprasanth A, Saravanan M. Synthesis and characterization of spirulina-mediated titanium dioxide nanoparticles: antimicrobial activity against multidrug-resistant bacteria. Nano-Struct Nano-Objects. 2024;39:101225. CrossRef
5. Annamalai KK, Selvaraj B, Subramanian K, Binsuwaidan R, Saeed M. Antibiofilm and antivirulence activity of selenium nanoparticles synthesized from cell-free extract of moderately halophilic bacteria. Micro Pathog. 2024;193:106740. CrossRef
6. Narayanan M, So?owski G, Özdemir FA. Green synthesis and applications of nanoparticles in biomedical chemistry. ChemistrySelect. 2024;9(35):e202402472. CrossRef
7. Francis AL, Namasivayam SKR, Samrat K. Potential of silver nanoparticles synthesized from Justicia adhatoda metabolites for inhibiting biofilm on urinary catheters. Microb Pathog. 2024;196:106957. CrossRef
8. Hetta HF, Ramadan YN, Al-Harbi AI, Ahmed EA, Battah B, Abd Ellah NH, et al. Nanotechnology as a promising approach to combat multidrug resistant bacteria: a comprehensive review and future perspectives. Biomedicines. 2023;11:413. CrossRef
9. Talib H, Mehmood A, Amjad MS, Mustafa A, Khan MAR, Raffi M, et al. Antibacterial, antioxidant, and anticancer potential of green fabricated silver nanoparticles made from Viburnum grandiflorum leaf extract. Bot Stud. 2024;65(1):4. CrossRef
10. Xu Y, Li H, Li X, Liu W. What happens when nanoparticles encounter bacterial antibiotic resistance? Sci Total Environ. 2023;876:162856. CrossRef
11. Alharbi NS, Alsubhi NS. Green synthesis and anticancer activity of silver nanoparticles prepared using fruit extract of Azadirachta indica. J Rad Res Appl Sci. 2022;15(3):335–45. CrossRef
12. Karunakaran G, Sudha KG, Ali S, Cho EB. Biosynthesis of nanoparticles from various biological sources and its biomedical applications. Molecules. 2023;28(11):4527. CrossRef
13. Mukherjee A, Sarkar D, Sasmal S. A review of green synthesis of metal nanoparticles using algae. Front Microbiol. 2021;12:693899. CrossRef
14. Putra NR, Fajriah S, Qomariyah L, Dewi AS, Rizkiyah DN, Irianto I, et al. Exploring the potential of Ulva lactuca: emerging extraction methods, bioactive compounds, and health applications-A perspective review. S Afr J Chem Eng. 2024;47(1):233–45. CrossRef
15. Amin MA, Chondra U, Mostafa E, Alam MM. Green seaweed Ulva lactuca, a potential source of bioactive peptides revealed by in silico analysis. Inform Med Unlocked. 2022;33:101099. CrossRef
16. Raj R, Anandan R, Asha KK, Mathew S, Ravishankar CN. Nutritional and human healthcare aspects of Ulva lactuca. J Chem Sci Rev. 2020;9(34):541–5. CrossRef
17. Ouahabi S, Daoudi NE, Loukili EH, Asmae H, Merzouki M, Bnouham M, et al. Investigation into the phytochemical composition, antioxidant properties, and in-vitro anti-diabetic efficacy of Ulva lactuca extracts. Mar Drugs. 2024;22(6):240. CrossRef
18. Ahila NK, Ramkumar VS, Prakash S, Manikandan B, Ravindran J, Dhanalakshmi PK, et al. Synthesis of stable nanosilver particles (AgNPs) by the proteins of seagrass Syringodium isoetifolium and its biomedicinal properties. Biomed Pharmacother. 2016;84:60–70. CrossRef
19. Missier MS, Ramakrishnan M, Paulpandian SSD, Rajeshkumar S, Tania M. Antibacterial, antioxidant and anti-inflammatory activity zinc-titanium dioxide nanocomposite. Bioinformation. 2023;19(5):638–43. CrossRef
20. Narayanan M, Srinivasan S, Gnanasekaran C, Ramachandran G, Chelliah CK, Rajivgandhi G, et al. Synthesis and characterization of marine seagrass (Cymodocea serrulata) mediated titanium dioxide nanoparticles for antibacterial, antibiofilm and antioxidant properties. Microb Pathog. 2024;189:106595. CrossRef
21. Amutha V, Deepak P, Kamaraj C, Balasubramani G, Aiswarya D, Arul D, et al. Mosquito-larvicidal potential of metal and oxide nanoparticles synthesized from aqueous extract of the seagrass, Cymodocea serrulata. J Cluster Sci. 2019;30:797–812. CrossRef
22. Sundar V, Balasubramanian B, Sivakumar M, Chinnaraj S, Palani V, Maluventhen V, et al. An eco-friendly synthesis of titanium oxide nanoparticles mediated from Syringodium isoetifolium and evaluate its biological activity and photocatalytic dye degradation. Inorg Chem Comm. 2024;161:112125. CrossRef
23. Balaraman P, Balasubramanian B, Liu WC, Kaliannan D, Durai M, Kamyab H, et al. Sargassum myriocystum-mediated TiO2-nanoparticles and their antimicrobial, larvicidal activities and enhanced photocatalytic degradation of various dyes. Env Res. 2022;204:112278. CrossRef
24. WHO. Bacterial priority pathogens list: bacterial pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance. Geneva, Switzerland: WHO; 2024.
25. Younis AB, Milosavljevic V, Fialova T, Smerkova K, Michalkova H, Svec P, et al. Synthesis and characterization of TiO2 nanoparticles combined with geraniol and their synergistic antibacterial activity. BMC Microbiol. 2023;23:207. CrossRef
26. Hassan MAA, Hassan NA, Khalaf SK. Electron microscope scanning, and antibacterial activity of TiO2 nanoparticles on pathogenic strains of Staphylococcus aureus and Escherichia coli. Diyala J Med. 2024;26(1):79–90. CrossRef
27. Salem W, El-Deen FN, Ebnalwaleed K, Badry M, Latef AAHA. UV-Induced antibacterial activity of green-synthesized TiO2 nanoparticles for the potential reuse of raw surface and underground water. J Plant Growth Regul. 2022;41(3):1344–58. CrossRef
28. Marsh PD, Devine DA. How is the development of dental biofilms influenced by the host? J Clin Periodontol. 2011;38:28–35. CrossRef
29. Venkatappa MM, Udagani C, Hanume Gowda SM, Venkataramaiah S, Casini R, Mouss IM, et al. Green synthesized TiO2 nanoparticles-mediated Terenna asiatica: evaluation of their role in reducing oxidative stress, inflammation and human breast cancer proliferation, Molecules 2023;28(13):5126. CrossRef
30. Bharathi D, AlSalhi MS, Devanesan S, Nandagopal JGT, Kim W, Ranjithkumar R. Photocatalytic degradation of Rhodamine B using green-synthesized ZnO nanoparticles from Sechium edule polysaccharides. Appl Nanosci. 2022;12(8):24772487. CrossRef
31. Kamal M, Abdel-Raouf N, Sonbol H, Abdel-Tawab H, Abdelhameed MS, Hammouda O, et al. In vitro assessment of antimicrobial, anti-inflammatory and schistolarvicidal activity of macroalgae-based gold nanoparticles. Front Mar Sci. 2022;9:1075832. CrossRef
32. Neacsu P, Mazare A, Schmuki P, Cimpean A. Attenuation of the macrophage inflammatory activity by TiO2 nanotubes via inhibition of MAPK and NF-κB pathways. Int J Nanomed. 2015;10:6455–67. CrossRef
33. Chakrabartty S, Alam MI, Bhagat S, Alam A, Dhyani N, Khan GA, et al. Inhibition of snake venom induced sterile inflammation and PLA2 activity by titanium dioxide nanoparticles in experimental animals. Sci Rep. 2019;9(1):11175. CrossRef
34. Garcia-Contreras R, Scougall-Vilchis RJ, Contreras-Bulnes R, Ando Y, Kanda Y, Hibino Y, et al. Effects of TiO2 nanoparticles on cytotoxic action of chemotherapeutic drugs against a human oral carcinoma cell line. In vivo. 2014;28(2):209–16.
35. Raja G, Cao S, Kim DH, Kim TJ. Mechanoregulation of titanium dioxide nanoparticles in cancer therapy. Mater Sci Eng C. 2020;107:110303. CrossRef