INTRODUCTION
The global prevalence of chronic wounds presents a significant healthcare challenge, affecting millions of patients and placing a substantial economic burden on healthcare systems worldwide [1]. Despite advancements in wound care management, there remains an urgent need for more effective, affordable, and accessible treatments, particularly in resource-limited settings [2]. In recent years, there has been a growing interest in exploring natural compounds for their potential wound-healing properties, with Persea americana Mill, commonly known as avocado, emerging as a promising candidate [3,4].
Persea americana has a long history of traditional medicinal use and has demonstrated various pharmacological activities, including anti-inflammatory [5], antioxidant [6], and antimicrobial properties [7]. Additionally, P. americana has demonstrated promising wound-healing properties in several studies. Nayak et al. [8] reported that a topical avocado fruit extract significantly accelerated wound closure in excision wounds of rats. Moreover, Hayati et al. [9] observed that an avocado leaf extract gel improved the healing process in mice. However, the complex molecular mechanisms underlying its potential wound-healing effects remain largely unexplored. This research gap hinders the development of evidence-based applications and the full exploitation of Persea americana’s therapeutic potential in wound management.
The integration of advanced computational approaches, such as network pharmacology and molecular docking, with in vivo studies offers a powerful strategy to elucidate the biological mechanisms of natural compounds [10–12]. This comprehensive approach can provide valuable insights into the multi-target, multi-component nature of herbal medicines, enabling a more holistic understanding of their therapeutic effects [13].
Our research seeks to bridge a crucial knowledge gap by utilizing a comprehensive strategy to explore how P. americana potentially functions as a wound-healing agent at the biological level. Through the integration of network pharmacology, molecular docking techniques, and in vivo experiments, we aim to decipher the intricate relationships between the active components found in P. americana and their corresponding molecular targets in wound healing pathways. The outcomes of this investigation could potentially catalyze the creation of innovative, nature-derived wound treatments and bolster the expanding scientific foundation supporting the integration of traditional plant-based remedies into contemporary medical practices.
MATERIALS AND METHODS
Sample preparation, extraction, and preliminary phytochemical screening
Fresh leaves of P. americana were harvested from South Kemering Ulu, South Sumatera-Indonesia. The sample was then botanical authenticated at PT. Mitra Dulur Sejahtera Palembang, with identification number 001/MDS-UKOT/IV/2023. The leaves were sorted, cleaned, and air-dried for 7 days, followed by pulverization using a laboratory mill. A 500 g of P. americana dry powder leaf was extracted using 2 l of 96% ethanol (technical grade, Brataco, Indonesia) in an isolated glass chamber for 48 hours at room temperature. The liquid extract was then filtered using Whathman filter paper No. 1 in a Buchner funnel (Buchi, Germany) equipped with a vacuum pump. The residues were re-extracted as much as three times using the same step previously. The menstruum was collected and evaporated using a vacuum rotary evaporator (Buchi, Germany) at 45°C with 90 radial per minute. The extract was subjected to preliminary phytochemical screening, according to the methods described by Ayu et al. [14].
In vivo study
Animal and study design
All experimental protocols strictly followed the guidelines outlined in the European Communities Council Directive (2010/63/EU, September 22, 2010). The study design received formal approval (Protocol Number: 002301/SITI KHADIJAH PALEMBANG/2024) from the National Peripheral Veterinary Authority Animal Ethics Committee, subsequent to a favorable review by the Animal Protocols Evaluation Committee. This ensured compliance with ethical standards and animal welfare regulations throughout the research process.
This study utilized male Wistar mice ranging from 1 to 4 months in age. The mice were sourced from the Department of Pharmacology, School of Pharmacy, Bandung Institute of Technology’s Small Animal Laboratory breeding stock. Environmental conditions in the animal facility were carefully controlled, maintaining a temperature of 25°C ± 1°C and humidity between 25% and 45%. Lighting was provided by yellow fluorescent tubes on a 12-hour light-dark cycle. The animals were given unlimited access to both standard chow and fresh water throughout the study period.
The experimental protocol involved dividing the mice into five distinct groups, each containing 5 animals. These groups were subjected to different treatments, with three groups receiving topical applications of various extract-containing ointments, one group receiving topical povidone iodine-containing ointments, and one group serving as a negative control. Specifically, the treatment groups (groups 1–3) were administered ointments containing 5%, 10%, and 15% (b/v) of P. americana leaf extract, respectively. The fourth group was administered povidone iodine-containing ointments, and the fifth group received no treatment, acting as a baseline for comparison. This experimental design allowed for the systematic evaluation of the effects of different extract-based ointments concentration against an untreated condition, providing a comprehensive assessment of their potential efficacy or impact.
Across all trials, wounds underwent a standardized daily cleaning protocol: a 1:1 soap-saline solution wash, followed by a saline rinse. Post-cleaning, wounds were either topically treated with extract preparations or left untreated as a negative control.
Wound infliction
Mice underwent anesthesia using a 3:1 ketamine (100 mg/ml) to xylazine (20 mg/ml) mixture. A 2 cm dorsal skin section was surgically removed. Post-excision, the wound was cleansed with a 1:1 soap-saline solution, followed by a saline rinse. Extract preparation (20 mg total) was applied topically, with 10 mg on the wound and 10 mg on the surrounding area. Subjects were then placed in individual cages under warming lamps and monitored until full recovery from anesthesia.
Clinical evaluation
Mice underwent daily clinical assessments, which included monitoring the total wound area, tracking healing progress, evaluating skin inflammation intensity, and observing mobility. A vernier caliper was used to measure the wound area precisely. Wound closure was defined and quantified as the reduction in wound size, with results expressed as a percentage of the original wound area. This approach allowed for a comprehensive daily record of each subject’s healing process and overall condition.
Network pharmacology study
Target identification
SMILES notations for P. americana phytoconstituents, as reported by Park et al. [15], were retrieved from PubChem (Table 1). These were analyzed using Super-PRED’s Homo sapiens mode to predict potential targets. The STRING database was then employed to standardize protein IDs and eliminate duplicates. Inflammation and fibrinolytic-associated targets were extracted from GeneCards. Finally, VENNY 2.1 was utilized to identify both unique and shared targets between P. americana phytocompounds and inflammation and fibrinolytic.
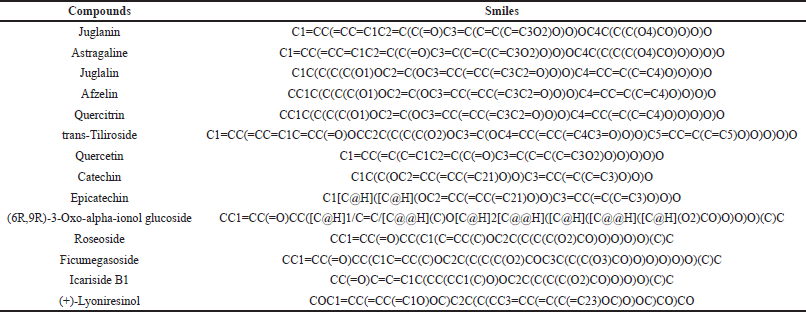 | Table 1. Chemical compound of P. americana along with their canonical SMILES. [Click here to view] |
Construction of protein-protein interaction
Previously identified proteins underwent analysis using STRING’s multiple protein feature. The analysis parameters were set to Homo sapiens as the organism, full STRING network type, high confidence score (0.400), and medium false discovery rate (FDR) stringency (5%). The resulting protein-protein interaction (PPI) data were exported in TSV format via the “explore” option. Subsequently, these data were imported into Cytoscape 3.10.1 software for network visualization and construction. This process enabled a comprehensive analysis of the protein interactions, from initial parameter setting to final network visualization.
Network construction and topological analysis
Cytoscape 3.10.1 software was used to process the TSV-formatted PPI data for topological analysis. The network analyzer tool performed topology parameter analysis. In the resulting network, nodes represented targets, pathways, and compounds, while edges showed their interactions. Node significance was determined by connectivity degree, with more connected nodes considered more influential.
Gene ontology (GO) and kyoto encyclopaedia of genes and genomes (KEGG) analysis
The functional roles of the identified targets were analyzed using the ShinyGO 0.80 tool. GO categories, including biological processes, cellular components, and molecular functions, as well as KEGG pathways, were encompassed in this analysis. A diFDR threshold of 0.05 was applied. The significant pathways and processes associated with the intersecting targets were then visualized through bar plot maps, which were generated from the analysis results.
Molecular docking study
All 14 compounds reported by Park et al. [15] were subjected to molecular docking to confirm their potential wound-healing activity on the core target NF-KB1 obtained from the network pharmacology. To prepare the molecules for docking, the 2D structures of all 14 ligands were sketched d using ChemDraw Ultra 12.0 and then converted into 3D structures using Chem 3D Pro. The ligand structures were then optimized through MM2 energy minimization and saved in Protein Data Bank (PDB) format. The X-ray crystal structure of the target macromolecule, NF-κB1, was retrieved from the PDB database with the PDB ID 4DN5 [16]. The macromolecule structure was then processed using Biovia Discover Studio Visualizer, where the ligand, water, heteroatoms, and unnecessary chains were removed, and polar hydrogen atoms were added. The final macromolecule structure was saved in PDB format, ready for the molecular docking analysis.
After ligand and macromolecule preparation, molecular docking was implemented. The prepared macromolecule was input in PyRx and converted into pdbqt format, while the ligands were input, processed, and converted into pdbqt format using OpenBabel platform in PyRx. The docking coordinate was set to x: -9.1978, y: 30.9392, y: -3.66568 with an adjustment of the gridbox dimensions (x: 17.5161, y: 9.3864, z: 11.2432). The molecular docking used Autodock Vina platform in a PyRx 0.8 version software with an exhaustiveness of 200 to assess the binding affinity between ligands and macromolecules. The docking score was then saved into CSV format and presented as free binding energy (kcal/mol).
The three best compounds (quercetin, catechin, and epicatechin) were further analyzed for their potential synergistic effect by employing multi-structural molecular docking study as reported by Setiawansyah et al. [17]. The best conformation of each compound obtained from the initial docking simulation was attached to the NF-KB1 co-crystallized structure. The complex ligand-NFKB1 structure was then used as the target macromolecules in the docking simulation employing the same protocols as in the initial docking process.
Toxicological assessment
The toxicity profile of P. americana compounds was evaluated using Biosig pkCSM web server (https://biosig.lab.uq.edu.au/pkcsm/prediction). The canonical SMILES of each compound were used to obtain the toxicity data including skin sensitization, LD50, mutagenicity, and hepatotoxicity.
Statistical analysis
Statistical analysis of wound healing data was undertaken using GraphPad Prism version 10.0.1. The data were presented as mean ± SD (n = 5) and the significance of the result was assessed using Two-Way ANOVA followed by a Tukey’s test.
RESULT AND DISCUSSION
Extraction and preliminary phytochemical screening
The maceration process yielded 246 g of crude extract, corresponding to 49.2% of the sample’s dry weight. This result not only meets but significantly exceeds the standards set by the Indonesian Herbal Pharmacopoeia, which stipulates that plant extracts obtained through extraction should yield no less than 10%. The substantial extract yield achieved in this study is indicative of a highly efficient extraction process. The effectiveness of ethanol as a solvent for extracting bioactive compounds from P. americana leaves has been well-documented in previous studies. For instance, Utami et al. [18] reported that ethanol extraction from avocado leaves resulted in a high yield of crude extract and natural dyes compared to other solvents (i.e., water, methanol, and acetone). Their study, which utilized 70% ethanol, demonstrated the solvent’s capacity to extract a wide range of polar and semi-polar compounds. The choice of 96% ethanol in the current study aligns with research by Owolabi et al. [19], who found that higher concentrations of ethanol were particularly effective in extracting antioxidant compounds from avocado leaves. They reported extract yields ranging from 15 to 20%, which, while substantial, are lower than the yield achieved in the present study. In contrast, this work provided a higher yield compared to previous work reported by Hidayati et al. [20] which only yielded 25% of avocado leaf extract using 96% ethanol. This difference might be attributed to variations in extraction techniques, solvent, plant material quality and size, or environmental factors affecting the leaves’ composition [21,22].
The extract was then subjected to preliminary phytochemical screening to analyze the class compound present in the extract qualitatively. As summarized in Table 2, P. americana leaf extract used in the current work seemed to possess all class compounds tested, including alkaloids, flavonoids, phenols, and saponins. These results collectively indicate that P. americana leaf extract is rich in bioactive compounds, which may explain its traditional medicinal uses and potential therapeutic applications. The positive results across all tested metabolite classes underscore the potential of avocado leaf extract as a source of diverse phytochemicals. This aligns with the growing interest in plant-based natural products for pharmaceutical and nutraceutical applications.
 | Table 2. Preliminary phytochemical screening result of P. americana leaf extract. [Click here to view] |
In vivo wound healing activity
The clinical evaluation of wound healing activity of P. americana leaf extract was observed for up to 14 days. As depicted in Figure 1, from day 1 to day 4, a reduction in incision length was observed in mice across several treatment groups: the positive control group (Povidone Iodine), and the 5%, 10%, and 15% dosage groups. Notably, the positive control group (Povidone Iodine) and the 15% dosage group showed visible improvements in wound condition. In these groups, some mice exhibited a decrease in swelling around the wound site. Similar effects were observed in the 10% dosage group, while the 5% dosage group still showed some swelling around the wound area. In contrast, the negative control group showed limited improvement. The wounds in this group merely dried out, with swelling still clearly visible. It was not until day 5 that the negative control group began to show a decrease in incision length. These observations suggest that the treatments, particularly at higher concentrations, may accelerate wound healing compared to no treatment. The positive control and the 15% dosage treatment appeared to be the most effective in reducing wound size and swelling in the early stages of healing.
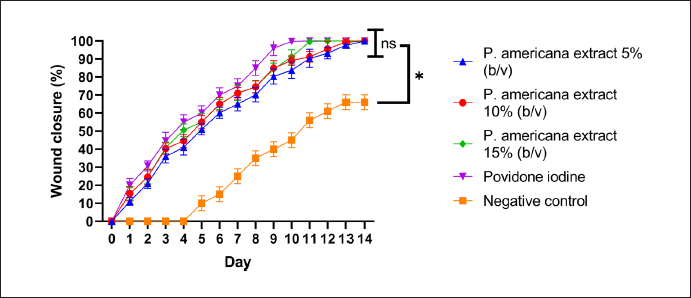 | Figure 1. Wound healing activity of P. americana on male Wistar mice. Data were presented as mean ± SD (n = 5) and statistically analyzed using Two Way ANOVA, Tukey’s. test; ns = not significantly different (p > 0.05); *= significantly different (p < 0.05) [Click here to view] |
By day 8, distinct differences became apparent among the treatment groups. The positive control group treated with Povidone iodine showed promising results, with wound closure was 85%. In the 5% dosage group, signs of inflammation, particularly swelling around the wound area, had subsided. Notably, the 10% and 15% dosage groups demonstrated significant improvement, with a marked increase in the percentage of wound closure (70% and 75%). These higher concentration treatments appeared to accelerate the healing process more effectively than the lower dose. In contrast, the negative control group exhibited slower progress. While the wounds in this group had dried out and swelling had slightly diminished, overall healing appeared less advanced compared to the treated groups (wound closure only 30%). These observations suggest that the treatments, especially at higher concentrations, significantly enhance wound healing rates compared to no treatment. The positive control and the higher dosage treatments (10% and 15%) seemed particularly effective in promoting wound closure and reducing inflammatory signs by the eighth day of the study.
By day 10, the positive control group treated with Povidone Iodine demonstrated complete wound closure, marking the fastest healing time among all groups. The 15% dosage group followed closely, with wounds fully closing by day 11. This was followed by the 10% dosage group, which achieved complete wound closure on day 12. The 5% dosage group took slightly longer, with wounds fully healing by day 13. In stark contrast, the negative control group showed the slowest healing progression. By day 14, wounds in this untreated group had not yet closed completely. These observations suggest a dose-dependent effect of the treatment on wound healing rates. Higher concentrations of the treatment appeared to accelerate wound closure, with effectiveness approaching that of the positive control. Overall, all concentrations of P. americana leaf extract (5%–15% b/v) demonstrated wound healing activity comparable to the positive control, povidone iodine (no significance different, p value > 0.05).
The result is particularly noteworthy, as it suggests that natural extracts from P. americana leaves could serve as a potential alternative or complementary treatment for wound healing. The effectiveness of P. americana leaf extract can be attributed to its rich phytochemical composition, which includes alkaloids, flavonoids, phenols, and saponins. These bioactive compounds contribute to various pharmacological activities crucial for wound healing. Alkaloids possess antimicrobial and anti-inflammatory properties [23,24], while flavonoids and phenols exhibit potent antioxidant effects, scavenging free radicals and modulating the inflammatory response [25,26]. Saponins, on the other hand, enhance wound contraction and epithelialization [27]. Therefore, the wound healing mechanisms of P. americana leaf extract were multifaceted, encompassing antimicrobial activity to prevent infection, anti-inflammatory effects to reduce excessive inflammation, antioxidant properties to protect against oxidative stress, and enhanced collagen synthesis for tissue repair. The comparable efficacy to povidone-iodine is particularly intriguing, as the extract offers additional benefits through its anti-inflammatory and antioxidant properties, potentially providing a more holistic approach to wound healing. While povidone-iodine effectively prevents wound infection, it has been reported to have cytotoxic effects on fibroblasts and keratinocytes, which may delay healing [28]. In contrast, P. americana leaf extract not only provides antimicrobial protection but also offers additional benefits that could enhance the overall healing process. The promising results of in vivo studies, coupled with the extract’s rich phytochemical profile, underscore the potential of P. americana leaf extract as a natural alternative in wound care.
Network pharmacology study
Initially, gene mining was employed to observe the target gene of all compounds followed by cross-matching with inflammation and fibrinolytic-related genes retrieved from the GeneCards. Approximately 282 genes were obtained for 14 compounds from P. americana and 1,066 for inflammation along with 268 genes for fibrinolytic-related genes. Cross-matching observed 39 (3.2%) common genes directly associated with P. americana, inflammation, and fibrinolytic, as illustrated in Figure 2.
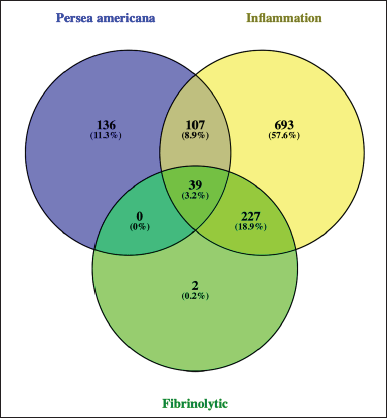 | Figure 2. Venn diagram of cross-matching genes from P. americana with inflammation and fibrinolytic-related genes. [Click here to view] |
These genes undergo PPI analysis and data was visualized and subjected to topological analysis. The resulting network demonstrated three nodes with high connectivity, including NF-KB1, SRC, and HIF1A as depicted in Figure 3. This high degree of interaction indicated that these genes may play essential roles in wound healing activity. The 39 genes were further processed for disease prediction, gene ontology biological process, and KEGG pathway analyses. As illustrated in Figure 4, the disease prediction from DAVID database obtained ten wound-related diseases with the core disease was plasminogen activator inhibitor 1 followed by bacteremia and wounds and injuries. The GO biological process annotation also supported this result that illustrated the genes were mainly for wound healing, regulation of body fluid levels, as well as response to wounding. Furthermore, platelet activation was observed as one of the pathways to how P. americana acts as a wound healing agent, resulting from the KEGG analysis.
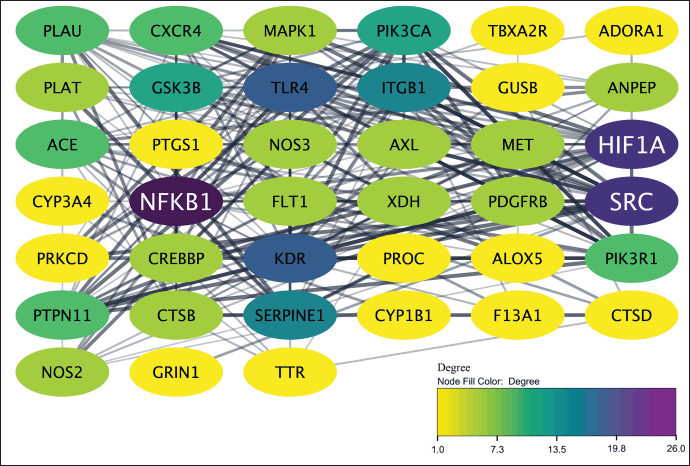 | Figure 3. The PPI illustration of all target genes. The color of the target nodes transitions from yellow to purple, indicating a shift from low to high degree values in the network. [Click here to view] |
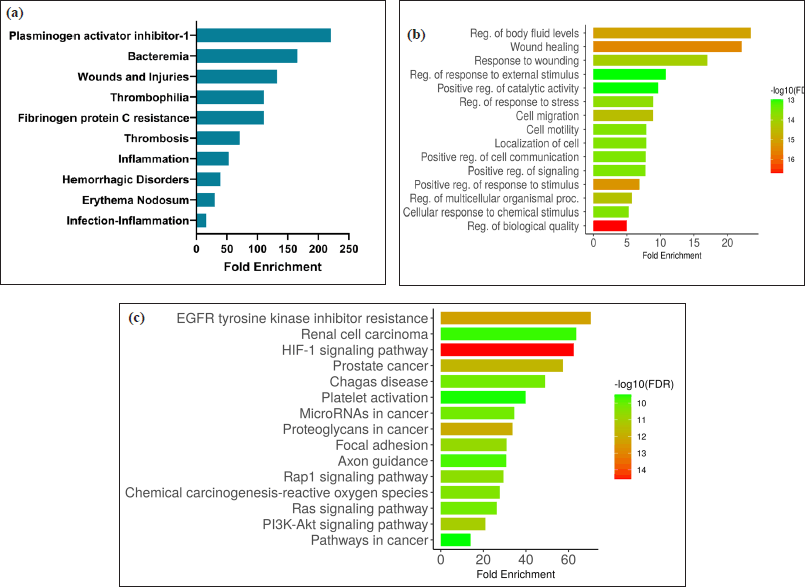 | Figure 4. Enrichment analysis. (a) Disease prediction, (b) gene ontology biological process, and (c) KEGG pathway related to P. americana gene targets. [Click here to view] |
The complex interplay between compounds found in P. americana, their biological targets, and associated diseases has been visualized through sophisticated network analysis (Fig. 5), revealing compelling insights into its potential wound healing properties, where key molecular targets like NF-KB1, SRC, and HIF1A emerge as crucial regulators orchestrating various stages of wound repair from inflammation to tissue remodeling. During the initial stages of wound healing, the activation of NF-κB1 is triggered by various stimuli, such as the release of proinflammatory cytokines, growth factors, and the detection of pathogen-associated molecular patterns or damage-associated molecular patterns [29]. This activation leads to the upregulation of a wide range of genes involved in the inflammatory response, including those encoding cytokines, chemokines, and adhesion molecules [30]. Furthermore, the activation of NF-κB1 increases MMP9 overexpression, leading to the destruction of extra cellular matrix [12]. Additionally, NF-κB1 could also be modulated by SRC, which not only fine-tuning the inflammatory response, but also increases the MMP expression [31]. Thus, inhibition of NF-κB1 and SRC complex could decrease the production of MMP9, resulting in the remodeling cellular matrix and increasing wound healing activity, as illustrated in Figure 6 [32,33].
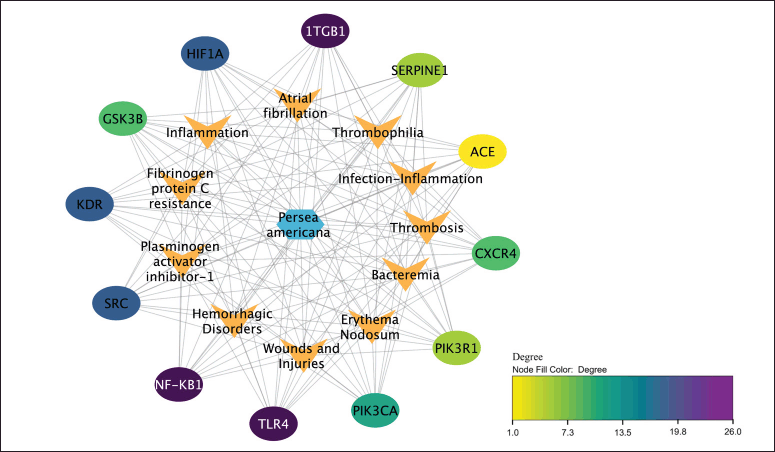 | Figure 5. Network diagram of P. americana core targets-diseases. The color of the core target nodes transitions from yellow to purple, indicating a shift from low to high degree values in the network. [Click here to view] |
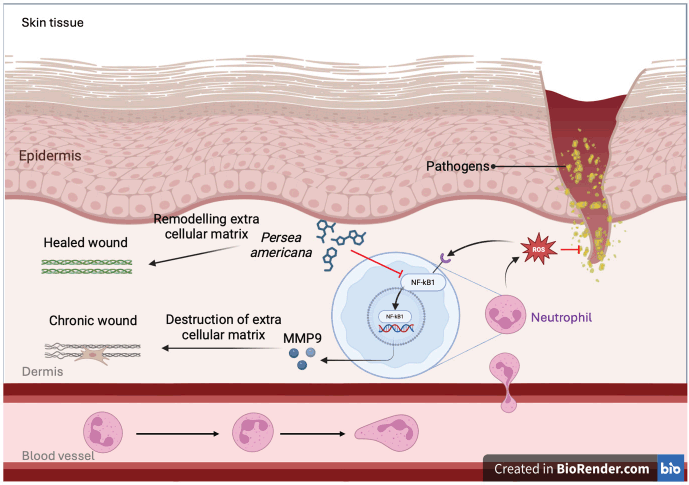 | Figure 6. Purpose mechanism of P. americana in wound-healing process. [Click here to view] |
Furthermore, another core target is HIF1A, which is known as a key transcription factor that regulates cellular responses to hypoxia, a condition commonly associated with tissue injury and the wound-healing process. Its involvement in various aspects of wound repair has been extensively studied, revealing its importance in promoting angiogenesis, cell migration, and extracellular matrix remodeling. One of the primary functions of HIF1A in wound healing is its ability to stimulate angiogenesis, the formation of new blood vessels. A recent study by Wen et al. [34] demonstrated that HIF1A activation in endothelial cells significantly enhanced their proliferation and tube formation capacity, leading to improved vascularization in wound sites. This increased blood supply is crucial for delivering oxygen and nutrients to the healing tissue, thereby accelerating the repair process. Moreover, HIF1A has been shown to play a vital role in keratinocyte migration and re-epithelialization. Research by Gao et al. [35] revealed that HIF1A upregulation in keratinocytes enhanced their motility and proliferation, facilitating faster wound closure. The study also noted that HIF-1α promoted the expression of key proteins involved in cell-cell adhesion and extracellular matrix interactions, which are essential for effective epithelial restoration.
In addition to its effects on angiogenesis and re-epithelialization, HIF1A has been implicated in modulating the inflammatory response during wound healing. A comprehensive review by Hong et al. [36] highlighted how HIF1A influences the recruitment and activation of immune cells, particularly macrophages, in the wound microenvironment. The authors noted that HIF-1α-mediated signaling can shift macrophages towards a pro-regenerative phenotype, promoting tissue repair while mitigating excessive inflammation. Recent research has also explored the potential of targeting HIF1A as a therapeutic strategy for enhancing wound healing. A promising study by Botusan et al. [37] and Li et al. [38] demonstrated that topical application of a HIF-1α stabilizer accelerated wound closure in a diabetic mouse model, suggesting its potential for treating chronic wounds associated with metabolic disorders.
Molecular docking simulation
To confirm the wound-healing mechanism of P. americana, molecular docking was performed on the target protein NF-κB1, as it was identified as the primary target. The validation of the docking process was an important initial step, which involved re-docking the native ligand. This step is crucial for ensuring the reliability and accuracy of the docking results. The key metric used for the validation was the root mean square deviation (RMSD) between the predicted binding pose and the experimental structure. Generally, an RMSD value below 2 Angstroms is considered acceptable for a successful docking protocol validation [17,39]. This threshold indicates that the predicted binding poses are reasonably close to the experimental structures, taking into account the inherent flexibility of proteins and the approximations made in the docking algorithms (Fig. 7). In this work, the obtained RMSD value was 1.273 ?, justifying the docking protocol could be employed for test ligands.
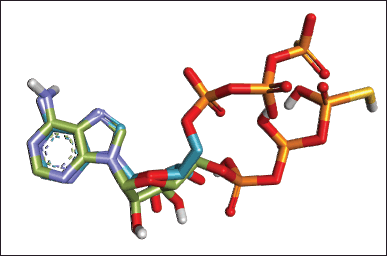 | Figure 7. Superimposed of the best re-docking of NF-KB1 native ligand with RMSD value of 1.273 ? (Original: green; Re-docked: blue). [Click here to view] |
Figure 8 depicts six of fourteen phytoconstituents from P. americana exhibit excellent binding affinity, illustrated by the comparable free binding energy with the native ligand. Epicatechin, catechin, and quercetin stand out as the most potential compounds from P. americana with the most negative free binding energy (−8.58, −8.51, and 8.53 kcal/mol) among others, even better than the native ligand (−7.87 kcal/mol). The wound-healing activity of P. americana is hypothesized to be attributed to these three key compounds. This hypothesis is further supported by numerous studies demonstrating the potential of flavonoids to inhibit NF-κB activation. Of particular interest is the research conducted by Lakshmi et al. [40] and Mackenzie et al. [41], which focused on catechin and epicatechin, prominent flavonoids. Their studies revealed that catechin and epicatechin effectively inhibit NF-κB-p65 transcriptional activity in normal human bronchial epithelial cells. This inhibition occurs through a mechanism that prevents NF-κB-p65 from binding to κBs. They discovered that catechin directly interacts with NF-κB-p65 through a covalent reaction, involving covalent reaction via enones within the catechin ring structure, preventing 1, 4-addition reactions, blocked adduct-forming reaction of biotinylated catechin with NF-κB-p65 [40]. On the other hand, quercetin also can hinder the activation of NF-κB, further lowering the MMP9 production and other pro-inflammatory agents including IL-1β, IL-6, IL-8, and TNF-α [42].
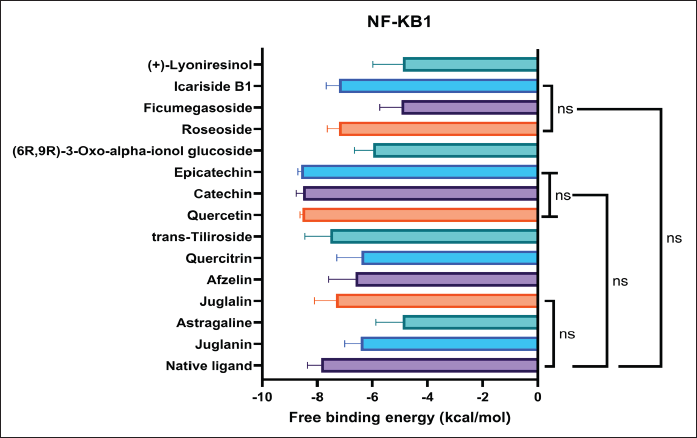 | Figure 8. Docking score of bioactive compounds from P. americana to NF-KB1. Data are presented as mean ± SD (n = 10) and statistically analyzed using One Way ANOVA, Dunnett’s test with the native ligand as control group. ns = not significantly different (p > 0.05). [Click here to view] |
The multi-structural molecular docking was employed to observe the potential synergistic effect of the three best compounds (quercetin, catechin, and epicatechin) in inhibiting NF-KB1. The results from the multi-compound molecular docking study provide interesting insights into the binding energies and interactions of quercetin, catechin, and epicatechin with NF-KB1. Figure 9 shows the free binding energies of various combinations of these compounds. Interestingly, the combination of quercetin and catechin exhibits the lowest free binding energy (–8.81 kcal/mol), suggesting the strongest binding affinity to the target protein. This synergistic effect is notable, as it implies that the presence of these two compounds together may enhance their overall binding strength. On the other hand, the combination of all three compounds (quercetin, catechin, and epicatechin) shows a consistent free binding energy with their individual states. However, the combination affinity was not statistically different with their individual affinities.
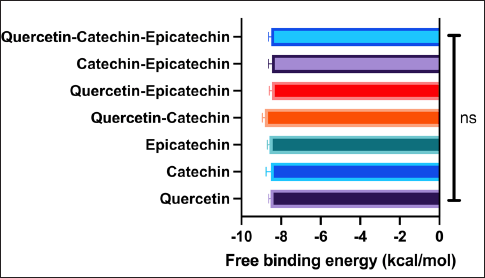 | Figure 9. Docking score of multi-component docking. Data are presented as mean ± SD (n=10) and statistically analyzed using One Way ANOVA, Dunnett’s test with the native ligand as control group. ns = not significantly different (p>0.05). [Click here to view] |
Figure 10 demonstrates how the presence of multiple compounds affects their binding modes. For instance, catechin in the presence of quercetin shows a slightly different interaction pattern compared to catechin alone. Similarly, epicatechin’s binding mode is influenced by the presence of catechin and quercetin. This observation supports the synergistic effect seen in the free-binding energy calculations. These results align with a study reported by Setiawansyah et al. [17] which demonstrated presence of one or more other ligands might cause conformational changes in the binding groove of the macromolecule, affecting their interaction with the active site. The synergistic binding of these compounds is particularly interesting from a pharmacological perspective. The enhanced binding when present together might explain some of the health benefits associated with consuming these natural products. These results suggest that the combination of quercetin and catechin may have enhanced effects on the target protein compared to the individual compounds. This finding could have implications for developing more effective nutraceuticals or pharmaceutical formulations that leverage these synergistic interactions. Future research could explore the effects of different ratios of these compounds, investigate potential allosteric effects, and examine how these interactions translate to biological activity. Additionally, molecular dynamics simulations could provide further insights into the stability and dynamic nature of these interactions over time.
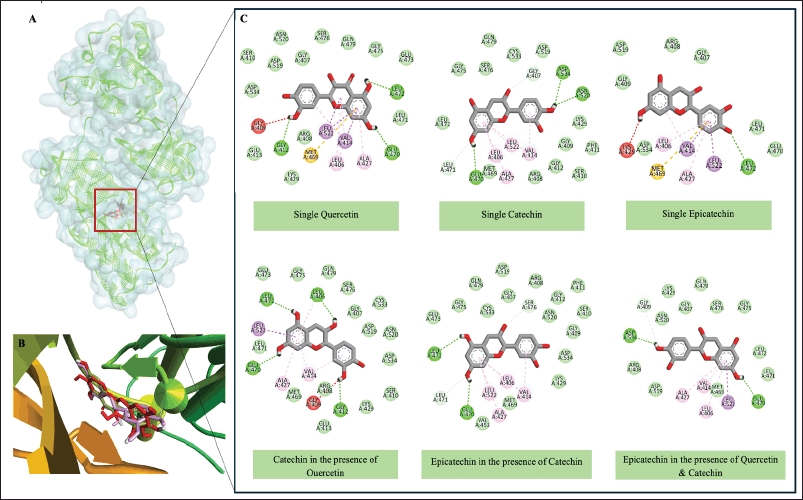 | Figure 10. Molecular interactions of Quercetin, Catechin, and Epicatechin with NF-KB1. (a) Molecular surface of NF-KB1 attached by Quercetin, Catechin, and Epicatechin, (b) Overview of Quercetin, Catechin, and Epicatechin in NK-KB1 binding site, (c) Two-dimensional interaction of Quercetin, Catechin, and Epicatechin with essential amino acid residues of NF-KB1 in single states and in combination states. [Click here to view] |
Our findings not only shed light on the specific molecular interactions involved in P. americana’s bioactive compounds’ inhibitory effect on NF-κB but also strengthen the overall hypothesis regarding the wound-healing properties of P. americana. Furthermore, since the wound-healing activity of P. americana is attributed to quercetin, catechin, and epicatechin, rises an important question whether other natural products with similar chemical compounds also demonstrated similar wound-healing activity or not. For instance, quercetin enhances fibroblast migration and proliferation, crucial steps in wound closure [43]. Comparatively, quercetin is also found in other plants known for wound healing, such as Allium cepa. Research by Mounir et al. [44] demonstrated that onion extract rich in quercetin promoted faster wound closure in a rat model. In addition, catechin and epicatechin, both flavanols present in P. americana, have shown significant wound-healing potential. A comprehensive review by Carvalho et al. [45] and Zulkefli et al. [46] highlighted that these compounds enhance collagen synthesis and reduce oxidative stress in wound sites, crucial for effective healing. When comparing to other sources, green tea (Camellia sinensis) is notably rich in catechins. Studies by Jia et al. [47] and Xu et al. [48] found that green tea extract accelerated wound healing in diabetic rats, primarily attributed to its high catechin content. However, the synergistic effects of quercetin with catechin and epicatechin which are all found in P. americana may provide a unique advantage. As illustrated in multi-components molecular docking, quercetin and catechin demonstrated the synergistic effect in inhabiting NF-KB1, enhancing their wound healing potential. Furthermore, these three compounds show a self-stabilized effect when used in combination, maintaining their individual affinities.
Toxicological insight
The toxicological profile of P. americana compounds presented in Table 3 reveals a generally favorable safety profile, with LD50 values ranging from 704.77556 to 1532.621 mg/kg BW, indicating relatively low acute toxicity. The compounds with LD50 of 500 mg/kg BW to 5,000 mg/kg BW are considered to be less toxic [49]. Notably, all compounds tested negative for mutagenicity, hepatotoxicity, and skin sensitization, further supporting the P. americana safety for clinical application. This data aligns with avocado’s widespread recognition as a nutritious food and suggests the potential for therapeutic applications of its constituents. Padilla-Camberos et al. [50] evaluated the toxicity and genotoxicity of P. americana extract. The result showed that P. americana exhibited LD50 value of 1200.75 mg/kg and demonstrated no mutagenicity on peripheral blood cells, illustrating P. americana is relatively safe to be used in clinical applications, particularly for wound healing management.
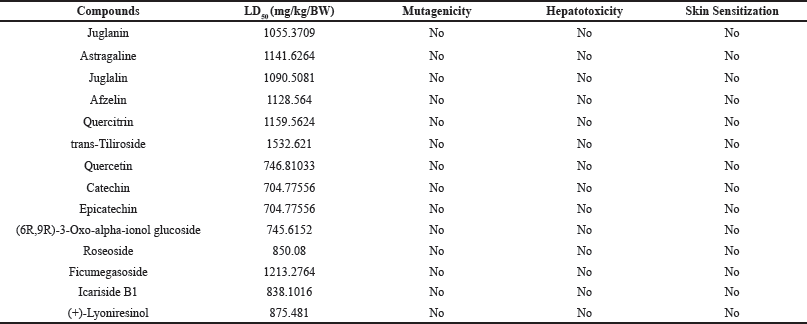 | Table 3. Toxicological profile of Persea americana. [Click here to view] |
CONCLUSION
This study demonstrates the efficacy of P. americana leaf extract in promoting wound healing through a multi-faceted approach combining in vivo studies, network pharmacology analysis, and molecular docking simulations. Our in vivo experiments revealed that P. americana leaf extract at concentrations ranging from 5% to 15% exhibited wound healing activity comparable to povidone iodine, a standard antiseptic treatment. This finding underscores the potential of P. americana as a natural alternative in wound management. Network pharmacology analysis identified NF-κB1 as a core molecular target in the wound-healing process mediated by P. americana. This result was further corroborated by molecular docking studies, which highlighted epicatechin, catechin, and quercetin as key compounds likely responsible for the observed wound-healing activity. The synergistic approach of in vivo testing, network pharmacology analysis, and molecular docking simulation provide a comprehensive understanding of P. americana’s wound healing mechanisms. These findings not only validate the traditional use of P. americana in wound treatment but also pave the way for developing novel, plant-based wound healing formulations. Further research is warranted to optimize the application of P. americana extract in clinical settings. This study contributes valuable insights to the field of natural product-based wound healing therapies and highlights the potential of P. americana as a promising candidate for future wound care applications. However, translating these promising results into clinical practice presents several practical challenges that need to be addressed. One significant challenge is the standardization of the extraction process to ensure consistent potency and quality of the P. americana leaf extract. Variations in growing conditions, harvest times, and extraction methods can significantly affect the concentration of active compounds. To overcome this, it is crucial to develop and validate a standardized extraction protocol that can be reliably reproduced on a larger scale. This may involve implementing strict quality control measures and potentially utilizing advanced extraction technologies to optimize yield and purity. Another challenge lies in the cost-effectiveness of producing P. americana leaf extract at a scale suitable for widespread clinical use. The current extraction methods may be labor-intensive and time-consuming, potentially leading to high production costs. To address this, research into more efficient extraction methods, such as supercritical fluid extraction or microwave-assisted extraction, could be explored. Additionally, investigating the potential for cultivating P. americana varieties with higher concentrations of the identified active compounds could improve yield and reduce costs.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This work receives no external funding.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study design received formal approval from the National Peripheral Veterinary Authority Animal Ethics Committee, subsequent to a favorable review by the Animal protocols Evaluation Committee with ethical approval number 002301/SITI KHADIJAH PALEMBANG/2024.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, et al. Human skin wounds: a major and snowballing threat to public health and the economy: perspective Article. Wound Repair and Regeneration 2009;17:763–71.
2. Järbrink K, Ni G, Sönnergren H, Schmidtchen A, Pang C, Bajpai R, et al. The humanistic and economic burden of chronic wounds: a protocol for a systematic review. Syst Rev. 2017;6(15):1–7.
3. Moses RL, Prescott TAK, Mas-Claret E, Steadman R, Moseley R, Sloan AJ. Evidence for natural products as alternative wound-healing therapies. Biomolecules. 2023;13(3):444–470.
4. Hürkul MM, Sar?alt?n SY, Köro?lu A, Çoban T. In vitro inhibitory potential of avocado fruits, Persea americana (Lauraceae) against oxidation, inflammation and key enzymes linked to skin diseases. Revis Biol Trop. 2021;69(2):472–81.
5. Al-Otaibi T, Hawsah MA, Alojayri G, Mares MM, Aljawdah HMA, Maodaa SN, et al. In vivo anticoccidial, antioxidant, and anti-inflammatory activities of avocado fruit, Persea americana (Lauraceae), against Eimeria papillata infection. Parasitol Int. 2023;95:102741.
6. Alkhalaf MI, Alansari WS, Ibrahim EA, ELhalwagy MEA. Antioxidant, anti-inflammatory and anti-cancer activities of avocado (Persea americana) fruit and seed extract. J King Saud Univ Sci. 2019;31(4):1358–62.
7. Salazar-López NJ, Domínguez-Avila JA, Yahia EM, Belmonte-Herrera BH, Wall-Medrano A, Montalvo-González E, et al. Avocado fruit and by-products as potential sources of bioactive compounds. Food Res Int. 2020;138:109774.
8. Nayak BS, Raju SS, Chalapathi Rao AV. Wound healing activity of Persea americana (avocado) fruit: a preclinical study on rats. J Wound Care. 2008;17(3):123–5.
9. Hayati W, Raif MA, Ginting CN, Ikhtiari R. Antioxidant and wound healing potential of Persea americana Mill. leaves extract. In: 2021 international conference on artificial intelligence and mechatronics systems (AIMS). 2021. pp. 1–5.
10. Anatasya Putri S, Rani Gustia Maharani A, Luthfiana D, Chinecherem Nweze L, Setiawansyah A, Susanti G. Integrating the network pharmacology and molecular docking to uncover the potential mechanism of rutin in fighting diabetes mellitus. Ad-Dawaa’ J Pharm Sci. 2024;7(1):39–52.
11. Zhang R, Zhu X, Bai H, Ning K. Network pharmacology databases for traditional Chinese medicine: review and assessment. Frontiers in Pharmacol. 2019;10:123.
12. Luthfiana D and Utomo DH. Network pharmacology reveals the potential of Dolastatin 16 as a diabetic wound healing agent. In Silico Pharmacol. 2023;11(1):23.
13. Noor F, Tahir Ul Qamar M, Ashfaq UA, Albutti A, Alwashmi ASS, Aljasir MA. Network pharmacology approach for medicinal plants: review and assessment. Pharmaceuticals. 2022;15(5):572.
14. Ayu DS, Pratama HA, Sari NP, Febrianti N, Komalasari NR, Adelia P, et al. Perbandingan uji mukolitik ekstrak dan fraksi daun lamtoro (Leucaena leucocephala (Lam) de Wit) halus dan kasar secara in vitro. J Ilmiah Sain Tek. 2024;2(4):12–20.
15. Park SJ, Nam YH, Rodriguez I, Park JH, Kwak HJ, Oh Y, et al. Chemical constituents of leaves of Persea americana (avocado) and their protective effects against neomycin-induced hair cell damage. Revis Brasileira de Farmacog. 2019;29(6):739–43.
16. Liu J, Sudom A, Min X, Cao Z, Gao X, Ayres M, et al. Structure of the nuclear factor κB-inducing kinase (NIK) kinase domain reveals a constitutively active conformation. J Biol Chem. 2012;287(33):27326–34.
17. Setiawansyah A, Susanti G, Alrayan R, Hadi I, Arsul MI, Luthfiana D, et al. Aaptamine enhanced doxorubicin activity on b-cell lymphoma 2 (bcl-2): a multi-structural molecular docking study. Ad-Dawaa’ J Pharm Sci. 2024;7(1):1–10.
18. Utami H, Agustin VT, Novirianti L, Darni Y, Lesmana D, Takagi R. The leaching of natural dyes from avocado (Persea americana Mill) seeds using the ultrasonic-assisted extraction method and its application to cellulose fibers. J Rekay Kim Ling. 2021;16(2):100–8.
19. Owolabi MA, Coker HAB, Jaja SI. Bioactivity of the phytoconstituents of the leaves of Persea americana. J Med Plant Res. 2010;4(12):1130–5.
20. Hidayati N, Fadillah Amanda P, Setiawansyah A. Utilization of avocado leaves (Persea americana Mill) ethanol extract in acne spot gel formulation. Indonesian J Cosmet. 2024;2(1):1–9.
21. Setiawansyah A, Widiyawati AT, Sari MSD, Reynaldi MA, Hidayati N, Alrayan R, et al. FT-IR-based fingerprint combined with unsupervised chemometric analysis revealed particle sizes and aqueous-ethanol ratio alter the chemical composition and nutraceutical value of Daucus carota. Nat Prod Res. 2024; In Press:1–9.
22. Utami N, Susianti S, Bakri S, Kurniawan B, Setiawansyah A. Cytotoxic activity of Cyperus rotundus L. rhizome collected from three ecological zones in Lampung-Indonesia against HeLa cervical cancer cell. J Appl Pharm Sci. 2023;13(10):141–8.
23. Li S, Liu X, Chen X, Bi L. Research progress on anti-inflammatory effects and mechanisms of alkaloids from Chinese medical herbs. Evidence-based Complement and Alternat Med. 2020;1303524.
24. Yan Y, Li X, Zhang C, Lv L, Gao B, Li M. Research progress on antibacterial activities and mechanisms of natural alkaloids. Antibiotics. 2021;10(3):318.
25. Zeb A. Concept, mechanism, and applications of phenolic antioxidants in foods. J Food Biochem. 2020;44(9):e13394.
26. Muflihah YM, Gollavelli G, Ling YC. Correlation study of antioxidant activity with phenolic and flavonoid compounds in 12 Indonesian indigenous herbs. Antioxidants. 2021;10(10):1530.
27. Ibrahim NI, Wong SK, Mohamed IN, Mohamed N, Chin KY, Ima-Nirwana S, et al. Wound healing properties of selected natural products. Int J Env Res Pub Health. 2018;15(11):2360.
28. Bigliardi PL, Alsagoff SAL, El-Kafrawi HY, Pyon JK, Wa CTC, Villa MA. Povidone iodine in wound healing: a review of current concepts and practices. Int J Surge. 2017;44:260–8.
29. Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18(5):309–24.
30. Mussbacher M, Salzmann M, Brostjan C, Hoesel B, Schoergenhofer C, Datler H, et al. Cell type specific roles of nf-kb linking inflamation and thrombosis. Front Immunol. 2019;10:5.
31. Euskirchen GM, Auerbach RK, Davidov E, Gianoulis TA, Zhong G, Rozowsky J, et al. Diverse roles and interactions of the SWI/SNF chromatin remodeling complex revealed using global approaches. PLoS Genet. 2011;7(3):e1002008.
32. Wang T, Jin X, Liao Y, Sun Q, Luo C, Wang G, et al. Association of NF-κB and AP-1 with MMP-9 overexpression in 2-chloroethanol exposed rat astrocytes. Cells. 2018;7(8):96.
33. Wu X, Yang L, Zheng Z, Li Z, Shi J, Li Y, et al. Src promotes cutaneous wound healing by regulating MMP-2 through the ERK pathway. Int J Mol Med. 2016;37(3):639–48.
34. Wen W, Yang L, Wang X, Zhang H, Wu F, Xu K, et al. Fucoidan promotes angiogenesis and accelerates wound healing through AKT/Nrf2/HIF-1α signalling pathway. Int Wound J. 2023;20(9):3606–18.
35. Gao Y, Xie Z, Ho C, Wang J, Li Q, Zhang Y, et al. LRG1 promotes keratinocyte migration and wound repair through regulation of HIF-1α stability. J Invest Dermatol. 2020;140(2):455–464.e8.
36. Hong WX, Hu MS, Esquivel M, Liang GY, Rennert RC, McArdle A, et al. The role of hypoxia-inducible factor in wound healing. Adv Wound Care (New Rochelle). 2014;3(5):390–9.
37. Botusan IR, Sunkari VG, Savu O, Catrina AI, Grünler J, Lindberg S, et al. Stabilization of HIF-1α is critical to improve wound healing in diabetic mice. Proceedings of the National Academy of Sciences; 2008;105(49):19426–31.
38. Li G, Ko CN, Li D, Yang C, Wang W, Yang GJ, et al. A small molecule HIF-1α stabilizer that accelerates diabetic wound healing. Nat Commun. 2021;12(1):3363.
39. Reynaldi MA, Setiawansyah A. Potensi anti-kanker payudara tanaman songga (Strychnos lucida R.Br): Tinjauan interaksi molekuler terhadap reseptor estrogen-α in silico. Sasambo J Pharm. 2022;3(1):30–5.
40. Lakshmi SP, Reddy AT, Kodidhela LD, Varadacharyulu NC. The tea catechin epigallocatechin gallate inhibits NF-κB-mediated transcriptional activation by covalent modification. Arch Biochem Biophys. 2020;695:108620.
41. Mackenzie GG, Carrasquedo F, Delfino JM, Keen CL, Fraga CG, Oteiza PI. Epicatechin, catechin, and dimeric procyanidins inhibit PMA-induced NF-kappaB activation at multiple steps in Jurkat T cells. FASEB J. 2004;18(1):167–9.
42. Das D, Banerjee A, Mukherjee S, Maji BK. Quercetin inhibits NF-kB and JAK/STAT signaling via modulating TLR in thymocytes and splenocytes during MSG-induced immunotoxicity: an in vitro approach. Mol Biol Rep. 2024;51(1):277.
43. Doersch KM, Newell-Rogers MK. The impact of quercetin on wound healing relates to changes in αV and β1 integrin expression. Exp Biol Med. 2017;242(14):1424–31.
44. Mounir R, Alshareef WA, El Gebaly EA, El-Haddad AE, Ahmed AMS, Mohamed OG, et al. Unlocking the power of onion peel extracts: antimicrobial and anti-inflammatory effects improve wound healing through repressing Notch-1/NLRP3/Caspase-1 Signaling. Pharmaceuticals. 2023;16(10).
45. Carvalho MTB, Araújo-Filho HG, Barreto AS, Quintans-Júnior LJ, Quintans JSS, Barreto RSS. Wound healing properties of flavonoids: a systematic review highlighting the mechanisms of action. Phytomedicine. 2021;90:153636.
46. Zulkefli N, Che Zahari CNM, Sayuti NH, Kamarudin AA, Saad N, Hamezah HS, et al. Flavonoids as potential wound-healing molecules: emphasis on pathways perspective. Int J Mol Sci. 2023;24(5):4607.
47. Jia G, Li Z, Le H, Jiang Z, Sun Y, Liu H, et al. Green tea derivative-based hydrogel with ROS-scavenging property for accelerating diabetic wound healing. Mater Des. 2023;225:111452.
48. Xu FW, Lv YL, Zhong YF, Xue YN, Wang Y, Zhang LY, et al. Beneficial effects of green tea EGCG on skin wound healing: a comprehensive review. Molecules. 2021; 26(20):6123.
49. Loomis TA, Hayes AW. CHAPTER 12—principles of biological tests for toxicity. In: Loomis TA, Hayes AW, editors. Loomis’s essentials of toxicology (fourth edition). San Diego, CA, USA: Academic Press; 1996. pp. 167–204.
50. Padilla-Camberos E, Martínez-Velázquez M, Flores-Fernández JM, Villanueva-Rodríguez S. Acute toxicity and genotoxic activity of avocado seed extract (Persea americana Mill., c.v. Hass). Sci World J. 2013;2013:245828.