INTRODUCTION
Wounds are very common and happen at any time to anyone daily. Wounds are the cuts or breaks in the continuity of tissues that are caused in different ways, such as physical, microbial, chemical, or by immunological insult in the soft tissues [1], and the condition is very prone to microbial contamination. Those injuries that were repaired by any other means are considered as wound healing. Though wound healing is an intricate method, the processes of healing of wounds are not equal for all people where skin repairs automatically after injury. The healing process mainly depends on various factors. If there are abnormalities in the immune system, any malnutrition condition, diabetic condition, any ischemia, or aging, in such cases healing of wounds is delayed. Mainly, three phases are followed in wound healing: initially, blood flow is increased, followed by increased capillary permeation near the affected area, which is an inflammatory stage; then decreased in epithelialization, and contraction of wounds, which is a proliferative stage; and finally the repair and strengthening of the soft tissues, i.e., the remodeling stage [2]. There are wide ranges of synthetic medicines available in the market for wound healing activity, but they have many side effects, mainly scar formation, allergic reactions, and so on. Therefore, an alternate source is required to cure the wounds with less or no side effects. With this view of that, herbal sources are reliable and safe natural medicines that cure many types of wounds without any toxicity or very minimal side effects [3]. Many known herbals already reported their wound-healing activity using human subjects and also in vivo animal experimentation [4,5].
Of late, in the present study, two Memecylon species, i.e., Memecylon lushingtonii (ML) and Memecylon malabaricum (MM) (F: Melastomataceae), were selected. There are more than 300 species available for the Memecylon genus, of which 40 species are available in India, and among them, 21 species are already endemics [6,7]. Some species are very important with respect to traditional uses and various pharmacological activities, viz. Memecylon umbellatum is used for snake bite treatment, skin, and stomach disorders, herpes, polyuria, dysentery, bacterial infection, chicken pox, and so on [8], MM is applied for controlling male sterility [9], Memecylon capitellatum is used for diabetic treatment [10], Memecylon angustifolium is used as a tonic and refrigerant, whereas ML has used in post citoal contraceptives [11]. Among the species, IUCN has reported that Memecylon lawsonii, ML, Memecylon royenii, M. umbellatum, Memecylon edule, and Memecylon flavescens are endangered species [12]. These activities were reported due to the presence of some important bioactive compounds, namely, M. umbelletum contains sitosterol, ursolic acid, and beta amyrin [13], Memecylon talbotianum contains flavonoid derivatives such as Kaempferol 3-O feruloylhexosyl rhamnoside, isorhamnetin-3-O-glycoside -7-O-glycoside, and synapoyl-hexose formic acid, etc. [14]. Related to the above-selected species, there are neither reports on any chemical constituents nor any reports on wound healing activity. Hence, based on the traditional applications, these two species, ML and MM (F: Melastomataceae), were selected. This was the first time we were reporting the wound healing activity based on the above three phases of wound repair with the proper scientific evidence.
MATERIALS AND METHODS
Collection and authentication of plant
Both ML and MM leaves (5 kg of fresh leaves from each plant) of the tree were collected from Horsley Hills station of Thirupathi during August 2023 (average temperature 25°C to 30°C) and authenticated by Dr K Madhava Chetty, Professor, Department of Botany, Sri Venkateswara University, Thirupathi (Voucher no: 0893 and 0591, respectively).
Extraction of leaves
Ethanol solvent was used for the extraction of the ML and MM leaves using a soxhlet apparatus. 500 g of leaves were used for extraction, and before extraction, the leaves were cleaned with running water, followed by 28 days of shade drying and coarsely grinding by a mixer grinder. Soxhlet extraction was carried out for 24 hours by maintaining an oven temperature of 40°C. The extracted liquid was dried using a rotary flash evaporator for 45 minutes at 40°C. Viscous liquid was collected and calculated for practical yield. Then, the extracts were collected separately and preserved in small glass bottles in refrigeration at 4°C.
Screening of phytochemicals
The above-stored extract of ML and MM leaves were further screened for the presence of various phytoconstituents by chemical tests [15,16]. Various tests were performed as per the standard methods, and routine analysis was carried out to know about the presence of a group of compounds.
Formulation of ointment
Three different formulations were prepared. The formulation was carried out with both individual plant extracts and one formulation with combined extracts of ML and MM. Paraffin wax was melted in a water bath, and in that 1.5 g each of lanolin, cetostearyl alcohol, and paraffin wax, and 24.5 g of petroleum jelly were mixed to make a homogenous mixture. To this mixture, 1 g of each ML and MM extracts were separately added, and two different ointments (30 g each) were made using mortar and pestle. Similarly, 1 g each of ML and MM extracts, together mixed with 23.5 g of the final ointment base, formed the ointment (30 g) using a mortar and pestle. Without extracts, only an ointment base was used for control preparation.
Animal experimentation
Initially, healthy rabbits (2 kg b.wt.) were selected for skin irritation tests, and a total of 30 Wistar albino rats (wt. 180–200 g) of either sex were used for wound healing efficacy. Before experimentation, all animals were housed in clean and dry cages, and rats were fed with rat pellets and water ad libitium. The rats were randomly divided into five groups for the wound healing model. The protocol was approved by the institutional animal ethics committee, Nirmala College of Pharmacy, Atmakur, Mangalagiri, AP (Approval No: IAEC/NCPA/2023-24/PhD/005). The room temperature was 25°C ± 3°C, humidity 45%–55%, and a light period of 12 hours was maintained. The whole experiment was conducted as per the guidelines of the Committee for the purpose of Control and Supervision of Experiments on Animals.
Skin irritation test
The rabbit’s fur was removed before 24 hours after the experimentation by trimming the dorsal region of their trunks on each side of the spinal cords with the sterile electric razor. Six rabbits were used for proper statistical analysis. In two rabbits, on one side, 0.5 g of ointment with ML extract was applied with gauze and tape, and on the other side, a control ointment was applied. In a similar manner, in another two rabbits, one side was ointment with MM extract and the other side with a control ointment, and in the other two rabbits, one side was treated with an ointment containing both ML and MM extracts, and the other side with a control ointment. The formulations were removed using sterile distilled water after the required 4 hours of exposure time. All the rabbits were observed for adverse reactions. After 1, 24, 48, and 72 hours, the skin was examined for any erythema, redness, and oedema formation, and the responses were recorded based on the criteria [17].
Excision wound formation
A total of 30 rats were divided into 5 groups, and in each group, 6 animals were kept. Animals were anesthetized by using 10% chloral hydrate solution [18]. Gr-I was treated with ointment base without the drug (placebo- negative control), Gr-II was treated with marketed formulation (Standard- Positive control); Gr-III was treated with an ointment containing ML extract; Gr-IV was treated with ointment containing MM extract; Gr-V was treated with ointment containing ML and MM extracts. After anesthesia, the dorsal fur of animals was removed; the anticipated area (~ 500 mm2) of wound to be created was outlined on the back of animals. A biopsy punch was used to remove a predefined amount of dorsal interscapular skin measured at 10 mm thickness and was left undressed in the open environment. All the grouped rats were separately kept in cages after the creation of the wound. The contraction, healing time, and epithelialization phases of the wound were monitored until the wound was fully healed, the ointments were applied daily and observed with the applied marketed ointment. The wound area was measured on the 0th, 4th, 8th, 12th, 16th, and 20th day for the progressive changes [19].
Histological analysis
Rat skin tissues were collected and preserved in 10% neutral buffered formalin for 24 hours at 4°C. Furthermore, skins were dehydrated with alcohol followed by xylene, and embedded in paraffin wax (58° mp). Around 8 µm of tissue section was de-paraffinized and further stained with hematoxylin and counterstained with eosine. Then finally mounted on a microscope [20]. Collagen density was measured by a scoring system using a high-magnified digital camera (400 X) and was measured by the local pathological lab in Madanapally, AP. The scoring system was carried out based on a score of 0 to 4 (low to high). “0” means no collagen formation, whereas score “4” indicates collagen formed properly with high density [21].
Statistical data analysis
Data were expressed as mean ± SD (standard deviation) for all the groups of animals. Graph Pad Prism 5 software was used for the determination of statistical data. Statistical analysis was made using one way analysis of variance followed by Tukey’s post hoc test. p < 0.05 was considered statistically significance. Furthermore, collage density was determined with SPSS software using Mann-Whitney analysis.
RESULTS AND DISCUSSION
The investigation on the percent yield for the ML and MM leaves was determined and resulted in 16.8 and 18.4 g yields, respectively. Therefore, the calculated percent yields were 3.36 and 3.68, respectively. Thereafter, various chemical tests were performed for the detection of various groups of chemicals. The tests resulted in the presence of glycosides, tannins, alkaloids, terpenoids, phenolics, flavonoids, and sterols from both the ML and MM extracts (Table 1). The result may be due to the use of solvent ethanol. Ethanol is the best suitable solvent for any extraction due to its high dielectric constant and non-toxic nature so maximum bioactive compounds were dissolved in the ethanol solvent [22,23].
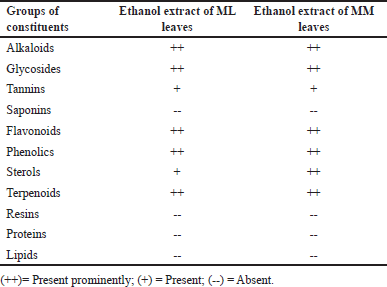 | Table 1. Various phytochemical tests for ML and MM leaves extract. [Click here to view] |
A skin irritation test was performed using rabbits as per OECD 404 guidelines for acute dermal toxicity study. The study showed no signs of erythema, skin irritation, or redness at the end of the study period of 72 hours (Fig. 1). The score of the acute dermal irritation study was tabulated in Table 2. Finally, the result concluded that all the sole ML, MM, and combined extracts of ML and MM ointment were safe and ready to perform for therapeutic efficacy as wound healing activity on rats.
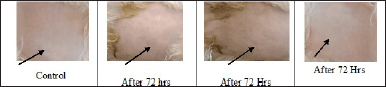 | Figure 1. Skin irritation test for all the three formulated ointment in rabbits skin. [Click here to view] |
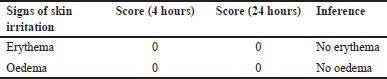 | Table 2. Sign of erythema and oedema. [Click here to view] |
Wound healing study
The wound contraction is depicted in Table 3. The result showed that after the 20th day, wounds were totally decreased for all the ointments, but interestingly it was observed that the ointment with the combined extract showed a satisfactory effect in minimized wound area (3.14 ± 9.34*** mm2) (p < 0.001) which was effective than the marketed sample (6.39 ± 3.18*** mm2) (p < 0.001, when compared with the control group). There was no such decrease in wounds in the control group (97.72 ± 8.51 mm2).
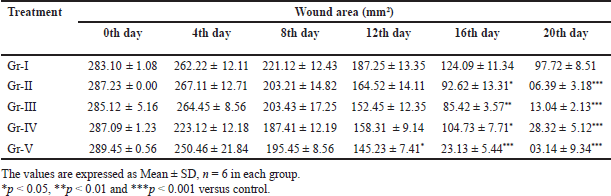 | Table 3. Contraction of wound area with ointment prepared by ML, MM and combined ML and MM extracts. [Click here to view] |
Figure 2 revealed the decreased period of epithelialization with the ointment containing the combined extract. Even though there was also a decreased period of epithelialization that occurred for the ointment prepared by sole extracts than the control sample (21 days). The marketed sample also decreased the same (15 days) but was less effective than the ointment of combined extracts of MM and ML (13 days).
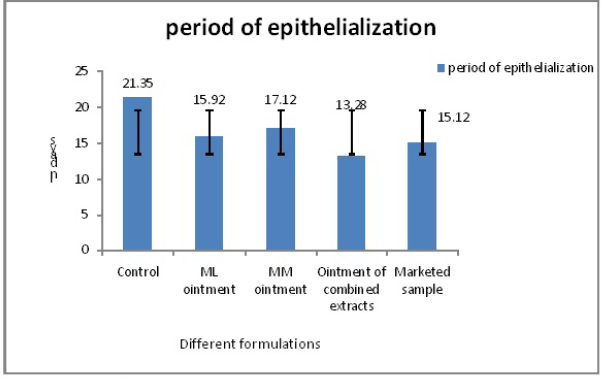 | Figure 2. Period of epithelialization for all the ointments with marketed sample. [Click here to view] |
Thereafter, the percent wound closure was also evaluated. It also showed a similar trend as the earlier analysis. It resulted that the percentage of wound closure increased with increased days. The marketed sample showed 96.13% wound closure after 20 days, whereas the ointment containing both the extracts ML and MM showed better results (98.26%) than the control as even the marketed sample. Even ointment containing a single extract showed better results than the control sample. ML ointment showed 96.16% and MM ointment showed 94.76%, which were more than the control sample after 20 days of study (Fig. 3).
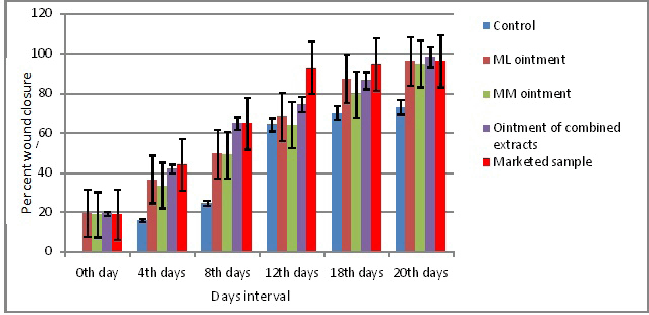 | Figure 3. Wound healing activity of herbal ointments in days interval. [Click here to view] |
Figure 4, showed after 20 days, ointment containing ML and MM extracts cured the wound, whereas the market ointment sample showed little wound area.
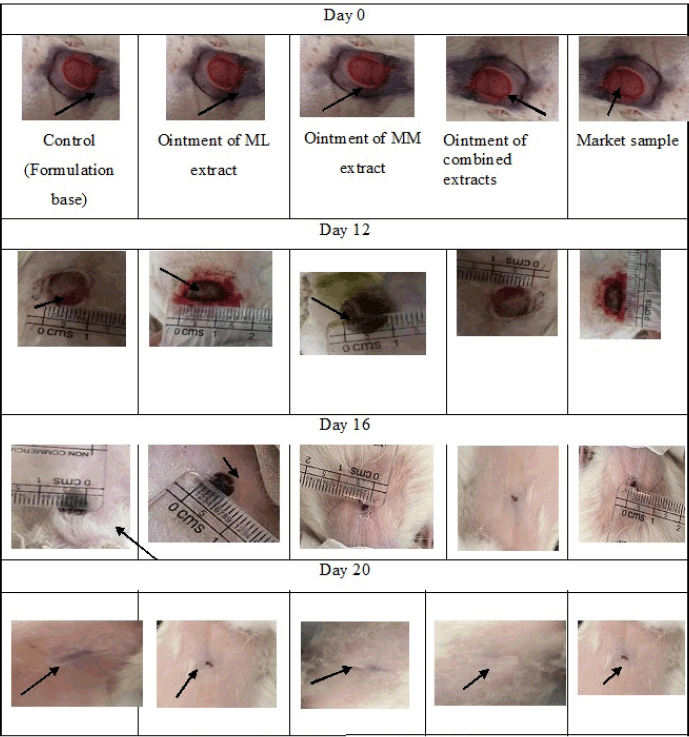 | Figure 4. Wound area contraction with days. [Click here to view] |
The above Figure 5 shows the histopathology of rat skin with magnification X 200 µm. In all the figures, collagen fibers showed pink color, cytoplasm stained with purple color, blood vessels stained with cherry red, whereas nuclei stained with blue. Wound healing mainly depends upon the synthesis of collagen, which is present in the extracellular matrix and provides strength [2]. Synthesis of collagen was seen more in the case of ointment containing ML and MM extracts than marketed ointment. It was concluded that the score of Group –V, showed 4.23 ± 1.24 after 20 days (n = 3) than the control group (2.0 ± 0.0), and the increased rate of contraction of wounds in herbal ointments ML and MM extracts treated animals might be due to increase in proliferation and transformation of fibroblast to myofibroblast. After 20 days of study, the combined treatment ointment group showed dense collagen formation that may be due to the presence of quercetin and luteolin in the ML and MM leaves [24] and responsible for the formation of collagen by tissue damage inhibition [25,26]. In the present study, herbal extracts showed the presence of many bioactive compounds through chemical tests. Earlier literature reported that wound contraction may be due to the presence of flavonoids and phytosterols, which are responsible for the increased synthesis of collagen due to the release of cytokines [27,28]. These constituents also play an immense role in homeostasis and epithelialization in the later stages of wound healing [29] and hence after 20 days of study, complete wound healing was observed with the ointment containing ML and MM extract. The possible mechanism of the isolated flavonoids (quercetin and luteolin) may be due to positively regulating matrix metalloproteinase-2 enzyme activity and also the Ras/Raf/MEK/ERK, PI3K/Akt and NO pathways to reduce inflammation and heal wounds [30]. The activity was also higher than that of marketed ointment which may be due to the synergistic action of the bioactive constituents present in both the extracts.
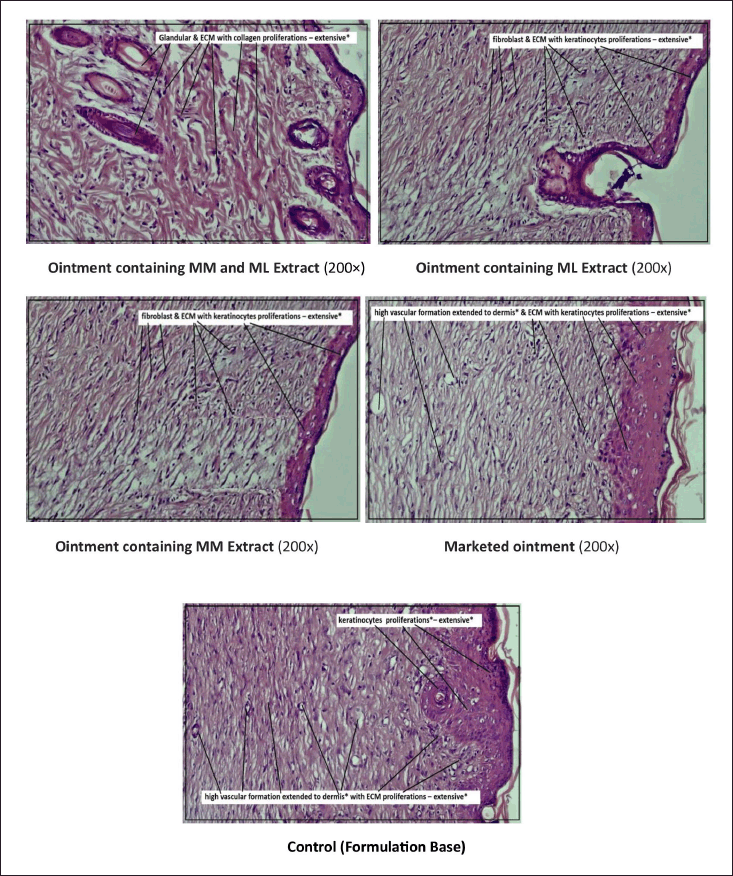 | Figure 5. Histopathology of rats skin for all the treated rats by different ointments (After 20 days). [Click here to view] |
CONCLUSION
In conclusion, herbal ointment containing ML and MM extracts and also ointment containing combined ML and MM extracts showed potential wound healing activity. The study was compared with the marketed ointment and resulted in combined extracts. Formulated ointment was more efficient and gave synergistic action with respect to increased wound contraction, improved collagen synthesis, and higher percent of wound closure than control as well as marketed samples. The result was maybe due to the presence of combined flavonoids, viz., quercetin and luteolin, in both the plant leaf extracts that support the synthesis of collagen faster in the extracts than others with the mechanism of formation of collagen by repairing the damaged tissue. This is the first time we have reported wound-healing activity of Memecylon species, especially for ML and MM. Furthermore, the isolation of phytoconstituents, followed by the exact mechanism of action, is needed to establish the wound healing activity.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The protocol was approved by the institutional animal ethics committee, Nirmala College of Pharmacy, Atmakur, Mangalagiri, Andhra Pradesh (Approval No: IAEC/ NCPA/2023-24/PhD/005).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. 1.Bhimani B, Prajapati C, Sabale P, Sabalea V. An overview of medicinal plants as wound healers. J Appl Pharm Sci. 2012;2(11):143–50.
2. Gunasekaran S, Nayagam AAJ, Natarajan R. Wound healing potentials of herbal ointment containing Calendula officinalis Linn. on the alteration of immunological markers and biochemical parameters in excision wounded animals. Clin Phytosci. 2020;6:77. CrossRef
3. Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014;4:177. CrossRef
4. Das K. Wound healing potential of aqueous crude extract of Stevia rebaudiana in mice. Rev Bras Farmacogn. 2013;23(2):351–57. CrossRef
5. Sharma A, Khanna S, Kaur G, Singh I. Medicinal plants and their components for wound healing applications. Futur J Pharm Sci. 2021;7:53. CrossRef
6. Viswanathan MB, Manikandan U. A new species, Memecylon mundanthuraianum, of melastomataceae from India. Nord J Bot. 2001;21:259–62.
7. Murugan C, Gopalan R. Four new additions to Indian Memecylon L. (Melastomataceae) from South India. Indian J Forestry. 2006;29:105–8.
8. Kshirsagar RD, Singh NP. Some less known ethnomedicinal uses from Mysore and Coorg districts, Karnataka state, India. J Ethnopharmacol. 2001;75:231–8.
9. Reddy MV. Wildlife biodiversity conservation: Proceedings of the “National Seminar on Wildlife Biodiversity Conservation”, 13 to 15 October 2006, a Seminar Conducted During the “bi-decennial celebrations” of Pondicherry University title endemic medicinal plants used by tribal people in Tirunelveli hills the Western Ghats; 2008.
10. Kottaimuthu R, Vasudevan N, Saravanan A. On the occurrence of Memecylon capitellatum (Memecylaceae) in India. Taprobanica. 2015;7:114–7.
11. Kirtikar KR, Basu BD. Indian medicinal plants. Dehra Dun, India: Bishen Singh Mahendra Pal Singh; 1918. Vol. 2.
12. Sivu AR, Narayanan MKR, Pradeed NS, Kumar SEK, Pandurangan AG. A new species of Memecylon (Melastomataceae) from the Western Ghats, India. Phytotaxa. 2014;162:44–50.
13. Joshi H, Joshi AB, Sati H, Gururaja MP, Shetty PR, Subrahmanyam EVS, et al. Fatty acids from Memecylon umbellatum (Burm.). Asian J Res Chem. 2009;2:178–80.
14. Bharathi TR, Prakash HS. UPLC-ESI-MS-based metabolite analysis of Memecylon talbotianum Brandis. and its potential bioactive paper presented in Metabolomics Conference IISC Bangalore, Bangalore; 2015.
15. Dubale S, Kebebe D, Zeynudin A, Abdissa N, Suleman S. Phytochemical screening and antimicrobial activity evaluation of selected medicinal plants in Ethiopia. J Exp Pharmacol. 2023;15:51–62. CrossRef
16. Das K, Iyer KR, Orfali R, Asdaq SMB, Alotaibi NS, Alotaibi FS, et al. In silico studies and evaluation of in vitro antidiabetic activity of berberine from ethanol seed extract of Coscinium fenestratum (Gaertn.) Colebr. J King Saud Univ Sci. 2023;35(5):102666.
17. Das K, Dang R, Machale MU. Formulation and evaluation of a novel herbal gel of stevia extract. Iran J Dermatol. 2009;12(4):117–22.
18. Al-Nadaf AH, Awadallah A, Thiab S. Superior rat wound-healing activity of green synthesized silver nanoparticles from acetonitrile extract of Juglans regia L: Pellicle and leaves. Heliyon. 2024;10(2):e24473. CrossRef
19. Ganesan S, Parasuraman S, Maheswaran SU, Gnanasekar N. Wound healing activity of Hemidesmus indicus formulation. J Pharmacol Pharmacother. 2012;3(1):66–7.
20. Sasidharan S, Logeswaran S, Latha LY. Wound healing activity of Elaeis guineensis leaf extract ointment. Int J Mol Sci. 2012;13(1):336–47. CrossRef
21. Hakim RF, Fakhrurrazi F, Andini YR. Effects of broccoli extract (Brassica oleracea var. Italica) on collagen images in wound on collagen fibers density in wound healing process. Padj J Dent. 2023;35(2):147–52.
22. Das K, Dang R, Sivaraman G, Ellath RP. Phytochemical screening for various secondary metabolites, antioxidant, and anthelmintic activity of Coscinium fenestratum fruit pulp: a new biosource for novel drug discovery. Turk J Pharm Sci. 2018;15(2):156–65. CrossRef
23. Das K, Singirikonda S. Elemental impact on antibacterial study of hydroalcoholic leaves extract of Belosynapsis vivipara. Not Sci Biol. 2023;15(1):11409. CrossRef
24. Ratna Manjula R, Thirumal M. Phytochemical screening, isolation and characterization of bioactive compounds from ethanol extract of Memecylon lushingtonii Gamble. Asian J Chem. 2024;36(12):2967–71.
25. Polerà N, Badolato M, Perri F, Carullo G, Aiello F. Quercetin and its natural sources in wound healing management. Curr Med Chem. 2018;25:1–22.
26. Chen LY, Cheng HL, Kuan YH, Liang TJ, Chao YY, Lin HC. Therapeutic potential of luteolin on impaired wound healing in streptozotocin-induced rats. Biomedicines. 2021;9:761.
27. Agnel Arul John N, Shobana G, Keerthana K. Wound healing efficacy of herbal ointment containing Oldenlandia herbacea Roxb. on excision wounded animals. Int Res J Pharm. 2018;9(8):95–9.
28. Muley BP, Khadabadi SS, Banarase NB. Phytochemical constituents and pharmacological activities of Calendula officinalis L. (Asteraceae): a review. Trop J Pharm Res. 2009;8:455–65.
29. Landsman A, Taft D, Riemer K. The role of collagen bioscaffolds, foamed collagen, and living skin equivalents in wound healing. Clin Podiatr Med Surg. 2009;26:525–33.
30. Carvalho MTB, Araújo-Filho HG, Barreto AS, Quintans-Júnior LJ, Quintans JSS, Barreto RSS. Wound healing properties of flavonoids: a systematic review highlighting the mechanisms of action. Phytomedicine. 2021;90:153636. CrossRef