INTRODUCTION
Atherosclerosis is a multistep progressive vascular condition that includes lipid buildup due to hyperlipidemia and inflammation that may lead to lethal consequences [1–2]. Linked to that, the anti-hypercholesterolemic effect of omega-3 polyunsaturated fatty acids (n-3FAs) has been reported as one of the potential advantages of the supplement in the prevention of cardiovascular disease (CVD) [3–4]. However, this may be accomplished by medical treatment including healthful dietary components in daily routine. It is well known that n-3FA supplements contain eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in various ratios. These two dietary supplements alone or in their combination form n-3FA are endorsed as efficient supplements in the prevention of CVD through modulation of blood lipids and lipoproteins [5–6]. Nevertheless, their anti-lipidemic effects as a secondary prevention of CAD are still mixed [7–8]. Likewise, the results of prior studies concerning the effect of n-3FA supplementation on serum triglycerides (TGs) and low-density lipoprotein (LDL) levels are still controversial. Hence, this discrepancy in lipidemic outcomes has directed studies to resolve that throughout non-high-density lipoprotein cholesterol (NHC) levels [9–10].
The lack of n-3FA benefit has been attributed to the low ratio of EPA to DHA [11–12]. Therefore, the supplements of n-3FA (EPA and DHA), which do not produce a synergistic effect when combined should not be generalized [13]. Concerning Lipoprotein(a) Lp(a) levels which are causally linked to an increased CVD risk through a variety of pathways [14–15]. It has been reported that a high dose of n-3FA decreased Lp (a) and stable CAD via, a direct arterial effect of EPA [16]. Furthermore, dyslipidemia was observed to be more heightened in diabetic patients supplemented with DHA, and therefore, it seems that the n-3FA ratio modulates atherogenic biomarkers [17]. Nevertheless, no comparative studies between the anti-hyperlipidemic effect of EPA and DHA have been conducted yet in a controlled animal model.
On the other hand, despite the relevance of oxidized-low-density lipoprotein (Ox-LDL) levels in relationship to cardiovascular events has not been proven yet, it has been suggested that Ox-LDL is a promising purpose for future studies that illuminate the mysterious events in the progression of atherosclerosis [2]. The reduction in the levels of Ox-LDL recorded was partially like some earlier studies that indicated a decrease in Ox-LDL levels in response to n-3FA supplementation [18]. However, it has been reported that EPA differs from DHA in modifying membrane lipid structure and antioxidant properties [19–20]. Hence, the comparison between the effect of n-3FA mixture supplementation may clarify mysterious points resulting from the potential changes in serum non-HDL and Ox-LDL-C levels.
Despite the widespread use of n-3FA supplements among Jordanians, there are no prior studies that assessed or compared the combined effect of n-3FA mixture (EPA and DHA) on serum Ox-LDL-C levels in high diet fat models in experimental rats. To date, this is the first experimental study that investigates the combined effect of n-3FAs (EPA and DHA) on serum Ox-LDL-C levels in a high-diet fat model in experimental rats.
MATERIALS AND METHODS
Study design
Ethical approval
All procedures were performed in accordance with the international regulations for the care and use of laboratory animals. Ethical approval on the study was obtained by the Institutional Review Board at Applied Science Private University, Amman, Jordan, (Approval ID# 2022-PHA-35).
Animals setting groups
Male Wistar rats (n = 48, 190–220 g) were kept in metabolic cages and maintained under standard housing conditions (21°C–22°C and 12 hours light/dark cycles) for 7 days before starting the experiment. All study animals were equally provided with diet and water. In this experiment, based on the daily diet for 6 weeks, animals were distributed into six (6) groups (6 rats/group). In addition to Group 1: SD, negative control rats fed with a standard diet (SD) of 150 g/kg/day, five animal groups were exposed to the high-fat diet (FD) for 6 weeks and categorized as follows:
Group 2: FD, high fat-fed control group, and did not receive any treatment.
Group 3: atorvastatin (ATV), FD fed, were daily administered 2.5 mg/kg ATV for 4 weeks.
Group 4: n-3FA, FD fed, were daily administrated with omega-3 (Wild Salmon and Fish oil complex 1,000 mg; contains 180 mg as EPA and 120 mg as DHA)for 4 weeks.
Group 5: FD fed EPA: were daily administrated with Eicosatetraenoic acid (EPA), (1,000 mg/kg) for 4 weeks.
Group 6: FD fed DHA: were daily administrated with DHA, (1,000 mg/kg) for 4 weeks.
At baseline and final (at the end of week 6), the blood samples were collected by heart puncture for determination of Ox-LDL, Lp(a), TC, LDL, high-density lipoprotein (HDL), TG, aspartate transferase (AST), and alanine transferase (ALT). Non-HDL levels were calculated using the following equation:
(non-HDL = TC-HDL).
Induction of FD model in Wistar rats
To induce hyperlipidemia, animals were fed with FD; a mixture of lamb fat with an SD as conducted in a similar study design [21] for 14 days to elevate TC levels. The rats were confirmed to have higher serum TC levels than in the SD control group (TC = 50 ± 5 vs. 30 ± 5 mg/dl).
Supplements dosing
Animal equivalent dose (AED) for n-3FA has initially been calculated based on the following equation [22]:
HED (daily dose /kg) = 1,000 mg /60 (IU/kg) × 6.17 (converting factor for rodents ) = 102.8 mg /kg.
However, based on the preliminary experiments, the mentioned calculated dose was not effective. Because the design of the supplementation duration was relatively short, the dose was increased several times to be consistent with previous related studies [23–24]. Therefore, a high dose (1,000 mg/kg ) was recognized and used in the current study.
Dietary supplements
Eicosapentaenoic acid (EPA®), Garlson, USA. Each capsule (volume = 1.5 ml) contains 1,000 mg of EPA. Docosahexaenoic acid (DHA®), California Gold nutrition. Each capsule (volume = 1 ml) contains 1,000 mg of DHA. n-3FAs (Jamieson Laboratories, Canada N8W 585). Each soft gel capsule contains Wild Salmon and Fish oil complex 1,000 mg (n-3FAs = 300 mg) providing 180 mg as EPA and 120 mg as DHA.
Biochemical analysis
The biochemical analysis was undertaken in a quality control registered at the laboratories of Applied Science Private University-Faculty of Pharmacy. OX-LDL levels were determined by serum with an ELISA kit (MyBiosource, San Diego, CA, USA). Lp(a), TC, LDL, HDL, TGs, AST, and ALT levels were assayed using the HumaStar 200 (Germany).
Statistical analysis
The sample size calculation was done by using a crude method based on the law of diminishing return with the equation of E = total number of animals-total number of groups. After the calculation with (6 groups × 6 rats/group)—(6 groups) = 30, suggesting the sample size for this study was adequate and 6 rats were used for each group [25]. The statistical analysis was performed using a Statistical Package for the Social Sciences, version 27.0 for Windows (Chicago, IL, USA). P-value for two independent sample t-tests at baseline. To evaluate if there are any significant differences in the mean values between the study groups, at the end of the experiment ANOVA test was used. The post-hoc multiple comparisons by Dunnett’s test were used to find out any significant differences in each mean parameter between EPA or DHA supplemented and other study groups, a p-value < 0.05 is statistically significant.
RESULTS
Baseline assessment of body weight and lipid profile in SD and FD diet animals
The BW and lipid profile in control SD and FD groups are represented in Table 1. All lipid profile parameters were significantly elevated after 14 days of hyperlipidemia induction by daily high-fat diet feeding (p < 0.05).
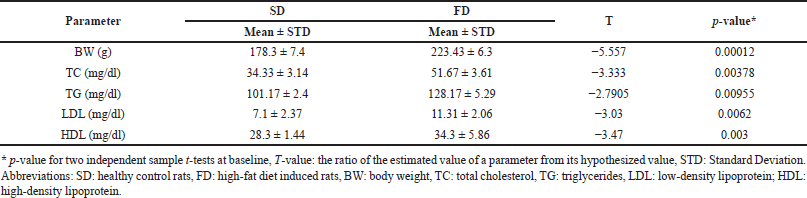 | Table 1. Baseline (day 14) means of the BW and lipid profile in SD and FD study groups. [Click here to view] |
Assessment of final changes in body weight and lipid profile parameters
Changes in body weight
A significant lower in the mean BW was observed between study groups as shown in Table 2. There were significantly higher mean BW in EPA and DHA-supplemented groups than in the SD group (p < 0.05) whereas, compared with FD, a higher mean BW was noted in the DHA as well as EPA-supplemented groups (p < 0.05) as presented in Figure 1.
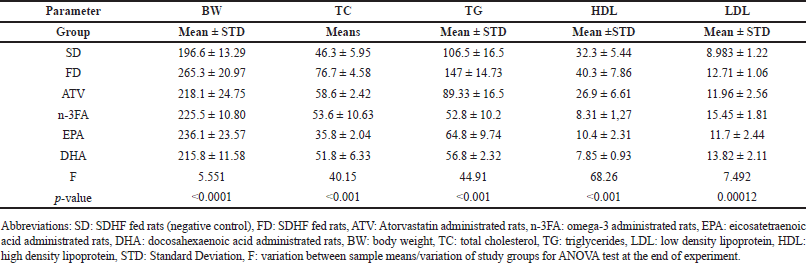 | Table 2. The mean differences of BW and lipid profile parameters at the end of the experiment (day 28). [Click here to view] |
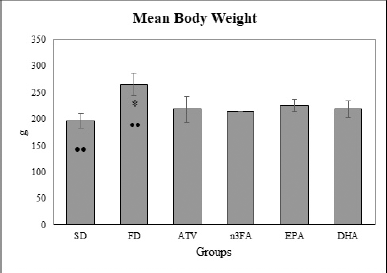 | Figure 1. The final mean body weight at the end of the experiment (day 28). [Click here to view] |
Changes in total cholesterol levels
At the end of the experiment, there were significant differences between the mean TC levels in all study groups (F = 40.15, p < 0.001). The post-hoc multiple comparisons by Dunnett’s test, as presented in Figure 2, indicated that mean TC levels were significantly lower in the EPA group than in all FD-study groups. The mean TC levels were significantly higher in the DHA group than in EPA (p < 0.001). In addition, lower mean TC levels were noted in the EPA group than in the n-3FA group (p < 0.01).
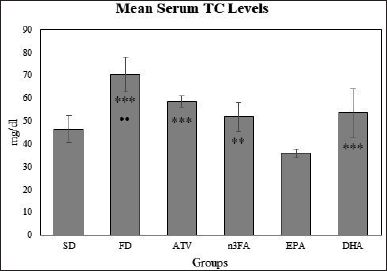 | Figure 2. The final mean TC levels at the end of the experiment (day 28). [Click here to view] |
Changes in Triglyceride levels
There was a significant difference in the mean TG levels between study groups (F = 44.91, p < 0.001). The post-hoc multiple comparisons test showed lower means TG levels in the EPA and DHA, p < 0.001 as well as in n-3FA and ATV-treated group than in the FD control group. No significant difference was observed in the mean levels of TG between EPA, DHA, and n-3FA study groups (Fig. 3).
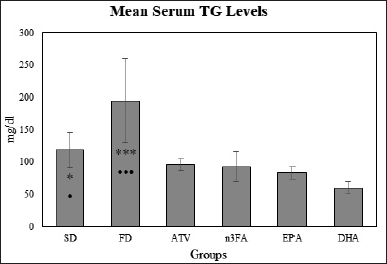 | Figure 3. The final mean TG levels at the end of the experiment (day 28). [Click here to view] |
Changes in HDL level
There was no significant difference in the mean HDL levels between EPA and DHA groups (t = −0.953, p = 0.983). The post-hoc multiple comparisons by the Tukey test indicated that the mean HDL levels for the control (FD) group were significantly higher than other groups (EPA, DHA, and 3-nFA) (Fig. 4).
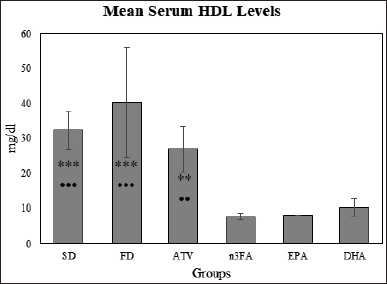 | Figure 4. The final mean HDL levels at the end of the experiment (day 28). [Click here to view] |
Changes in LDL level
As shown in Table 2 and Figure 5, the mean serum LDL levels were significantly higher in the DHA than in the SD control group (13.82 ± 2.11 vs. 8.983 ± 1.22 mg/dl, T = 6.07, p < 0.05). Compared with the SD group, the mean serum LDL levels were significantly higher in the n-3FA group (15.45 ± 1.81vs. 8.983 ± 1.22 mg/dl, T = 8.121, p < 0.001). The post-hoc multiple comparisons also, indicated that the mean LDL levels for the n-3FA group were significantly higher than in EPA and ATV groups (p < 0.05).
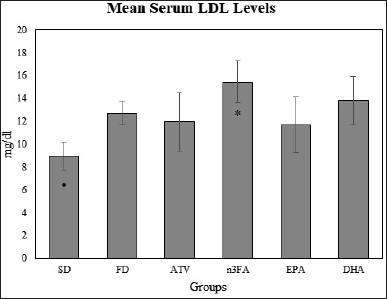 | Figure 5. The final mean LDL levels at the end of the experiment (day 28). [Click here to view] |
Changes in selected atherogenesis progression predictors
As presented in Table 3, using ANOVA analysis significant differences were detected in the mean levels of selected thermogenesis progression predictors (NHCL, Lp (a), and Ox-LDL) between supplemented and other groups (p < 0.001).
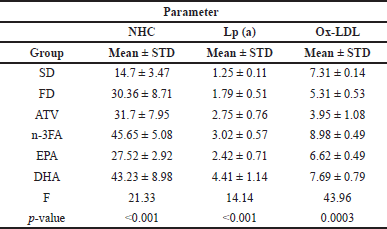 | Table 3. The assessment of non-HDL, lipoprotein A, and oxidized LDL levels at the end of experiment of all study groups. [Click here to view] |
Changes in Non-HDL (NHC) levels
At the end of the experiment, there was a significant difference in mean NHC levels (F = 21.62, p < 0.001) among the control and treatment groups as represented in Table 3. The post-hoc multiple comparisons test showed a significantly higher mean level in DHA and n-3FA, compared with EPA-supplemented rats (27.52 ± 2.92 vs. 43.23 ± 8.98 and 45.65 ± 5.08, respectively, p < 0.001) as shown in Figure 6. A higher mean NHC level was observed in n-3FA than in ATV-treated rats (t = 5.6103, p < 0.05).
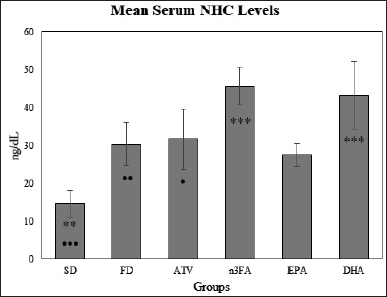 | Figure 6. The final mean NHC levels at the end of the experiment (day 28). [Click here to view] |
Changes in Lipoprotein (A) levels
As shown in Table 3, there was a significant difference in mean Lp (a) level between study groups (F = 12.68, p < 0.001). Based on the post-hoc multiple comparisons tests, there was significantly lower mean Lp(a) levels in EPA than in the DHA group (2.42 ± 0.71 vs. 4.41 ± 1.14 ng/dl, p < 0.001). Compared with other study groups, the DHA group has shown also higher mean Lp(a) levels (Fig. 7).
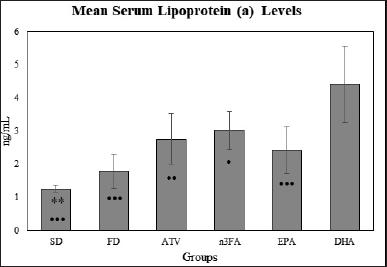 | Figure 7. The final mean Lp (a) levels at the end of the experiment (day 28). [Click here to view] |
Changes in Ox-LDL level
Table 3 showed a significant difference in the mean Ox-LDL levels (p < 0.001) between study groups. These differences are shown by the post-hoc multiple comparisons test. Higher mean Ox-LDL levels in n-3FA than EPA or DHA-supplemented groups (Fig. 8). The most significant lower mean Ox-LDL was shown in the ATV group. No significance in mean Ox-LDL levels was observed between EPA and DHA study groups (t = 3.62, p = 0.1387).
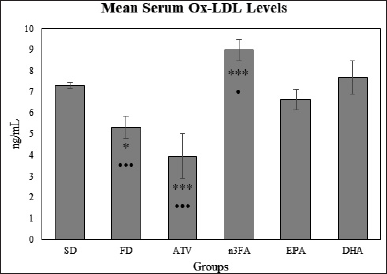 | Figure 8. The changes in mean Ox-LDL levels at the end of the experiment (day 28). [Click here to view] |
The assessment of liver function tests
Table 4 demonstrated that there was a significant difference in the mean levels of AST (p < 0.001) between study groups. No significant differences in the mean levels of both AST and ALT were observed between DHA and EPA-supplemented groups (t = 0.9431, 1.6989, p = 0.9843, 0.8327, respectively). Compared with EPA, a remarkable elevation in the mean ALT levels was observed in the n-3FA supplemented group (t = 5.53, p = 0.006).
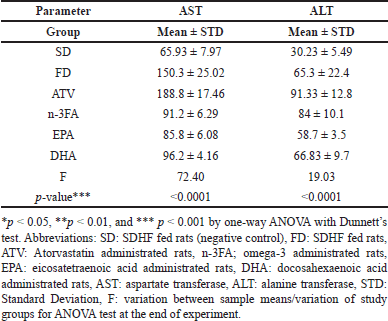 | Table 4. The assessment of liver function test at the end of experiment of all study groups. [Click here to view] |
DISCUSSION
The current study was designed to assess whether high doses of EPA, DHA, and their combination n-3FA induce any negative consequences on lipid profile and some atherogenic biomarkers in a hyperlipidemic-induced animal model. It showed significantly higher mean TC, non-HDL, and Lp (a) in the DHA-supplemented group than in the EPA-supplemented group. These findings were consistent with prior studies that revealed the n-3FA ratio modulates serum levels of atherogenic biomarkers [9,17].
Omega-3 supplementation has been reported to improve lipid profile, particularly TG levels [25–26]. In this study, except for LDL, all lipid profile parameters were significantly lower in the n-3FA-supplemented group than in the FD control group. Regardless of treatment protocol, these observations were consistent with previous studies. For example, elevated LDL levels have been noted with a significant reduction in TC and TG levels in response to 2 g/day of n-3FAs for 8 weeks in hyperlipidemic subjects [7]. A significant increase in TC, HDL, and LDL levels was also seen in diabetic men after using 3 g/ day of n-3FAs for 8 weeks [27]. It seems that the TG-lowering effect of high-dose n-3FA is accompanied by elevated LDL levels. Nevertheless, inconsistency in lipidemic outcomes about omega-3 supplementation has directed studies to resolve that throughout NHC levels. Based on this, except in the DHA group, the current study showed that the mean NHC levels in n-3FA were significantly higher than in other study groups. Overall, the intake of high doses of n-3FA in a formulation (EPA 180: DHA 120) might be not effective to counteract dyslipidemia consequences which was consistent with our results [28].
Accordingly, these findings are consistent with the data revealing DHA supplements have been shown to cause a negative impact on atherogenic biomarkers as noted in the current study. Furthermore, Allaire et al. [27] demonstrated that, EPA and DHA molecules had a detrimental impact on elevating LDL levels. Similar findings were found in a previous investigation which indicated that EPA did not raise LDL levels and the DHA group had a higher level of LDL [29]. Furthermore, high-dose DHA has more profound effects on LDL-related features than high-dose EPA [27]. In a systematic review examined, the effect of DHA among 485 healthy individuals, DHA in an average dose of 1.68 g/day may reduce TG levels and increase HDL and LDL levels [30]. Furthermore, in an important related clinical trial for 1-year follow-up, high doses of EPA (4 g/day) alone significantly decreased non-HDL levels [31]. Similarly, elevated LDL levels have been observed only in DHA-supplemented men with no change in their peer-supplemented with EPA [27]. Linked to elevated LDL levels in the DHA-supplemented group, similar observations for Lp (a) levels which is known as a form of LDL, were noted in the current study. These findings confirmed Nicholls et al. [28] claim who have hypothesized that the lack of cardiovascular benefit with (omega-3 CA) could reflect adverse effects from coadministration of DHA. Nevertheless, Nevertheless, the two predictors are not proportionally related where statin therapy lowers actual LDL but not Lp (a) [14–15]. Likewise, the present study has shown no significant in the mean difference of LDL levels between EPA and DHA groups whereas the mean Lp (a) levels were significantly higher in DHA than in APA groups. A potential negative lipidemic effect of DHA supplementation might be more obvious when it is linked to NHC changes which were more consistent with Lp than LDL changes. It has been reported that a high dose of n-3FA decreased Lp (a) and stable CAD via a direct arterial effect of EPA [16]. Furthermore, dyslipidemia was observed to be more heightened in diabetic patients supplemented with DHA, and therefore, the n-3FA ratio is responsible for the inconsistency in the changes of atherogenic biomarkers [17].
Our findings are consistent with these results, where an equal dose of EPA when compared to DHA, has shown better laboratory findings concerning selected lipidemic and atherogenic biomarkers. Results of a previous study demonstrated that pretreatment with EPA had pleiotropic effects on rapid pacing-induced atrial remodeling, including improving anticoagulant activity [32]. It has shown that the intake of omega-3 formulation EPA:DHA 6:1 by rats for 2 weeks improved endothelium-dependent relaxations [33]. In addition, EPA supplementation has been shown to alter the progression of thrombosis via a potential mechanism related to the lipid metabolic pathway [34]. On the other hand, the risk of high doses of n-3FA in terms of the dyslipidemic effect observed in this study may be related to the potential thrombotic risk of n-3FA supplementation for patients with COVID-19 [35].
Studies on animal models have demonstrated that n-3FA inhibits fatty acid synthesis and stimulates fatty acid oxidation in the liver, which would reduce the availability of fatty acids for TG synthesis [36,,37]. The increase in fatty acid oxidation is due to omega-3 fatty acids activating peroxisome proliferation-activated receptors alpha, which stimulates fatty acid oxidation in the liver and other tissues [38,,39]. This way may potentially be in accordance with our results concerning elevated ox-LDL in the omega-3 supplemented group. The elevation of Ox-LDL levels in the omega-3 group might be correlated as a possible consequence of high doses of the supplement. Linked to that, elevated serum liver enzyme was observed in the DHA and EPA groups of the current study which have been previously reported in animal models that received high doses of omega-3 [40]. Furthermore, elevated serum liver enzyme levels have been associated with liver injury that is accompanied by an increase in DHA and EPA levels [41]. Consistent with our findings, elevated AST and ALT levels with significant depletion in hepatic glutathione were induced by high doses of EPA in dyslipidemic patients [42].
Several lines of evidence led researchers to hypothesize that EPA differs from DHA in modifying membrane lipid structure and antioxidant properties. It interferes with lipid oxidation and several pathways leading to endothelial dysfunction [5]. According to a recent systematic review, only EPA, at a pharmacologic dosage equal to 4 g/day, can interfere with atherosclerosis mechanisms. It reduced TG and Ox-LDL-C levels in subjects with normal LDL levels [19]. Nevertheless, elevated serum Ox-LDL-C levels are still a disagreement point among researchers, whether it is considered an advantage or disadvantage for atherogenesis. Previous studies have demonstrated that the sex-dependent differences in the metabolism of omega-3 polyunsaturated fatty acids in both humans and animals explain the differential regulation of hemostasis in men and women [43––46]. Therefore, the current study just focused on males, excluding females, due to the potential gender-related confounders that might be responsible for the inconsistency of the results seen in the prior related studies. Nevertheless, the current study model was based on male rats only, and the duration of the experiment was relatively short. These factors were considered as the experiment’ limitations. Besides these limitations, the measurement of HDL subfractions in both genders and whether these factors are correlated to the effects of EPA and DHA should be taken into consideration as a part of the future research scope.
CONCLUSION
The effect of equal doses of EPA and DHA on serum NHC and LP(a) levels was inconsistent. While the DHA supplementation has shown a dyslipidemic effect, but the EPA supplement does not ameliorate the risk of atherosclerosis. Our findings do not recommend the intake of high doses of DHA supplements alone, or in n-3FA formulation (EPA 180:DHA 120) to prevent or treat dyslipidemia or adverse CAD risk.
ACKNOWLEDGMENTS
The authors are grateful to the Applied Science Private University (ASU), Amman, Jordan, for the full support for this research project.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Ethical approval details given in the 'Material and Methods section'.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Abu-Taha M, Dagash R, Mohammad BA, Basheiti I, Abu-Samak MS. Combined effect of coffee consumption and cigarette smoking on serum levels of vitamin B12, folic acid, and lipid profile in young male: a cross-sectional study. Int J Gen Med. 2019;12:421–32. CrossRef
2. Poznyak AV, Nikiforov NG, Markin AM, Kashirskikh DA, Myasoedova VA, Gerasimova EV, et al. Overview of OxLDL and its impact on cardiovascular health: focus on atherosclerosis. Front Pharmacol. 2020;11:613780. CrossRef
3. Stewart J, Manmathan G, Wilkinson P. Primary prevention of cardiovascular disease: a review of contemporary guidance and literature. JRSM Cardiovasc Dis. 2017;6:2048004016687211. CrossRef
4. Wang HH, Garruti G, Liu M, Portincasa P, Wang DQ. Cholesterol and lipoprotein metabolism and atherosclerosis: recent advances in reverse cholesterol transport. Ann Hepatol. 2017;16(Suppl. 1: s3-105.):s27–s42. CrossRef
5. Kelsey MD, Pagidipati NJ. Should we “RESPECT EPA” more now? EPA and DHA for cardiovascular risk reduction. Curr Cardiol Rep. 2023;25(11):1601–9. CrossRef
6. Sherratt SC, Libby P, Bhatt DL, Mason P.Eicosapentaenoic acid (EPA) inhibits low-density lipoprotein (LDL) oxidation compared to docosahexaenoic acid (DHA) and mineral oil in vitro. Circulation. 2022;146(1):A13685. CrossRef
7. Zibaeenezhad MJ, Ghavipisheh M, Attar A, Aslani A. Comparison of the effect of omega-3 supplements and fresh fish on lipid profile: a randomized, open-labeled trial. Nutr Diabetes. 2017;7(12):1–8. CrossRef
8. Manson JE, Cook NR, Lee I-M, Christen W, Bassuk SS, Mora S, et al. Marine n−3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2018;380(1):23–32. CrossRef
9. Mehdawi A, Mohammad BA, Mosleh I, Khader HA, Habash M, Nassar RI, et al. Combined effect of omega-3 fatty acid and vitamin D3 on oxidized LDL-C and non–HDL-C levels in people with vitamin D deficiency: a randomized controlled trial. J Cardiovasc Pharmacol. 2023;81(4):251–8. CrossRef
10. Awwad S, Abu-Zaiton A, Issa R, Said R, Sundookah A, Habash M, et al. The effect of excessive coffee consumption, in relation to diterpenes levels of medium-roasted coffee, on non-high-density lipoprotein cholesterol level in healthy men. Pharmacia. 2023;70(1):49–59. CrossRef
11. Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, et al. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77?917 individuals. JAMA Cardiol. 2018;3(3):225–34. CrossRef
12. Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, et al. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med. 2018;379(16):1540–50. CrossRef
13. Nissen SE, Lincoff AM, Wolski K, Ballantyne CM, Kastelein JJ, Ridker PM, et al. Association between achieved ω-3 fatty acid levels and major adverse cardiovascular outcomes in patients with high cardiovascular risk: a secondary analysis of the STRENGTH trial. JAMA Cardiol. 2021;6(8):910–7. CrossRef
14. Scipione CA, Koschinsky ML, Boffa MB. Lipoprotein(a) in clinical practice: new perspectives from basic and translational science. Crit Rev Clin Lab Sci. 2018;55(1):33–54. CrossRef
15. Schrock CG. Lipoprotein(a): it is not the cholesterol content: it is the apolipoprotein(a)! Eur Heart J. 2019;40(43):3576. CrossRef
16. Ward NC, Ying Q, Chan DC, Pang J, Mori TA, Schultz CJ, et al. Improved arterial inflammation with high dose omega-3 fatty acids in patients with elevated lipoprotein(a): selective effect of eicosapentaenoic acid? J Clin Lipidol. 2023;17(5):694–9. CrossRef
17. Gouaref I, Bouazza A, Abderrhmane SA, Koceir EA. Lipid profile modulates cardiometabolic risk biomarkers including hypertension in people with type-2 diabetes: a focus on unbalanced ratio of plasma polyunsaturated/saturated fatty acids. Molecules. 2020;25(18):4315. CrossRef
18. Gajos G, Zalewski J, Mostowik M, Konduracka E, Nessler J, Undas A. Polyunsaturated omega-3 fatty acids reduce lipoprotein-associated phospholipase A2 in patients with stable angina. Nutr Metab Cardiovasc Dis. 2014;24(4):434–9. CrossRef
19. Mason RP. New insights into mechanisms of action for omega-3 fatty acids in atherothrombotic cardiovascular disease. Curr Atheroscler Rep. 2019;21(1):2. CrossRef
20. Sezai A, Unosawa S, Taoka M, Osaka S, Obata K, Kanno S, et al. Long-term comparison of ethyl icosapentate versus. Omega-3-acid ethyl in patients with cardiovascular disease and hypertriglyceridemia (DEFAT Trial). Circ J. 2019;83(6):1368–76. CrossRef
21. Al-Bayati M, Issa R, Abu-Samak M, Alnsour L, Awwad S. Phytochemical analysis and evaluation of anti-hyperlipidaemic effect for ethanolic leaf extract of Equisetum ramosissimum L.: in vivo study on rats’ models. Pharmacia. 2023;70(3):557–68. CrossRef
22. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016 Mar;7(2):27–31. CrossRef
23. Oda SS. The influence of Omega3 fatty acids supplementation against aluminum-induced toxicity in male albino rats. Environ Sci Pollut Res Int. 2016;23(14):14354–61. CrossRef
24. de Sales Guilarducci J, Marcelino BAR, Konig IFM, Orlando TM, Varaschin MS, Pereira LJ. Therapeutic effects of different doses of prebiotic (isolated from Saccharomyces cerevisiae) in comparison to n-3 supplement on glycemic control, lipid profiles and immunological response in diabetic rats. Diabetol Metab Syndr. 2020;12:69. CrossRef
25. Charan J, Kantharia ND. How to calculate sample size in animal studies? J Pharmacol Pharmacother. 2013;4(4):303–6. CrossRef
26. Mason RP, Sherratt SCR, Eckel RH. Rationale for different formulations of omega-3 fatty acids leading to differences in residual cardiovascular risk reduction. Metabolism. 2022;130:155161. CrossRef
27. Allaire J, Couture P, Leclerc M, Charest A, Marin J, Lépine MC, et al. A randomized, crossover, head-to-head comparison of eicosapentaenoic acid and docosahexaenoic acid supplementation to reduce inflammation markers in men and women: the Comparing EPA to DHA (ComparED) Study. Am J Clin Nutr. 2016;104(2):280–7. CrossRef
28. Nicholls SJ, Lincoff AM, Garcia M, Bash D, Ballantyne CM, Barter PJ, et al. Effect of high-dose omega-3 fatty acids versus corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324(22):2268–80. CrossRef
29. Asztalos IB, Gleason JA, Sever S, Gedik R, Asztalos BF, Horvath KV, et al. Effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular disease risk factors: a randomized clinical trial. Metabolism. 2016;65(11):1636–45. CrossRef
30. Bernstein AM, Ding EL, Willett WC, Rimm EB. A meta-analysis shows ythat docosahexaenoic acid from algal oil reduces serum triglycerides and increases HDL-cholesterol and LDL-cholesterol in persons without coronary heart disease. J Nutr. 2012;142(1):99–104. CrossRef
31. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11–22. CrossRef
32. Tong M, Wang J, Ji Y, Chen X, Wang J, Wang S, et al. Effect of eicosapentaenoic acid and pitavastatin on electrophysiology and anticoagulant gene expression in mice with rapid atrial pacing. Exp Ther Med. 2017;14(3):2310–6. CrossRef
33. Farooq MA, Gaertner S, Amoura L, Niazi ZR, Park SH, Qureshi AW, et al. Intake of omega-3 formulation EPA:DHA 6:1 by old rats for 2 weeks improved endothelium-dependent relaxations and normalized the expression level of ACE/AT1R/NADPH oxidase and the formation of ROS in the mesenteric artery. Biochem Pharmacol. 2020;173:113749. CrossRef
34. Adili R, Hawley M, Holinstat M. Regulation of platelet function and thrombosis by omega-3 and omega-6 polyunsaturated fatty acids. Prostag Oth Lipid M. 2018;139:10–18. CrossRef
35. Rogero MM, Leão MC, Santana TM, Pimentel MVMB, Carlini GCG, da Silveira TFF, et al. Potential benefits and risks of omega-3 fatty acids supplementation to patients with COVID-19. Free Radic Biol Med. 2020;156:190–9. CrossRef
36. Green CJ, Pramfalk C, Charlton CA, Gunn PJ, Cornfield T, Pavlides M, et al. Hepatic de novo lipogenesis is suppressed and fat oxidation is increased by omega-3 fatty acids at the expense of glucose metabolism. BMJ Open Diabetes Res Care. 2020;8(1):e000871. CrossRef
37. ?ebrowska A, Hall B, Stolecka-Warzecha A, Stanula A, Sadowska-Kr?pa E. The effect of omega-3 fatty acid supplementation on serum adipocytokines, lipid profile and biochemical markers of inflammation in recreational runners. Nutrients. 2021;13(2):456. CrossRef
38. Xie X, Liu X, Li R, Fan L, Huang F. ω3 fatty acids in atherosclerotic cardiovascular disease (Review). Biomed Rep. 2024;20(6):94. CrossRef
39. Dubois V, Eeckhoute J, Lefebvre P, Staels B. Distinct but complementary contributions of PPAR isotypes to energy homeostasis. J Clin Invest. 2017;127:1202–14. CrossRef
40. Ketsa OV, Marchenko MM. [The effect of diet ratio of polyunsaturated fatty acids of omega-3 and omega-6 families on activity of aminotransferases and gamma-glutamyltransferase in rat blood serum]. Vopr Pitan. 2014;83(1):27–32.
41. Vatsalya V, Song M, Schwandt ML, Cave MC, Barve SS, George DT, et al. Effects of sex, drinking history, and omega-3 and omega-6 fatty acids dysregulation on the onset of liver injury in very heavy drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2016;40(10):2085–93. CrossRef
42. Fraser DA, Wang X, Lund J, Nikoli? N, Iruarrizaga-Lejarreta M, Skjaeret T, et al. A structurally engineered fatty acid, icosabutate, suppresses liver inflammation and fibrosis in NASH. J Hepatol. 2022;76(4):800–11. CrossRef
43. Lin YH, Brown JA, DiMartino C, Dahms I, Salem N Jr, Hibbeln JR. Differences in long chain polyunsaturates composition and metabolism in male and female rats. Prostaglandins Leukot Essent Fatty Acids. 2016;113:19–27. CrossRef
44. Childs CE, Romeu-Nadal M, Burdge GC, Calder PC. Gender differences in the n-3 fatty acid content of tissues. Proc Nutr Soc. 2008;67(1):19–27. CrossRef
45. Sibbons CM, Brenna JT, Lawrence P, Hoile SP, Clarke-Harris R, Lillycrop KA, et al. Effect of sex hormones on n-3 polyunsaturated fatty acid biosynthesis in HepG2 cells and in human primary hepatocytes. Prostaglandins Leukot Essent Fatty Acids. 2014 Feb-Mar;90(2-3):47–54. CrossRef
46. Phang M, Scorgie FE, Seldon M, Garg ML, Lincz LF. Reduction of prothrombin and Factor V levels following supplementation with omega-3 fatty acids is sex dependent: a randomised controlled study. J Nutr Biochem. 2014 Oct;25(10):997–1002. CrossRef