INTRODUCTION
Parkinson’s disease (PD) is a neurodegenerative disorder that affects multiple body systems and leads to a wide range of motor and non-motor symptoms. These symptoms arise from significant disturbances in the neurotransmitter system, particularly in the dopamine system. In PD, there is a notable loss of neurons in specific regions of the brain, such as the substantia nigra pars compacta (SNpc). This neuronal loss results in a deficiency of dopamine in another brain region known as the striatum, which plays a crucial role in motor control [1–3].
Moreover, PD is characterized by the presence of abnormal structures within cells, specifically the accumulation of protein aggregates containing α-synuclein. These intracellular inclusions are considered primary neuropathological hallmarks of PD. The combination of dopamine deficiency in the striatum and the presence of α-synuclein aggregates in neurons contributes to the development of motor symptoms and other manifestations observed in individuals with PD [1–3].
Based on information from the World Health Organization, as of 2019, approximately 8.5 million people are living with PD, with 5.8 million of these individuals experiencing disability. Additionally, the mortality rate associated with the disease reached 329 thousand, which represents an increase of more than 100% compared to data from 2000 [4]. According to the Parkinson Foundation, although Parkinson’s is more common in older populations, an estimated 4% of all cases are diagnosed in individuals under the age of 50. This has a significant impact, especially on patients who are still in their productive years [5].
The treatment of PD primarily focuses on addressing dopamine deficiency in the brain through dopamine replacement therapy, which involves administering medications to supplement dopamine levels and alleviate motor symptoms. Non-dopaminergic strategies are also important for managing symptoms that are not directly related to dopamine deficiency and for improving overall quality of life. Deep brain stimulation is an option for individuals with motor complications or levodopa-related fluctuations, where electrodes are implanted in the brain to modulate activity and control symptoms while reducing medication side effects [6–9].
Current treatment approaches for PD can lead to clinical improvements in some individuals. However, they often carry the risk of consequential complications and are only effective for a limited time in a subset of patients. Additionally, these treatments do not address the underlying neuropathological processes that result in progressive cell death in patients with PD. This limitation highlights the need for alternative therapeutic strategies that target the root cause of the disease and provide long-term benefits [10,11].
Mesenchymal stem cell (MSC)-based approaches have been identified as potentially effective therapeutic alternatives for neurodegenerative conditions, such as PD. MSCs possess unique characteristics that make them attractive candidates for use in regenerative medicine. These cells can differentiate into various cell types and secrete bioactive molecules that promote tissue repair and regeneration, modulate immune responses, and exhibit anti-inflammatory properties. By harnessing these inherent qualities, MSC-based therapies offer a novel approach to alleviate symptoms and potentially modify disease progression by addressing the mechanisms underlying neurodegeneration in PD [12–15].
Initially, the therapeutic benefits of MSCs in PD were thought to stem from their ability to engraft into damaged tissues and differentiate into various cell types, including dopaminergic neurons. However, recent research has shown that the long-term survival of implanted MSCs is limited and that their therapeutic effects are primarily mediated by their paracrine activity rather than by their ability to directly replace damaged cells [16,17].
Paracrine activity refers to the release of bioactive molecules such as growth factors, cytokines, and extracellular vesicles by MSCs into the surrounding environment. These secretory factors play a crucial role in modulating the cellular microenvironment, promoting tissue repair, reducing inflammation, and supporting cell survival and regeneration. This paracrine mechanism allows MSCs to indirectly exert their therapeutic effects by influencing neighboring cells and tissues [16–19].
Recent research has highlighted the significance of the secretome, which refers to the diverse collection of physiologically active compounds secreted by MSCs. These bioactive molecules include growth factors, cytokines, extracellular vesicles, and other signaling molecules that collectively play crucial roles in mediating the therapeutic effects of MSCs. Studies have shown that secretome-derived products from MSCs possess neuroregulatory properties that can influence key pathological processes associated with maintaining cellular balance and function, including cellular differentiation and proliferation, blood vessel formation and regeneration, inflammatory processes, and regulation of oxidative stress [16,20–22].
Research related to the use of secretomes for the treatment of neurodegenerative diseases in recent years has advanced. However, there are still many differences among the various studies conducted such as the type of initial stem cell used as a source of secretome, the experimental animals used, and the method of secretome production. Previous reviews discussing the use of secretome in neurodegenerative diseases have been published before, but they did not focus on PD specifically [22,23,24]. In addition, the existing reviews are primarily narrative in nature. In this new review, the scoping review method was utilized to cover a wide range of studies and provide comprehensive information from the existing studies on the use of the secretome in PD.
Therefore, this study aims to determine the various utilizations of the secretome from existing studies in PD cases, how the secretome provides benefits for the treatment of these diseases, and the molecular mechanisms underlying these effects, as well as the latest developments related to secretome research in PD.
METHODS
Study design
This was a scoping review based on the Preferred Reporting Items for Scoping Reviews protocol (PRISMA-ScR). The assessment results of the checklist-based writing adequacy of the PRISMA-ScR are shown in Supplementary Table 1. This scoping review aimed to determine the development of secretome use in PD therapy, particularly regarding the updates in research and the molecular mechanisms associated with the effects of secretome on the repair or prevention of cell degeneration in PD.
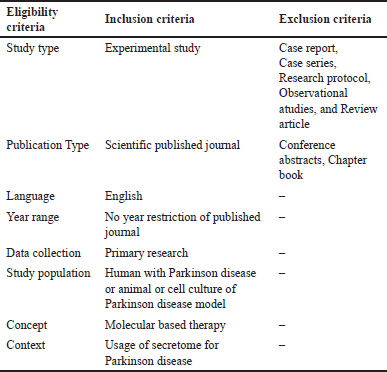 | Table 1. Eligibility criteria. [Click here to view] |
Eligibility criteria
The eligibility criteria for the journals included in this study encompass several assessments, with the inclusion and exclusion criteria outlined in Table 1. In this study, there were no time limitations for the included journals, allowing for more comprehensive results. This scoping review will also focus on the utilization of molecular therapy in the management of PD, particularly regarding the use of secretome as a novel therapeutic approach.
Search strategy
The journals used for this study were obtained from the PubMed Central, ScienceDirect, and Scopus databases. The search covered journals from their inception until March 27, 2024. The keywords for this study included “Parkinson,” “Secretome,” “Exosome,” “therapy,” and “treatment.” A more detailed search strategy for this review is provided in Supplementary Table 2.
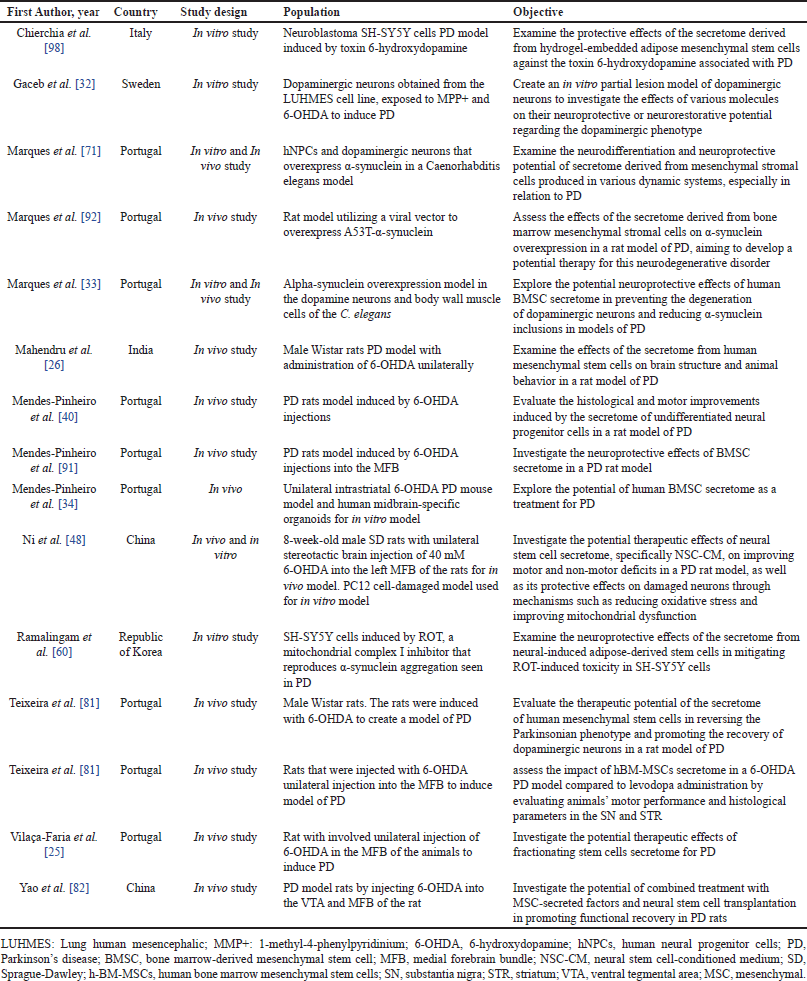 | Table 2. Summary of included studies. [Click here to view] |
Study selection
Two authors independently reviewed the abstracts, followed by a full-text assessment of the studies that met the inclusion criteria. Any discrepancies were resolved through discussion. The inclusion criteria were based on the population, concept, and context framework, specifically focusing on studies related to PD or PD models, the use of secretome as an intervention, and the examination of the molecular therapies underlying the outcomes
Data extraction
The journals obtained from the full-text analysis will be utilized in the data extraction process. The results of the data extraction will include information on the first author, year of publication, country where the study was conducted, study design, study population, study objectives, route of secretome administration, outcomes of the study, and the molecular mechanisms underlying the results.
RESULTS
Based on the screening results, 15 journals (Fig. 1) were included in this study. All included studies were conducted either in vitro or in vivo. No human studies related to the use of the secretome in the management of PD were found during the search. Most studies on the use of the secretome were conducted in Portugal (n = 9), followed by China (n = 2). In other countries, such as Italy, Sweden, India, and the Republic of Korea, only one study per country was conducted. In in vivo studies, most models utilized mice, although some studies also employed Caenorhabditis elegans models. A complete summary of the studies included in this review is presented in Table 2. Several sources of the secretome used in the studies were identified, and various routes of secretome administration were observed. The types of secretome sources and administration routes are listed in Table 3.
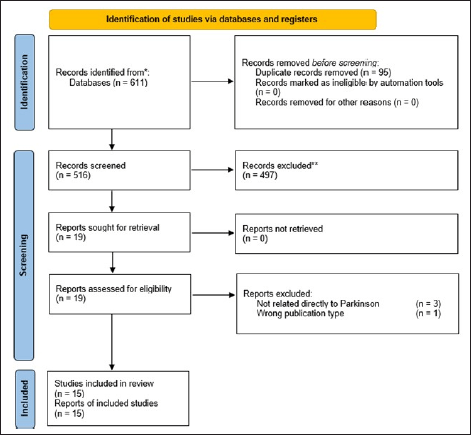 | Figure 1. PRISMA-ScR flow diagram of study selection. [Click here to view] |
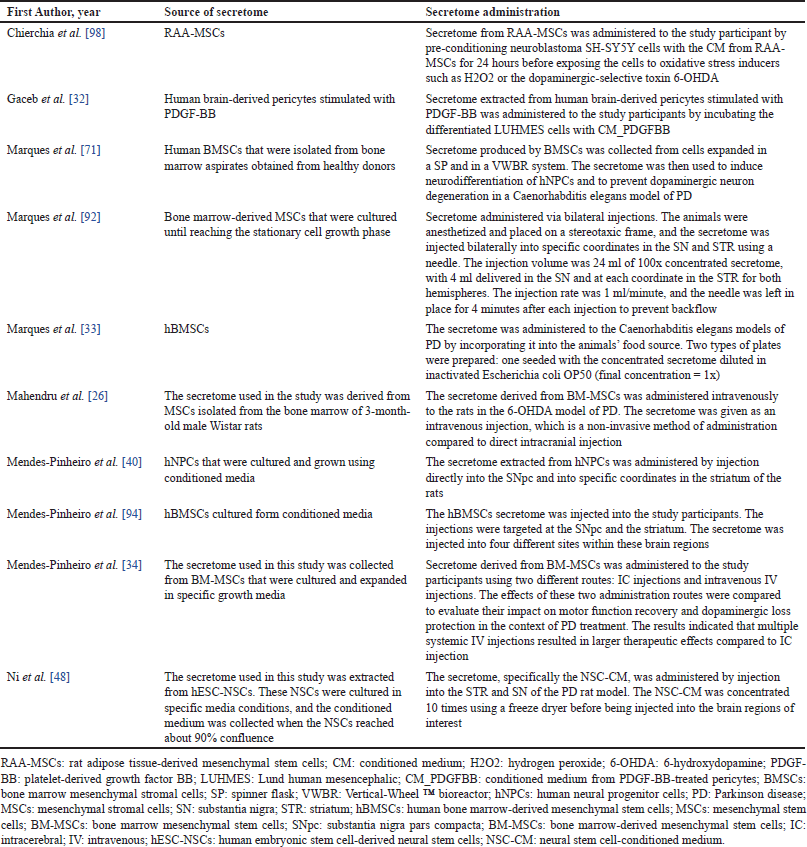 | Table 3. Details of secretome therapy and administration. [Click here to view] |
DISCUSSION
Secretome characteristics and molecular effect
An in vitro study using the secretome of hBM-MSCs applied to hNPCs demonstrated that progenitor cells were transformed into mature cells (MAP-2-positive) and immature neurons (DCX-positive). When comparing the effects of the whole secretome and its vesicular fraction to those of the protein fraction, it was observed that both the secretome and its vesicular fraction induced a higher differentiation rate of hNPCs than the protein fraction alone [25].
BDNF, NGF, and VEGF were found to be produced by MSCs in the conditioned media [26]. Additionally, other cell types, such as secretome-producing pericytes, exhibited increased production of pro-regenerative molecules following stimulation with PDGF-BB, which binds to the PDGFRβ receptor [27,28]. An in vitro study of parkinsonism using LUHMES, a type of human-derived cell culture, demonstrated that PDGF-BB-treated pericytes (CMPDGFBB) resulted in a significant increase in markers of DA neurons, specifically TH and Dopa-β-H, compared to LUHMES that received PDGF-BB alone [29–32].
In an in vivo study using C. elegans to assess the effects of the secretome from hBMSCs on a model with α-syn overexpression, it was found that secretome administration helped maintain a higher percentage of DA neurons in the study subjects compared to the controls, with results of 44%–10%, respectively. The secretome primarily exerted neuroprotective effects on neurons in the anterior deirid and cephalic regions. Additionally, the C. elegans group treated with the secretome exhibited a 13% reduction in α-syn inclusion bodies compared to the control group [33].
Another study utilized a novel in vitro model based on 6-OHDA-induced neurotoxicity in hMOs, which allowed for three-dimensional mask and skeleton analysis to examine the complexity of DA neurons. The results indicated that treatment with the secretome led to significantly less neurite fragmentation compared to the untreated group, suggesting a protective effect on the DA neuronal network [34].
The secretome contains several proteins that can enhance function and strengthen the connections between dopaminergic DA neurons in the nigrostriatal region. Notably, various components of the secretome exhibit anti-proteotoxic effects against α-syn, including BDNF, CFL1, CLU, CST3, HSPA8, HSPB1, IGF1, LGALS1, MMP2, DJ-1 or PARK7, UBE3A, UCHL1, and VEGFB [33,35–38].
The use of the secretome has also revealed molecular effects associated with the apoptotic process in cells. Analysis indicated an increase in the expression of BCL-2 and a decrease in CASP3 [26,39]. Furthermore, the secretome demonstrated a modulating effect on the differentiation of neuronal cells into dopaminergic neurons, particularly in the context of PD. This effect is linked to the Dickkopf 3 molecule, which influences the Wnt/β-catenin signaling pathway [40,41].
Effect of secretome on mitochondrial function
Impaired mitochondrial function is associated with the pathogenesis of PD. Patients with PD exhibit impaired mitochondrial biosynthesis, which is related mtDNA replication and transcription. This disruption affects the mitochondrial ETC, resulting in the accumulation of free radicals [42,43]. Under normal circumstances, mitochondria can counteract the accumulation of reactive oxygen species (ROS); however, this function is compromised when the structure and function of the mitochondria are impaired. Additionally, mitochondrial dysfunction triggers the import of α-synuclein into the mitochondria. The accumulation of α-synuclein in mitochondria contributes to mitochondrial damage, creating a detrimental cycle that exacerbates mitochondrial dysfunction and cellular damage in PD [44–46].
The use of secretome has been shown to reduce mitochondrial energy generation, mitochondrial membrane potential, and mitochondrial mass in PD cells. By preserving some of these mitochondrial parameters, the secretome can mitigate the effects of oxidative stress and apoptosis that play a role in the development of PD [47]. Important molecules involved in this process include PARK7 and Sirt1 [48]. The PARK7 protein, also known as DJ-1, functions as a cellular oxidative stress sensor and plays a crucial role in maintaining cellular homeostasis [49]. PARK7 has several protective functions, including inhibiting the accumulation of glycosylated α-synuclein, increasing the expression of antioxidant enzymes to reduce ROS production, and enhancing ATP production in dopaminergic neurons [50–53]. Furthermore, studies have established a connection between PARK7 and Sirt1.
Research indicates that PARK7 can directly bind to and activate Sirt1, leading to the inhibition of p53 acetylation and a reduction in cell apoptosis [54,55]. One study demonstrated that Sirt1 levels were upregulated following exogenous recombinant PARK7 treatment, although this finding remains preliminary [48]. Sirt1, a member of the sirtuin family of proteins, plays a crucial role in regulating various physiological processes, particularly in the mitochondria. It is a NAD+-dependent lysine deacetylase that is abundant in the brain [47]. Sirt1 is closely linked to mitochondrial function, as it promotes mitochondrial biogenesis and energy synthesis by activating transcription factors such as p53, CREB, PGC-1α, and HIF-1α. Additionally, Sirt1 is involved in inhibiting oxidative stress and facilitating the degradation of α-synuclein oligomers, which have been implicated in PD pathology [56,57].
In a study utilizing rotenone (ROT) a molecule that functions as a mitochondrial complex I inhibitor and causes an increase in α-synuclein, the protective effect of secretomes produced by NI-ADSC-SM was observed [58–60]. The protective effect of the NI-ADSC-SM secretome was found to be superior to that of human ADSC-SM in general [60]. Cells treated with ROT exhibited an increase in calcium levels, which can affect the incidence of cell damage [60,61]. The secretome of NI-ADSC-SM showed a decrease in calcium levels compared to the secretome of ADSC-SM [60].
In examining the protein expression of LRRK2, PINK1, parkin, soluble and insoluble Ub proteins, DJ-1, and TOM20, it was found that NI-ADSC-SM secretome administration decreased the expression of LRRK2 and insoluble Ub proteins, which play a role in the progression of PD [60,62]. For the expression of parkin, soluble Ub protein, DJ-1, and TOM20, an increase in expression was observed, although no increase in PINK1 expression was noted [60].
PINK1 and parkin are essential for mitophagy, targeting damaged mitochondria for degradation. Decreased levels of PINK1 and parkin induced by ROT exposure led to increased mitochondrial fragmentation, potentially resulting in apoptotic cell death due to ROS-induced stress [60,63–65]. The activation of mitophagy aims to clear ubiquitinated substrates, such as misfolded α-synuclein protein aggregates, for cellular clearance [59]. ROT exposure also decreased soluble Ub levels while increasing insoluble Ub levels in cell lysates [60]. This accumulation of Ub conjugates under oxidative stress may lead to the aggregation of misfolded proteins, which are targeted for degradation through mitophagy mechanisms [60,66].
Decreased levels of DJ-1, a protein crucial for mitigating α-synuclein aggregation and maintaining the mitochondrial function, after ROT exposure, may be linked to increased ROS production, promoting α-synuclein aggregation and mitochondrial dysfunction [60,67]. ROT exposure also decreased TOM20 levels, a protein involved in mitochondrial protein import and function, potentially impairing mitochondrial function. The modulation of TOM20 and DJ-1 levels by NI-ADSC-SM treatment may contribute to improved mitochondrial function, counteract α-synuclein aggregation, and enhance overall neuronal health in the context of PD-associated pathologies [60,68].
Effect of dynamic system in secretome production
Research is currently exploring the use of system dynamics for the large-scale production of secretomes, particularly through bioreactor systems. These systems can significantly influence the molecular expression of secretomes compared to static culture models [36,69–71]. A study compared two dynamic models, specifically the SP and VWBR, using two different culture media: AlphaMEM for SP1 and VWBR1, and Neurobasal-A for SP2 and VWBR2 [71]. Each model operates with distinct mechanisms and agitation speeds [72].
The results from the bioreactor experiments indicated fold expansion rates of 4.19 times for VWBR1, 2.93 times for SP1, 2.58 times for VWBR2, and 2.45 times for SP2. The doubling times were 2.24 days for VWBR1, 1.92 days for SP1, 2.90 days for VWBR2, and 2.23 days for SP2. Cells cultured in the dynamic bioreactor system maintained their MSC phenotype and multilineage differentiation capabilities, along with the ability to induce neurodifferentiation. However, the secretomes produced from these dynamic systems exhibited varying neuroprotective effects, with those from the SP model showing superior neuroprotective properties compared to those from VWBR. This variation is attributed to the different secretory profiles generated by the secretomes in the distinct dynamic models and culture conditions. Notably, secretome from SP1 demonstrated a higher secretory intensity overall, with MCP-1 and IL-8 being highly expressed across all secretome types. In contrast, higher levels of TNF-β, MMP3, and IL-6 were observed in SP, while VWBR1 showed increased osteopontin expression, and VWBR2 had higher levels of HB-EGF, GCSF, NGFβ, and IL-13 [71].
The differences in neuroprotective effects may be linked to the shear stress experienced in the two bioreactor types, with SP exhibiting higher shear stress levels [72–74]. Additionally, the glucose concentration was lower in SP (3.4 ± 1.1 mM) compared to VWBR (9.5 ± 3.4 mM) [71]. This suggests that higher stress conditions may enhance the secretory production of the secretome, particularly concerning cytokines with anti-inflammatory properties, such as oncostatin M, TGF-β1, IL-4, and IL-6 [71,75–80]. However, the precise effects of stress on these outcomes remain largely unexplored.
Effect of secretome on behavioral and motor parameters
In studies involving PD models treated with secretome, various behavioral and motor function tests have been employed, including rotational behavior, the MWM, rotarod, staircase, motor swimming, beam balance walk, and pole tests. Results indicate that animals receiving secretome therapy generally performed better than those treated with other therapies, such as levodopa or hNPC transplantation alone [25,26,34,40,81,82].
Notably, in experiments where a combination of NSC transplantation and secretome therapy was administered, significant motor improvements were observed. In contrast, the group receiving only transplantation did not show enhancements in motor coordination or fine motor skills in the rotarod and staircase tests compared to the untreated group. This suggests that combining transplantation with secretome therapy is more effective for recovering motor function and cognitive abilities in PD model rats [40,82]. Furthermore, another study reported that animals treated with the secretome derived from hBM-MSCs exhibited significant improvements in motor functionality, as evidenced by an increased success rate in retrieving food pellets on the affected side [25].
The route of secretome administration also plays a crucial role in the outcomes of motor and behavioral functions. Research has shown that IV administration yields better results than IC administration [34].
It is essential to note that while the rotarod test evaluates motor coordination, it does not specifically assess manipulation skills involving individual paws, which is a focus of the staircase test. The staircase test is designed to provide a more precise evaluation of performance, as its configuration prevents animals from compensating by using their unaffected paw, even when the affected side is presumed to be impaired. This distinction is not captured by the rotarod test [83,84].
Effect of secretome in pro-inflammatory cytokine levels
The administration of secretome in PD models has demonstrated several beneficial effects on oxidative stress and inflammation. Specifically, secretome treatment resulted in a significant reduction in MDA levels, an increase in antioxidant molecules such as glutathione, SOD, and catalase, and a notable decrease in IL-1β levels in rat models of PD [26]. These changes are indicative of a response to oxidative stress and inflammation [85].
Molecular analyses of the secretome have identified several key molecules, including Prdx1, fibronectin, and cadherin-2 [40]. Prdx1, when overexpressed in DA neuronal cell lines, has been shown to mitigate the effects of 6-OHDA by functioning as a ROS scavenger, which enhances the survival and protection of DA neurons [86].
SOD enzymes play a critical role as the first line of defense against ROS, catalyzing the dismutation of superoxide radicals to protect cells, including DA neurons, from oxidative damage [87,88]. Fibronectin exhibits dual roles in the nervous system, contributing both to neuroinflammation and neuroprotection. It can bind to integrins and growth factor receptors, such as the IGF-1 receptor, activating intracellular signaling pathways like the PI3K/AKT pathway. This activation promotes neuroprotective actions akin to those of growth factors, helping to limit apoptosis, prevent microglial activation and neuroinflammation, and regulate ROS accumulation, thereby maintaining oxidative stress levels [89,90].
Histological effect of secretome
In the PD-induced group, shrunken pyknotic and hypoxic changes were observed. Similar morphological alterations were noted in the levodopa-treated group; however, the secretome-treated group exhibited intact neuronal cell architecture, with only occasional pyknosis. This indicates milder neurodegenerative changes compared to both the disease and levodopa-treated groups [26].
In another study involving rats, NSCs were transplanted into the MFB and VTA of PD model rats, which subsequently received secretome therapy derived from MSCs CM or hNPCs CM. The results demonstrated an increased ratio of TH-positive neurons, indicating a rise in dopaminergic neurons in the group that received secretome therapy [40,82]. By week eight, the expansion and migration of neuronal cells in the CM-NSCs group that received secretome therapy showed superior results in histological examinations compared to the group with transplanted NSCs alone [82].
Although a study using secretome from hNPCs reported only a slight increase in TH-positive cells in the SNpc, most studies suggest that secretome administration enhances the number of TH-positive cells and neuronal density in various brain regions, including the SNpc [34,40,81,82,91]. Furthermore, secretome application via direct IC injection resulted in a greater increase in TH-positive cells compared to IV administration, although this increase did not directly correlate with motor function improvements [34].
A comparative study of protein and vesicular fractions of secretomes derived from hBM-MSCs yielded similar results, showing increased TH intensity and survival rates of dopaminergic neurons in the SNpc. However, the increases observed with protein or vesicular fractions alone were not as pronounced as those achieved with the direct application of secretome derived from hBM-MSCs [25].
Additionally, a recent study utilizing the viral vector AAV1/2-A53T-a-syn to induce overexpression of the A53T mutant form of alpha-synuclein in animal models found that treatment with MSC(M)-derived secretome resulted in a higher percentage of TH-positive cells in the SNpc compared to vehicle-treated animals [92–94]. This secretome treatment also led to a reduction in microglial reactivity, suggesting its role in maintaining microglial homeostasis and neuroprotective functions while managing the alpha-synuclein burden in the injured SNpc [92].
Effect of coating material on secretome release
The application of secretome in treating neurodegenerative diseases has shown promising results; however, direct administration often fails to produce long-lasting effects, which is particularly important in chronic conditions like PD [95–97]. To enhance the retention time of the secretome in tissues, a study utilized hydrogels with a semi-interpenetrated polymer system, incorporating COLL, PEG, and LMWHA as coating materials. Two hydrogel combinations were developed: COLL/PEG2000 and COLL/LMWHA. Both formulations demonstrated prolonged availability of the secretome in the tissue, lasting up to 48 hours. Furthermore, the use of these hydrogels for secretome administration resulted in improvements of 26% for the COLL/PEG2000 combination and 24% for the COLL/LMWHA combination following induction with 6-OHDA [98].
Effect of administration route on secretome efficacy
Several studies have explored various administration routes for the secretome, which is typically delivered directly to the brain region. Notably, IV administration, which is less invasive, has been shown to produce effects comparable to those of direct intracranial administration [26]. One study found that administering the secretome via IV resulted in more significant improvements in motor function. However, histological examinations revealed that IC administration led to a greater number of dopaminergic cells [34]. This suggests that while IV administration may be effective for enhancing motor function, IC administration might be more beneficial for promoting dopaminergic cell survival.
Potential of secretome therapy in psychosocial aspect
Motor impairment is one of the most prevalent symptoms associated with PD; however, its effects extend beyond physical limitations to include significant psychosocial challenges. Patients with PD may experience a range of psychosocial disorders, including anxiety, depression, social isolation, and feelings of stigmatization. These perceptions can profoundly affect both the patients and their families [99,100]. Research indicates that such perceptual issues can also hinder social interactions between PD patients and their communities [101].
Recent developments in secretome research have demonstrated promising biomolecular effects and improvements in motor function in animal studies [33,34,40,71,81,91,92,102]. This suggests that secretome therapy has the potential to alleviate motor dysfunction, which is a primary symptom of PD, and may also enhance patients’ self-perception. However, it is important to note that there are currently no clinical trials investigating the use of secretome in humans with PD, leaving a gap in objective data regarding its psychosocial effects.
Comparison of secretome and levodopa in Parkinson’s treatment
Until now, the use of levodopa is still the mainstay of therapy for PD. The use of levodopa works by increasing the amount of dopamine in the striatum that has degenerated due to PD [103]. Although it has been widely studied, the use of levodopa as a PD therapy still shows some side effects in its use [103,104]. This is possible due to the combination of oxidative and antioxidant effects of levodopa, which in the use of levodopa can occur autooxidation which increases quinones and hydrogen peroxide [104,105]. The existence of this effect causes the use of levodopa to require precise dose adjustments, besides that the improvement effect of levodopa only appears after prolonged use [106,107]. Another factor to consider is how many DA cells are left when levodopa is used because this condition also affects the improvement of the patient’s condition [108].
When compared to levodopa, secretome showed a faster effect on motor improvement. This may be because secretome works on multiple pathways [81]. In addition, the neuroregenerative effect produced by secretome increases the potential for cell differentiation in the brain into new DA cells so that the potential for dopamine production increases. However, the use of secretome in PD patients in humans has not been studied so there is still not much data related to the potential of this therapy in humans.
Perspective of current challenge and future recommendation
Research utilizing the secretome has demonstrated promising preclinical data for its application as a therapy for PD. However, significant variations exist in the types of trials conducted, both in vitro and in vivo. These variations include the source of MSCs, the methods of secretome production, the routes of secretome administration, and the types of experimental animals used to examine the effects of the secretome.
Differences in the types of experimental animals employed in various studies can lead to differing outcomes in secretome therapy. The choice of animal model often depends on the study’s objectives; for instance, cell cultures or organoids can facilitate the analysis of molecular mechanisms, while more complex interactions may require the use of experimental animals such as C. elegans or mice. Currently, research has focused more on the molecular effects of the secretome, indicating a need for further studies involving both experimental animals and human trials to better understand the secretome’s effects.
In this review, it was noted that MSCs derived from neural-induced stem cells yielded better results compared to those derived from adipose tissue. However, many studies also utilize other stem cell sources, such as bone marrow. Future research should compare the effectiveness and safety of various stem cell types to identify the most suitable progenitor source for secretome production. Additionally, exploring dynamic models for secretome production and employing coating materials to extend the half-life of the secretome is an intriguing area for further investigation, as there is currently limited research on this topic.
Most studies included in this review applied the secretome via intra-cerebral administration in animal research. While this method may complicate its application in human studies, some research has explored intravenous administration. Therefore, expanding studies that utilize intravenous routes may be beneficial. Furthermore, investigating the combination of secretome therapy with existing PD treatments could reveal potential additive effects that enhance current management guidelines.
To fully assess the benefits of the secretome in PD management, further human studies are essential. Phase one clinical trials should be conducted to evaluate the safety, therapeutic dosage, and efficacy of secretome therapy in PD. Human assessments will also allow for a broader evaluation of factors that cannot be addressed in in vivo trials, such as psychosocial effects.
Limitation
In searching for journals related to the use of secretome in Parkinson’s interventions, a broad keyword search was conducted. However, there are still limited search results on this topic because research on the use of secretome in Parkinson’s cases is still in its early stages. The existing results are primarily preclinical studies conducted in vivo and in vitro, with no studies conducted on humans. Scoping reviews aim to provide a broad overview of a topic, but for this specific topic, research is still sparse. However, this scoping review can already highlight developments in research related to the use of secretome in Parkinson’s cases, including biomolecular mechanisms, development of production methods, implementation assessments, histological effects, and more complex effects observed in animal trials, particularly in assessing motor effects. This broad discussion on the use of secretome provides an overview that is not limited to one aspect, aligning with the scoping review’s function of presenting a comprehensive view of the topic’s development.
Currently, research on the secretome remains primarily in the preclinical phase. Additionally, there are numerous variations in the sources of cell progenitors used to produce secretome, as well as in the research subjects involved. These variations contribute to inconsistencies in research findings, as there is no standardized protocol for secretome production in studies related to PD. Furthermore, there have been no studies investigating the use of secretome in humans with PD, which limits the assessment of its potential in clinical settings.
CONCLUSION
The use of the secretome as a novel therapeutic approach in PD research has demonstrated promising results. This is supported by molecular effects associated with neuroprotection, neurodifferentiation, and anti-inflammatory responses, all of which play crucial roles in the progression of neurodegenerative diseases. Additionally, the application of the secretome has led to improvements in motor and behavioral functions that are often impaired in Parkinsonian models. However, current research on secretome therapy for PD is primarily limited to laboratory studies involving experimental animals. To advance the clinical application of secretome therapy for patients with PD, further research, particularly phase one clinical trials in humans, is essential.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This study received no external funding.
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest.
ETHICS APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Pires AO, Teixeira FG, Mendes-Pinheiro B, Serra SC, Sousa N, Salgado AJ. Old and new challenges in Parkinson’s disease therapeutics. Progress Neurobiol. 2017;156:69–89. CrossRef
2. Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease. Nat Rev Dis Primers. 2017;3:17013. CrossRef
3. Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. 2021;397:2284–303. CrossRef
4. World Health Organization. Parkinson Disease: a Public Health Approach. Technical Brief. 1st ed. Geneva, Switzerland: World Health Organization; 2022.
5. Parkinson’s Foundation. Understanding Parkinson’s. Miami, FL: Parkinson’s Foundation; 2022. https://www.parkinson.org/understanding-parkinsons/statistics
6. Vijverman AC, Fox SH. New treatments for the motor symptoms of Parkinson’s disease. Expert Rev Clin Pharmacol. 2014;7:761–77. CrossRef
7. Dong J, Cui Y, Li S, Le W. Current pharmaceutical treatments and alternative therapies of Parkinson’s disease. CN. 2016;14:339–55. CrossRef
8. Lindholm D, Mäkelä J, Di Liberto V, Mudò G, Belluardo N, Eriksson O, et al. Parkinson’s disease: towards better preclinical models and personalized treatments. Cell Mol Life Sci. 2016;73:1383–5. CrossRef
9. Krack P, Martinez-Fernandez R, Del Alamo M, Obeso JA. Current applications and limitations of surgical treatments for movement disorders. Movement Disorders. 2017;32:36–52. CrossRef
10. Onofrj M, Bonanni L, Thomas A. An expert opinion on safinamide in Parkinson’s disease. Expert Opin Investig Drugs. 2008;17:1115–25. CrossRef
11. Stoker TB, Barker RA. Recent developments in the treatment of Parkinson’s Disease. F1000Res. 2020;9:862. CrossRef
12. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–22. CrossRef
13. Chen Y, Shao JZ, Xiang LX, Dong XJ, Zhang GR. Mesenchymal stem cells: a promising candidate in regenerative medicine. Int J Biochem Cell Biol. 2008;40:815–20. CrossRef
14. Gao F, Chiu SM, Motan DAL, Zhang Z, Chen L, Ji HL, et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016;7:e2062. CrossRef
15. Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal stem cells for regenerative medicine. Cells. 2019;8:886. CrossRef
16. Vizoso F, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. IJMS. 2017;18:1852. CrossRef
17. Merimi M, El-Majzoub R, Lagneaux L, Moussa Agha D, Bouhtit F, Meuleman N, et al. The therapeutic potential of mesenchymal stromal cells for regenerative medicine: current knowledge and future understandings. Front Cell Dev Biol. 2021;9:661532. CrossRef
18. Yang D, Wang W, Li L, Peng Y, Chen P, Huang H, et al. The relative contribution of paracine effect versus direct differentiation on adipose-derived stem cell transplantation mediated cardiac repair. PLoS One. 2013;8:e59020. CrossRef
19. Brown C, McKee C, Bakshi S, Walker K, Hakman E, Halassy S, et al. Mesenchymal stem cells: cell therapy and regeneration potential. J Tissue Eng Regen Med. 2019;13:1738–55. CrossRef
20. Teixeira FG, Carvalho MM, Sousa N, Salgado AJ. Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration? Cell Mol Life Sci. 2013;70:3871–82. CrossRef
21. Marques CR, Marote A, Mendes-Pinheiro B, Teixeira FG, Salgado AJ. Cell secretome based approaches in Parkinson’s disease regenerative medicine. Expert Opin Biol Ther. 2018;18:1235–45. CrossRef
22. Pinho AG, Cibrão JR, Silva NA, Monteiro S, Salgado AJ. Cell secretome: basic insights and therapeutic opportunities for CNS disorders. Pharmaceuticals. 2020;13:31. CrossRef
23. Müller T. View point: disease modification and cell secretome based approaches in Parkinson’s disease: are we on the right track? BTT. 2021;15:307–16. CrossRef
24. Giovannelli L, Bari E, Jommi C, Tartara F, Armocida D, Garbossa D, et al. Mesenchymal stem cell secretome and extracellular vesicles for neurodegenerative diseases: risk-benefit profile and next steps for the market access. Bioactive Mat. 2023;29:16–35. CrossRef
25. Vilaça Faria H, Marote A, Lages I, Ribeiro C, Mendes-Pinheiro B, Domingues AV, et al. Fractionating stem cells secretome for Parkinson’s disease modeling: is it the whole better than the sum of its parts? Biochimie. 2021;189:87–98. CrossRef
26. Mahendru D, Jain A, Bansal S, Malik D, Dhir N, Sharma AR, et al. Neuroprotective effect of bone marrow-derived mesenchymal stem cell secretome in 6-OHDA-induced Parkinson’s disease. Regen Med. 2021;16:915–30. CrossRef
27. Gaceb A, Özen I, Padel T, Barbariga M, Paul G. Pericytes secrete pro-regenerative molecules in response to platelet-derived growth factor-BB. J Cereb Blood Flow Metab. 2018;38:45–57. CrossRef
28. Paul G, Sullivan AM. Trophic factors for Parkinson’s disease: where are we and where do we go from here? Eur J Neurosci. 2019;49:440–52. CrossRef
29. Lindh B, Hökfelt T. Chapter 20 Structural and functional aspects of acetylcholine peptide coexistence in the autonomic nervous system. Progress Brain Res. 1990;84:175–91. CrossRef
30. Lotharius J, Barg S, Wiekop P, Lundberg C, Raymon HK, Brundin P. Effect of mutant α-synuclein on dopamine homeostasis in a new human mesencephalic cell line. J Biol Chem 2002;277:38884–94. CrossRef
31. Lotharius J, Falsig J, Van Beek J, Payne S, Dringen R, Brundin P, et al. Progressive degeneration of human mesencephalic neuron-derived cells triggered by dopamine-dependent oxidative stress is dependent on the mixed-lineage kinase pathway. J Neurosci. 2005;25:6329–42. CrossRef
32. Smirnova L, Harris G, Delp J, Valadares M, Pamies D, Hogberg HT, et al. A LUHMES 3D dopaminergic neuronal model for neurotoxicity testing allowing long-term exposure and cellular resilience analysis. Arch Toxicol. 2016;90:2725–43. CrossRef
33. Gaceb A, Barbariga M, Paul G. An in vitro partial lesion model of differentiated human mesencephalic neurons: effect of pericyte secretome on phenotypic markers. J Mol Neurosci. 2020;70:1914–25. CrossRef
34. Marques CR, Pereira-Sousa J, Teixeira FG, Sousa RA, Teixeira-Castro A, Salgado AJ. Mesenchymal stem cell secretome protects against alpha-synuclein-induced neurodegeneration in a Caenorhabditis elegans model of Parkinson’s disease. Cytotherapy. 2021;23:894–901. CrossRef
35. Mendes-Pinheiro B, Campos J, Marote A, Soares-Cunha C, Nickels SL, Monzel AS, et al. Treating Parkinson’s disease with human bone marrow mesenchymal stem cell secretome: a translational investigation using human brain organoids and different routes of in vivo administration. Cells. 2023;12:2565. CrossRef
36. Pires AO, Mendes-Pinheiro B, Teixeira FG, Anjo SI, Ribeiro-Samy S, Gomes ED, et al. Unveiling the differences of secretome of human bone marrow mesenchymal stem cells, adipose tissue-derived stem cells, and human umbilical cord perivascular cells: a proteomic analysis. Stem Cells Dev. 2016;25:1073–83. CrossRef
37. Teixeira FG, Panchalingam KM, Assunção-Silva R, Serra SC, Mendes-Pinheiro B, Patrício P, et al. Modulation of the mesenchymal stem cell secretome using computer-controlled bioreactors: impact on neuronal cell proliferation, survival and differentiation. Sci Rep. 2016;6:27791. CrossRef
38. Teixeira FG, Carvalho MM, Panchalingam KM, Rodrigues AJ, Mendes-Pinheiro B, Anjo S, et al. Impact of the secretome of human mesenchymal stem cells on brain structure and animal behavior in a rat model of Parkinson’s Disease. Stem Cells Transl Med. 2017;6:634–46. CrossRef
39. Teixeira FG, Vilaça-Faria H, Domingues AV, Campos J, Salgado AJ. Preclinical comparison of stem cells secretome and levodopa application in a 6-hydroxydopamine rat model of Parkinson’s Disease. Cells. 2020;9:315. CrossRef
40. Hussar P. Apoptosis regulators Bcl-2 and caspase-3. Encyclopedia. 2022;2:1624–36. CrossRef
41. Mendes-Pinheiro B, Teixeira FG, Anjo SI, Manadas B, Behie LA, Salgado AJ. Secretome of undifferentiated neural progenitor cells induces histological and motor improvements in a rat model of Parkinson’s Disease. Stem Cells Transl Med. 2018;7:829–38. CrossRef
42. Fukusumi Y, Meier F, Götz S, Matheus F, Irmler M, Beckervordersandforth R, et al. Dickkopf 3 promotes the differentiation of a rostrolateral midbrain dopaminergic neuronal subset in vivo and from pluripotent stem cells in vitro in the mouse. J Neurosci. 2015;35:13385–401. CrossRef
43. Kilbride SM, Telford JE, Davey GP. Complex I controls mitochondrial and plasma membrane potentials in nerve terminals. Neurochem Res. 2021;46:100–7. CrossRef
44. Umeno A, Biju V, Yoshida Y. In vivo ROS production and use of oxidative stress-derived biomarkers to detect the onset of diseases such as Alzheimer’s disease, Parkinson’s disease, and diabetes. Free Radical Res. 2017;51:413–27. CrossRef
45. Lin KJ, Lin KL, Chen SD, Liou CW, Chuang YC, Lin HY, et al. The overcrowded crossroads: mitochondria, alpha-synuclein, and the endo-lysosomal system interaction in Parkinson’s Disease. IJMS. 2019;20:5312. CrossRef
46. Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–95. CrossRef
47. Martínez JH, Fuentes F, Vanasco V, Alvarez S, Alaimo A, Cassina A, et al. Alpha-synuclein mitochondrial interaction leads to irreversible translocation and complex I impairment. Arch Biochem Biophys. 2018;651:1–12. CrossRef
48. Li X, Feng Y, Wang XX, Truong D, Wu YC. The critical role of SIRT1 in Parkinson’s Disease: mechanism and therapeutic considerations. Aging Dis. 2020;11:1608. CrossRef
49. Ni W, Zhou J, Ling Y, Lu X, Niu D, Zeng Y, et al. Neural stem cell secretome exerts a protective effect on damaged neuron mitochondria in Parkinson’s disease model. Brain Res. 2022;1790:147978. CrossRef
50. Dolgacheva LP, Berezhnov AV, Fedotova EI, Zinchenko VP, Abramov AY. Role of DJ-1 in the mechanism of pathogenesis of Parkinson’s disease. J Bioenerg Biomembr. 2019;51:175–88. CrossRef
51. Sharma N, Rao SP, Kalivendi SV. The deglycase activity of DJ-1 mitigates α-synuclein glycation and aggregation in dopaminergic cells: role of oxidative stress mediated downregulation of DJ-1 in Parkinson’s disease. Free Radical Biol Med. 2019;135:28–37. CrossRef
52. Zeng J, Zhao H, Chen B. DJ-1/PARK7 inhibits high glucose-induced oxidative stress to prevent retinal pericyte apoptosis via the PI3K/AKT/mTOR signaling pathway. Exp Eye Res. 2019;189:107830. CrossRef
53. Yin L, Li H, Liu Z, Wu W, Cai J, Tang C, et al. PARK7 Protects against chronic kidney injury and renal fibrosis by inducing SOD2 to reduce oxidative stress. Front Immunol. 2021;12:690697. CrossRef
54. Zhang F, Yan Y, Peng W, Wang L, Wang T, Xie Z, et al. PARK7 promotes repair in early steroid-induced osteonecrosis of the femoral head by enhancing resistance to stress-induced apoptosis in bone marrow mesenchymal stem cells via regulation of the Nrf2 signaling pathway. Cell Death Dis. 2021;12:940. CrossRef
55. Takahashi-Niki K, Ganaha Y, Niki T, Nakagawa S, Kato-Ose I, Iguchi-Ariga SMM, et al. DJ-1 activates SIRT1 through its direct binding to SIRT1. Biochem Biophys Res Commun. 2016;474:131–6. CrossRef
56. Xu RY, Xu XW, Deng YZ, Ma ZX, Li XR, Zhao L, et al. Resveratrol attenuates myocardial hypoxia/reoxygenation-induced cell apoptosis through DJ-1-mediated SIRT1-p53 pathway. Biochem Biophys Res Commun. 2019;514:401–6. CrossRef
57. Yuan Y, Cruzat VF, Newsholme P, Cheng J, Chen Y, Lu Y. Regulation of SIRT1 in aging: roles in mitochondrial function and biogenesis. Mech Ageing Dev. 2016;155:10–21. CrossRef
58. Fang Y, Wang X, Yang D, Lu Y, Wei G, Yu W, et al. Relieving cellular energy stress in aging, neurodegenerative, and metabolic diseases, SIRT1 as a therapeutic and promising node. Front Aging Neurosci. 2021;13:738686. CrossRef
59. Ramalingam M, Jang S, Jeong HS. Neural-induced human adipose tissue-derived stem cells conditioned medium ameliorates rotenone-induced toxicity in SH-SY5Y cells. IJMS. 2021;22:2322. CrossRef
60. Ramalingam M, Jeong HS, Hwang J, Cho HH, Kim BC, Kim E, et al. Autophagy signaling by neural-induced human adipose tissue-derived stem cell-conditioned medium during rotenone-induced toxicity in SH-SY5Y cells. IJMS. 2022;23:4193. CrossRef
61. Ramalingam M, Jang S, Hwang J, Kim B, Cho HH, Kim E, et al. Neuroprotective effects of the neural-induced adipose-derived stem cell secretome against rotenone-induced mitochondrial and endoplasmic reticulum dysfunction. Int J Mol Sci. 2023;24:5622. CrossRef
62. Cerella C, Diederich M, Ghibelli L. The dual role of calcium as messenger and stressor in cell damage, death, and survival. Int J Cell Biol 2010;2010:1–14. CrossRef
63. Xiong Y, Dawson TM, Dawson VL. Models of LRRK2-associated Parkinson’s disease. In: Rideout HJ, editor. Leucine-Rich Repeat Kinase 2 (LRRK2). Cham, Switzerland: Springer International Publishing; 2017. vol. 14, pp 163–91. CrossRef
64. Van Laar VS, Otero PA, Hastings TG, Berman SB. Potential role of Mic60/Mitofilin in Parkinson’s disease. Front Neurosci. 2019;12:898. CrossRef
65. Xu L, Wang X, Tong C. Endoplasmic reticulum–mitochondria contact sites and neurodegeneration. Front Cell Dev Biol. 2020;8:428. CrossRef
66. Yamano K, Youle RJ. PINK1 is degraded through the N-end rule pathway. Autophagy. 2013;9:1758–69. CrossRef
67. McLelland GL, Goiran T, Yi W, Dorval G, Chen CX, Lauinger ND, et al. Mfn2 ubiquitination by PINK1/parkin gates the p97-dependent release of ER from mitochondria to drive mitophagy. eLife. 2018;7:e32866. CrossRef
68. Irrcher I, Aleyasin H, Seifert EL, Hewitt SJ, Chhabra S, Phillips M, et al. Loss of the Parkinson’s disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum Mol Genet. 2010;19:3734–46. CrossRef
69. Di Maio R, Barrett PJ, Hoffman EK, Barrett CW, Zharikov A, Borah A, et al. α-synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson’s disease. Sci Transl Med. 2016;8:342ra78. CrossRef
70. Das R, Roosloot R, Van Pel M, Schepers K, Driessen M, Fibbe WE, et al. Preparing for cell culture scale-out: establishing parity of bioreactor- and flask-expanded mesenchymal stromal cell cultures. J Transl Med. 2019;17:241. CrossRef
71. Pinto DS, Ahsan T, Serra J, Fernandes-Platzgummer A, Cabral JMS, Da Silva CL. Modulation of the in vitro angiogenic potential of human mesenchymal stromal cells from different tissue sources. J Cell Physiol. 2020;235:7224–38. CrossRef
72. Marques CR, Fuzeta MA, Dos Santos Cunha RM, Pereira-Sousa J, Silva D, Campos J, et al. Neurodifferentiation and neuroprotection potential of mesenchymal stromal cell-derived secretome produced in different dynamic systems. Biomedicines. 2023;11:1240. CrossRef
73. Croughan MS, Giroux D, Fang D, Lee B. Novel single-use bioreactors for scale-up of anchorage-dependent cell manufacturing for cell therapies. In: Cabral JMS, da Silva CL, Chase LG, Diogo MM, editors. Stem cell manufacturing. Amsterdam, The Netherlands: Elsevier BV; 2016. pp. 105–39. CrossRef
74. Santos FD, Andrade PZ, Abecasis MM, Gimble JM, Chase LG, Campbell AM, et al. Toward a clinical-grade expansion of mesenchymal stem cells from human sources: a microcarrier-based culture system under xeno-free conditions. Tissue Eng Part C Methods. 2011;17:1201–10. CrossRef
75. De Almeida Fuzeta M, Bernardes N, Oliveira FD, Costa AC, Fernandes-Platzgummer A, Farinha JP, et al. Scalable production of human mesenchymal stromal cell-derived extracellular vesicles under serum-/xeno-free conditions in a microcarrier-based bioreactor culture system. Front Cell Dev Biol. 2020;8:553444. CrossRef
76. Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45:27–37. CrossRef
77. Ismadi MZ, Hourigan K, Fouras A. Experimental characterisation of fluid mechanics in a spinner flask bioreactor. Processes. 2014;2:753–72. CrossRef
78. Jossen V, Pörtner R, Kaiser SC, Kraume M, Eibl D, Eibl R. Mass production of mesenchymal stem cells—impact of bioreactor design and flow conditions on proliferation and differentiation. In: Eberli D, editor. cells and biomaterials in regenerative medicine. London, UK: InTech; 2014. CrossRef
79. Diaz M, Evans S, Olson S, Cox C, Wenzel P. A co-culture assay to determine efficacy of TNF-α suppression by biomechanically induced human bone marrow mesenchymal stem cells. Bio-Protoc. 2017;7:e2513. CrossRef
80. Diaz MF, Vaidya AB, Evans SM, Lee HJ, Aertker BM, Alexander AJ, et al. Biomechanical forces promote immune regulatory function of bone marrow mesenchymal stromal cells. Stem Cells. 2017;35:1259–72. CrossRef
81. Jones SA, Jenkins BJ. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. 2018;18:773–89. CrossRef
82. Yao Y, Huang C, Gu P, Wen T. Combined MSC-secreted factors and neural stem cell transplantation promote functional recovery of PD rats. Cell Transplant. 2016;25:1101–13. CrossRef
83. Baird AL, Meldrum A, Dunnett SB. The staircase test of skilled reaching in mice. Brain Res Bull. 2001;54:243–50. CrossRef
84. Whishaw IQ, Woodward NC, Miklyaeva E, Pellis SM. Analysis of limb use by control rats and unilateral DA-depleted rats in the Montoya staircase test: movements, impairments and compensatory strategies. Beha Brain Res. 1997;89:167–77. CrossRef
85. Khan Z, Ali SA. Oxidative stress-related biomarkers in Parkinson’s disease: a systematic review and meta-analysis. Iran J Neurol. 2018;17:137–44.
86. Lee YM, Park SH, Shin DI, Hwang JY, Park B, Park YJ, et al. Oxidative modification of peroxiredoxin is associated with drug-induced apoptotic signaling in experimental models of Parkinson Disease. J Bio Chem. 2008;283:9986–98. CrossRef
87. Filograna R, Godena VK, Sanchez-Martinez A, Ferrari E, Casella L, Beltramini M, et al. Superoxide dismutase (SOD)-mimetic M40403 is protective in cell and fly models of paraquat toxicity. J Biol Chem. 2016;291:9257–67. CrossRef
88. Zhou C, Huang Y, Przedborski S. Oxidative stress in Parkinson’s Disease: a mechanism of pathogenic and therapeutic significance. Ann N Y Acad Sci. 2008;1147:93–104. https://doi.org/10.1196/annals.1427.023
89. Wang J, Yin L, Chen Z. Neuroprotective role of fibronectin in neuron-glial extrasynaptic transmission. Neural Regen Res. 2013;8:376–82. CrossRef
90. Jha SK, Jha NK, Kar R, Ambasta RK, Kumar P. p38 MAPK and PI3K/AKT Signalling Cascades inParkinson’s Disease. Int J Mol Cell Med. 2015;4:67–86.
91. Mendes-Pinheiro B, Anjo SI, Manadas B, Da Silva JD, Marote A, Behie LA, et al. Bone marrow mesenchymal stem cells’ secretome exerts neuroprotective effects in a Parkinson’s disease rat model. Front Bioeng Biotechnol. 2019;7:294. CrossRef
92. Marques CR, Campos J, Sampaio-Marques B, Antunes FF, Dos Santos Cunha RM, Silva D, et al. Secretome of bone marrow mesenchymal stromal cells cultured in a dynamic system induces neuroprotection and modulates microglial responsiveness in an α-synuclein overexpression rat model. Cytotherapy. 2024;26(7):700–13. CrossRef
93. Ip CW, Klaus LC, Karikari AA, Visanji NP, Brotchie JM, Lang AE, et al. AAV1/2-induced overexpression of A53T-α-synuclein in the substantia nigra results in degeneration of the nigrostriatal system with Lewy-like pathology and motor impairment: a new mouse model for Parkinson’s disease. Acta Neuropathol Commun. 2017;5:11. CrossRef
94. Koprich JB, Johnston TH, Reyes MG, Sun X, Brotchie JM. Expression of human A53T alpha-synuclein in the rat substantia nigra using a novel AAV1/2 vector produces a rapidly evolving pathology with protein aggregation, dystrophic neurite architecture and nigrostriatal degeneration with potential to model the pathology of Parkinson’s disease. Mol Neurodegeneration. 2010;5:43. CrossRef
95. Uccelli A, Milanese M, Principato MC, Morando S, Bonifacino T, Vergani L, et al. Intravenous mesenchymal stem cells improve survival and motor function in experimental amyotrophic lateral sclerosis. Mol Med. 2012;18:794–804. CrossRef
96. Yousefi F, Ebtekar M, Soudi S, Soleimani M, Hashemi SM. In vivo immunomodulatory effects of adipose-derived mesenchymal stem cells conditioned medium in experimental autoimmune encephalomyelitis. Immunol Lett. 2016;172:94–105. CrossRef
97. Cui Y, Ma S, Zhang C, Cao W, Liu M, Li D, et al. Human umbilical cord mesenchymal stem cells transplantation improves cognitive function in Alzheimer’s disease mice by decreasing oxidative stress and promoting hippocampal neurogenesis. Behav Brain Res. 2017;320:291–301. CrossRef
98. Chierchia A, Chirico N, Boeri L, Raimondi I, Riva GA, Raimondi MT, et al. Secretome released from hydrogel-embedded adipose mesenchymal stem cells protects against the Parkinson’s disease related toxin 6-hydroxydopamine. Eur J Pharm Biopharm. 2017;121:113–20. CrossRef
99. Jonasson SB, Rantakokko M, Franzén E, Iwarsson S, Nilsson MH. Prediction of life satisfaction in people with Parkinson’s disease. Parkinson’s Dis. 2020;2020:1–7. CrossRef
100. Vardanyan R, König HH, Hajek A. Association between Parkinson’s disease and psychosocial factors: results of the nationally representative german ageing survey. JCM. 2022;11:4569. CrossRef
101. Salazar RD, Weizenbaum E, Ellis TD, Earhart GM, Ford MP, Dibble LE, et al. Predictors of self-perceived stigma in Parkinson’s disease. Parkinsonism Related Disorders. 2019;60:76–80. CrossRef
102. Cervenka J, Tylecková J, Kupcová Skalníková H, Vodicková Kepková K, Poliakh I, Valeková I, et al. Proteomic characterization of human neural stem cells and their secretome during in vitro differentiation. Front Cell Neurosci. 2020;14:612560. CrossRef
103. Zahoor I, Shafi A, Haq E. Pharmacological treatment of Parkinson’s disease. In: Stoker TB, Greenland JC, editors. Chapter 7. Parkinson’s disease: pathogenesis and clinical aspects [Internet]. Brisbane, Australia: Codon Publications; 2018 Dec 21. Available from: https://www.ncbi.nlm.nih.gov/books/NBK536726/ CrossRef
104. Colamartino M, Santoro M, Duranti G, Sabatini S, Ceci R, Testa A, et al. Evaluation of levodopa and carbidopa antioxidant activity in normal human lymphocytes in vitro: implication for oxidative stress in Parkinson’s disease. Neurotox Res. 2015;27:106–17. CrossRef
105. Tayarani-Binazir KA, Jackson MJ, Fisher R, Zoubiane G, Rose S, Jenner P. The timing of administration, dose dependence and efficacy of dopa decarboxylase inhibitors on the reversal of motor disability produced by L-DOPA in the MPTP-treated common marmoset. Eur J Pharmacol. 2010;635:109–16. CrossRef
106. Thirugnanasambandam N, Grundey J, Paulus W, Nitsche MA. Dose-dependent nonlinear effect of l-DOPA on paired associative stimulation-induced neuroplasticity in humans. J Neurosci. 2011;31:5294–9. CrossRef
107. Brod LS, Aldred JL, Nutt JG. Are high doses of carbidopa a concern? a randomized, clinical trial in Parkinson’s disease. Movement Disorders. 2012;27:750–3. CrossRef
108. Teixeira FG, Gago MF, Marques P, Moreira PS, Magalhães R, Sousa N, et al. Safinamide: a new hope for Parkinson’s disease? Drug Discovery Today. 2018;23:736–44. CrossRef