INTRODUCTION
Severe dengue is a potentially fatal complication, due to plasma leakage, fluid accumulation, respiratory problems, bleeding, and organ damage, thereby increasing the risk of death [1]. In dengue virus (DENV) infection, the early immune responses play a dual role, involving innate immunity and an inflammatory response. Both collaborate to mediate the body’s protection, while at the same time, they can worsen the severity of the infection, namely when excessive cytokine production occurs and triggers a cytokine storm [2]. Cytokine storms directly affect endothelial cells in vascular tissue by increasing capillary permeability resulting in plasma leakage [3]. Therefore, the most effective antiviral treatment not only involves agents that can inhibit virion formation, but also can inhibit the development of cytokine storm [4].
In DENV infection, activation of the NFκB pathway also occurs when pattern recognition receptors recognize pathogen-associated molecular patterns. This activation induces the transcription of proinflammatory cytokines, chemokines, and additional inflammatory mediators in various immune cell types. There are various pro-inflammatory cytokines produced from the NFκB pathway, namely IL-1, IL-2, IL-6, IL-8, IL-12, and TNF-α [5]. Some of them are cytokines that have an essential role in the severity of DENV infection, such as TNF-α, IL-8, IL-6, and IFN-γ, which can disrupt of adhesion molecules [6–8], adherence junction [9], and tight junctions [10]. This disturbance results in increased endothelial permeability [11], allowing plasma to reach the intercellular cell junctions below, resulting in leakage and plasma being able to escape from the blood vessels [12,13]. IL-10 is an anti-inflammatory cytokine that is a marker for DENV infection [14]. IL-10 exerts anti-inflammatory effects by inhibiting the production of pro-inflammatory cytokines by the Janus kinase 1/ Signal transducer and activator of transcription 3 (JAK1/STAT3 pathway) [15].
Cassia alata is an herbaceous plant from the Leguminoceae family that is widespread and cultivated for medicinal purposes such as diabetes, diarrhea, microbial infections, malaria, cure fever, healing dermal wounds, and so on. Several scientific studies have also been carried out regarding the therapeutic activity of C. alata as an anti-fungal [16,17], anti-bacterial [18], antioxidant, antiviral [19], anti-diabetic [20], anti-inflammatory, anti-lipogenic, anti-hyperlipidemic, anthelmintic, and anti-malaria [21]. These potentials are supported by the content of bioactive chemical compounds such as phenol (rhein, chrysophanol, kaempferol, aloeemodin, and glycosides), anthraquinones (alatinone and alatonal), fatty acids (oleic, palmitic, and linoleic acids), steroids, and terpenoids (sitosterol, stigmasterol, and campesterol) [21,22].
Angelina et al. [23] show that the ethanol fraction of C. alata leaves originating from Jakarta has the most robust DENV inhibitory ability at each stage of virus replication with an average inhibition >95% [23]. In vitro studies on Huh-7it-1 cells infected with DENV-2 showed that the butanol fraction of C. alata leaves originating from Jakarta (containing active compounds in the form of kaempferol-3,7-diglucoside, tetrahydroxy-flavone, and deoxycholic acid) had activity potent antiviral with an IC50 value of 6.47 g/ml and SI 98.29 g/ml), making it a potential candidate for DENV antiviral [24]. This study investigated the anti-inflammatory properties of the butanol fraction derived from the ethanol extract of C. alata leaves, cultivated in Solo, Java Island, Indonesia, in the context of DENV-1 infection. The NFκB pathway was examined, which is crucial for producing several pro-inflammatory cytokines such as IL-6 and TNF-α that lead to plasma leakage. The expression of IL-10, an anti-inflammatory cytokine, was also considered. Therefore, this research focused on analyzing NFκB, TNF-α, IL-6, and IL-10 expression levels.
MATERIAL AND METHODS
Materials
Chemicals and extract
The butanol fraction of C. alata leaves ethanol extract used in this study was obtained from PT. Konimex, Solo, Indonesia, dissolved in dimethyl sulfoxide (DMSO; Thermo Scientific, USA). The stock with a final 100 mg/ml concentration was stored at 4ºC until use. In the inhibitory concentration 50% (IC50) and cytotoxicity concentration 50% (CC50) tests, the extract was diluted with 2% FBS MEM (Gibco, USA); and in the anti-inflammatory activity test, the extract was diluted with 2% FBS RPMI 1640 (Gibco, USA) until the desired concentration was obtained.
Cells and viruses
The cells used for DENV propagation, IC50 and CC50 determination were Vero E6 C1008 cells from African green monkey kidneys cultured in MEM medium (Gibco, USA) supplemented with Fetal Bovine Serum (Sigma, USA) and Antibiotic Antimycotic Solution (10,000 units penicillin, 10 mg streptomycin and 25 μg Amphotericin B per ml) (Sigma-Aldrich, USA). The cells used for anti-inflammatory test were peripheral blood mononuclear cells (PBMC) from a donor who was not being infected with DENV, cultured in RPMI 1,640 media (Gibco, USA) (ethical clearance from Health Research Ethics Committee, Dr. Cipto Mangunkusumo General Hospital, Faculty of Medicine, Universitas Indonesia number KET-1449/UN2.F1/ETIK/PPM.00.02/2023). The virus used was DENV serotype-1 (DENV-1) strain IDS 11/2010 (collection of Department of Microbiology, Faculty of Medicine, Universitas Indonesia), propagated in Vero E6 C1008 cells.
Methods
The IC50, CC50, and selectivity index determination
To determine IC50, a dose-dependent test was carried out. It is necessary to determine the dose to be used in the anti-inflammatory test study and follow published procedures [24] with slight modifications. Vero cells were seeded in 96-well plates with a concentration of 2 × 104 cells in 100 μl/well then incubated for 24 hours at 37ºC, 5% CO2, until they reached 80% confluence. Virus at multiplicity of infection (MOI) 0.5 FFU/cell were mixed with C. alata extract butanol fraction with various concentrations (2, 1, 0.25, and 0.125 μg/ml) in triplicate. DMSO at 0.1% was used as a negative control.
To determine the DENV-1 titer, a focus assay was carried out using Vero E6 C1008 cells in 96-well plates [24,25]. After incubation for 2 days, the cells were stained and foci were counted under an inverted microscope [25].
Determination of the CC50 value was carried out using the MTT ((3-(4,5-dimetiltiazol-2-il)-2,5-difeniltetrazolium bromida) test based on references from previous research [23,26], in two independent experiments. CC50 is commonly used in toxicology studies as an indicator of a compound’s cytotoxicity to a cell population [27]. The initial experiment utilized 320, 160, 80, and 40 μg/ml dose ranges. The subsequent experiment employed 240, 120, 60, and 30 μg/ml doses. The absorbance readings were carried out at a wavelength of 490 nm with blank wells without cells (added with 100% DMSO) as background. The cell viability of the treatment group was compared with the control group in percentage form using the formula:
From the IC50 and CC50 values, the selectivity index (SI) value can be determined which is the quotient of CC50/IC50 [28].
Anti-inflammation assay
PBMC Isolation
Twelve ml of blood was taken in EDTA tubes from a donor who met the inclusion criteria (no fever for 3 weeks, NS1 negative, and IgM anti-DENV negative). The PBMC isolation procedure was based on references [29,30] with slight modifications by using the cell scrapper to detach the adherent PBMC from the bottom of the flask. PBMCs were incubated in a T75 flask at 37°C with 5% CO2. After 2 hours of incubation, non-adherent PBMC (those not sticking to the flask) were discarded. Then, 4 ml of RPMI with 10% FBS was added to the flask, and using a cell scrapper the adherent PBMC was collected and transfered to a 15 ml tube. The adherent PBMC was counted using an improved Neubauer hemocytometer The PBMC was then planted in a 96-well flat bottom microplate at a concentration of 2 × 105 cells/well and incubated at 37°C with 5% CO2. After 24 hours, the medium was removed and treated according to the treatment groups.
Anti-inflammatory test
The anti-inflammatory experiment was done on PBMC infected by DENV-1 based on the ADE model (Widodo et al., manuscript under review for publication). PBMC cells were infected with DENV-1 final MOI 0.5 FFU/cell, 50 μl/well, then incubated for 2 hours at 37ºC with 5% CO2 and agitation every 30 minutes. After that, C. alata extract treatment with a final concentration of 2 × IC50 in 50 μl/well was added, followed by 2 hours and 24 hours incubation. The RNA from the cells was then extracted to determine cytokine mRNA expression. Concanavalin A 1 μg/ml was used as a positive control (data not shown). PBMC without DENV infection was used as a negative control.
RNA extraction
RNA extraction was performed using the Total RNA Mini Kit (Blood/Cultured Cell) (Geneaid, Taiwan), following the manufacturer’s instructions. RNA was eluted in 40 μl RNase-free water. RNA concentration was measured by nanodrop at wavelengths of 230 nm, 260 nm, and 280 nm. The purity index was measured as 260/280 and 260/230. Next, cDNA synthesis was immediately carried out, the remaining RNA was stored at −80ºC.
cDNA synthesis
cDNA was synthesized with the RevertAid First Strand cDNA Synthesis kit (Thermo Scientific, USA). The amount of RNA template used for cDNA synthesis was 40 ng. A total volume of cDNA synthesis mix (20 μl) consisted of 40 ng RNA template, 1 μl random hexamer primer (Thermo Scientific, USA), nuclease-free water (NFW), 4 μl 5X reaction buffer, 1 μl RiboLock RNase Inhibitor, 2 μl 10 mM dNTP Mix, and 1 μl RevertAid M-MuLV RT. cDNA synthesis program followed the manufacturer’s instructions. The cDNA product was then stored at −20ºC and used as real time PCR template.
Real-time PCR assay
Real-time PCR was carried out to analyze target cytokine mRNA using the PowerUp SYBR Green Master Mix kit (Applied Biosystem, USA). Cytokine mRNA (TNF-α, IL-6, IL-10, and NFκB) was quantified by real-time PCR using specific primers and previously synthesized cDNA templates. A total of 1 μl of cDNA was added to 10 μl of master mix, with 1.25 μl of forward primer and 1.25 μl of reverse primer for each target gene (Table 1), as well as 6.5 μl of NFW. Real-Time PCR conditions included: (i) pre-incubation at 95°C for 5 minutes; (ii) 40 amplification cycles of denaturation at 95°C for 10 seconds, annealing at 60°C for 10 seconds (same annealing temperature for all primers), and extension at 72°C for 20 seconds; and (iii) melting curve and cooling step.
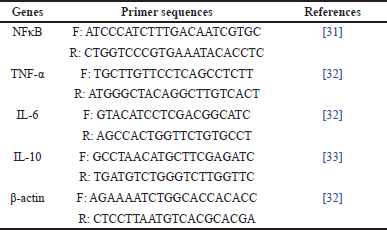 | Table 1. The primer sequences of inflammation-related genes utilized in real-time PCR. [Click here to view] |
Relative mRNA expression was analyzed by the delta delta CT method (ΔΔCT) so that the fold changes value is obtained. The fold change value is obtained from several stages of analysis starting from calculating ΔCT. The ΔCT value is the difference in CT value between the target gene and the reference gene (in this study the reference gene used was β-actin). Then continued by calculating ΔΔCT. ΔΔCT is the difference in ΔCT between the target sample (treatment sample) and the reference sample (negative control) [34]. The final result obtained is the fold changes of target gene expression in the target sample relative to the reference sample, normalized to the reference gene. The relative gene expression is made to 1 for the reference sample because ΔΔCT is equal to 0 and, therefore, 20 is equal to 1 [35]. From the results of ΔΔCT obtained, the value of 2-ΔΔCT is then determined, to determine the fold changes of target gene expression in the sample.
Data analysis
Statistical analysis was carried out using GraphPad Prism 9 software. Statistical differences between groups were analyzed using T-Test and one-way analysis of variance to determine the significance of mean differences between the treatment group and the control group.
RESULTS
IC50, CC50, and selectivity index of C. alata butanol fraction
The IC50 value obtained was 5.46 μg/ml. This concentration was used as the extract dose in the anti-inflammatory test (we used 2 × IC50 concentration). From 2 independent experiments, we found that the average of CC50 value was 101.96 μg/ml. The SI was determined by calculating the ratio between CC50 and IC50. The SI value for the butanol fraction of C. alata leaf extract in Vero E6 C1008 cells in this study was 18.67.
Anti-inflammatory activity of C. alata butanol fraction in PBMC cells
The anti-inflammatory activity of the butanol fraction of C. alata was analyzed using delta delta CT (ΔΔCT) to obtain the fold changes value of the target cytokine expression.
At 2 hours of treatment, it was observed that there was a downregulation in the expression of NFκB) and all cytokines (TNF-α, IL-6, and IL-10) in the group treated with a butanol fraction of C. alata leaf extract 2 × IC50. The downregulation in expression of the transcription factor NFκB was slight (0.084-fold change). The cytokines TNF-α, IL-6, and IL-10 were down-regulated by 0.528; 3.519; and 0.445-fold change, respectively (Fig. 1). However, these downregulations were not statistically significant. The most substantial downregulation observed is in IL-6, suggesting that the fraction of C. alata leaf extract 2 × IC50 exerts a particularly strong influence on IL-6 levels within the first 2 hours of treatment. This indicates that the butanol extract may be highly effective in rapidly reducing IL-6 expression during this time frame.
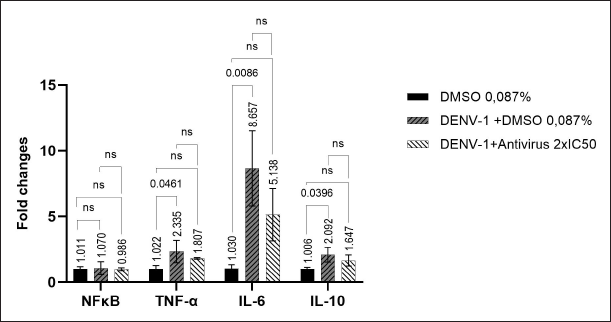 | Figure 1. The expression of NFκB and cytokines in 2 hours post-treatment butanol fraction of C. alata leaf extract 2 × IC50. Relative mRNA expression was analyzed by the delta delta CT method (ΔΔCT). [Click here to view] |
At 24 hours of treatment, it was observed that there was a slight downregulation on the expression of NFκB in the group treated with a butanol fraction of C. alata leaf extract 2 × IC50, while TNF-α, IL-6, and IL-10 were slightly up-regulated (Fig. 2). This shows that the effect of the butanol fraction of C. alata leaf extract was more observed at 2 hours post-treatment compared to 24 hours post-treatment.
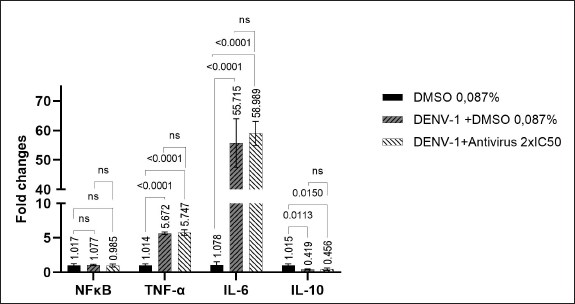 | Figure 2. The expression of NFκB and cytokines in 24 hours post-treatment butanol fraction of C. alata leaf extract 2 × IC50. Relative mRNA expression was analyzed by the delta delta CT method (ΔΔCT). [Click here to view] |
Based on the results, it has been observed that the levels of TNF-α and IL-6 in the group infected with DENV-1 (with or without C. alata treatment) were elevated at 24 hours post-treatment compared to 2 hours post-treatment. In contrast, the expression of IL-10 decreased at 24 hours post-treatment relative to 2 hours post-treatment. The expression of NFκB remained relatively constant between 2 hours and 24 hours post-treatment (Figs. 1 and 2).
DISCUSSION
This research studied the anti-inflammatory activity of the butanol fraction of C. alata leaves on DENV-1 infection in vitro. The C. alata leaf extract itself has been widely studied in previous studies and shows that the active compounds in C. alata leaves have DENV antiviral activity both in vitro and in vivo [23–25,36]. From prior experiments (data not shown), it was found that the total flavonoid content in the butanol fraction of C. alata leaves was 31.34%, which is higher compared to the total flavonoid content in the crude or ethanol extracts of C. alata, which was 6.82%. From IC50 and CC50, the SI value of the butanol fraction of C. alata leaves was 18.67. The SI is usually used as a pre-clinical screening strategy by determining the toxic and effective therapeutic concentrations ratio. A higher SI value is an early indicator of drug efficacy [37], but the value cannot be extrapolated to doses administered in vivo [38]. However, the SI value is a relevant indicator to use in screening procedures for further evaluation of drug candidates [37]. In the dose dependent test (CC50) and toxicity test (IC50), the cells used were Vero E6 cells. Vero E6 cells are a continuous cell line in the form of epithelial cells obtained from the kidneys of African green monkeys (Cercopithecus aethiops) which are extensively used in virology research [39], for example, to evaluate the activity of anti-DENV compounds [40,41] and to produce viruses in the development of DENV vaccines [42]. One of the key limitations of this study is that the IC50 value was derived from Vero cells and used as a reference to assess the anti-inflammatory effects in PBMCs. This approach was adopted because conducting dose-dependent assays directly on PBMCs presents challenges, primarily due to the fact that PBMCs are not a continuous cell line, making them more difficult to work with compared to Vero cells.
DMSO at a concentration of 0.087% was used as a negative control because the 2 × IC50 butanol fraction of C. alata leaves extract contained 0.087% DMSO. Employing DMSO as a negative control helped to minimize bias in the data collected. Administration of the butanol fraction of C. alata leaf extract at 2 × IC50 resulted in a downregulation at 2 hours post-treatment of NFκB expression and TNF-α, IL-6, and IL-10, but not statistically significant. A study with α-mangostin extract showed the suppression of mRNA TNF-α, IL-6, and IL-10 expression by a dose-dependent manner [32]. In this study, we used one dose, i.e., 2 × IC50, and 1 time addition, therefore it is still necessary to explore the optimal dose and time of addition. While this dose was selected to provide initial insights, it may not capture the full range of the compound’s effects. Future studies could benefit from using a more robust experimental design, incorporating multiple doses or higher concentrations. This approach would allow for a more comprehensive understanding of dose-dependent effects and potentially yield more pronounced and reliable results.
In this study, we found that the RNA expression of NFκB after treatment with C. alata were slightly changed, while the expression of TNF-α and IL-6 was decreased at 2-hours post treatment. Cassia alata contain 5’-Tetrahidrox-flavone, Daturametelin H and kaempferol-3,7-diglukoside [23]. This study is concomitant with a previous study that demonstrated that kaempferol inhibits the expression of cytokines induced by NFκB and AP-1 in human umbilical vein endothelial cells (HUVECs) [43]. Flavonoids are also known to inhibit IκBα phosphorylation and degradation and NFκB p65 translocation in HEK 293 cells [44].
In this study, the expression of anti-inflammatory cytokines IL-10 was decreased, meanwhile, the ratio between TNF-α and IL-6 towards IL-10 in C. alata treatment group was slightly lower than in the untreated group (data not shown). The ability of C. alata and kaempferol as anti-inflammatory agents that can suppress TNF-α expression in DENV infection has not been reported, but several previous studies have shown that kaempferol can significantly inhibit TNF-α expression in aged rat gingival tissues by attenuating NFκB nuclear binding activity [45]. Other studies also show that kaempferol at a concentration of 30 µM can significantly decrease the TNF-α expression and secretion of RAW-264.7 macrophages [46]. Kaempferol diminished the overproduction of TNF-α, IL-1β, and IL-6 in inflammation induced by the H9N2 influenza virus both in vivo and in vitro by inhibiting several pathways such as TLR4, NF-κB p65 DNA binding activity, MyD88, and MAPKs [47].
In this study, we evaluate the expression of NFκB, TNF-α, IL-6, and IL-10 in 2 time points (2-hours and 24-hours post-treatment based on the kinetic of NFκB; NFκB function as transcription factor so we expect that the effect of C. alata will be better observed in early phase of stimulation. The results showed that the effect of C. alata leaves butanol fraction was stronger in downregulating the expression of TNF-α, IL-6, and IL-10 at 2 hours post treatment.
In blood vessels, IL-6 can directly or indirectly affect vascular endothelial cells. IL-6 is able to increase the disassembly of VE-cadherin through the induction of VEGF and the expression of the C5a receptor, which causes blood vessel permeability. IL-6 directly stimulates vascular endothelial cells to produce proinflammatory cytokines through trans-signaling [48]. With its ability to stimulate pro-inflammatory cytokines, IL-6 is very influential in plasma leakage. The decrease in IL-6 expression by the butanol fraction of C. alata 2 × IC50 is very useful in preventing the severity of DENV infection. On the other hand, with the nature of the butanol fraction of C. alata which is nontoxic, has anti-DENV activity with a low IC50 value, and shows anti-inflammatory activity by reducing IL-6 very significantly, the butanol fraction of C. alata has potential as an anti-inflammatory candidate in the future.
Further study is needed to evaluate the potential and mechanism of C. alata as anti-inflammation. In another hand, this study reflects the immune response of only one individual, as the PBMC donor was a single person. Future research should involve more subjects for a better understanding of the immune response across multiple individuals and to obtain more reliable data.
CONCLUSION
These results suggest the butanol fraction of C. alata leaf extract has more pronounced effects at 2 hours post-treatment compared to 24 hours. Butanol fraction of C. alata leaf extract potentially used as an antiviral agent with other advantages of potential anti-inflammation effect.
ACKNOWLEDGMENT
Special thanks to Fithriyah, Mayuri Tarasuk, Hidayati Desti, Chrecentia Hanna Swestikaputri, Fathur Luthfiano, Lovendo Ilham Widodo, Sabighoh Zanjabila, Regita Aulia, and Resda Akhra Syahrani for technical supports.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research was partly supported by PUTI Pasca-sarjana 2023 Grant no. NKB-154/UN2.RST/HKP.05.00/2023 and Indonesia Endowment Fund for Education Agency.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The anti-inflammatory test used PBMC from a donor who was not being infected with DENV. The study protocol was approved by the Health Research Ethics Committee, Dr. Cipto Mangunkusumo General Hospital, Faculty of Medicine, Universitas Indonesia, Indonesia (Approval No.KET-1449/UN2.F1/ETIK/PPM.00.02/2023).
DATA AVAILABILITY
The data presented in this study are available in this article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Bhatt P, Sabeena SP, Varma M, Arunkumar G. Current understanding of the pathogenesis of dengue virus infection. Curr Microbiol. 2021;78:17–32.
2. Flint J, Rall GF, Racaniello VR, Hatziioannou T, Skalka AM. Principle of virology volume I: molecular biology. 4th ed. Washington, DC: ASM Press; 2015. doi: https://doi.org/978-1-55581-933-0
3. Nanaware N, Banerjee A, Bagchi SM, Bagchi P, Mukherjee A. Dengue virus infection: a tale of viral exploitations and host responses. Viruses. 2021;13:1–20.
4. Roy SK, Bhattacharjee S. Dengue virus: epidemiology, biology, and disease aetiology. Can J Microbiol. 2021; 67(10):687–702. doi: https://doi.org/10.1139/cjm-2020-0572
5. Malavige GN, Ogg GS. Pathogenesis of vascular leak in dengue virus infection. Immunology. 2017;151:261–9.
6. Nakao S, Kuwano T, Ishibashi T, Kuwano M, Ono M. Synergistic effect of TNF-alpha in soluble VCAM-1-induced angiogenesis through alpha 4 integrins. J Immunol. 2003;170:5704–11.
7. Inyoo S, Suttitheptumrong A, Pattanakitsakul S. Synergistic effect of TNF-a and dengue virus infection on adhesion molecule reorganization in human endothelial cells. Jpn J Infect Dis. 2017;70:186–91.
8. Dewi BE, Takasaki T, Sudiro TM, Nelwan RH, Kurane I. Elevated levels of soluble tumour necrosis factor receptor 1, thrombomodulin and soluble endothelial cell adhesion molecules in patients with dengue haemorrhagic fever. Dengue Bull. 2007;31:103–10.
9. Dewi BE, Takasaki T, Kurane I. Peripheral blood mononuclear cells increase the permeability of dengue virus-infected endothelial cells in association with downregulation of vascular endothelial cadherin. J Gen Virol. 2008;89:642–52.
10. Talavera D, Castillo AM, Dominguez MC, Gutierrez AE, Meza I. IL8 release, tight junction and cytoskeleton dynamic reorganization conducive to permeability increase are induced by dengue virus infection of microvascular endothelial monolayers. J Gen Virol. 2004;85:1801–13.
11. Dewi BE, Takasaki T, Kurane I. In vitro assessment of human endothelial cell permeability: effects of inflammatory cytokines and dengue virus infection. J Virol Methods. 2004;121:171–80.
12. Yong YK, Wong WF, Vignesh R, Chattopadhyay I, Velu V, Tan HY, et al. . Dengue infection—recent advances in disease pathogenesis in the Era of COVID-19. Front Immunol. 2022;13:1–17.
13. Kasilo OM, Lusamba Dikassa PS, Mwikisa Ngenda C, Trapsida JM. An overview of the traditional medicine situation in the African region. Afr Health Monit 2010;14:7–15.
14. Butthep P, Chunhakan S, Yoksan S, Tangnararatchakit K, Chuansumrit A. Alteration of cytokines and chemokines during febrile episodes associated with endothelial cell damage and plasma leakage in dengue hemorrhagic fever. Pediatr Infect Dis J. 2012;31:232–8.
15. Riley JK, Takeda K, Akira S, Schreiber RD. Interleukin-10 receptor signaling through the JAK-STAT pathway. J Biol Chem. 1999;274:16513–21.
16. Damodaran S, Venkataraman S. A study on the therapeutic efficacy of Cassia alata, Linn. leaf extract against Pityriasis versicolor. J Ethnopharmacol. 1994;42:19–23.
17. Edegbo E, Okolo ML, Adegoke AS, Omatola CA, Idache BM, Abraham JO, et al. Phytochemical screening and antifungal activity of Cassia alata (Linn.) crude leaf extracts. African J Microbiol Res. 2023;17:176–83.
18. Toh SC, Lihan S, Bunya SR, Leong SS, . In vitro antimicrobial efficacy of Cassia alata (Linn.) leaves, stem, and root extracts against cellulitis causative agent Staphylococcus aureus. BMC Complement Med Ther. 2023;23(1):85. doi: https://doi.org/10.1186/s12906-023-03914-z
19. Yang PS, Dai JM, Gu XJ, Xiong W, Huang DQ, Qiu SY, et al. Indole alkaloids and chromones from the Stem Bark of Cassia alata and their antiviral activities. Molecules. 2022;27:3129.
20. Varghese GK, Bose LV, Habtemariam S. Antidiabetic components of Cassia alata leaves: identification through α-glucosidase inhibition studies. Pharm Biol. 2012;51:345–9.
21. Oladeji OS, Adelowo FE, Oluyori AP, Bankole DT. Ethnobotanical description and biological activities of Senna alata. Hindawi Evid-Based Complement Altern Med. 2020;2020:2580259.
22. Henri, Sari DP, Hakim L. Medicinal plants for traditional treatment used by the Malays in South Bangka Regency, Indonesia. Biosaintifika. 2022;14:125–34.
23. Angelina M, Hanafi M, Suyatna FD, Dewi BE. Drug of action Cassia Alata leaves extract as antiviral to dengue virus serotype-2 in vitro. Pharmacogn J. 2020;12:864–71.
24. Angelina M, Hanafi M, Suyatna FD, Ratnasari S, Dewi BE. Antiviral effect of sub fraction Cassia alata leaves extract to dengue virus serotype-2 strain New Guinea C in human cell line Huh-7 it-1. IOP Conf Ser Earth Environ Sci. 2017;101:012004. doi: https://doi.org/10.1088/1755-1315/101/1/012004
25. Angelina M, Hanafi M, Yuliani T, Suyatna F, Soediro TM, Dewi BE. The inhibitory activity of Cassia alata leaves extract on denv-2 replication infected mice. Pharmacia. 2022;69:821–6.
26. Tresnaningtyas SA, Sjatha F, Dewi BE. Infectivity and viability of dengue virus infected hepatocytes cocultured with peripheral blood mononuclear cells from a healthy subject. Med J Indones. 2020;29:260–7.
27. Ugwu DI, Conradie J. Anticancer properties of complexes derived from bidentate ligands. J Inorg Biochem. 2023;246:112268.
28. Widiandani T, Tandian T, Zufar BD, Suryadi A, Purwanto BT, Hardjono S. In vitro study of pinostrobin propionate and pinostrobin butyrate: Cytotoxic activity against breast cancer cell T47D and its selectivity index. J Public Heal Afr. 2023;14:2516.
29. Kleiveland CR. Peripheral blood mononuclear cells. In: Verhoeckx K, Cotter P, López-Expósito I, Kleiveland C, Lea T, Mackie A, et al. editors. The impact of food bioactives on health: in vitro and ex vivo models. Berlin, Germany: Springer; 2015. Available from: https://www.ncbi.nlm.nih.gov/books/NBK500148
30. Nadhira M, Puspitasari RL, Moegni KF, Rosadi I, Rosliana I.. Profil peripheral blood mononuclear cells (PBMC) pasien dengan Berbagai Usia Menggunakan Flow Cytometry di Klinik Hayandra. J Al-Azhar Indones Seri Sains Dan Teknol. 2018;4:208–16.
31. Yi B, Hu X, Zhang H, Huang J, Liu J, Hu J, et al. Nuclear NF-κB p65 in peripheral blood mononuclear cells correlates with urinary MCP-1, RANTES and the severity of type 2 diabetic nephropathy. PLoS One. 2014;9(6):e99633. doi: https://doi.org/10.1371/journal.pone.0099633
32. Tarasuk M, Songprakhon P, Chieochansin T, Choomee K, Na-Bangchang K, Yenchitsomanus PT. Alpha?mangostin inhibits viral replication and suppresses nuclear factor kappa B (NF?κB)?mediated inflammation in dengue virus infection. Sci Rep. 2022;12:16088.
33. Staples KJ, Smallie T, Williams LM, Foey A, Burke B, Foxwell BM, et al. IL-10 Induces IL-10 in primary human monocyte-derived macrophages via the transcription factor Stat3. J Immunol. 2007;178:4779–85.
34. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8.
35. Rao X, Huang X, Zhou Z, Lin X. An improvement of the 2ˆ(–delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath. 2013;3:71–85.
36. Prasetya SI, Tanujaya A, Ratnasari S, Desti H, Louisa M, Angelina M, et al. Antiviral activity of Cassia Alata and Cosmos Caudatus Leaf extract to dengue virus serotype-2 new Guinea C strain in Huh7-it1 cell. Adv Sci Lett. 2018;24:6388–92.
37. Ali M, van Gent ME, de Waal AM, van Doodewaerd BR, Bos E, Koning RI, et al. Physical and functional characterization of PLGA nanoparticles containing the antimicrobial peptide SAAP-148. Int J Mol Sci. 2023;24:2867.
38. Kaminsky R, Schmid C, Brun R. An ‘In vitro selectivity index’ for evaluation of cytotoxicity of antitrypanosomal compounds. In Vitro Toxicol. 1996;9:315–23.
39. Ammerman NC, Beier-Sexton M, Azad AF. Growth and maintenance of Vero cell lines. Curr Protoc Microbiol. 2008;Suppl. 11:A.4E.1–A.4E.7. doi: https://doi.org/10.1002/9780471729259.mca04es11
40. Ansori AN, Fadholly A, Proboningrat A, Hayaza S, Susilo RJ, Naw SW, et al. In vitro antiviral activity of Pinus merkusii (Pinaceae) stem bark and cone against dengue virus type-2 (DENV-2). Res J Pharm Technol. 2021;14:3706–9.
41. Safitri M, Kholifah SN, Roza RM. Mangifera foetida L. (Macang) Source of Potent Antiviral Activity of Against Dengue Virus Serotype 2 (Anti DENV2). J Phys Conf Ser. 2021;2049:012018.
42. Piccini LE, Castilla V, Damonte EB. Dengue-3 virus entry into vero cells: role of clathrin-mediated endocytosis in the outcome of infection. PLoS One. 2015;10:e0140824.
43. Crespo I, García-Mediavilla MV, Gutiérrez B, Sánchez-Campos S, Tuñón MJ, González-Gallego J. A comparison of the effects of kaempferol and quercetin on cytokine-induced pro-inflammatory status of cultured human endothelial cells. Br J Nutr. 2008;100:968–76.
44. Lee SH, Kim YJ, Kwon SH, Lee YH, Choi SY, Park JS, et al. Inhibitory effects of flavonoids on TNF-α-induced IL-8 gene expression in HEK 293 cells. BMB Rep. 2009;42:265–70.
45. Kim HK, Park HR, Lee JS, Chung TS, Chung HY, Chung J. Down-regulation of iNOS and TNF-alpha expression by kaempferol via NF-kappaB inactivation in aged rat gingival tissues. Biogerontologi. 2007;8:399–408.
46. Palacz-Wrobel M, Borkowska P, Paul-Samojedny M, Kowalczyk M, Fila-Danilow A, Suchanek-Raif R, et al. Effect of apigenin, kaempferol and resveratrol on the gene expression and protein secretion of tumor necrosis factor alpha (TNF-α) and interleukin-10 (IL-10) in RAW-264.7 macrophages. Biomed Pharmacother. 2017;93:1205–12.
47. Zhang R, Ai X, Duan Y, Xue M, He W, Wang C, et al. Kaempferol ameliorates H9N2 swine influenza virus-induced acute lung injury by inactivation of TLR4/MyD88-mediated NF-κB and MAPK signaling pathways. Biomed Pharmacother. 2017;89:660–72.
48. Kang S, Kishimoto T. Interplay between interleukin-6 signaling and the vascular endothelium in cytokine storms. Exp Mol Med. 2021;53:1116–23.