INTRODUCTION
Warfarin (trade name Coumarin) is an oral anticoagulant prescribed to prevent thrombosis in patients with hereditary and acquired homeostasis disorders. Warfarin is characterized by a narrow therapeutic index: even a small change in its plasma concentration can lead to unpredictable drug reactions or ineffective treatment. Therapy with this drug has one of the highest rates of severe adverse reactions.
There is wide individual variability in the dose of warfarin required to achieve the target level of anticoagulation and maintain the coagulation level of the international normalized ratio (INR) within therapeutic limits, which ensures the effectiveness and safety of treatment [1]. The INR is a standardized measurement of prothrombin time that indicates the level of blood clotting. The initial and maintenance dose of warfarin should be selected individually for each patient with regular monitoring of the INR. Approximately 50% of the individual response to warfarin treatment is caused by clinical factors, lifestyle, comorbidities and their treatment, race, and genetic characteristics (for example, polymorphisms in the vitamin K epoxide reductase complex 1 (VKORC1), the cytochrome P450 2C9 (CYP2C9), the cytochrome P450 4F2 (CYP4F2), and the cytochrome P450 2C19 (CYP2C19) genes) [2]. In the study of warfarin pharmacogenetics, the relationship between carriers of various genetic polymorphisms and the pharmacodynamics and pharmacokinetics of warfarin was confirmed. Patients with unfavorable variants of the studied genes need to titrate warfarin doses to achieve an adequate anticoagulant effect [1].
Among the genes associated with warfarin dose response CYP2C9 and VKORC1 gene are the two most important, with genetic polymorphisms accounting for 30%–40% of the variation in the required warfarin dose. The inclusion of genetic testing for identification of the main polymorphisms associated with warfarin pharmacogenetics in the clinical algorithm for predicting the safe and effective dose of this drug has improved the safety and efficacy of anticoagulant therapy [3]. Despite the reliable pharmacological basis, various clinical trials of warfarin dosing depending on genotype have presented ambiguous and contradictory results, which limits the widespread use of genetic testing in warfarin therapy [4–6]. The therapeutic dose of warfarin may need to be adjusted depending on the identified genotypes and the predicted phenotype. Thus, patients with high genetically determined sensitivity to warfarin are recommended to significantly reduce the dose of warfarin or switch to another similar drug.
This comprehensive review offers several novel contributions to understanding ethnic differences in warfarin pharmacogenetics. While earlier research concentrated on particular populations, this work synthesizes data from a wide variety of ethnic groups worldwide. It builds upon prior findings by analyzing not only the well-known polymorphisms in CYP2C9 and VKORC1, but also the growing significance of CYP4F2, CYP2C19, and GGCX genes in different populations. This more comprehensive genetic profile enables a more nuanced comprehension of inter-ethnic variability in warfarin response. Furthermore, the review incorporates clinical, demographic, and lifestyle factors that affect warfarin dose across cultural contexts in addition to genetic considerations. Clinicians worldwide can benefit from this study’s practical resource, which compiles prescribed genetic panels and dose algorithms customized for various ethnic groups, helping them to optimize warfarin therapy. This work fills a vital void in the era of precision medicine by bridging the gap between theoretical pharmacogenetics and practical clinical application.
Sridharan and Sivaramakrishnan [7] conducted a meta-analysis in Bahrain involving 7898 patients and evaluated the results of considering VKORC1 and CYP2C9 genotypes in warfarin prescription. However, their study did not consider CYP2C19 and gamma-glutamyl carboxylase (GGCX) genotypes. Same with Xie et al. [8] and Guo et al. [9]. Both studies compared the effectiveness of predicting different warfarin dosing algorithms based on Chinese patients with CYP2C9, VKORC1, and CYP4F2 genotypes but did not consider other nations and patients with CYP2C19 and GGCX genotypes. Chumnumwat et al. [10] compared the predictive accuracy of existing pharmacogenetic warfarin dosing algorithms derived from Caucasian and Asian populations to determine the appropriate algorithm for the Thai population. The researchers considered patients only with genotypes CYP2C9 and VKORC1. Chang and Tan [11] demonstrated Singapore’s experience in using pharmacogenetic testing to improve warfarin therapy. Their studies were concentrated on CYP2C9, VKORC1, and CYP4F2 genotypes.
It is worth noting that the presented works describe only certain ethnic groups. Therefore, the study aims to identify, cover, and structure all relevant data on warfarin pharmacogenetics in different ethnic populations.
MATERIALS AND METHODS
Before performing the presented literature review, a thorough structured systematic search for relevant information on the dosing of warfarin (trade name Coumarin) in different ethnic groups was conducted in PubMed, Embase, Scopus, Google Scholar, and Web of Science databases.
All relevant articles with the necessary information (clinical trials, randomized controlled trials, reviews, systematic reviews, and meta-analyses) published from 2011 to 2023 in peer-reviewed journals in English, French, Polish, Chinese, and German were found and reviewed. The necessary data were searched using a combined set of keywords: “warfarin”, “coumarin”, “genotype”, “pharmacokinetics”, “pharmacogenetics”, “ethnic groups”, “indirect oral anticoagulants”, “international normalized ratio”, “thrombosis”, “homeostasis”, “CYP2C9”, “VKORC1”, “CYP2C19”, “GGCX”, “CYP4F2”, “warfarin side effect”, “warfarin adverse reaction”, “dosing algorithms”, and “therapeutic dose”. The sources of information obtained were carefully studied, processed, and analyzed, selected by titles and abstracts, and based on relevance, time of publication, and appropriate level of evidence for the case studies. To obtain additional information sources, the reference lists for the relevant articles were examined and the relevance of their content was assessed. Further, duplicates and those that did not correspond to the selected period since publication were removed. From the identified list of papers, the following information was processed: basic data on the study, the period of publication and the region where the study was conducted; characteristics of the study population (race, ethnicity, gender, age, lifestyle, body mass index, comorbidities, vitamin K intake, access to quality diagnosis and treatment, and level of economic development). The available articles were then evaluated against the inclusion and exclusion criteria. Papers that did not meet the inclusion criteria in part or in full were immediately removed. Articles that contained outdated, unconfirmed, and irrelevant information; unreliable, poorly conducted work and its results; descriptions of the study conducted only on animal models were not included in this study. The literature review included studies from the last 13 years (2011 to 2023) with up-to-date relevant information on the pharmacokinetics and pharmacogenetics of warfarin; the relationship between warfarin dosing and ethnicity; development of side effects and adverse reactions in case of incorrectly selected warfarin dosage; the necessity to adjust the warfarin dose depending on the ethnic group represented; available genotypes associated with the response to warfarin administration.
A careful systematic selection process resulted in 45 relevant articles that fully met the inclusion criteria. To avoid errors, the selected sources of information were re-evaluated, processed, and verified. The results of the selected studies were cited in the literature review in compliance with all necessary technical requirements and copyrights.
RESULTS AND DISCUSSION
Polymorphisms of genes associated with warfarin pharmacodynamics and pharmacokinetics
CYP2C9 (representative of cytochrome P450 family 2, subfamily C) is highly polymorphic, with about 60 alleles described. Warfarin prescribed to patients with alleles 2 and 3 is metabolized more slowly and remains in circulation longer [7]. The CYP2C9*1 allele has a high population frequency and is associated with a normal phenotype in the carrier (i.e., patient). The two allelic variants CYP2C9*2 and CYP2C9*3 occur with a frequency of about 13% and 7%. Compared to the normal phenotype, patients who inherit one or two copies of CYP2C9*2 or CYP2C9*3 are more sensitive to warfarin and require lower doses due to an increased risk of bleeding [12]. Population frequencies of CYP2C9 gene alleles vary in different ethnic groups. Both CYP2C9*2 and CYP2C9*3 variants in the same genotype are very rare (<2%) and mainly in people of African or Asian descent. The CYP2C9*5, CYP2C9*6, CYP2C9*8, and CYP2C9*1 alleles are significantly more common in people of African descent [13].
CYP4F2 is involved in the metabolism of vitamin K in the liver and metabolizes reduced vitamin K to hydroxylated vitamin K1, thereby removing vitamin K from the process of activation of blood coagulation factors affected by warfarin. The influence of the CYP4F2 gene on warfarin dose is controversial, as indicated by studies [14,15]. When studying the effect of the rs210862 CYP4F2 polymorphism on warfarin dosing, it was found that the homozygous T/T genotype of the European race requires a warfarin dose of approximately 1 mg/day higher than that of individuals with the normal C/C genotype. Carriers of the minor T allele require an increase in warfarin dose by approximately 8%–11% compared with the normal C/C genotype. The influence of the rs210862 CYP4F2 polymorphism carrier on the required therapeutic dose of warfarin in African Americans has not been proven. No significant association between the rs2108622 CYP4F2 polymorphism carrier and the need for warfarin dose adjustment has been described in cohorts of patients from Korea and India [14]. At the same time, Choi et al. [16] found that Koreans carrying the T/T genotype of the CYP4F2 gene required the highest mean warfarin dose compared with carriers of other genotypes. Therefore, CYP4F2 was recommended to be included in the pharmacogenetic prediction algorithm for warfarin dose for patients in Korea.
CYP2C19 is a gene with 9 exons. Polymorphisms of this gene are associated with different abilities to metabolize xenobiotics [17,18]. The frequency of the minor allele of the T rs3814637 polymorphism is almost twice as high in the African population as in the European population (11.6% vs. 6.7%). The meta-analysis presented by Wang et al. [19] demonstrated that the carriage of the minor allele of the T rs3814637 polymorphism of CYP 2C19 is associated with a reduced need for warfarin. At the same time, regression analysis showed that VKORC1, CYP2C9, weight, age, and gender were factors that significantly affected warfarin dosing, whereas CYP2C19 polymorphism was not associated with the need for dose adjustment [20]. Characteristics of the genotypes mentioned above are presented in Table 1.
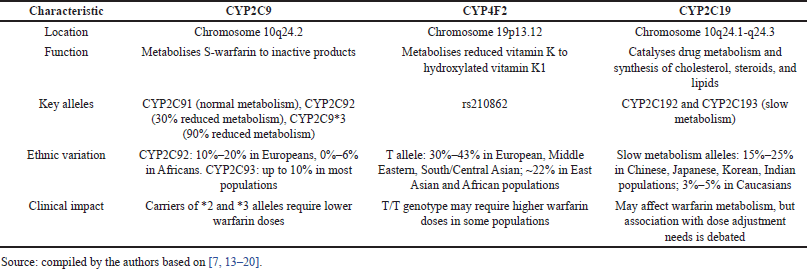 | Table 1. Characteristics of CYP2C9, CYP4F2, and CYP2C19 genotypes. [Click here to view] |
VKORC1 encodes subunit-1 of the vitamin K epoxide reductase complex, a small transmembrane protein of the endoplasmic reticulum that is part of the vitamin K cycle and a target for warfarin therapy. The rs9923231 polymorphism in the VKORC1 promoter leads to reduced gene expression, which can cause an accumulation of warfarin in the blood serum and an increase in INR values above the therapeutic range. Patients who carry the minor T rs9923231 polymorphism are more sensitive to warfarin, and lower dosages are recommended. The VKORC1-1639G>A polymorphism has a minor allele frequency ranging from 41% to 47% in European and Middle Eastern populations, while in East Asian populations it reaches 88%, but decreases to 13% in populations of African descent, and to 15% in South/Central Asian populations. Other tested polymorphisms and haplotypes of VKORC1 did not demonstrate genetically determined sensitivity to warfarin. The allelic frequencies of this polymorphism vary in different races: 37% of Caucasians, 90% of Asians, and 14% of Africans carry the minor T allele. In the Chinese population, more than 83% of the population carries the T/T genotype, which should be accounted for when dosing warfarin to patients in this group [21,22]. Genetic variability in the CYP2C9 and VKORC1 genes are the most important genetic factors affecting warfarin dosing [23]. Carriers of the above two alleles should reduce warfarin dosing by 19% and 33%, respectively. Carriers of the minor T allele of the rs9923231 polymorphism of the VKORC 1 gene should reduce the average dose of warfarin by 28%. Standard warfarin dosing algorithms in carriers of CYP2C9*2 and CYP2C9*3 alleles cause a twofold increase in the risk of severe hemorrhagic complications.
The GGCX gene is located on chromosome 2p12 and occupies a genomic region of ≈13 bp, consisting of 15 exons. The enzyme of the same name, GGCX, oxidizes reduced vitamin K to vitamin K-2 and 3-epoxide by adding a carboxyl residue to the gamma carbon of glutamic acids, resulting in the formation of functional coagulation factors II, VII, IX, and X. Minor G allele of the GGCX rs11676382 polymorphism is associated with a significant reduction in warfarin dose by 6.1% [24].
Factors affecting warfarin dose titration
Effect of ethnicity
Ethnicity affects the average daily dose of warfarin due to differences in the population frequencies of minor alleles of genes associated with warfarin pharmacogenetics. African Americans require a higher dose of warfarin compared to other ethnic groups. The therapeutic dose of warfarin in patients of Asian descent is approximately 30–40% lower than in patients of European race. These differences are explained by genetic polymorphisms in the CYP2C9 and VKORC1 genes [25,26].
Asians of different ethnic/racial backgrounds also possess a VKORC1 polymorphism other than the commonly studied VKORC1 (1639 G>A) [27]. Several studies have reported inter- and intra-population differences in VKORC1 susceptibility and pharmacokinetics in different ethnic groups [28–30]. Variants in VKORC1, CYP2C9 genotyping, and other clinical variables explain approximately 30%–50% of the difference in required warfarin doses. The genetic polymorphisms of CYP2C9 and VKORC1 together can account for up to 30% of the total variation in warfarin dose, while the genetic polymorphisms of VKORC1 and CYP2C9 alone can account for 25% and 9%, respectively. Although warfarin dosing remains a challenge, significant progress has been made using a modeling approach [31,32]. For instance, Wright and Duffull [33] proposed the individualization of warfarin dose according to a Bayesian scheme, which allowed for maintaining a steady state of INR from 65% to 80%. A study of the CYP2C9 (rs1057910) and VKORC1 (rs9923231) genotypes in 120 Chinese patients with atrial fibrillation with a target INR of 2 to 3 demonstrated that the required effective amount of warfarin was lower in patients with CYP2C9*1/*3 genotype compared to CYP2C9*1/*1 carriers (p < 0.05) and higher in patients with the AG and GG VKORC1 genotypes (p < 0.05) [34–36]. The study determined that age, height, and CYP2C9 and VKORC1 genotypes were the main variables in the assessment of the dose of the above drug. Based on the CYP2C9 and VKORC1 genotypes, a new warfarin dosing algorithm was developed, which reduced the time to titrate the dose to the required therapeutic dose in patients with atrial fibrillation and heart valve replacement.
Effect of comorbidities
The role of genetic and nongenetic factors in warfarin dose selection in cardiac patients in North India was studied in the study by Kaur et al. [37]. It was shown that the carriage of CYP2C9 and VKORC1 gene alleles was associated with a lower warfarin dose, with an additive effect in the presence of haplotypes in two alleles in the CYP2C9*3 and VKORC1 genes. One patient with the CYP2C93/*3 haplotype with the C/T genotype for VKORC 1 was eligible for the lowest dose among the study group (5 mg/week). At the same time, a patient with the *3/*3 haplotype and a normal C/C genotype for VKORC 1 required a higher dosage of 11 mg/week. In patients with variant alleles, the probability of INR exceeding the permissible range is significantly higher, which is accompanied by a high incidence of hemorrhagic complications. Genotyping of CYP2C9*2 (c. 430 C>T), CYP2C9*3 (c. 1075 A>C), CYP4F2 (c. 1297 G>A), and VKORC 1 (−1639 G>A) in 152 Colombians on warfarin therapy showed that the average weekly dose of warfarin was lower in patients with VKORC 1-1639 AA and CYP2C9*2/*2, CYP2C9*2/*3 genotypes [38,39].
In a study of 108 Korean patients, patients with VKORC 1 (C/C) genotypes were found to require lower warfarin doses than those with VKORC 1 genotypes (2.95 vs. 4.63 mg/day). An algorithm based on CYP2C9, VKORC1, and CYP4F2 genotyping with weight, amiodarone use, and diuretics explained 45% of the variation in individual warfarin requirements in Korean patients, but 55% of the variation remained unexplained [27]. In the current study, it was confirmed that the inclusion of the CYP4F2 genotype in the proposed algorithm improved the accuracy of warfarin dose prediction in Korean patients with various diseases. A study by Mak et al. [40] showed that VKORC1, CYP2C9*2, CYP2C9*3, and CYP4F2 carriers requiring the highest warfarin doses were Europeans; slightly lower for Latin Americans and the lowest for Asians. Homozygous carriage of the minor allele rs9923231 of the VKORC 1 gene polymorphism was found in 15% of Europeans, 15% of Latin Americans, and 79% of Asians. The frequency of CYP2C9*2 and CYP2C9*3 alleles was higher in Europeans than in Latin Americans and almost absent in Asians. The frequency of CYP4F2 gene polymorphisms was similar for all ethnic groups, but their effect on warfarin dose requirements was insignificant. Clinical factors, such as age, body surface area, history of coronary heart disease, deep vein thrombosis, or atrial fibrillation, had a different impact on the required warfarin dose in the ethnic groups studied. The study demonstrated significant differences between the three ethnic groups in warfarin dosing and the influence of clinical factors.
The study by Pirmohamed et al. [41] compared 227 patients who received genotype-specific warfarin doses on days 1–5 with 228 patients who received the standard clinical loading dose of warfarin. 353 patients were from the United Kingdom and 102 from Sweden, most of them were of European ethnic background. Genetic factors included CYP2C9 and VKORC1 genotyping, and nongenetic factors included age, height, weight, and amiodarone use. The time to reach the therapeutic window was higher in the group with known genotypes compared with the standard treatment group (67.4% vs. 60.3%, respectively). There were no significant differences in the incidence of hemorrhagic and thromboembolic complications between the groups.
Genetic warfarin dosing panels for different ethnic populations
There are two types of warfarin dosing recommendations in the warfarin leaflet: with known or unknown CYP2C9 and VKORC1 genotypes. The Dutch Working Group on Pharmacogenetics in its published guidelines noted the need to reduce the dose of warfarin for carriers of CYP2C9 genotypes and two minor alleles in the homozygous VKORC1 T/T genotype [42].
In the African American population, the influence of CYP2C9*5, CYP2C9*6, CYP2C9*8, and CYP2C9*11 alleles on warfarin dosing is as important as the influence of CYP2C9*2 and CYP2C9*3 alleles on warfarin dosing in Europeans, but the inclusion of these additional CYP2C9 alleles in the expanded genotyping algorithm is only being discussed. The CYP2C9*2 and CYP2C9*3 variants have been undetectable in Asian populations. Therefore, genotyping of this polymorphism in this population is irrelevant. Polymorphisms of the CYP4F2 gene did not have a significant effect on warfarin dose and were not a clinically useful test [43]. The guidelines on warfarin dosing stated that in the absence of genetic information about the patient, a clinical dosing algorithm or a standard dosing approach based on the INR should be used [44]. Genotyping-based warfarin dose adjustment is most convincing for populations of European and East Asian descent but may not always be correctly defined for other nations. From the self-identified African ancestry, 45%–50% of people carry the slow alleles CYP2C9*5, CYP2C9*6, CYP2C9*8, and CYP2C9*11, which require clinical warfarin doses. An alternative drug should be considered in patients with slower metabolizing genotypes: CYP2C9*3/*3, CYP2C9*2/*3, with C/T and T/T genotypes of the VKORC1 gene rs9923231 polymorphism.
Recommendations for a minimal (level 1) and an expanded panel of polymorphic alleles (level 2) are presented to assist clinical laboratories in the development of pharmacogenetic tests for warfarin therapy. These recommendations are based on the functional impact of polymorphisms, allele frequency in multinational populations, and technical aspects of genotyping. The allelic variants of the selected genes were classified based on three criteria: allele/polymorphism studied and known to be functionally significant; common in at least one population; public availability of a confirmatory test. Polymorphisms that met all three of these criteria were included in level 1, and those that met one or more of the three criteria were classified in level 2 [17–19,28].
All existing recommendations of professional organizations include genetic testing of CYP2C9*2, CYP2C9*3, and VKORC1 c. -1639G>A alleles when prescribing warfarin as allele 1. In the 2017 guidelines, genetic analysis of CYP2C9*5, CYP2C9*6, CYP2C9*8, CYP2C9*11, and CYP4F2*3 alleles is included as an additional level 2 recommendation for non-African American patients for warfarin dose adjustment. The CYP4F2*3 allele is associated with moderate sensitivity to warfarin and was found to be useful for warfarin dose adjustment in some ethnic groups, such as Europeans and Asians, but not in Africans, due to the low frequency of this allele in this population. In the absence of reliable information, this allele was included in the level 2 list as an insufficiently studied functional allele. According to the Genetic Testing Registry, a panel of CYP2C9 and VKORC genes was proposed for determining warfarin sensitivity. Not only does the number of genes recommended for genetic testing vary, but also the methods of genotyping—from targeted genotyping of several genes to exon sequencing. In total, there are more than 26 complete variants of the VKORC1 gene associated with warfarin resistance that have been identified in patients with a warfarin requirement of >10 mg/day or warfarin resistance. These enzymes have been shown to pre-bind the warfarin inhibitor to the VKORC1 enzyme, two of which are included in the list of level 2 alleles—VKORC 1 c. 196 G>A (p. Val 66 Met) and c.106 G>A (p. Asp 36 Tyr). The updated 2017 guidelines include additional polymorphisms that are predictors of warfarin dose requirements in patients of African descent [44].
The GGCX and CALU genes are considered candidates for inclusion in warfarin dose adjustment recommendations. The GGCX enzyme is associated with glutamate proteins, catalyzes the biosynthesis of vitamin K-dependent coagulation factors, and the CALU enzyme acts as a chaperone of γ-carboxylation systems. The more common GGCX variants, including rs699664, rs12714145, and rs11676382, necessitate warfarin dose adjustment in different populations. The internal CALU variant rs339097 (NM_001199671.1: c. 606+133A>G) is common in populations of African descent (MAF–11%–14%) but rarely found in European populations (MAF<1%). Carriage of the minor allele rs339097G is associated with the need for higher doses of warfarin in African Americans and Egyptians, but not in people of European descent [45]. A system based on three different daily doses (5–7 mg, 3–4 mg, or 0.5–2 mg) based on the combination of VKORC1 and CYP2C9 alleles is recommended [45–48]. All patients who have not previously taken warfarin should also be tested for the presence of CYP2C9*2 and CYP2C9*3 alleles, as well as VKORC1 (−1639G>A). It has been shown that genotyping results and clinical changes should be interpreted using a pharmacogenetic warfarin dosing algorithm; however, this does not substitute INR monitoring [46,49]. In patients of African descent, it is recommended to administer the clinical dose if the status of CYP*5, CYP*6, CYP*8, and CYP*11 is unknown without regard to genotype. If all the necessary data are available, it is recommended to use proven pharmacogenetic algorithms that include CYP2C9*2 and CYP2C9*3 alleles and VKORC1 status and reduce the dose by 15%–30%. If patients are carriers of two alleles that affect warfarin metabolism, a reduction in the standard dosage of up to 40% is required. A 10%–25% dose reduction is recommended for African Americans with the rs12777823 A/G or A/A genotype [44,50,51].
The genetic basis of warfarin dose requirements varies significantly throughout ethnic groups, requiring customized algorithms for dosing. Generally speaking, individuals of Asian origin require 30%–40% lower warfarin doses than those of European ancestry, whereas African Americans typically require greater doses than patients of other ethnicities. The main cause of these changes is the frequency of polymorphisms in genes like CYP2C9, VKORC1, and CYP4F2. For example, 88% of East Asian populations have the VKORC1-1639G>A polymorphism, which is linked to higher warfarin sensitivity, whereas in African and South/Central Asian groups only 13%–15% of the population have it. Europeans are more likely than Asians to carry the CYP2C9*2 and 3 polymorphisms, which are associated with decreased warfarin metabolism. Furthermore, several alleles (such as CYP2C95, *6, *8, and *11) are especially important for dosing in African people. Thus, when prescribing warfarin, it is imperative to consider the presented dosing algorithms to improve the therapeutic effect and reduce the risk of side effects in patients.
CONCLUSION
Characteristics of warfarin (Coumarin) pharmacogenetics were studied in detail. The study determined that this drug is widely used in anticoagulant therapy, has a narrow therapeutic range, and high variability, and often causes the development of adverse reactions. However, warfarin’s significant benefits include low cost and availability of the drug, and the possibility of prescribing it in case of contraindications to the use of direct anticoagulants (for example, in patients with replaced artificial mechanical valves and mitral stenosis).
The study determined that genotype-specific warfarin dosing significantly affects the efficacy and safety of anticoagulant therapy. Genotype-specific warfarin prescribing resulted in a reduction in the incidence of side effects, improved control of the anticoagulant system, stabilization of the therapeutic dose, and increased accuracy and efficacy of the drug during the treatment period. At the same time, a literature review demonstrated that the use of genotype-based warfarin dosing algorithms does not always improve anticoagulant control in the first few weeks of therapy. The ambiguity of the results is not only due to genetic factors. It is also important to consider the individual variability of the therapeutic dose of warfarin, including clinical factors (concomitant diseases of the gastrointestinal tract, excretory system, the need for daily vitamin K intake), demographic factors (age and gender, access to quality medical examination and necessary care), and personal factors (diet, race, body mass index, and bad habits).
The study noted that the features and consequences of warfarin use have been studied much more in the European race. At the same time, few studies have been conducted on the relationship between therapeutic dose and pharmacological effects in Asians. Given the significant diversity of ethnic and genetic characteristics of the population, further randomized trials involving all ethnic populations are needed to develop high-quality warfarin treatment protocols.
AUTHOR CONTRIBUTIONS
All authors have made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines’.
FUNDING
The work was carried out within the framework of the project—state registration number 0123PK01103, AP19677439 “Pharmacogenetics of indirect-acting anticoagulants in patients with heart surgery”.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVAL
This study does not involve the use of animal or human subjects.
DATA AVAILABILITY
All data generated or analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Lee KE, Yee J, Lee GY, Chung Je, Seong JM, Chang BC, et al. Genotype-guided warfarin dosing may benefit patients with mechanical aortic valve replacements: randomized controlled study. Sci Rep. 2020 Apr 24;10(1):6988. CrossRef
2. Fahmi AM, El Bardissy A, Saad MO, Elshafei MN, Bader L, Mahfouz A, et al. Clinical versus fixed warfarin dosing and the impact on quality of anticoagulation (The ClinFix trial). Clin Transl Sci. 2024 Jun;17(6):e13797. CrossRef
3. Zhang S, Zhao M, Zhong S, Niu J, Zhou L, Zhu B, et al. Association between CYP2C9 and VKORC1 genetic polymorphisms and efficacy and safety of warfarin in Chinese patients. Pharmacogenet Genomics. 2024 Jun 1;34(4):105–16. CrossRef
4. Wang X, Zhao D, Ma J, Wang X, Liu J. Correlation between metabolic parameters and warfarin dose in patients with heart valve replacement of different genotypes. Rev Cardiovasc Med. 2024 Apr;25(4):128. CrossRef
5. Hirai T, Aoyama T, Tsuji Y, Itoh T, Matsumoto Y, Iwamoto T. Kinetic-pharmacodynamic model of warfarin for prothrombin time-international normalized ratio in Japanese patients. Br J Clin Pharmacol. 2024 Mar;90(3):828–36. CrossRef
6. Anand A, Hegde N, Chhabra P, Purohit J, Kumar R, Gupta A, et al. Pharmacogenetic guided versus standard warfarin dosing for routine clinical care with its pharmacoeconomic impact: a randomized controlled clinical trial. Ann Hematol. 2024 Jun;103(6):2133–44. CrossRef
7. Sridharan K, Sivaramakrishnan G. A network meta-analysis of CYP2C9, CYP2C9 with VKORC1 and CYP2C9 with VKORC1 and CYP4F2 genotype-based warfarin dosing strategies compared to traditional. J Clin Pharm Ther. 2021 Jun;46(3):640–8. CrossRef
8. Xie C, Xue L, Zhang Y, Zhu J, Zhou L, Hang Y, et al. Comparison of the prediction performance of different warfarin dosing algorithms based on Chinese patients. Pharmacogenomics. 2020 Jan;21(1):23–32. CrossRef
9. Guo C, Kuang Y, Zhou H, Yuan H, Pei Q, Li J, et al. Genotype-guided dosing of warfarin in Chinese adults: a multicenter randomized clinical trial. Circ Genom Precis Med. 2020 Aug;13(4):e002602. CrossRef
10. Chumnumwat S, Yi K, Lucksiri A, Nosoongnoen W, Chindavijak B, Chulavatnatol S, et al. Comparative performance of pharmacogenetics-based warfarin dosing algorithms derived from Caucasian, Asian, and mixed races in Thai population. Cardiovasc Ther. 2018 Apr;36(2):e12315. CrossRef
11. Chang GSW, Tan DSY. Using pharmacogenetic testing to tailor warfarin therapy: the Singapore experience and what the future holds. Eur Cardiol. 2020 Jun 29;15:e53. CrossRef
12. Vogl S, Lutz RW, Schönfelder G, Lutz WK. CYP2C9 genotype versus metabolic phenotype for individual drug dosing—a correlation analysis using flurbiprofen as probe drug. PLoS One. 2015 Mar 16;10(4):e0126329. CrossRef
13. Asiimwe IG, Pirmohamed M. Ethnic diversity and warfarin pharmacogenomics. Front Pharmacol. 2022 Apr 4;13:866058. CrossRef
14. Rathore SS, Agarwal SK, Pande S, Singh SK, Mittal T, Mittal B. Therapeutic dosing of acenocoumarol: proposal of a population specific pharmacogenetic dosing algorithm and its validation in north Indians. PLoS One. 2012 May 22;7(5):e37844. CrossRef
15. Tong HY, Borobia AM, Quintana-Díaz M, Fabra S, González-Viñolis M, Fernández-Capitán C, et al. Acenocoumarol pharmacogenetic dosing algorithm versus usual care in patients with venous thromboembolism: a randomised clinical trial. J Clin Med. 2021 Jun 30;10(13):2949. CrossRef
16. Choi JR, Kim JO, Kang DR, Yoon SA, Shin JY, Zhang XH, et al. Proposal of pharmacogenetics-based warfarin dosing algorithm in Korean patients. J Hum Genet. 2011 Apr;56(4):290–5. CrossRef
17. Bauer T, Bouman HJ, Werkum JW, Ford NF, ten Berg JM, Taubert D. Impact of CYP2C19 variant genotypes on clinical efficacy of antiplatelet treatment with clopidogrel: systematic review and meta-analysis. BMJ. 2011 Aug 4;343:d4588. CrossRef
18. Luo W, Luo X, Chen S, Li J, Huang X, Rao Y, et al. Chinese stroke patients with atrial fibrillation using Robert’s age-adjusted warfarin loading protocol achieved good INR results within therapeutic range. Sci Rep. 2023 Oct 25;13:18230. CrossRef
19. Wang D, Yong L, Zhang Q, Chen H. Impact of CYP2C19 gene polymorphisms on warfarin dose requirement: a systematic review and meta-analysis. Pharmacogenomics. 2022 Nov;23(16):903–11. CrossRef
20. Khalighi K, Cheng G, Mirabbasi S, Khalighi B, Wu Y, Fan W. Opposite impact of Methylene tetrahydrofolate reductase C677T and Methylene tetrahydrofolate reductase A1298C gene polymorphisms on systemic inflammation. J Clin Lab Anal. 2018 Jun;32(5):e22401. CrossRef
21. Li S, Zou Y, Wang X, Huang X, Sun Y, Wang Y, et al. Warfarin dosage response related pharmacogenetics in Chinese population. PLoS One. 2015 Jan 16;10(1):e0116463. CrossRef
22. Pirmohamed M. Pharmacogenomics: current status and future perspectives. Nat Rev Genet. 2023 Jun;24(6):350–62. CrossRef
23. Chong K. Warfarin dosing and VKORC1/CYP2C9. [Internet]. Newark, NJ, US: Medscape [cited 2024 Apr 10]. Available from: https://emedicine.medscape.com/article/1733331-overview?form=fpf
24. Huang SW, Xiang DK, Huang L, Chen BL, An BQ, Li GF, et al. Influence of GGCX genotype on warfarin dose requirements in Chinese patients. Thromb Res. 2011 Feb;127(2):131–4. CrossRef
25. Wang D, Wu H, Dong M, Zhang Q, Zhao A, Zhao X, et al. Clinical significance of the series of CYP2C9*non3 variants, an unignorable predictor of warfarin sensitivity in Chinese population. Front Cardiovasc Med. 2022 Nov 24;9:1052521. CrossRef
26. Wang D, Wu H, Zhang Q, Zhou X, An Y, Zhap A, et al. Optimisation of warfarin-dosing algorithms for Han Chinese patients with CYP2C9*13 variants. Eur J Clin Pharmacol. 2023 Oct;79(10):1315–20. CrossRef
27. Zhu Y, Swanson KM, Rojas RL, Wang Z, St Sauver JL, Visscher SL, et al. Systematic review of the evidence on the cost-effectiveness of pharmacogenomics-guided treatment for cardiovascular diseases. Genet Med. 2020 Mar;22(3):475–86. CrossRef
28. Kubo K, Ohara M, Tachikawa M, Cavallari LH, Lee MTM, Wen MS, et al. Population differences in S-warfarin pharmacokinetics among African Americans, Asians and whites: their influence on pharmacogenetic dosing algorithms. Pharmacogenomics J. 2017 Dec;17(6):494–500. CrossRef
29. Al-Mahayri ZN, Khasawneh LQ, Alqasrawi MN, Altoum SM, Jamil G, Badawi S, et al. Pharmacogenomics implementation in cardiovascular disease in a highly diverse population: initial findings and lessons learned from a pilot study in United Arab Emirates. Hum Genomics. 2022 Sep 25;16(1):42. CrossRef
30. de Lara DV, de Melo DO, Araújo Silva LC, Gonçalves TS, Júnior Lima Santos PC. Pharmacogenetics of clopidogrel and warfarin in the treatment of cardiovascular diseases: an overview of reviews. Pharmacogenomics. 2022 May;23(7):443–52. CrossRef
31. Cai X, Chen J, Chen M, Xia X, Fang G, Zhang J. Application of a warfarin dosing calculator to guide individualized dosing versus empirical adjustment after fixed dosing: a pilot study. Front Pharmacol. 2023 Aug 17;14:1235331. CrossRef
32. Falkenhagen U, Knöchel J, Kloft C, Huisinga W. Deriving mechanism-based pharmacodynamic models by reducing quantitative systems pharmacology models: an application to warfarin. CPT Pharmacometrics Syst Pharmacol. 2023 Apr;12(4):432–43. CrossRef
33. Wright DFB, Duffull SB. A Bayesian dose-individualization method for warfarin. Clin Pharmacokinet. 2013 Jan;52(1):59–68. CrossRef
34. Jiang NX, Ge JW, Xian YQ, Huang SY, Li YS. Clinical application of a new warfarin-dosing regimen based on the CYP2C9 and VKORC1 genotypes in atrial fibrillation patients. Biomed Rep. 2016 Apr;4(4):453–8. CrossRef
35. Asiimwe IG, Pirmohamed M. Drug-drug-gene interactions in cardiovascular medicine. Pharmacogenomics Pers Med. 2022 Nov 2;15:879–911. CrossRef
36. Zhu Y, Moriarty JP, Swanson KM, Takahashi PY, Bielinski SJ, Weinshilboum R, et al. A model-based cost-effectiveness analysis of pharmacogenomic panel testing in cardiovascular disease management: preemptive, reactive, or none? Genet Med. 2021 Mar;23(3):461–70. CrossRef
37. Kaur N, Pandey A, Shafiq N, Gupta A, Das R, Singh H, et al. Genetic and nongenetic determinants of variable warfarin dose requirements: a report from North India. Public Health Genomics. 2022 Jan;25(1–2):52–60. CrossRef
38. Farzamikia N, Sakhinia E, Afrasiabirad A. Pharmacogenetics-based warfarin dosing in patients with cardiac valve replacement: the effects of CYP2C9 and VKORC1 gene polymorphisms. Lab Med. 2018 Feb;49(1):25–34. CrossRef
39. Galvez JM, Restrepo CM, Contreras NC, Alvarado C, Calderón-Ospina CA, Peña N, et al. Creating and validating a warfarin pharmacogenetic dosing algorithm for Colombian patients. Pharmacogenomics Pers Med. 2018 Oct 16;11:169–78. CrossRef
40. Mak M, Lam C, Pineda SJ, Lou M, Xu LY, Meeks C, et al. Pharmacogenetics of warfarin in a diverse patient population. J Cardiovasc Pharmacol Ther. 2019 Nov;24(6):521–33. CrossRef
41. Pirmohamed M, Burnside G, Eriksson N, Jorgensen AL, Toh CH, Nicholson T, et al. A randomized trial of genotype-guided dosing of warfarin. N Engl J Med. 2013 Dec 12;369(24):2294–303. CrossRef
42. Baldacci A, Saguin E, Balcerac A, Mouchabac S, Ferreri F, Gaillard R, et al. Pharmacogenetic guidelines for psychotropic drugs: optimizing prescriptions in clinical practice. Pharmaceutics. 2023 Oct 27;15(11):2540. CrossRef
43. Pratt VM, Turner A, Broeckel U, Dawson DB, Gaedigk A, Lynnes TC, et al. Characterization of reference materials with an association for molecular pathology pharmacogenetics working group tier 2 status: CYP2C9, CYP2C19, VKORC1, CYP2C cluster variant, and GGCX: A GeT-RM collaborative project. J Mol Diagn. 2021 Aug;23(8):952–8. CrossRef
44. Johnson JA, Caudle KE, Gong L, Whirl-Carrillo M, Stein CM, Scott SA, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for pharmacogenetics-guided warfarin dosing: 2017 update. Clin Pharmacol Ther. 2017 Sep;102(3):397–404. CrossRef
45. Fahmi AM, Elewa H, El Jilany I. Warfarin dosing strategies evolution and its progress in the era of precision medicine, a narrative review. Int J Clin Pharm. 2022 Jun;44(3):599–607. CrossRef
46. Hippman C, Nislow C. Pharmacogenomic testing: clinical evidence and implementation challenges. J Pers Med. 2019 Aug 7;9(3):40. CrossRef
47. Sridharan K, Al Banna R, Malalla Z, Husain A, Sater M, Jassim G, et al. Influence of CYP2C9, VKORC1, and CYP4F2 polymorphisms on the pharmacodynamic parameters of warfarin: a cross-sectional study. Pharmacol Rep. 2021 Oct;73(5):1405–17. CrossRef
48. Anand A, Kumar R, Gupta A, Vijayvergiya R, Mehrotra S, Lad D, et al. Development of an interview-based warfarin nomogram predicting the time spent in the therapeutic INR range: a cost-effective, and non-invasive strategy building from a cross sectional study in a low resource setting. Indian Heart J. 2022 May-Jun;74(3):245–8. CrossRef
49. Sun B, Ma S, Xiao F, Luo J, Liu M, Liu W, et al. Integrated analysis of clinical and genetic factors on the interindividual variation of warfarin anticoagulation efficacy in clinical practice. BMC Cardiovasc Disord. 2023 May 31;23(1):279. CrossRef
50. Stevens SM, Woller SC, Kreuziger LB, Bounameaux H, Doerschug K, Geersing GJ, et al. Executive summary: antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021 Dec;160(6):2247–59. CrossRef
51. Lawal OD, Aronow HD, Hume AL, Shobayo F, Matson KL, Barbour M, et al. Venous thromboembolism, chronic liver disease and anticoagulant choice: effectiveness and safety of direct oral anticoagulants versus warfarin. Res Pract Thromb Haemost. 2024 Jan;8(1):102293. CrossRef