INTRODUCTION
Originally derived from Alpinia galanga, acetoxychavicol acetate (ACA) (Fig. 1a) has recently drawn much interest as a bioactive constituent [1,2]. Historically, A. galanga has been utilized for the treatment of various diseases, including digestive disorders, such as indigestion and bloating; respiratory conditions, including cough and asthma; and infections, owing to its antibacterial and antifungal properties [3]. Moreover, it has attracted interest due to its prospective anticancer properties, particularly due to its active component, ACA, which has demonstrated cytotoxicity against multiple cancer cell lines [4]. Additional therapeutic applications encompass its anti-inflammatory, antioxidant, and neuroprotective properties, making it pertinent for ailments such as arthritis, metabolic disorders, and neurodegenerative diseases [1,3,5]. Alpinia galanga, especially for ACA, has been recognized for its multifaceted pharmacological activities, supporting its use in traditional medicine, particularly in Southeast Asian herbal treatments [3,6,7]. Following the advancement of science and technology, ACA has been effectively isolated, purified, and isolated for future investigation into its bioactive properties, including its anticancer, anti-inflammatory, and antimicrobial effects [5,8]. In a wide range of scientific fields, ACA has been the subject of substantial research because of its possible anticancer properties, as well as its function in modifying immunological responses [9]. Multiple studies have emphasized the anti-inflammatory [10], antioxidant [11], and antibacterial [12,13] properties of this compound. Recent studies have demonstrated that the antimicrobial substance ACA targets the cell membrane, reducing its stability and initiating bacterial defense mechanisms by altering the expression of crucial membrane proteins [13]. These molecular mechanisms not only support the use of ACA as a naturally preserving food but also show a promising interest in the development of novel therapies and health supplements.
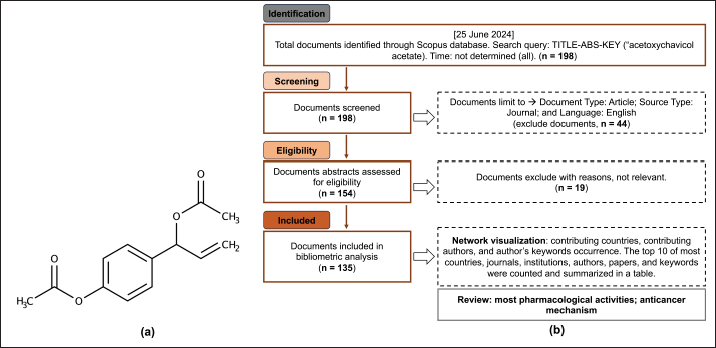 | Figure 1. Chemical structure of ACA (a) and study flowchart (b). [Click here to view] |
Multiple studies have extensively examined the anticancer properties of ACA, revealing potent cytotoxicity against various human cancer cell lines, including breast cancer (4T1, T47D, MDA-MB-231, and MCF-7), cervical cancer (HeLa), colon cancer (WiDr), hepatocellular carcinoma (HepG2), lung cancer (A549), and prostate cancer (PC-3) [8,14]. ACA has been shown to induce apoptosis, a controlled signaling pathway of cell death that is critical for inhibiting cancer cell proliferation in HeLa and T47D cells [15]. Acknowledged for its anticancer properties, ACA exhibits immunomodulatory effects [9]. Furthermore, the antioxidant properties of ACA are crucial for its wide range of pharmacological benefits, including anti-inflammatory, antidiabetic, neuroprotective, and gastroprotective properties [1]. The unsaturated double bond and acetyl groups of ACA play significant roles in activating the AMP-activated protein kinase (AMPK) pathway, highlighting its pivotal role in signal transduction and mitigating the aforementioned diseases [16]. Ongoing research into the mechanisms of action and potential applications of ACA continues to highlight its therapeutic potential, positioning it as a significant player in the field of natural products and pharmacology.
There is still a lack of bibliometric studies on the ACA despite the growing research interest and several published review articles on this topic. Using the extensive Scopus database, this study methodically examines the current state of ACA research, including publishing trends, collaboration networks, citation patterns, topic areas, and future directions. This study aimed to provide evidence-based approaches to extend our understanding of the use of ACA in medicine and other disciplines by elucidating the related research dynamics.
METHODS
Search strategy
A comprehensive literature search was conducted, and data were obtained from Scopus on August 1, 2024. The search phrase “ACA” yielded a total of 198 articles. Using a screening procedure that restricted the selection to relevant articles based on the following criteria: Document Type: Article; Language: English; and Source Type: Journal, we obtained a total of 135 documents (Fig. 1) [17–19].
Data analysis
Citation analyses and author keywords were conducted using VOSviewer v.1.6.18 [20]. To make the VOSviewer analysis more accurate, we cleaned the data using a thesaurus file that combined many different forms of the word ACA, including A. galanga, A. galanga (l.) Willd., and A. galangal. A scoping review of articles related to ACA was conducted in detail.
RESULTS
Trends in publishing articles on ACA
The Scopus database yielded 135 articles published between 1976 and 2024 (Fig. 2). Each year’s document number was a single digit, with the highest number of documents published in a single year being 9. The number of documents experienced fluctuations from 1996 to 2023, culminating in 2017 at its highest level. We accessed the Scopus database in the middle of the year (August), which partially accounts for the limited number of documents obtained in 2024. We used a number of important bibliometric indicators, such as impact factor (IF), H-index, and citation analysis, to identify the most important papers and authors in ACA research. These metrics are necessary to understand the landscape of ACA research, identify the best journals and authors, and determine how certain studies will affect future research.
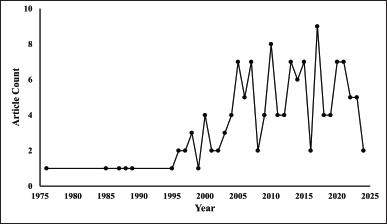 | Figure 2. Publication trends by the years of “ACA” publications (n = 135). [Click here to view] |
Planta Medica had the most publications, with the most recent being in 2022 (Table 1) [4]. However, the Journal of Ethnopharmacology had the highest average number of citations (AC = 83.33) [21], followed by the Chemical and Pharmaceutical Bulletin (AC = 61.75) [22] and the Japanese Journal of Cancer Research (AC = 59.50) [23]. The Journal of Agricultural and Food Chemistry had the highest IF (Table 1). Since the IF measures the average citations per article in a journal, it indicates the Journal of Agricultural and Food Chemistry’s overall influence within the ACA’s research. The 135 articles were published from 38 different countries, with Japan (n = 71), Malaysia (n = 22), the United States (n = 20), India (n = 17), Thailand (n = 16), China (n = 8), Indonesia (n = 4), Saudi Arabia (n = 4), South Korea (n = 4), and the United Kingdom (n = 3) having the top 10 publications (Table 2). Nonetheless, the United Kingdom placed the first rank in terms of citations per article published, followed by Japan and the United States. Table 3 shows the top 10 institutions by publication count, with Japan (Kyoto University, Kinday University, Osaka Metropolitan University, Kyoto Pharmaceutical University, and Gifu University) and Malaysia (University Malaya, Institute of Biological Sciences, and Universiti Sains Malaysia) dominating. Gifu University published the most cited paper (AC = 69.83) in 2009, focusing on research on the genetic connection of Thai galangal using Random Amplified Polymorphic DNA [24]. Houghton et al.’s [25] 2007 paper, which investigated the anticancer action of ACA, placed first among the top 10 most referenced publications, with antimicrobial and anti-allergy subjects close behind (Table 4). Conversely, Matsuda et al. [26], affiliated with Kyoto Pharmaceutical University in Japan, emerged as the most prolific author, as evidenced by an H-index of 89, consistent with Japan’s status as the most productive country. The H-index is a metric that indicates both the productivity and citation influence of a researcher or institution, offering a comprehensive evaluation of research-based contributions [19]. We also studied the average citation count for each characteristic to illustrate the impact and distribution of certain papers (Tables 1–3) while also revealing major patterns in ACA’s research into influential works. The results showed that ACA possesses a range of pharmacological properties and has been extensively studied, with a specific focus on its anticancer properties.
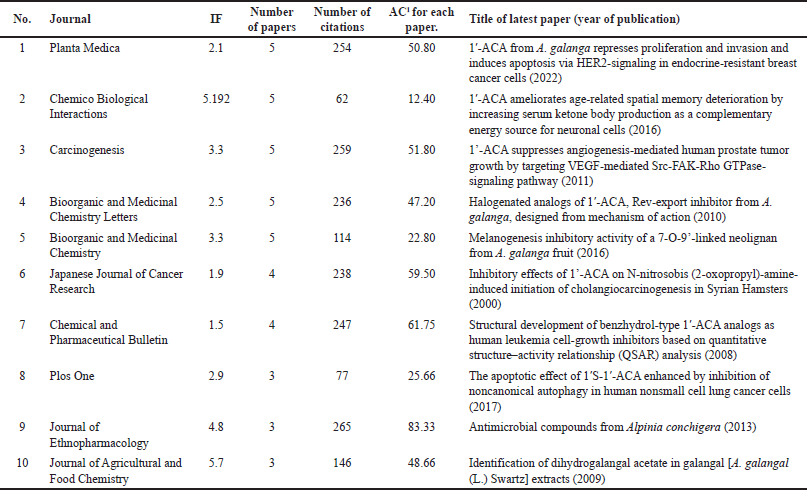 | Table 1. The top 10 journals with the most articles. [Click here to view] |
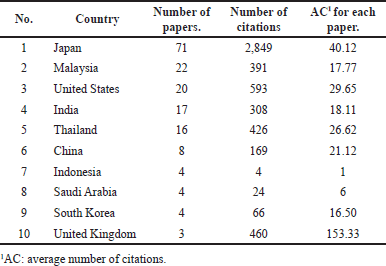 | Table 2. Top 10 countries with the highest number of publications in Scopus journals. [Click here to view] |
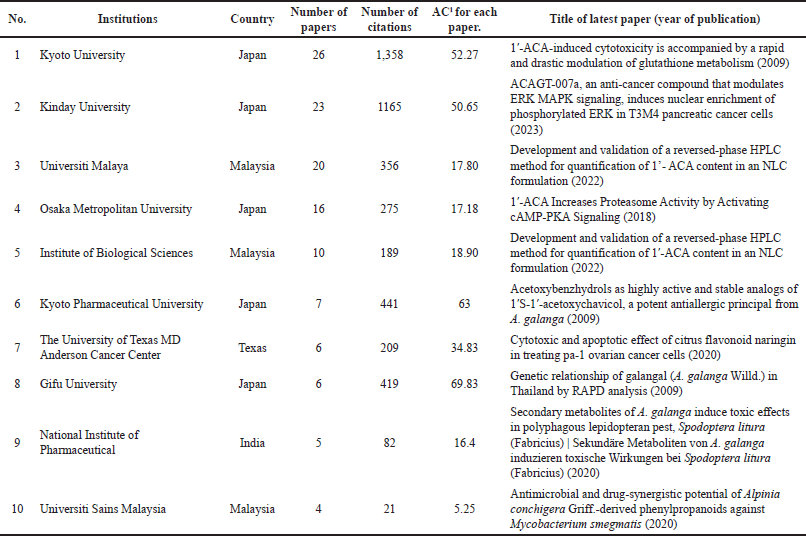 | Table 3. The top 10 institutions with the most articles. [Click here to view] |
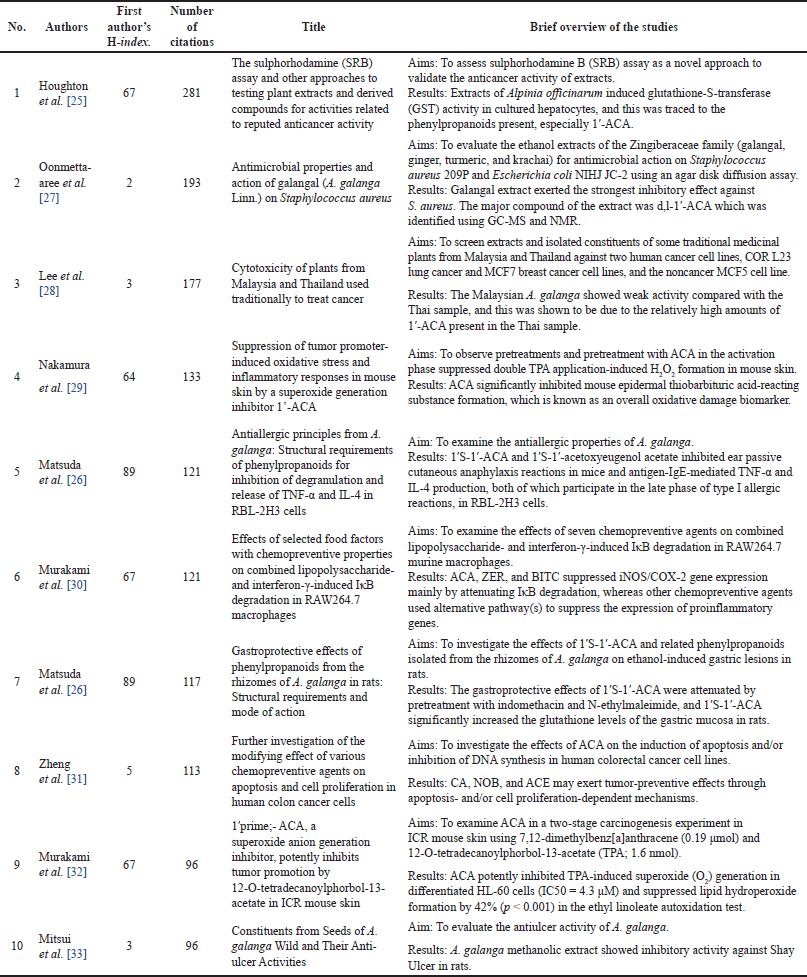 | Table 4. Top 10 most-cited articles. [Click here to view] |
Co-occurrence of author’s keyword related to ACA publications
Graphical representation co-occurrence keyword analysis is a precise tool that provides a valuable understanding of the most common subjects in publications within a certain study landscape, as well as fluctuations in their frequencies over time [8]. Figure 3 used size coding to represent the frequency of node recurrence, and the lines connecting these nodes indicated their co-occurrence within the same publication. The more frequently two keywords appeared together, the smaller the gap between the two distinct nodes. The analysis revealed a total of 294 items, 35 clusters, 1,051 linkages, and 1,205 overall link strengths. The data presented in Table 5 revealed three keywords: ACA (n = 73), A. galanga (n = 33), and apoptosis (n = 13). Furthermore, it is important to emphasize the pharmacological properties of ACA, including its anticancer, antibacterial, anti-TB, and antidiabetic effects. Moreover, the researchers focused on specific subjects from 2015 to 2020 (Fig. 3b). The purple nodes in the graph represent earlier terms, whereas the yellow nodes represent more current keywords. Keywords, including apoptosis, antimicrobial, cervical cancer, NF-κB, and breast cancer, occurred with greater frequency between 2015 and 2020. Figure 4 highlights the relationships between ACAs and various cancer types. ACAs serve as the central node in this network, underscoring their pivotal role in the analyzed studies. Visualization reveals direct associations of 61 connections between ACAs and different cancer types with varying connection strengths. Strong links, indicated by thick edges, suggest high research intensity or strong evidence of ACA’s anticancer effects. Notable cancer types with significant connections include breast, prostate, and cervical cancers, which are likely driven by ACA’s known mechanisms, such as apoptosis induction, cell cycle arrest, and pathway inhibition [e.g., NF-κB and vascular endothelial growth factor (VEGF)]. Moderate connections, such as those with lung and colon cancers, highlight areas for further exploration. This visualization reflects key research trends, demonstrating that ACA has garnered the most attention in breast and prostate cancers, where its cytotoxic and antiangiogenic effects have been well-documented. These findings emphasize the need for continued exploration of the underexplored applications of ACA, particularly in cancers with limited data. Researchers have also identified an emerging keyword associated with compound limitations and formulations as well as the underlying anticancer mechanisms. The documented change in the simultaneous presence of the author’s keywords over time probably indicates the changing patterns and research focus in ACA.
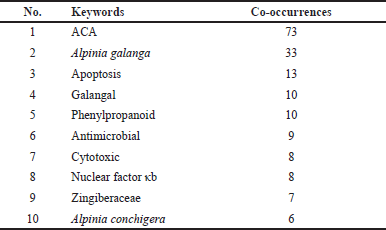 | Table 5. Top 10 authors’ keywords with the most co-occurrences. [Click here to view] |
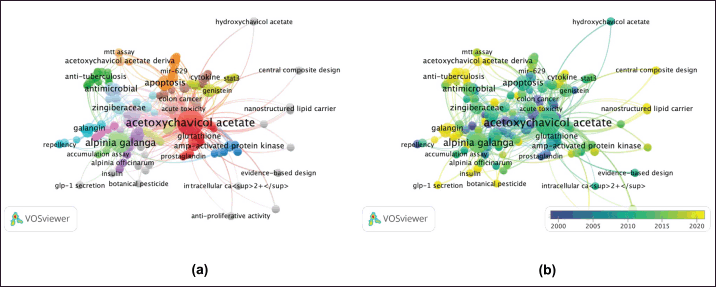 | Figure 3. Network visualization (a) and overlay visualization (b) of authors’ keywords co-occurrence of “ACA” publications (n = 135). [Click here to view] |
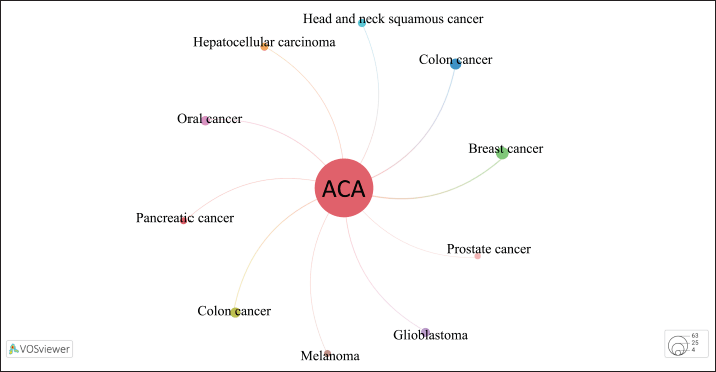 | Figure 4. Visualization of cancer-type associations in ACA studies. [Click here to view] |
DISCUSSION
Within the field of anticancer research, the bibliometric study of 1’-ACA indicates a number of significant discoveries and developing trends. Publications have increased significantly in recent years, indicating a rising interest in natural chemicals as potential anticancer agents. However, one limitation is that our bibliometric analysis relies solely on the Scopus database, which may miss relevant literature from non-English sources or less prominent journals, and the analysis only reflects trends up to August 2024, which means that ongoing research could change these trends. This analysis only included original research articles to maintain the comprehensiveness of the research that has been conducted and to map the research pattern. Acknowledging these limitations is crucial for gaining a comprehensive understanding of the dynamics of ACA research.
The study of ACA’s mechanisms of action is increasingly interdisciplinary, integrating insights from pharmacology, molecular biology, and phytochemistry. There is a growing emphasis on the potential of ACA to target critical molecular pathways, including apoptosis, metastasis, and AMPK signaling, which are essential for cancer progression and treatment. Preclinical research is increasingly using computer modeling, with emphasis on the use of in silico methods to predict the therapeutic potential of ACA and optimize its use. In addition, recent research has investigated the synergistic effects of ACAs when used in conjunction with conventional chemotherapy, which has the potential to improve treatment efficacy and minimize adverse effects.
The relevance of ethnomedicine in the process of influencing modern scientific inquiry is demonstrated by the fact that a significant amount of research emanates from locations where A. galanga, the source of ACA, is traditionally used locally. Furthermore, the increasing interest in ACAs, as evidenced by patents and agreements with pharmaceutical firms, suggests their potential for development into innovative cancer therapeutics. Despite these advancements, obstacles, such as limited clinical trials and gaps in translational research, persist, indicating the need for more studies to establish ACA’s safety and effectiveness in people. Overall, these results show that ACA research is expanding and that natural product-based cancer therapies are becoming increasingly popular. They also provide a solid foundation for future research that aims to improve ACA’s therapeutic role in oncology.
Most of the studied ACA pharmacological activities
The pharmacological activities of ACA among 135 articles were multifaceted, of which the top 10 included anticancer, antimicrobial, anti-allergy, anti-tuberculosis, anti-inflammation, anti-diabetic, antifungal, gastroprotective, antioxidant, and neuroprotective (Fig. 5). The most prominent research on ACA’s anticancer activity of ACA appeared in the Scopus database. Antioxidants are among the most extensively influential antioxidants, and they reveal several mutually supportive pharmacological activities. As an antioxidant, ACA inhibits xanthine oxidase, an enzyme involved in purine metabolism that generates reactive oxygen species (ROS) as a by-product, thereby reducing oxidative stress and enhancing its anti-inflammatory, anticancer, antimicrobial, antidiabetic, gastroprotective, and neuroprotective activities [11,26,34–36]. A recent study found that ACA increases Nuclear factor-erythroid-2-related factor (Nrf2) [37,38], a transcription factor that controls the activity of antioxidant enzymes, such as glutathione and NADPH quinone oxidoreductase 1 (NQO1). By targeting these enzymes, ACA can simultaneously mediate ROS detoxification and maintain cellular redox balance [39,40].
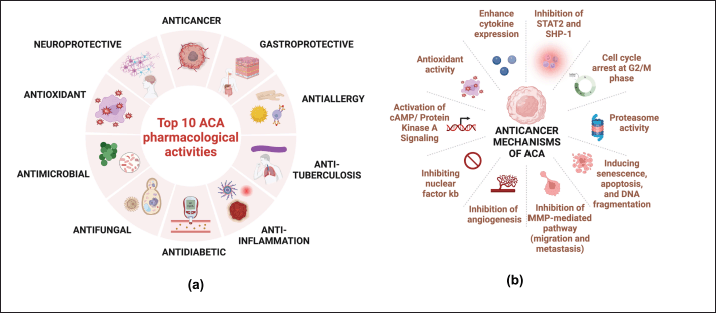 | Figure 5. Top 10 ACA pharmacological activities based on the Scopus database (a) and the most common anticancer mechanisms (b) (n = 135). [Click here to view] |
ACA inhibits NF-κB activation, a transcription factor implicated in inflammatory reactions, resulting in the reduced production of proinflammatory cytokines, including TNF-α and IL-1β [30,41,42]. This effect sheds light on its anticancer capabilities because inflammation is one of cancer’s hallmarks [43]. ACA also improves the immune system by reducing the production of cytokines associated with allergic responses and by lowering the levels of Th2 cytokines (IL-4 and IL-13) and Th1 cytokines (IL-12α and interferon-γ) in mice with asthma caused by ovalbumin [30,41]. Cytokines contribute to the development of asthma and other allergies [44]. Thus, there might be any potential association between anti-inflammatory, anticancer, and antiallergic capabilities in the emergence of immunotherapy medicines.
Numerous studies on ACA have reported that this compound effectively combats bacterial, mycobacterial, and fungal infections [45–47], highlighting its effectiveness as a natural antibacterial agent. Alpinia galanga acetone extract, which contains ACA, kills Salmonella typhi and Escherichia coli, as well as vancomycin-resistant bacteria [48]. ACA’s activity has also been demonstrated against a variety of fungi, including dermatophytes like Trichophyton mentagrophytes [46,49]. The antimicrobial characteristics of ACA are attributed to its capacity to disrupt cellular processes and structures, thereby affecting the bacterial shape and compromising the cell membrane integrity, which is essential for bacterial survival [12,21,50]. One of ACA’s antibacterial mechanisms is its ability to inhibit important cellular activities. ACA inhibits the generation of ROS in the mitochondria, thereby preventing the activation of the NLRP3 inflammasome [51], a key player in inflammatory reactions. This inhibits the release of oxidized mitochondrial DNA, which activates the inflammasome and reduces inflammation, leading to improved microbial resistance. A recent investigation on ACA’s anti-TB properties found substantial antimycobacterial activity against Mycobacterium tuberculosis strains H37Ra and H37Rv [52–54]. These broad-spectrum antimicrobial effects highlight the efficacy of ACA for treating microbial infections.
Beyond its anti-inflammatory and anticancer properties, ACA has also been investigated for its therapeutic benefits on obesity and metabolism [55]. It stops the formation of fat by decreasing the activity of GPDH and the levels of PPARγ, C/EBPα, and phosphorylated AMPK [56]. This adipogenesis suppression decreases visceral fat accumulation in animal models, suggesting its potential in treating metabolic illnesses associated with obesity. Furthermore, researchers have recognized the gastroprotective characteristics of ACA, indicating its potential to shield the gastrointestinal system from various types of injury [26]. ACA reduces ROS production in mitochondria and stops the release of oxidized mitochondrial DNA. This prevents caspase-1 activation and IL-1 production, thereby reducing inflammation in the digestive tract [51]. Combining ACA with other constituents enhances its gastroprotective properties [56,57]. A recent study showed that the combination of ACA and sodium butyrate has a synergistic effect on triggering apoptosis in cancer cells [57], suggesting its possible use in preventing gastrointestinal malignancies.
In terms of neuroprotection, ACA regulates cell signaling pathways, increases proteasome activity, and provides alternative energy sources. Proteasome activity in PC12 cells that have differentiated into neurons is increased by ACA [58], which maintains protein homeostasis and halts neurodegenerative processes. Proteasome activity is facilitated by stimulation of the cAMP-PKA signaling pathway [59]. The neuroprotective properties of ACA are also shown by the decreased cell viability of amyloid protein, which is a critical factor in the development of Alzheimer’s disease [60]. Its neuroprotective effects are attributed to its antioxidant and anti-inflammatory properties. Although the ACA has promise in these areas, it is critical to consider the entire context of its pharmacological activities. Additional confirmation of the compound’s efficacy and safety through toxicological investigations and clinical trials is necessary. It is also critical to understand the molecular processes responsible for its multiple benefits to further develop it as a therapeutic agent. Implementing this comprehensive research will ensure the advancement of ACA’s potential in treating numerous diseases.
Mechanism underlying ACA’s anticancer
Given the main areas of research on ACA in the field of anticancer, particularly breast, cervical, colon, head and neck, and prostate cancers, this study aimed to shed light on the intricate mechanisms involved. Almost all targets associated with the ACA cancer hallmarks have been studied. ACA is an effective cytotoxic agent against a variety of human cancer cells by inducing cellular senescence and apoptosis by enhancing cleaved PARP, p53, and Bax expressions while reducing Bcl-2 and Bcl-xL expression [4,61–63]. ACA also inhibits cancer cell proliferation and migration by interrupting the integrin-1 signaling pathway and preventing the production of adhesion molecules like ICAM-1 [4,16,63]. As a result of inhibiting NF-κB activation, ACA increases apoptosis and minimizes cellular invasion. Inactivation of this pathway by ACA leads to a decrease in the expression of pro-tumorigenic factors, such as the chemokine receptor CXCR4 and VEGF, which are linked to tumor growth and metastasis [64–66]. Induction of apoptosis via oxidative stress pathways is another mechanism by which ACA exerts its anticancer effects. ACA induces oxidative stress that exacerbates pro-apoptotic effect. Antioxidants mitigate stress, emphasizing the interplay between oxidative stress and apoptosis in anticancer mechanisms. ACA may also trigger apoptosis and cell death in cancer cells by regulating ROS generation over the threshold, inducing cellular senescence, and activating autophagy pathways [67]. Therefore, it is necessary to validate the effects of ACA as a dual antioxidant for cancer treatment.
ACA regulates the cell cycle to trigger G1 and G2/M-phase arrest [15,68,69] by altering the phosphorylation status of Rb and p27kip1. In tumor cells, the enantiomers of ACA, (S)-ACA and (R)-ACA, demonstrate distinct mechanisms. (S)-ACA induces G1 phase arrest by decreasing phosphorylated Rb and increasing p27kip1, whereas (R)-ACA induces G2 phase arrest by increasing hyperphosphorylated Rb and p27kip1 phosphorylation. ACA also inhibits angiogenesis-mediated tumor growth by targeting the Src-FAK-Rho GTPase signaling pathway mediated by VEGF. This inhibition results in decreased cell viability and angiogenic factor production, which, in turn, contributes to the arrest of the cell cycle and inhibition of tumor growth.
Controlling the protein balance is an essential component of the initiation of cancer, and the proteasome is a key part of the system that breaks down proteins [70]. The involvement of proteasomes in biochemical processes, such as cell cycle control, apoptosis, and protein degradation, highlights their significance in cancer [71]. Cancer cells often exhibit increased proteasome activity compared with normal tissues, promoting the accelerated breakdown of proteins that control cell growth and survival [19]. Specifically, this increase occurs in the chymotrypsin-like function of the proteasome, which has the potential to function as a biomarker for the diagnosis and prognosis of cancer [43,72,73]. It has been shown that ACA enhances proteasome activity by activating the cAMP-PKA signaling pathway [59,74]. The function of proteasomes in cancer is further confounded by their interaction with cancer stem cells, which are believed to be responsible for promoting tumor development and recurrence. Furthermore, these cells often exhibit modified proteasome activity, which may enhance their resistance to traditional treatments. Although the anticancer potential of ACAs is substantial, their effects may differ based on specific cancer types and cellular environments.
Challenges in ACA development
The complexity of ACA’s biological activities and physicochemical attributes creates several obstacles that limit its therapeutic potential. Low water solubility complicates the distribution of drugs, particularly in vivo, where absorption and bioavailability are critical for therapeutic efficacy [16]. To increase the solubility and bioavailability of ACA, a recent study reported that the formulation of ACA-nanostructured lipid carriers (NLCs) improved solubility and absorption, making it more effective as a treatment for prostate cancer models [75]. Another concern is the lack of specificity of the ACAs and other anticancer drugs. Given the lack of specificity, large doses may cause systemic toxicity and immunosuppression. The clinical use of ACA as a medicinal agent requires balancing the effectiveness and safety.
Formulating the ACA into an efficient delivery method remains a challenge. The development of NLCs is improving ACA’s pharmacokinetic profile, but these formulations require extensive validation and testing. ACA must undergo extensive preclinical and clinical research to determine its safety and effectiveness, similar to any novel drug candidate. The procedure is lengthy and expensive, and negative results may postpone or halt development. In addition, plant-derived chemical licensing regulations could potentially slow down market entry. Although ACA has the potential to become an anticancer drug candidate, its solubility, specificity, formulation, regulatory barriers, and research gaps impede its development. To advance ACA’s clinical applicability as an innovative drug candidate, innovative formulation methodologies and rigorous research are needed. Further research is needed to improve these approaches and ensure the safety and efficacy.
CONCLUSION AND FUTURE DIRECTIONS
Our bibliometric study reveals that ACA has promising potential as an anticancer agent, with growing research interest in its mechanisms of action, molecular pathways, and therapeutic efficacy. However, the current research landscape is limited by a lack of clinical trials and comprehensive studies on bioavailability, pharmacokinetics, and long-term safety. Moving forward, focused efforts on clinical validation, improving drug delivery systems, and exploring personalized approaches will be crucial to fully realizing ACA’s potential in clinical applications, especially cancer treatment. These findings underscore the need for continued interdisciplinary research and collaboration in order to translate ACA from preclinical success into effective clinical application.
The co-citation patterns showed the main study groups and topics, such as molecular pathways, drug discovery, and personalized medicine. These findings may guide future research directions and clinical applications. Our investigation revealed shortcomings in the incorporation of cutting-edge technologies, such as AI and machine learning, into cancer research. Future studies might examine how these technologies help accelerate drug development and improve treatment options. Furthermore, the low cocitation of developing themes, such as immunotherapy and nanomedicine, indicates the need for further multidisciplinary cooperation and attention to these potential areas.
ACA’s preclinical success is not being translated into concrete clinical benefits, and the lack of evidence from clinical trials indicates that this is a crucial gap. Well-designed clinical studies are required to establish ACA’s safety and effectiveness in humans. These studies would also assist in identifying appropriate doses and treatment regimes, paving the way for their use in clinical cancer. Developing innovative drug delivery methods is critical for improving drug absorption and stability, which may increase the usefulness of drugs in clinical settings. Targeted delivery techniques, such as nanoparticles or conjugates, may be very useful for decreasing toxicity and enhancing the selectivity of ACAs for cancer cells, resulting in greater therapeutic effectiveness and fewer adverse effects. Furthermore, interest in changing the structure of ACAs to generate more effective molecules has increased dramatically. Addressing these challenges and concentrating on these research areas might help ACA’s drug candidate development, perhaps leading to its approval and usage in clinical settings to treat various malignancies.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This study was funded by International Collaborative Research Scheme (303.28/A.3-III/LRI/X/2023) by Universitas Muhammadiyah Surakarta.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments with animals or humans.
DATA AVAILABILITY
All data are available in the supplementary materials or provided by the authors upon request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Priyono QAP, Yusniasari PA, Alifiansyah MRT, Suryanto GY, Widyowati R, Herdiansyah MA, et al. Ethnomedical potentials, phytochemicals, and medicinal profile of Alpinia galanga L.: a comprehensive review. BIO Integr. 2024;5(1):1–9.
2. Sankaran S, Selvaraj J, Pottabathula SS, Namboori K, Venkidasamy B, Alharbi NS, et al. Alpinia galanga bioactive constituents as multi-target inhibitors of SARS-CoV-2 proteins: a molecular docking, molecular simulation and ADMET analysis. Traditional MedRes. 2024;9(4):24.
3. David EM, Parthasarathi T, Selvaraj CI. Phytochemistry and bioactive potential of Galangal (Alpinia galanga (L.) Willd.). In: Pullaiah T, editor. Phytochemical composition and pharmacy of medicinal plants: 2-volume set. Palm Bay, FL: Apple Academic Press; 2023. pp. 165–74.
4. Pradubyat N, Giannoudis A, Elmetwali T, Mahalapbutr P, Palmieri C, Mitrpant C, et al. 1′-acetoxychavicol acetate from Alpinia galanga represses proliferation and invasion, and induces apoptosis via HER2-signaling in endocrine-resistant breast cancer cells. Planta Med. 2022;88(2):163–78.
5. Liu P, Wu SL, Wang T, Zhang XM, Geng CA. Four new phenolic compounds from the fruits of Alpinia galanga. Phytochem Lett. 2023;55:75–9.
6. Yit KH, Zainal-Abidin Z. Antimicrobial potential of natural compounds of Zingiberaceae plants and their synthetic analogues: a scoping review of in vitro and in silico approaches. Curr Top Med Chem. 2024;24(13):1158–84.
7. Ahmad A, Riaz S, Farooq R, Ahmed M, Hussain N. Alpinia officinarum (Galangal): a beneficial plant. J Med Public Health. 2023;4:1057.
8. Ramanunny AK, Wadhwa S, Gulati M, Gupta S, Porwal O, Jha NK, et al. Development and validation of RP-HPLC method for 1-Acetoxychavicol acetate (ACA) and its application in optimizing the yield of ACA during its isolation from Alpinia galanga extract as well as its quantification in nanoemulsion. South Afr J Bot. 2022 Sep;149:887–98.
9. Alif I, Utomo RY, Ahlina FN, Nugraheni N, Hermansyah D, Putra A, et al. Immunopotentiation of galangal (Alpinia galanga L.) when combined with T-cells against metastatic triple-negative breast cancer, MDA-MB 231. J Appl Pharm Sci. 2021;11(11):53–61.
10. Ong GH, Ori D, Kawasaki T, Kawai T. Inhibition of lipopolysaccharide-induced inflammatory responses by 1′-acetoxychavicol acetate. Genes Cells. 2022;27(7):482–92.
11. Higashida M, Xu S, Kojima-Yuasa A, Kennedy DO, Murakami A, Ohigashi H, et al. 1′-Acetoxychavicol acetate-induced cytotoxicity is accompanied by a rapid and drastic modulation of glutathione metabolism. Amino Acids. 2009;36(1):107–13.
12. Humaidi SNIC, Shalan NSN, Taib MNAM, Al-Shammary AAK, Anuar N, Awang K, et al. Antimicrobial and drug-synergistic potential of Alpinia conchigera Griff.-derived phenylpropanoids against Mycobacterium smegmatis. Malaysia J Microbiol. 2020;16(6):511–8.
13. Zhang D, Zou L, Wu DT, Zhuang QG, Li HB, Mavumengwana V, et al. Discovery of 1’-acetoxychavicol acetate (ACA) as a promising antibacterial compound from galangal (Alpinia galanga (Linn.) Willd). Ind Crops Prod. 2021;171:113883.
14. Ketkomol P, Songsak T, Jongrungruangchok S, Madaka F, Pradubyat N. The effect of 1’-acetoxychavicol acetate on A549 human non-small cell lung cancer. J Curr Sci Tech. 2024;14(2):43.
15. Hua HY, Jiang YJ, You XY, Ye QX. Effects of 1’-acetoxychavicol acetate submicron emulsion on proliferation and apoptosis of HeLa cells. Chin Trad Herbal Drugs. 2012;43(4):729–33.
16. Kojima-Yuasa A, Matsui-Yuasa I. Pharmacological effects of 1′-acetoxychavicol acetate, a major constituent in the rhizomes of Alpinia galanga and Alpinia conchigera. J Med Food. 2020;1;23(5):465–75.
17. Cardona- Galeano W, Ramirez- Malule H, Gómez- Ríos D. Hybrids based on coumarins and their anticancer activities: a bibliometric analysis. J Appl Pharm Sci. 2023;13(9):204–12. .
18. Omar N, Othman Z, Abdul Halim AS, Ahmad R, Md Lazin Md Lazim MR, Shafin N, et al. Unveiling the therapeutic potential of ketamine in depression: a bibliometric analysis and research landscape overview. J Appl Pharm Sci. 2024;14:27–34.
19. Cardona- Galeano W, Ramirez- Malule H, Gómez- Ríos D. Anticancer activity of monastrol, hybrids and derivatives: a comprehensive bibliometric analysis of recent research. J Appl Pharm Sci. 2024;14:73–82.
20. Dagli N, Patel B, Dagli R, Adnan N, Ahmad R, Haque M, et al. Bibliometric analysis and visualization of research on nanotechnology in dentistry from 1999 to 2022. J Appl Pharm Sci. 2023;13:58–66.
21. Aziz AN, Ibrahim H, Rosmy Syamsir D, Mohtar M, Vejayan J, Awang K. Antimicrobial compounds from Alpinia conchigera. J Ethnopharmacol. 2013;145(3):798–802.
22. Misawa T, Aoyama H, Furuyama T, Dodo K, Sagawa M, Miyachi H, et al. Structural development of benzhydrol-type 1′-Acetoxychavicol Acetate (ACA) analogs as human leukemia cell-growth inhibitors based on quantitative structure-activity relationship (QSAR) analysis. Chem Pharm Bull. 2008;56(10):1490–5.
23. Miyauchi M, Nishikawa A, Furukawa F, Nakamura H, Son HY, Murakami A, et al. Inhibitory effects of 1’-acetoxychavicol acetate on N-nitrosobis (2-oxopropyl)-amine-induced initiation of cholangiocarcinogenesis in Syrian Hamsters. Jpn J Cancer Res. 2000;91(5):477–81.
24. Saritnum O, Suamsiri P, Minami M, Matsushlma KI, Nemoto K. Genetic relationship of galangal (Alpinia galanga Willd.) in Thailand by RAPD analysis. Sabrao J Breed Genet. 2009;41(1):69–76.
25. Houghton P, Fang R, Techatanawat I, Steventon G, Hylands PJ, Lee CC. The sulphorhodamine (SRB) assay and other approaches to testing plant extracts and derived compounds for activities related to reputed anticancer activity. Methods. 2007;42(4):377–87.
26. Matsuda H, Pongpiriyadacha Y, Morikawa T, Ochi M, Yoshikawa M. Gastroprotective effects of phenylpropanoids from the rhizomes of Alpinia galanga in rats: structural requirements and mode of action. Eur J Pharmacol. 2003;471(1):59–67.
27. Oonmetta-aree J, Suzuki T, Gasaluck P, Eumkeb G. Antimicrobial properties and action of galangal (Alpinia galanga Linn.) on Staphylococcus aureus. LWT Food Sci Technol. 2006;39(10):1214–20.
28. Lee CC, Houghton P. Cytotoxicity of plants from Malaysia and Thailand used traditionally to treat cancer. J Ethnopharmacol. 2005;100(3):237–43.
29. Nakamura Y, Murakami A, Ohto Y, Torikai K, Tanaka T, Ohigashi H. Suppression of tumor promoter-induced oxidative stress and inflammatory responses in mouse skin by a superoxide generation inhibitor 1’-acetoxychavicol acetate. Cancer Res. 1998;58(21):4832–9.
30. Murakami A, Matsumoto K, Koshimizu K, Ohigashi H. Effects of selected food factors with chemopreventive properties on combined lipopolysaccharide- and interferon-γ-induced IκB degradation in RAW264.7 macrophages. Cancer Lett. 2003;195(1):17–25.
31. Zheng Q, Hirose Y, Yoshimi N, Murakami A, Koshimizu K, Ohigashi H, et al. Further investigation of the modifying effect of various chemopreventive agents on apoptosis and cell proliferation in human colon cancer cells. J Cancer Res Clin Oncol. 2002;128(10):539–46.
32. Murakami A, Ohura S, Nakamura Y, Koshimizu K, Ohigashi H. 1’-Acetoxychavicol acetate, a superoxide anion generation inhibitor, potently inhibits tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in ICR mouse skin. Oncology. 1996;53(5):386–91.
33. Mitsui S, Kobayashi S, Nagahori H, Ogiso A. Constituents from seeds of Alpinia galanga Wild, and their anti-ulcer activities. Chem Pharm Bull (Tokyo). 1976;24(10):2377–82.
34. Haque AKMM, Leong KH, Lo YL, Awang K, Nagoor NH. In vitro inhibitory mechanisms and molecular docking of 1′-S-1′-acetoxychavicol acetate on human cytochrome P450 enzymes. Phytomedicine. 2017;31:1–9.
35. Kojima-Yuasa A, Yamamoto T, Yaku K, Hirota S, Takenaka S, Kawabe K, et al. 1′-acetoxychavicol acetate ameliorates age-related spatial memory deterioration by increasing serum ketone body production as a complementary energy source for neuronal cells. Chem-Biol Interact. 2016;257:101–9.
36. Korkina LG. Phenylpropanoids as naturally occurring antioxidants: from plant defense to human health. Cell Mol Biol. 2007;53(1):15–25.
37. Liu Y, Murakami N, Zhang S, Xu T. Structure-activity relationships of 1′-acetoxychavicol acetate homologues as new nuclear export signal inhibitors. Pharmazie. 2007;62(9):659–62.
38. Yaku K, Matsui-Yuasa I, Azuma H, Kojima-Yuasa A. 1′-acetoxychavicol acetate enhances the phase II enzyme activities via the increase in intranuclear Nrf2 level and cytosolic p21 level. Am J Chin Med. 2011;39(4):789–802.
39. Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24(5):981–90.
40. Trachootham D, Lu W, Ogasawara MA, Valle NRD, Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10(8):1343–74.
41. Ando S, Matsuda H, Morikawa T, Yoshikawa M. 1′S-1′-Acetoxychavicol acetate as a new type inhibitor of interferon-β production in lipopolysaccharide-activated mouse peritoneal macrophages. Bioorg Med Chem. 2005;13(9):3289–94.
42. Ohata T, Fukuda K, Murakami A, Ohigashi H, Sugimura T, Wakabayashi K. Inhibition by 1-acetoxychavicol acetate of lipopolysaccharide- and interferon-γ-induced nitric oxide production through suppression of inducible nitric oxide synthase gene expression in RAW264 cells. Carcinogenesis. 1998;19(6):1007–12.
43. Chen X, Wu X, Li L, Zhu X. Development of proteasome inhibitors for cancer therapy. IJDDP. 2024;3:100004.
44. Seo JW, Cho SC, Park SJ, Lee EJ, Lee JH, Han SS, et al. 1′-acetoxychavicol acetate isolated from Alpinia galanga ameliorates ovalbumin-induced asthma in mice. PLoS One. 2013;8(2):e56447.
45. Anuar N, Taib MNAM, Hanafiah KM, Shammary AAKA, Shalan NSN, Humaidi SNIC, et al. Synthesis of 1-Acetoxychavicol Acetate (ACA) analogues and their inhibitory activities against methicillin-resistant Staphylococcus aureus. J Phys Sci. 2020;31(3):101–11.
46. Janssen AM, Scheffer JJC. Acetoxychavicol acetate, an antifungal component of Alpinia galanga. Planta Med. 1985;51(6):507–11.
47. Weerakkody NS, Smith WM, Mikkelsen D, Waanders J, Kerven G, Caffin N, et al. Purified 1′acetoxychavicol acetate (1′ACA) from galangal spice affects membrane fatty acid composition and triggers a cell envelope stress response in Staphylococcus aureus. Int J Antimicrob Agents. 2012;39(3):269–71.
48. Chouni A, Paul S. A review on phytochemical and pharmacological potential of Alpinia galanga. Pharmacogn J. 2018;10(1):9–15.
49. Moghadamtousi SZ, Kadir HA, Hassandarvish P, Tajik H, Abubakar S, Zandi K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed Res Int. 2014;2014:1–12.
50. Shen CL, Wang R, Ji G, Elmassry MM, Zabet-Moghaddam M, Vellers H, et al. Dietary supplementation of gingerols- and shogaols-enriched ginger root extract attenuate pain-associated behaviors while modulating gut microbiota and metabolites in rats with spinal nerve ligation. J Nutr Biochem. 2022;100:108904.
51. Sok SPM, Ori D, Wada A, Okude H, Kawasaki T, Momota M, et al. 1′-acetoxychavicol acetate inhibits NLRP3-dependent inflammasome activation via mitochondrial ROS suppression. Int Immunol. 2021;33(7):373–86.
52. Gupta P, Bhatter P, D’souza D, Tolani M, Daswani P, Tetali P, et al. Evaluating the anti Mycobacterium tuberculosis activity of Alpinia galanga (L.) Willd. axenically under reducing oxygen conditions and in intracellular assays. BMC Complement Altern Med. 2014;14:84.
53. Phanumartwiwath A, Kesornpun C, Sureram S, Hongmanee P, Pungpo P, Kamsri P, et al. Antitubercular and antibacterial activities of isoxazolines derived from natural products: isoxazolines as inhibitors of Mycobacterium tuberculosis InhA. J Chem Res. 2021;45(11–12):1003–15.
54. Warit S, Rukseree K, Prammananan T, Hongmanee P, Billamas P, Jaitrong S, et al. In vitro activities of enantiopure and racemic 1’-acetoxychavicol acetate against clinical isolates of Mycobacterium tuberculosis. Sci Pharm. 2017;85(3):32.
55. Liang CH, Lin YS, Chiang SS. Regulation of adipogenesis and lipolysis by the rhizomes of Alpinia galanga in 3T3-L1 preadipocytes and high fat diet-induced obese BALB/c mice. Taiwanese J Agric Chem Food Sci. 2018;56(1–2):9–24.
56. Yaku K, Matsui-Yuasa I, Konishi Y, Kojima-Yuasa A. AMPK synergizes with the combined treatment of 1′-acetoxychavicol acetate and sodium butyrate to upregulate phase II detoxifying enzyme activities. Mol Nutr Food Res. 2013;57(7):1198–208.
57. Kato R, Matsui-Yuasa I, Azuma H, Kojima-Yuasa A. The synergistic effect of 1′-acetoxychavicol acetate and sodium butyrate on the death of human hepatocellular carcinoma cells. Chem-Biol Interact. 2014;212(1):1–10.
58. Chiu SP, Wu MJ, Chen PY, Ho YR, Tai MH, Ho CT, et al. Neurotrophic action of 5-hydroxylated polymethoxyflavones: 5-demethylnobiletin and gardenin A stimulate neuritogenesis in PC12 cells. J Agric Food Chem. 2013;61(39):9453–63.
59. Yaku K, Matsui-Yuasa I, Kojima-Yuasa A. 1′-acetoxychavicol acetate increases proteasome activity by activating cAMP-PKA signaling. Planta Med. 2018;84(3):153–9.
60. He P, Yan S, Zheng J, Gao Y, Zhang S, Liu Z, et al. Eriodictyol attenuates LPS-induced neuroinflammation, amyloidogenesis, and cognitive impairments via the inhibition of NF-κB in male C57BL/6J mice and BV2 microglial cells. J Agric Food Chem. 2018;66(39):10205–14.
61. Awang K, Nurul Azmi M, Lian Aun LI, Nazif Aziz A, Ibrahim H, Hasima Nagoor N. The apoptotic effect of 1’S-1’-acetoxychavicol acetate from Alpinia conchigera on human cancer cells. Molecules. 2010;15(11):8048–59.
62. Campbell CT, Prince M, Landry GM, Kha V, Kleiner HE. Pro-apoptotic effects of 1′-acetoxychavicol acetate in human breast carcinoma cells. Toxicol Lett. 2007;173(3):151–60.
63. Muangnoi P, Lu M, Lee J, Thepouyporn A, Mirzayans R, Le XC, et al. Cytotoxicity, apoptosis and DNA damage induced by Alpinia galanga rhizome extract. Planta Med. 2007;73(8):748–54.
64. Ghallab AM, Eissa RA, El Tayebi HM. CXCR2 Small-molecule antagonist combats chemoresistance and enhances immunotherapy in triple-negative breast cancer. Front Pharmacol. 2022;13:862125.
65. Pang X, Zhang L, Lai L, Chen J, Wu Y, Yi Z, et al. 1’-acetoxychavicol acetate suppresses angiogenesis-mediated human prostate tumor growth by targeting VEGF-mediated Src-FAK-Rho GTPase-signaling pathway. Carcinogenesis. 2011;32(6):904–12.
66. Guntarno NC, Rahaju AS, Kurniasari N. The role of MMP-9 and VEGF in the invasion state of bladder urothelial carcinoma. Indonesian Biomed J. 2021;13(1):61–7.
67. Aggarwal V, Tuli HS, Varol A, Thakral F, Yerer MB, Sak K, et al. Role of reactive oxygen species in cancer progression: molecular mechanisms and recent advancements. Biomolecules. 2019;9(11):735.
68. Ahlina FN, Nugraheni N, Salsabila IA, Haryanti S, Da’i M, Meiyanto E. Revealing the reversal effect of galangal (Alpinia galanga L.) extract against oxidative stress in metastatic breast cancer cells and normal fibroblast cells intended as a co- chemotherapeutic and anti-ageing agent. Asian Pac J Cancer Prev. 2020;21(1):107–17.
69. Azmi MN, Tan CS, Abdulameed HT, Kamal NNSNM, Kahar NEA, Omar MTC. Synthesis of benzhydrol analogues based on 1’-acetoxychavicol acetate (ACA), as a stable and potent antiproliferative agent on breast cancer cell lines, ADMET analysis and molecular docking study. Org Commun. 2024;17(2):99–114.
70. Shao X, Xing F, Zhang Y, Lok CN, Che CM. Integrative chemoproteomics reveals anticancer mechanisms of silver( i ) targeting the proteasome regulatory complex. Chem Sci. 2024;15(14):5349–59.
71. Sari AA, Munawaroh R, Sofyanita EN. Bibliometric analysis of antibacterial activity of Centella asiatica: a study based on Scopus database. J Appl Pharm Sci. 2023;13:1–15.
72. Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31–46.
73. Zhou X, Xu R, Wu Y, Zhou L, Xiang T. The role of proteasomes in tumorigenesis. Genes Dis. 2024;11(4):101070.
74. Sagawa M, Tabayashi T, Kimura Y, Tomikawa T, Nemoto-Anan T, Watanabe R, et al. TM -233, a novel analog of 1′-acetoxychavicol acetate, induces cell death in myeloma cells by inhibiting both JAK / STAT and proteasome activities. Cancer Sci. 2015;106(4):438–46.
75. Subramaniam B, Arshad NM, Malagobadan S, Misran M, Nyamathulla S, Mun KS, et al. Development and evaluation of 10-acetoxychavicol acetate (ACA)-loaded nanostructured lipid carriers for prostate cancer therapy. Pharmaceutics. 2021;13(4):439.
SUPPLEMENTARY MATERIAL
The supplementary material can be accessed at the journal’s website link here: [https://japsonline.com/admin/php/uploadss/4485_pdf.pdf]