INTRODUCTION
Alzheimer’s disease (AD) is a neurological condition that causes cognitive deficits to progress to the point where a person is unable to perform daily activities. It is the most common form of dementia, accounting for 60%–70% of all dementias [1]. The prevalence of AD is increasing rapidly and is projected to reach 16 million individuals by the year 2050 [2]. Dementia is expected to affect up to 24 million people worldwide, and its incidence is expected to rise every 20 years until at least 2040 [3]. The prevalence of AD increases exponentially with age, particularly after 65 years [3]. The burden of AD is significant, with a prediction for worsening trends in the United States and other countries [4]. Of the about 55 million people worldwide with dementia, 60%–70% are estimated to have AD [1]. The prevalence of dementia in adults 60 years of age and older was estimated to be 3.9% worldwide, with regional prevalences of 1.6% in Africa, 4.0% in China and the Western Pacific, 4.6% in Europe, and 8.0% in North America [1,5]. According to the Alzheimer’s Association, about 6.5 million adults 65 years of age and older have AD In the United States. Of these, almost 70% are 75 years and older [5].
Early diagnosis of AD is crucial for several reasons, including providing timely support and care, reducing the financial burden on healthcare systems, and possibly decreasing the progression of the disease [6]. The diagnosis rate for AD remains low, and there is an urgent need to improve diagnosis rates so that those at greatest risk can be identified [6]. The urgency of AD is also underscored by the growing body of research on risk factors and the need for effective prevention strategies [7,8]. Besides, there are currently only two fully approved non-modifying-disease therapies for AD, including N-methyl d-aspartate receptor antagonists and acetylcholinesterase inhibitors [9]. The FDA granted partial approval for Aducanumab and Lecanemab, two monoclonal antibodies that target amyloid [10–12]. However, these regiments have been questioned for their efficacy because of the significant risks of amyloid-related imaging abnormalities, such as hemorrhage or edema [13,14]. Furthermore, this class of drugs has no direct curative effect in AD [15].
Alzheimer’s is caused by neuronal death, which covers a large area of the central nervous system and is stimulated by the plaques formed by the deposition of amyloid-β (Aβ) peptides [16]. Insoluble Aβ fibrils are produced as a result of modified cleavage of the amyloid precursor protein (APP) by β- and γ-secretases, which then Insoluble Aβ fibrils oligomerize and interfere with synaptic signaling, contributing to neurodegeneration [17,18]. Additionally, the deposition of Aβ in the brain and the presence of NFTs lead to the gradual loss of synapses and impair mitochondrial function, cognition, and intracellular neurofibrillary tangles memory [17,19]. Other factors such as insulin resistance, oxidative stress, impaired energy metabolism, and the pathophysiology of AD are also linked to the activation of the inflammasome complex [17]. AD pathogenesis is characterized by a complex system of molecular and cellular mechanisms and involves multiple interconnected pathways, making it a challenging area for research and the development of effective treatments [20].
Additionally, it has been discovered that extracellular-vesicles (EVs) in the secretome contribute to the pathophysiology of AD, with EVs inducing pro-inflammatory effects in mixed cortical cultures [21]. The chemical composition of mesenchymal stem cell (MSC)-derived secretome, stem cell-derived exosomes, and EVs includes a variety of bioactive molecules that contribute to their therapeutic potency against AD. The MSC-derived secretome comprises soluble factors such as cytokines, chemokines, growth factors (e.g., VEGF, NGF, and BDNF), and extracellular matrix proteins, which collectively promote neuroprotection, neuroregeneration, and immunomodulation [22,23]. Stem cell-derived exosomes, a subtype of EVs, are enriched with proteins (e.g., tetraspanins and heat shock proteins), lipids (e.g., sphingomyelin and cholesterol), and nucleic acids (e.g., miRNA and mRNA) that facilitate intercellular communication and modulate inflammatory responses, oxidative stress, and Aβ aggregation [24–27]. These exosomes can cross the blood-brain barrier (BBB), delivering their cargo directly to neural cells, thereby reducing neuroinflammation, enhancing neurogenesis, and improving cognitive functions in AD models. Overall, the combined action of these bioactive components in MSC-derived secretomes and exosomes makes them potent candidates for AD therapy by targeting multiple pathological mechanisms simultaneously [28,29]. Previous studies of AD animal models have shown that the secretome derived from MSCs could reduce the amount of amyloid plaque and reactive gliosis, as well as increase hippocampal and cortical neuronal density, indicating potential positive effects on AD pathology [30,31].
Preclinical studies and clinical trials have highlighted the safety, disease-specific therapeutic potential, and neuroprotective effects of MSC secretome for AD treatment [22,32]. However, there are challenges and limitations, including the need to understand the impact of bioengineering advances, the development of large-scale good manufacturing protocol (GMP) secretome-based products, and the optimization of secretome-based therapy for clinical use [33]. Despite these challenges, secretome-based therapy shows promise as a potential treatment for AD, as evidenced by preclinical studies and ongoing clinical trials. Thus, this study aimed to determine information gaps comprehensively by evaluating the preclinical and clinical data for secretome-based therapy, including exosome and microvesicles in AD.
METHODS
Study design
This systematic review was carried out based on the Systematic Review Protocol for Animal Intervention Studies (SYRCLE). The systematic review protocol has been registered in PROSPERO (ID: CRD42024498742). We carried out a thorough search of academic databases, including Scopus, PubMed, ScienceDirect, and the Cochrane Library. Keywords generated from free texts and medical subject headings were combined in the search strategy (Table 1). We also searched by previous references of related review articles.
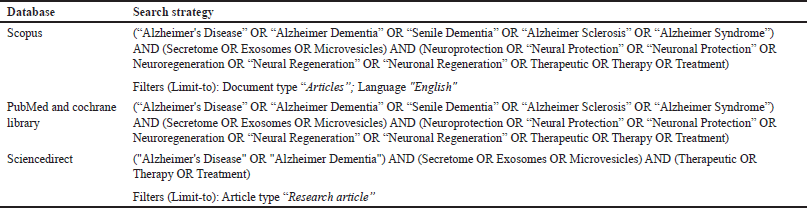 | Table 1. The search strategy for eligible articles. [Click here to view] |
Eligibility criteria
The inclusion criteria of this study were in vivo and clinical studies focused on stem cell-based therapy through the secretome, exosomes, and microvesicles. We also restricted the article language to English. Incompatible results were excluded and we also did not include review, case, or editorial studies.
Study selection
The results of the search were exported to rayyan.ai. After removing duplicate studies, the articles were examined by the titles and abstracts. Full-text of records were retrieved and screened based on eligibility criteria. The articles were independently reviewed by two reviewers and a third reviewer was used in any disagreements.
Quality assessment
The SYRCLE risk of bias (RoB) tool was used to measure the quality of the pre-clinical studies and Cochrane RoB 2.0 was used for clinical studies. Critical judgment was conducted by two reviewers and a third party was included if there were any disagreements. Traffic-light plot graphs were used to display the results of the RoB assessment, demonstrating whether risks were low, high, or unclear.
Data extraction and analysis
Data extraction was independently performed by three reviewers using predefined sheets that included the following information: general information regarding the authors, study design, subject’s characteristic data, and outcomes related to the efficacy and safety of secretome-based therapy in AD. We included outcome measures of immunological assays (Immunocytochemical analysis, Multielectrode array recording, Real-time polymerase chain reaction, Western blot, Immunohistochemistry, Immunofluorescence analysis, and so on), electron microscopy analysis, and behavior analysis. The data were analyzed qualitatively.
RESULTS
Study selection
A systematic search was conducted from 4 databases resulting 660 records. After the removal of duplicated articles, we screened 465 articles for eligible title and abstract, and 421 articles were excluded. From 44 articles, a total of 36 full-text retrieved articles were assessed for eligibility criteria. Finally, we included 21 in vivo studies, with details of 4 secretome studies, 13 study of exosomes studies, and 4 micro-vesicles studies (Fig. 1). In addition, we included 2 clinical studies, which mentioned from the eligible articles.
RoB assessment
The quality of the included studies was evaluated using the RoB methodology for animal research created by SYRCLE [34]. Ten criteria were evaluated to determine whether the RoB was low, high (if information was lacking), or unclear (if not enough information was available). A set of ten criteria were assessed to ascertain if the RoB was low (if details met the criteria), high (if information was lacking), or unclear (if not enough information was available). Based on the criteria assessment, there were a significant risk for randomization of sequence generation, housing, and outcome assessment with studies percentages of 71%, 76%, and 52%, respectively. Furthermore, the blinding of housing and outcome were lack of reporting, with percentages of 90%–71%, respectively. However, other criteria were at low RoB (Fig. 2).For clinical studies, Cochrane RoB 2.0 was used by measuring five domains and performed as per the prescribed algorithm [35]. We found that both studies have a high risk for the randomization process as they were open-labeled studies. RoB for the other four domains was low (Fig. 3).
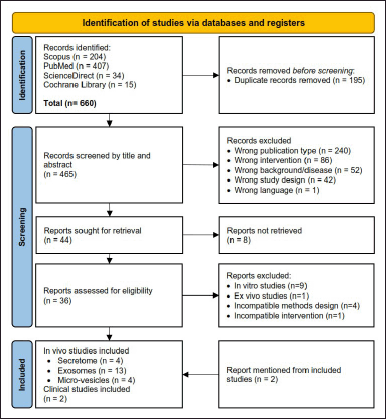 | Figure 1. PRISMA flowchart diagram. [Click here to view] |
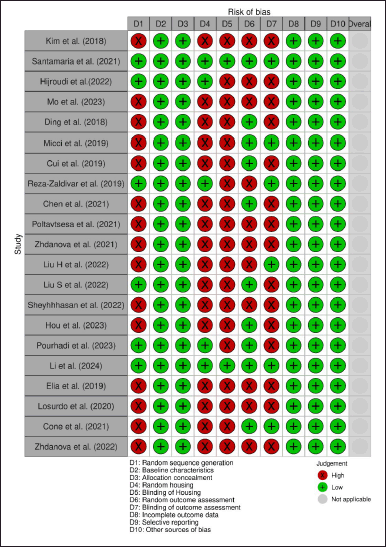 | Figure 2. Result of SYRCLE RoB assessment. [Click here to view] |
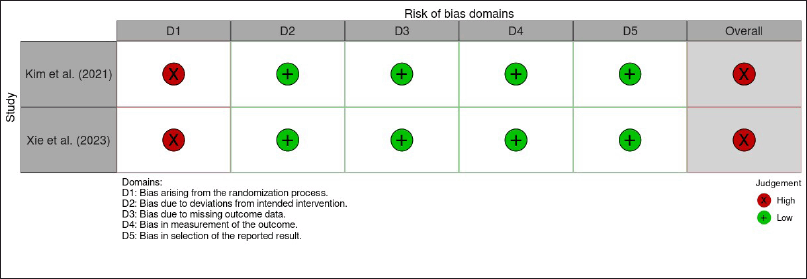 | Figure 3. Result of cochrane RoB 2.0. assessment. [Click here to view] |
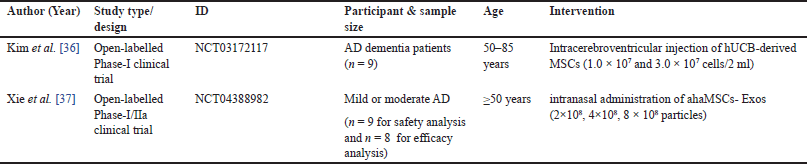 | Table 2. Characteristics of clinical trials. [Click here to view] |
Characteristics of animal studies
This systematic review included studies varying from 2018 to 2024. Study characteristics of in vivo and clinical studies were described in Table S1. Based on the results, different animal models were used in eligible studies, which were APP/PS1mice (n = 5), 5XFAD mice (n = 4), NMRI mice (n = 3), C57BL/6 mice with induction (n = 3), Wistar rats (n = 2), BALB/c mice (n = 1), triple-transgenic AD mice (n = 1), APP/PS1/SIRT1 CKO mice (n = 1), Nestin-δ-HSV-TK mice (n = 1), and J20 mouse model of AD (n = 1). The ages of animal models were ranged from 4 weeks to 22 months old. Male animal model was more often to be used (n = 12) than female (n = 2) or both sexes (n = 2). Induction was performed in non-genetical engineered animal using streptozotocin (n = 3) and Aβ aggregates or Aβ1–42 (n = 2).
Characteristics of clinical trials
From 21 included articles, two of which referred for further clinical trials as shown in Table 2. Both studies were designed as open-label, phase I/IIa clinical trial. Each study required AD patients, which were 9 patients, with age above 50 years old. A study by Kim et al. [36] performed intervention for three patients by low dose (1.0 × 107 cells/2 ml), and six patients by high dose (3.0 × 107 cells/2 ml) of human umbilical cord blood (hUCB)-derived MSCs. For all patients, three consecutive MSC injections were given at 4-week intervals. After the first hUCB-MSC injection, these patients were monitored for up to 12 weeks. In the extended observation phase, they were monitored for an additional 36 months.Click or tap here to enter text. However, a study by Xie et al. [37] used nasal spray devices to deliver allogenic human adipose-derived MSCs exosomes (ahaMSCs-Exos) (2 × 108, 4 × 108, and 8 × 108 particles) dissolved in saline (1 ml) twice a week for a total of 24 sessions [37]. Furthermore, the 3+3 design was applied in the three groups, which were split into low, medium, and high interventional dose groups.Click or tap here to enter text.
Data analysis of in vivo study results
Table S2 provides results summary from in vivo studies related to secretome-based therapies for AD. We include information on various parameters and outcomes assessed in these studies, such as cognitive performance, spatial memory, therapeutic efficacy, and brain region-specific effects. The results present data from a total of 21 in vivo studies, including 4 secretome studies, 13 exosomes studies, and 4 micro-vesicles studies. The studies varied in terms of the type of secretome-based therapy used, the administration route, and the specific outcomes measured. Some of the key findings reported in the table include improved spatial memory, enhanced cognitive performance, regulation of neuronal and astrocytic activity, and prevention of spatial memory deterioration.The study of secretome-based therapy explored the therapeutic potential of various types of secretomes derived from MSCs, neural stem cells (NSCs), and induced pluripotent stem cells in animal models of AD. These studies demonstrated promising results in mitigating AD symptoms and pathology through different mechanisms. For instance, Kim et al. [38] reported that hUCB-MSCs could rescue synaptic density loss induced by Aβ42 peptide in vivo, highlighting the protective effect of hUCB-MSCs against synaptic dysfunction mediated by thrombospondin-1 (TSP-1).Click or tap here to enter text. Santamaria et al. [39] observed memory recovery and a reduction in amyloid plaques in APP/PS1 mice following a single intravenous injection of MSC-derived conditioned serum (MSC-CS), suggesting that MSC-CS mimics the neuroreparative effects of MSCs through paracrine action.Click or tap here to enter text. Hijroudi et al. [40] found that NSCs conditioned medium (NSCs-CM) improved memory retention and reduced Aβ plaque formation in AD mice, indicating that NSCs-CM supports neuronal survival and function.Click or tap here to enter text. Finally, Mo et al. [41] showed that intranasal delivery of iPSC-derived central nervous system cells secretome (CNSC-SE) improved deficits in cognitive function and spatial memory in 5xFAD mice, with CNSC-SE promoting cortical neuron differentiation and reducing amyloidosis. These studies collectively underscore the potential of secretome-based therapies in addressing various aspects of AD pathology, including synaptic dysfunction, amyloidosis, neuroinflammation, and cognitive decline, through multiple mechanisms of action.In the study that described exosomes-based therapy, there were various tests and assays used to evaluate the efficacy of the exosome treatments, such as the Morris Water Maze test for learning and memory, immunostaining for cellular and molecular analysis, and Western Blot and ELISA for protein quantification. Most of the exosomes-studies highlighted decreased Aβ plaque deposition, enhancements of cognitive function, and changes in neuronal and synaptic markers that indicates neuroregeneration or synaptic plasticity [24,42–53]. In terms of action mechanism, these studies showed insights into how these exosome-based therapies might be working at a molecular level. Exosomes have been shown to modulate microglial activation states, reduce inflammation, and decrease levels of Aβ, which are implicated in AD pathology. Exosomes-based therapies also promoted neurogenesis, enhanced mitochondrial biogenesis, and activate signaling pathways like SIRT1-PGC1α, which are beneficial for neuronal health and function. Thus, exosome-based therapies have potential therapeutic actions in AD models, with effects on learning and memory, synaptic plasticity, and neuroinflammation.However, the study of EVs-based therapy derived from MSCs also demonstrated to be therapeutic agents for AD. The studies detailed in the table explore the potential benefits of administering MSC-derived EVs to animal models of AD. For instance, Elia et al. [16] found that administering bone marrow-derived MSC-EVs (BM-MSC-EVs) to APP/PS1 mice reduced AD pathology, including Aβ plaque area and dystrophic neurites. The proposed mechanism is that these EVs carry neprilysin, an enzyme that degrades Aβ, and inherit anti-inflammatory and neurotrophic properties from their parental BM-MSCs.Click or tap here to enter text. Similarly, Losurdo et al. [54] observed a decrease in microglia activation and an increase in the hippocampal dendritic spine density and other brain regions after treating mice with MSC-derived EVs, suggesting immunomodulatory and neuroprotective effects.Click or tap here to enter text. Cone et al. [55] reported improved cognitive performance and reduced Aβ plaque load in EV-treated mice, with the EVs exhibiting immunoprotective and immunomodulatory abilities and the capacity to cross the BBB.Click or tap here to enter text. Finally, Zhdanova et al. [56] demonstrated that intranasally administered vesicles could reach the hippocampus and neocortex, acting as nanocontainers for targeted delivery of compounds to brain regions affected by neurodegeneration.Click or tap here to enter text. These findings collectively suggested that MSC-derived EVs could be a promising Alzheimer’s therapeutic approach, offering benefits such as Aβ plaque reduction, cognitive performance improvement, and neuroprotection. These studies also highlight the potential mechanisms by which these effects are achieved, including direct Aβ degradation, immunomodulation, and the facilitation of cell-to-cell communication across the BBB.Overall, the included studies demonstrated that treatments involving secretomes, exosomes, and EVs from different cell sources can significantly improve cognitive functions, reduce amyloid plaque deposition, and modulate neuroinflammation in AD animal models. The mechanisms of action involved modulation of signaling pathways related to neuroprotection, neurogenesis, synaptic plasticity, and reduction of Aβ levels. Intranasal and intravenous administrations were also noted for their effectiveness while transporting these therapeutic agents to the brain, demonstrating the potential of secretomes-based therapies as promising strategies for AD management.
Safety and efficacy of MSCs for AD based on clinical studies
Table 3 showed a detailed comparison of the safety and efficacy of MSCs in treating AD, based on two distinct studies. The study by Kim et al. [36] reported several adverse events including fever in 9 participants, headache in 7, nausea in 5, and vomiting in 4, all of which subsided within 36 hours. Furthermore, two participants experienced three severe adverse events that were thought to be related to the experimental drug. Nevertheless, no dose-limiting toxicities were seen. Interestingly, five individuals finished a 36-month extended observation trial without experiencing any more severe side effects, indicating a long-term safety profile. However, the efficacy data for this study was not reported (N/R) [36]. Besides, the study by Xie et al. [37] in 2023 demonstrated a more promising outlook in terms of both safety and efficacy. No adverse events were reported, indicating the safety and tolerability of the treatment. The AD Assessment Scale-Cognitive section (ADAS-cog) scores in the medium-dose group showed improvements in cognitive function, with a 2.33-point reduction from the baseline, showing a decrease in cognitive deterioration. This suggested that the treatment was effective. Furthermore, there was a 2.38-point rise in the baseline Montreal Cognitive Assessment (MoCA-B) basic version scores, indicating improved cognitive function. The continuous improvement in ADAS-cog scores by 3.98 points until week 36 further supported the sustained cognitive benefits of the treatment. Despite the fact that the three dose groups did not significantly differ in terms of changed amyloid or tau deposition, the medium-dose arm exhibited less neurodegeneration, indicating potential neuroprotective effects[37].
DISCUSSION
Mesenchymal stromal cell-derived secretomes, which includes the use of exosomes and EVs, represents a novel and promising approach in the treatment of AD, an progressive neurodegenerative condition marked by memory loss and cognitive impairment [57]. We offer a comprehensive evaluation of the possible benefits of secretome-based therapies for AD. We highlight that secretome-based therapies, which include a complex mixture of proteins, nucleic acids, and lipids secreted by cells, have shown promise in targeting multiple disease pathways, promoting neuroprotection, and regeneration based on animal studies. Furthermore, a recent clinical trial suggested a reduction of cognitive decline and sustained cognitive benefits.Click or tap here to enter text.Secretome-based therapies, particularly those derived from MSCs, exert their effects through paracrine mechanisms that can modulate the microenvironment of the CNS. These therapies have been shown to promote neurogenesis, reduce oxidative stress, alleviate cognitive impairment, and increase the number of neuroblasts in the hippocampus region, which are crucial for memory and learning [22]. The MSC-derived secretome contains a variety of bioactive molecules (nerve growth factor and brain-derived neurotrophic factor), which are vital for neuronal survival and function [22,58,59]. However, stem cell-derived exosomes have also been found to reduce the load of Aβ plaque formation, inhibit neuronal death, and promote neurogenesis, thereby potentially ameliorating the cognitive deficits associated with AD [45,60]. Additionally, they might change the pro-inflammatory to anti-inflammatory phenotypes of microglia, which contributed reduce neuroinflammation as a key component of AD pathology [60]. Additionally, extracellular vesicles, a key component of the secretome, can mediate the propagation of tau aggregation and decrease Aβ plaques, addressing two major pathological hallmarks of AD [58,61]. Overall, included preclinical studies of this systematic review showed improvements in cognitive functions, reduced amyloid plaque deposition, and modulated neuroinflammation. Intranasal and intravenous administrations have been effective in delivering these therapeutic agents to the brain.Clinical trials have shown the safety and long-term safety profile of MSC secretome-based therapies, with some adverse events subsiding within 36 hours in intracerebroventricular injection [36,37]. But, clinical trial by Xie et al. [37] revealed no signficant changes in the accumulation of tau or amyloid among different dosage arms, although the medium-dose arm showed less hippocampal volume shrinkage, hinting at neuroprotection.Click or tap here to enter text. These two clinical trials were utilized MSCs derived therapy through intranasal and intracerebroventricular administrations. The results demonstrated that intranasal administration may provide lower adverse effects of the therapy. Compared to other developing therapies like gene therapy and small molecule drugs, secretome-based therapies can address multiple AD pathology aspects simultaneously. Due to their nanoscale size, these therapies are considered to have a higher safety profile, potentially offering a cell-free therapy option that could circumvent the risks related to the direct transplantation of cells, such as immune rejection and tumor formation [62]. In this systematic review, several challenges were addressed to bridge the gap between animal studies and clinical trials effectively. Differences in disease pathology between animal models and humans, as well as the need for well-designed clinical trials to assess therapeutic outcomes accurately, are critical challenges that need to be resolved [63]. A deeper understanding of how MSCs and their secretome exert their effects is crucial for optimizing therapeutic strategies and identifying biomarkers for treatment efficacy. Producing MSC-derived secretome, exosomes, and EVs in quantities sufficient for clinical trials while ensuring batch-to-batch consistency is challenging. Standardization of production methods, isolation, characterization, dosage, and route of administration is crucial for translating preclinical success into clinical settings [62]. The use of stem cells and their derivatives also faces regulatory and ethical scrutiny, varying significantly across countries. Ensuring compliance with regulatory requirements is essential for advancing these therapies from the laboratory to the clinic [64]. However, these challenges require extensive clinical validation to establish safety, efficacy, and practicality as an AD treatment option.This systematic review also acknowledged several limitations, including the high RoB in randomization process and outcome assessment in animal studies, as well as the randomization process in clinical studies due to their open-label design. We also highlighted the paucity of existing clinical evidence for secretome-based therapies in AD and the challenges in translating preclinical findings into clinical applications, such as the need for bioengineering advances and the development of large-scale GMP products. It may be difficult to synthesize data and reach strong conclusions regarding the safety and efficacy of secretome-based therapeutics due to the heterogeneity in study designs and results among the included studies. Further research and development are needed to address current challenges and advance these therapies towards clinical application.
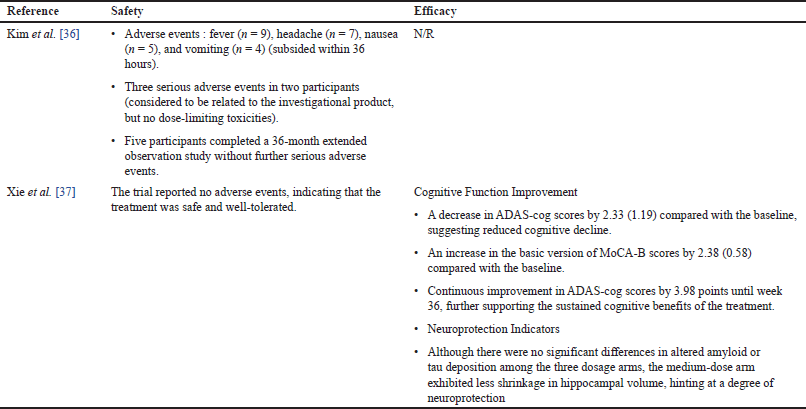 | Table 3. Safety and efficacy of MSC for AD. [Click here to view] |
CONCLUSION
Secretome-based therapies represent a promising frontier in the treatment of AD, which also involving exosomes and extracellular vesicles, in reducing amyloid plaque load, reactive gliosis, and enhancing neuronal density. These outcomes suggest mechanisms of action in neuroprotection, neuroregeneration, and inflammation modulation, which are critical in AD pathology. This therapeutical approach faced several challenges in translating preclinical findings into clinical settings, including the need for large-scale production, optimization of protocols, understanding biomarkers, and addressing the heterogeneity in administration methods. Despite these challenges, we highlighted that secretome-based therapies hold significant promise for AD treatment, emphasizing the need for further research and development to address the identified gaps and limitations.
ACKNOWLEDGMENTS
The authors would like to express their gratitude to the reviewers who contributed their time and expertise to the peer review process of this systematic review.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Not applicable.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci. 2009;11(2):111–28.
2. Lanctôt KL, Hahn-Pedersen JH, Eichinger CS, Freeman C, Clark A, Tarazona LRS, et al. Burden of Illness in People with Alzheimer’s disease: a systematic review of epidemiology, comorbidities and mortality. J Prev Alzheimer’s Dis. 2024;11(1):97–107.
3. Mayeux R, Stern Y. Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med. 2012 Aug;2(8):a006239.
4. Dharmarajan TS, Gunturu SG. Alzheimer’s disease: a healthcare burden of epidemic proportion. Am Health Drug Benefits. 2009 Jan;2(1):39–47.
5. Alzheimers Dement. 2023;19(4):1598–695. doi: 10.1002/alz.13016
6. Rasmussen J, Langerman H. Alzheimer’s Disease—why we need early diagnosis. Degener Neurol Neuromuscul Dis. 2019;9:123–30.
7. Passeri E, Elkhoury K, Morsink M, Broersen K, Linder M, Tamayol A, et al. Alzheimer’s disease: treatment strategies and their limitations. Int J Mol Sci. 2022;23:13954.
8. Hickman RA, Faustin A, Wisniewski T. Alzheimer disease and its growing epidemic: risk factors, biomarkers, and the urgent need for therapeutics. Neurol Clin. 2016 Nov;34(4):941–53.
9. Zemek F, Drtinova L, Nepovimova E, Sepsova V, Korabecny J, Klimes J, et al. Outcomes of Alzheimer’s disease therapy with acetylcholinesterase inhibitors and memantine. Expert Opin Drug Saf. 2014;13(6):759–74.
10. Hoy SM. Lecanemab: first approval. Drugs. 2023;83(4):359–65.
11. van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023 Jan;388(1):9–21.
12. Daly T, Epelbaum S. The accelerated approval of Aducanumab invites a rethink of the current model of drug development for Alzheimer’s disease. AJOB Neurosci. 2023;14(3):332–5.
13. Jeong SY, Suh CH, Shim WH, Lim JS, Lee JH, Kim SJ. Incidence of amyloid-related imaging abnormalities in patients with Alzheimer disease treated with anti-β-amyloid immunotherapy: a meta-analysis. Neurology. 2022 Nov;99(19):e2092–101.
14. Villain N, Planche V, Levy R. High-clearance anti-amyloid immunotherapies in Alzheimer’s disease. Part 1: Meta-analysis and review of efficacy and safety data, and medico-economical aspects. Rev Neurol (Paris). 2022 Dec;178(10):1011–30.
15. Reiss AB, Muhieddine D, Jacob B, Mesbah M, Pinkhasov A, Gomolin IH, et al. Alzheimer’s disease treatment: the search for a breakthrough. Medicina. 2023;59:1084.
16. Elia CA, Losurdo M, Malosio ML, Coco S. Extracellular vesicles from mesenchymal stem cells exert pleiotropic effects on amyloid-β, inflammation, and regeneration: a spark of hope for alzheimer’s disease from tiny structures? Bioessays. 2019 Apr;41(4):e1800199.
17. Rostagno AA. Pathogenesis of Alzheimer’s disease. Int J Mol Sci. 2022;24(1):107.
18. Tiwari S, Atluri V, Kaushik A, Yndart A, Nair M. Alzheimer’s disease: pathogenesis, diagnostics, and therapeutics. Int J Nanomedicine. 2019;14:5541–54.
19. Fan L, Mao C, Hu X, Zhang S, Yang Z, Hu Z, et al. New insights into the pathogenesis of Alzheimer’s disease [Internet]. Front Neurol. 2020;10:1312. Available from: https://www.frontiersin.org/articles/10.3389/fneur.2019.01312
20. Guo T, Zhang D, Zeng Y, Huang TY, Xu H, Zhao Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol Neurodegener [Internet]. 2020;15(1):40. CrossRef
21. Kuo CC, Chiang AWT, Baghdassarian HM, Lewis NE. Dysregulation of the secretory pathway connects Alzheimer’s disease genetics to aggregate formation. Cell Syst [Internet]. 2021;12(9):873–84.e4. Available from: https://www.sciencedirect.com/science/article/pii/S2405471221002088
22. Ghasemi M, Roshandel E, Mohammadian M, Farhadihosseinabadi B, Akbarzadehlaleh P, Shamsasenjan K. Mesenchymal stromal cell-derived secretome-based therapy for neurodegenerative diseases: overview of clinical trials. Stem Cell Res Ther [Internet]. 2023;14(1):1–20. CrossRef
23. M?zes G, Sipos F. Mesenchymal stem cell-derived secretome: a potential therapeutic option for autoimmune and immune-mediated inflammatory diseases. Cells. 2022 Jul;11(15):2300.
24. Reza-Zaldivar EE, Hernández-Sapiéns MA, Gutiérrez-Mercado YK, Sandoval-Ávila S, Gomez-Pinedo U, Márquez-Aguirre AL, et al. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer’s disease. Neural Regen Res. 2019;14(9):1626–34.
25. Tan F, Li X, Wang Z, Li J, Shahzad K, Zheng J. Clinical applications of stem cell-derived exosomes. Signal Transduct Target Ther [Internet]. 2024;9(1):17. CrossRef
26. Hade MD, Suire CN, Suo Z. Mesenchymal stem cell-derived exosomes: applications in regenerative medicine. Cells. 2021 Aug;10(8):1959.
27. Ghadami S, Dellinger K. The lipid composition of extracellular vesicles: applications in diagnostics and therapeutic delivery [Internet]. Front Mol Biosci. 2023;10:1198044. Available from: https://www.frontiersin.org/articles/10.3389/fmolb.2023.1198044
28. Khan MI, Jeong ES, Khan MZ, Shin JH, Kim JD. Stem cells-derived exosomes alleviate neurodegeneration and Alzheimer’s pathogenesis by ameliorating neuroinflamation, and regulating the associated molecular pathways. Sci Rep [Internet]. 2023;13(1):15731. CrossRef
29. Guo M, Yin Z, Chen F, Lei P. Mesenchymal stem cell-derived exosome: a promising alternative in the therapy of Alzheimer’s disease. Alzheimers Res Ther [Internet]. 2020;12(1):109. CrossRef
30. Hu J, Wang X. Alzheimer’s disease: from pathogenesis to mesenchymal stem cell therapy—bridging the missing link. Front Cell Neurosci. 2021;15:811852.
31. Tüshaus J, Müller SA, Kataka ES, Zaucha J, Sebastian Monasor L, Su M, et al. An optimized quantitative proteomics method establishes the cell type-resolved mouse brain secretome. EMBO J. 2020 Oct;39(20):e105693.
32. Giovannelli L, Bari E, Jommi C, Tartara F, Armocida D, Garbossa D, et al. Mesenchymal stem cell secretome and extracellular vesicles for neurodegenerative diseases: risk-benefit profile and next steps for the market access. Bioact Mater [Internet]. 2023;29(June):16–35. CrossRef
33. Teixeira FG, Salgado AJ. Mesenchymal stem cells secretome: current trends and future challenges. Neural Regen Res [Internet]. 2020;15(1):75–7. Available from: https://journals.lww.com/nrronline/fulltext/2020/15010/mesenchymal_stem_cells_secretome__current_trends.22.aspx
34. Hooijmans CR, Rovers MM, De Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14(1):1–9.
35. Higgins JP, Savovi? J, Page MJ, Sterne JAC. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2) [Internet]. 2019. p. 1–24. Available from: https://www.riskofbias.info/welcome/rob-2-0-tool/current-version-of-rob-2
36. Kim HJ, Cho KR, Jang H, Lee NK, Jung YH, Kim JP, et al. Intracerebroventricular injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer ’ s disease dementia?: a phase I clinical trial. Alzheimers Res Ther. 2021;13(154):1–11.
37. Xie X, Song Q, Dai C, Cui S, Tang R, Li S, et al. Clinical safety and efficacy of allogenic human adipose mesenchymal stromal cells-derived exosomes in patients with mild to moderate Alzheimer’s disease: a phase I/II clinical trial. Gen Psychiatr. 2023;36(5):1–11.
38. Kim DH, Lim H, Lee D, Choi SJ, Oh W, Yang YS, et al. Thrombospondin-1 secreted by human umbilical cord blood-derived mesenchymal stem cells rescues neurons from synaptic dysfunction in Alzheimer’s disease model. Sci Rep. 2018;8(1):1–13.
39. Santamaria G, Brandi E, Vitola P La, Grandi F, Ferrara G, Pischiutta F, et al. Intranasal delivery of mesenchymal stem cell secretome repairs the brain of Alzheimer’s mice. Cell Death Differ [Internet]. 2021;28(1):203–18. CrossRef
40. Hijroudi F, Rahbarghazi R, Sadigh?Eteghad S, Bahlakeh G, Hassanpour M, Shimia M, et al. Neural stem cells secretome increased neurogenesis and behavioral performance and the activation of Wnt-β-Catenin signaling pathway in mouse model of Alzheimer’s disease. Neuromolecular Med. 2022;24:424–36.
41. Mo H, Kim J, Kim JY, Kim JW, Han H, Choi SH, et al. Intranasal administration of induced pluripotent stem cell-derived cortical neural stem cell-secretome as a treatment option for Alzheimer’s disease. Transl Neurodegener [Internet]. 2023;12(1):1–19. CrossRef
42. Ding M, Shen Y, Wang P, Xie Z, Xu S, Zhu ZY, et al. Exosomes isolated from human umbilical cord mesenchymal stem cells alleviate neuroinflammation and reduce amyloid-beta deposition by modulating microglial activation in Alzheimer’s disease. Neurochem Res [Internet]. 2018;43(11):2165–77. CrossRef
43. Micci MA, Krishnan B, Bishop E, Zhang WR, Guptarak J, Grant A, et al. Hippocampal stem cells promotes synaptic resistance to the dysfunctional impact of amyloid beta oligomers via secreted exosomes. Mol Neurodegener. 2019;14(1):1–22.
44. Cui GH, Guo HD, Li H, Zhai Y, Gong Z Bin, Wu J, et al. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease. Immunity and Ageing. 2019;16(1):1–12.
45. Chen YA, Lu CH, Ke CC, Chiu SJ, Jeng FS, Chang CW, et al. Mesenchymal stem cell-derived exosomes ameliorate alzheimer’s disease pathology and improve cognitive deficits. Biomedicines. 2021;9(6):1–19.
46. Zhdanova DY, Poltavtseva RA, Svirshchevskaya EV, Bobkova NV. Effect of intranasal administration of multipotent mesenchymal stromal cell exosomes on memory of mice in Alzheimer’s disease model. Bull Exp Biol Med. 2021;170(4):575–82.
47. Poltavtseva RA, Bobkova NV, Zhdanova DY, Svirshchevskaya EV, Sukhikh GT. Alzheimer’s type neurodegeneration. Possible correction of memory impairment with intravenous administration of exosomes. Biochem (Mosc) Suppl Ser A Membr Cell Biol. 2021;15(4):306–18.
48. Liu S, Fan M, Xu JX, Yang LJ, Qi CC, Xia QR, et al. Exosomes derived from bone-marrow mesenchymal stem cells alleviate cognitive decline in AD-like mice by improving BDNF-related neuropathology. J Neuroinflammation [Internet]. 2022;19(1):1–20. CrossRef
49. Liu H, Jin M, Ji M, Zhang W, Liu A, Wang T. Hypoxic pretreatment of adipose-derived stem cell exosomes improved cognition by delivery of circ-Epc1 and shifting microglial M1/M2 polarization in an Alzheimer’s disease mice model. Aging. 2022;14(7):3070–83.
50. Sheykhhasan M, Amini R, Soleimani Asl S, Saidijam M, Hashemi SM, Najafi R. Neuroprotective effects of coenzyme Q10-loaded exosomes obtained from adipose-derived stem cells in a rat model of Alzheimer’s disease. Biomedicine and Pharmacotherapy [Internet]. 2022;152(May):113224. CrossRef
51. Hou X, Jiang H, Liu T, Yan J, Zhang F, Zhang X, et al. Depletion of gut microbiota resistance in 5×FAD mice enhances the therapeutic effect of mesenchymal stem cell-derived exosomes. Biomed Pharmacother. 2023;161(May):1–22.
52. Pourhadi M, Zali H, Ghasemi R, Faizi M, Mojab F, Soufi Zomorrod M. Restoring synaptic function: how intranasal delivery of 3D-cultured hUSSC exosomes improve learning and memory deficits in Alzheimer’s disease. Mol Neurobiol [Internet]. 2023;(0123456789). CrossRef
53. Li B, Chen Y, Zhou Y, Feng X, Gu G, Han S, et al. Neural stem cell-derived exosomes promote mitochondrial biogenesis and restore abnormal protein distribution in a mouse model of Alzheimer’s disease. Neural Regen Res. 2024;19(7):1593–601.
54. Losurdo M, Pedrazzoli M, D’Agostino C, Elia CA, Massenzio F, Lonati E, et al. Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer’s disease. Stem Cells Transl Med. 2020;9(9):1068–84.
55. Cone AS, Yuan X, Sun L, Duke LC, Vreones MP, Carrier AN, et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate Alzheimer’s disease-like phenotypes in a preclinical mouse model. Theranostics. 2021;11(17):8129–42.
56. Zhdanova D, Gomzikova M, Bobkova N, Starostina I, Kovalev V, Rizvanov A. Intranasal administration of microvesicles in the brain of mice with induced model of Alzheimer’s type of neurodegeneration. Bionanoscience [Internet]. 2022;12(2):685–92. CrossRef
57. Pinho AG, Cibrão JR, Silva NA, Monteiro S, Salgado AJ. Cell secretome: basic insights and therapeutic opportunities for CNS disorders. Pharmaceuticals (Basel). 2020 Feb;13(2):31.
58. Garcia-Contreras M, Thakor AS. Extracellular vesicles in Alzheimer’s disease: from pathology to therapeutic approaches. Neural Regen Res. 2023 Jan;18(1):18–22.
59. Hernández AE, García E. Mesenchymal stem cell therapy for Alzheimer’s disease. Stem Cells Int. 2021;2021:7834421.
60. Wang H, Huber CC, Li XP. Mesenchymal and neural stem cell-derived exosomes in treating Alzheimer’s disease. Bioengineering (Basel). 2023 Feb;10(2):253.
61. Polanco JC, Scicluna BJ, Hill AF, Götz J. Extracellular vesicles isolated from the brains of rTg4510 mice seed tau protein aggregation in a threshold-dependent manner. J Biol Chem. 2016 Jun;291(24):12445–66.
62. Lotfy A, AboQuella NM, Wang H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res Ther. 2023 Apr;14(1):66.
63. Si Z, Wang X. Stem cell therapies in Alzheimer’s disease: applications for disease modeling. J Pharmacol Exp Ther. 2021 May;377(2):207–17.
64. Gemayel J, Chaker D, El Hachem G, Mhanna M, Salemeh R, Hanna C, et al. Mesenchymal stem cells-derived secretome and extracellular vesicles: perspective and challenges in cancer therapy and clinical applications. Clin Transl Oncol. 2023 Jul;25(7):2056–68.
SUPPLEMENTARY MATERIAL
The supplementary material can be accessed at the journal's website: [https://japsonline.com/admin/php/uploadss/4396_pdf.pdf].