INTRODUCTION
Alpinia calcarata (Haw.) Roscoe, the Zingiberaceae family herb, has traditionally been used as medicine in Sri Lanka and is produced mainly in tropical regions of Asia [1]. Alpinia calcarata rhizome ethanolic extract Alpinia calcarata (Haw.) Roscoe rhizome ethanolic extract (ACREE) has been used to treat respiratory diseases such as asthma, bronchitis, and cough and as an anti-inflammatory agent [2]. ACR rhizome contains bioactive compounds such as flavonoids and terpenoids, phenolic compounds with antioxidant, anti-inflammatory, antidiabetic, antifungal, antibacterial, analgesic, and anticancer properties and was found to be an effective antinociceptive in rats [3–7]. Previous studies have also reported ACREE’s hypoglycaemic and antihyperlipidemic effects [8]. ACREE was also a possible source of antitumor agents [9]. Cancer is one of the most common causes of death worldwide. Cis-diamminedichloroplatinum II, also known as cisplatin, is an antineoplastic agent effective in cancer treatment [10]. However, it forms a DNA-platinum product, leading to neural damage by axonal modification [11] and significant myelinated neural fiber injury [12]. Cisplatin can react with mitochondrial DNA, generating reactive oxygen compounds affecting axonal transmission due to the axonal localization of mitochondria, leading to chronic neural weakness [13]. Other independent studies reported that mitochondrial irregularities are linked to neuronal and axonal degeneration [14]. Cisplatin is a platinum anticancer drug with severe side effects, including peripheral neuropathy [15] and a molecular affinity for the peripheral nervous system not protected by a vascular barrier [16]. It causes DNA crosslinking, leading to apoptosis, causing peripheral neural damage, and sensory dysfunction [17]. They have also been reported to reduce anti-angiogenesis effects, which may cause nerve damage [18]. Other toxicities of cisplatin, including nephron and cardio, were reported [19], while peripheral neurotoxicity is a disabling side effect affecting the living quality of the patient. The therapeutic findings of ACREE and the worldwide spread of cisplatin-induced peripheral neuropathy (CIPN) have garnered interest in exploring ACREE’s potential in treating CIPN, which has not been done so far. Plant-derived medicines represent a crucial aspect of proactive medicine, which focuses on preventing diseases and enhancing overall well-being rather than merely treating symptoms. If ACREE is proven to be an effective remedy for CIPN, it is likely to be seen as a gentler alternative to synthetic drugs and produce fewer side effects. Herbal medicines have gained popularity among patients seeking to improve their quality of life. This interest has also sparked significant attention from the scientific community, which is increasingly exploring the medicinal properties of plants to validate their effectiveness and discover new therapeutic compounds. This study evaluates physical, behavioral, and biochemical parameters, the direct measurement of nerve conduction velocity (NCV), and the histopathology study of sciatic nerves to explore the neuroprotective activity of ACREE in CIPN.
MATERIALS AND METHODS
Materials
Alpinia calcarata rhizomes were freshly collected in January 2021 from the Indian Institute of Horticultural Research, Bengaluru (Karnataka state, India), authenticated by Dr. P.E. Rajasekharan, Nodal Officer and Principal Scientist, GAC Division, Department of Plant Genetic Resources, Bengaluru (Karnataka state, India). Verified the plant name Alpinia calcarata with http://www.theplantlist.org/, accessed on Sep 20, 2023.
Cisplatin was procured from Hetero Healthcare Ltd. Diagnostic for the high sensitivity C reactive protein (hs-CRP) ELISA (enzyme-linked immunosorbent assay) kit and biochemical evaluations from Krishgen Biosystem, India. Cytokines assay Tumor necrosis factor-α (TNF-α) and IL-6 assessment commercially available ELISA kits, following manufacturer instructions from Pierce Endogen (Rockford, IL, USA). All the chemicals used were of analytical grade. Ethanolic extraction of A. calcarata rhizomeThe freshly collected Alpinia calcarata (Haw.) Roscoe (ACR) rhizomes were fragmented and air-dried under shade for 2 weeks. Five hundred grams of dried powder was extracted with 1.5 l of ethanol using the Soxhlet process for 4 hours. The extraction then was filtered, followed by concentration under diminished pressure at 55°C temp. The percentage yield was 18.5 w/w (dry weight). The extract was preserved at 4°C for further use. Then, a co-precipitate with Polyvinylpyrrolidone (PVP, 44,000 MW) in a 1:1 (w/w) ratio was used for the administration [3].
CIPN in rats
Wistar albino rats of either sex, weighing 180–200 g, were kept in clean polypropylene cages under suitable laboratory conditions (12 hours light/dark cycle, 25ºC ± 2ºC temp) provided with a nutritional diet and water ad libitum. Before commencing the experiment, an animal ethical clearance and approval were availed from KLE’s College of Pharmacy Bengaluru, Institutional Animal Ethics Committee (IAEC no. 03/HP/2021). Cisplatin [(2 mg/kg), intraperitoneal (i.p.)] was administered (followed by 2 ml of normal saline water as nephrotoxicity prevention) to form CIPN rat models [20,21]. The cisplatin dosage routine is mentioned in Table 1.
Experimental design
After a week’s acclimatation, a 5-week experiment followed by analysis was performed on rats (4 groups with N = 6), as detailed in Table 1. Groups I and II were (normal and diseased groups, respectively), and III and IV were treatment groups with daily ACREE dosages of 250 mg/kg and 500 mg/kg, respectively [3,5].
Parameters evaluation
To explore the neuroprotective activity of ACREE, physical (body weight), food and water intake, behavioral, electrophysiological, and biochemical parameters were analyzed using a one-way ANOVA technique and a histopathological study haematoxylin-eosin (H & E) was performed.
Physical
Changes in body weight and food and water intake profiles were recorded across animals used in the experiment.
Hot and cold hyperalgesia
To test hot and cold hyperalgesia, the tail was immersed in hot (46ºC, cut-off time 12 seconds) and cold (4ºC, cut-off time 10 seconds) water. The reaction time required to remove the tail was recorded as discomfort or reflexive action [22]. Tails were thoroughly dried in each rat sample post experiment [23].
Rota-rod performance (motor coordination)
The ability of the rat to maintain its position on a rotating rod (75 mm diameter) was measured using a rota-rod setup calibrated to a speed at which normal rats (Group I) could withstand >5 min on it. All the rats had three trial runs with a minimum 3-hour gap. The time spent by the rats on rotating rod at 15 and 25 rpm was recorded for each rat [20].
Grip strength
The rats were familiarised with the grip strength meter for a designated period. Each rat was suspended using its tail to measure grip strength, ensuring a roughly horizontal orientation above the apparatus grid. Then, an external force was applied to the tail base in alignment with the sensor axis, and the response force by the rat was recorded before leaving its grip [24].
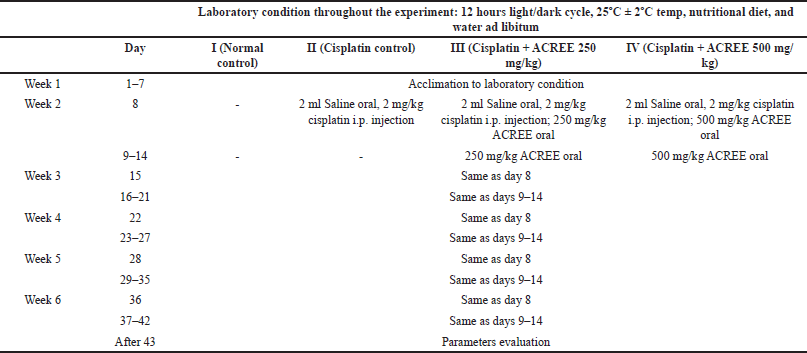 | Table 1. Experimental design to explore peripheral neuroprotection potential of ACREE. [Click here to view] |
Nerve conduction study
Non-invasively NCV was measured using a Power Lab data acquisition system. An anesthetic combination of Xylazine (5–10 mg/kg, i.p.) and Ketamine (80–100 mg/kg, i.p.) was administered during the measurement. The sciatic nerve was stimulated using a bipolar electrode with an 8V, 20Hz signal, and calculated the NCV by dividing the spacing between the stimulating and recording electrodes by proximal and distal latencies difference [25,26]. The stimulating electrode probed the sciatic notch and the recording to the second interosseous muscle of the hindfoot and the ground electrode to the lateral part of the hindfoot. Stimulus latency was measured from the stimulus artefact to the M wave peak. Then, the recording electrode was moved to the tendon, and the procedure was repeated to find the recording latency.
Biochemical (Inflammatory markers and oxidative stress parameters)
hs-CRP level in blood serum was done using hs-CRP ELISA kit [27], procured from Krishgen Biosystems Ltd. Cytokines assay IL-6 and TNF-α assessment was done using commercially available ELISA kits (Spectramax i3x, Molecular devices), following manufacturer instructions from Pierce Endogen (Rockford, IL, USA) and the method used by Whiteside [28]. To measure oxidative stress markers [Superoxide dismutase (SOD), Catalase (CAT), Malondialdehyde (MDA) and Glutathione (GSH)], the sciatic nerve was segregated, followed by homogenization in phosphate-buffered saline (pH 7.4, 0.1M) at ice-cold temp (IKA 3720000 T 18 Digital Ultra Turrax), further segregated into aliquots, getting post-nuclear and mitochondrial fractions [29]. The method elaborated by Paoletti et al. [30] was used for SOD assessment. CAT was assessed by measuring the hydrogen peroxide decomposition rate (mole/minute) [31]. Lipid peroxidation was determined by MDA assessment [32]. The tissue sulfhydryl group was assessed by GSH levels [33].
Histopathology
The separated sciatic nerve was immersed in pH-balanced formalin buffer (10%) and planted on paraffin wax for sectioning. Ultra-fine 5-μm transverse segments were taken with a microtome for staining with haematoxylin-eosin (H & E) for general morphology [34,35].
Statistical analysis
The comparison across groups (normal control, disease control, and treatments) was done using ANOVA. Post hoc Dunnett’s multiple comparison was done using Graph pad Prism software (v9), and data were represented in mean ± standard mean error (SEM).
RESULTS AND DISCUSSION
Cisplatin or ACREE doses during the study did not cause any mortality. The exploration of ACREE as a neuroprotectant has not been done so far, but multiple other allied therapeutic potentials, like anti-inflammatory, anti-oxidant, and antinociceptive of A. calcarata have been shown by studies [3,4,5]. The physical, behavioral, and biochemical parameters, sciatic NCV measurement results, and histopathology analysis are detailed in the following subsections.
Measurement of body weight, food intake, and water intake
Reduced food consumption and increased water intake in group II indicate loss of appetite (diseased condition) compared to group I. As a result, weight loss was observed in group II (p < 0.001), which was improved in ACREE treatment groups (III: p < 0.001 and IV: p < 0.05) (Fig. 1). Also, as mentioned in Table 2, the treatment groups showed balanced food and water intake with maintained body weight.
Motor coordination and grip strength
Rota rod time reduction by 25% and grip strength by 40% confirmed peripheral neuropathy in group II, which was significantly improved in treatment groups (rota-rod performance, Fig. 2c, p < 0.01 and grip strength, Fig. 2d, p < 0.05 and p < 0.001 for III and IV resp.) compared to group II. The grip strength reduction aligns with another study about peripheral neuropathy [36].
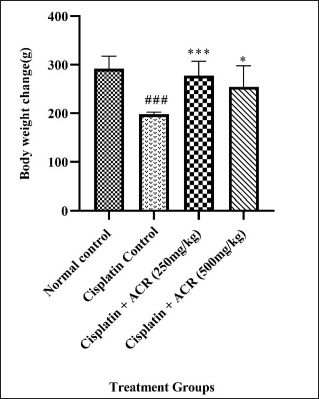 | Figure 1. Body weight across test groups. Values are in Mean ± SEM, n = 6. ###: p < 0.001 with respect to normal group, ***: p < 0.001, **: p < 0.01, *:p < 0.05 with respect to cisplatin group. [Click here to view] |
Hot and cold hyperalgesia
As listed in Table 2, reduced tail immersion time in hot (by 56%, p < 0.001) and cold (by 42%, p < 0.001) water in the cisplatin control group indicates an increased sensitivity to pain (Hyperalgesia), a known symptom of peripheral neuropathy [37]; improved with treatment (p < 0.001) in both the dosage groups III and IV (Fig. 2a and b). Hyperalgesia is one of the critical symptoms of neuropathic pain in patients, seen in 15%–50% of individuals with peripheral or central neuropathy [38]. According to the underlying mechanism, tissues get invaded, leading to signaling and expression changes of non-specific ionic and specific potassium and sodium channels [39–42].
Nerve conduction study
Severe reduction (p < 0.001) in sciatic NCV was found in the cisplatin control group over the normal group. The ACREE treatment, both 250 mg/kg and 500 mg/kg doses, showed improved NCV (p < 0.001) (Fig. 3) over cisplatin control, indicating nerve functionality reversal against cisplatin-induced deficits. NCV reduction could be due to myelin thickness reduction caused by cisplatin toxicity [43–45], and the myelin abnormality could be due to Schwann cells apoptosis by cisplatin [46]. The demyelination observed in these studies also explains the NCV reduction in the cisplatin group and the possibility of myelin regeneration with ACREE treatment showing increased NCV. Though the mechanisms underlying CIPN and NCV reduction are not fully revealed, the available evidence suggests oxidative stress was one of the major culprits. The reactive oxygen species (ROS) formation was elevated in cisplatin-exposed neurons [47,48]. Cisplatin also causes mitochondrial injuries in DRG neurons [49]. Higher ROS levels, along with mitochondrial dysfunction, cause oxidative stress. Therefore, it is also possible that the favorable effects of ACREE on these impairments in CIPN models are due to oxidative stress reduction. To support this, the oxidative stress parameters (SOD, CAT, MDA, and GSH) were evaluated in the current study.
Inflammatory markers and oxidative stress parameters
hs-CRP (Fig. 4), TNF-α, and Interleukin (IL6) (Fig. 5a and b) data showed severe inflammation with cisplatin-caused neuropathic damage indication [15]. Additionally, the NF-kß overexpression, resulting from increased protein kinase C, ROS, and advanced glycation end products caused by leukocytic infiltration, contributes to the peripheral neuron degeneration and reduction in TNF-α, IL6, neuronal growth factor, and IL1ß levels [50]. ACREE showed anti-inflammatory and analgesic properties via inhibiting NF-kß and TNF-α pathways [51]. ACREE treatment on CIPN models in this study showed inflammation reduction (hs-CRP, TNF-α, IL6) (p < 0.001), as detailed in Table 3.
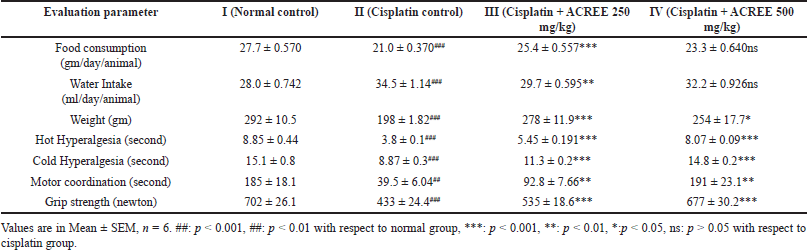 | Table 2. Effect of ACREE on food and water consumption, body weight, and behavioral parameters. [Click here to view] |
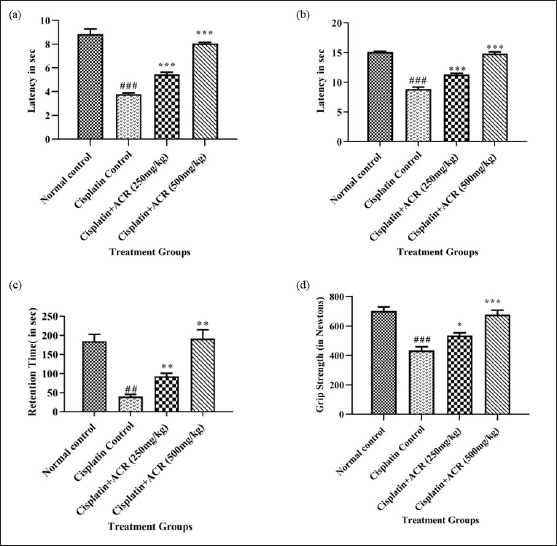 | Figure 2. Behavioural evaluation of ACREE treatment: a) Hot hyperalgesia, b) cold hyperalgesia, c) rota-rod performance, and d) grip strength. Values are in Mean ± SEM, n = 6. ###: p < 0.001 with respect to normal group, ***: p < 0.001, **: p < 0.01, *:p < 0.05 with respect to cisplatin group. [Click here to view] |
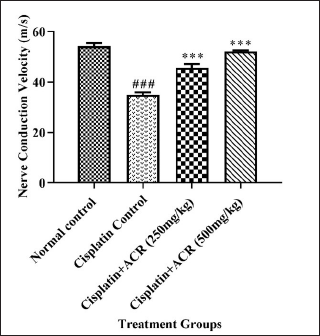 | Figure 3. Effect of ACREE treatment on NCV. Values are in Mean ± SEM, n = 6. ###: p < 0.001 with respect to the normal group, ***: p < 0.001 with respect to the cisplatin group. [Click here to view] |
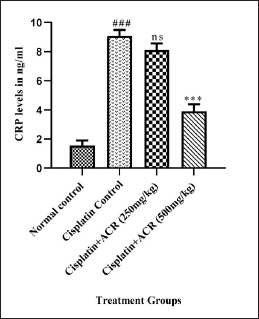 | Figure 4. ACREE treatment effect on hs-CRP level. Values are in Mean ± SEM, n = 6. ###: p < 0.001 with respect to the normal group, ns: p > 0.05, ***: p < 0.001, ns p > 0.05 with respect to the cisplatin group. [Click here to view] |
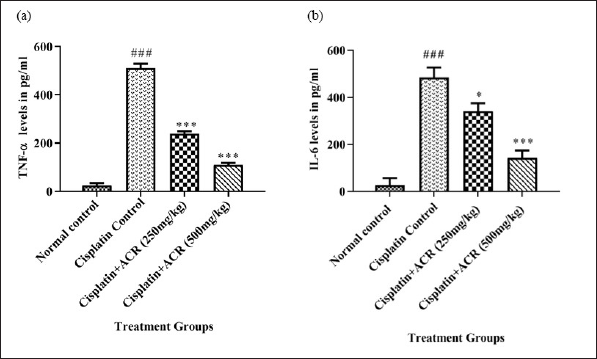 | Figure 5. ACREE effect on Inflammatory markers: a) TNF-α and b) IL-6. Values in Mean ± SEM, n = 6. ###: p < 0.001 with respect to normal group, *: p < 0.05, **: p < 0.01, ***: p < 0.001, with respect to cisplatin group. [Click here to view] |
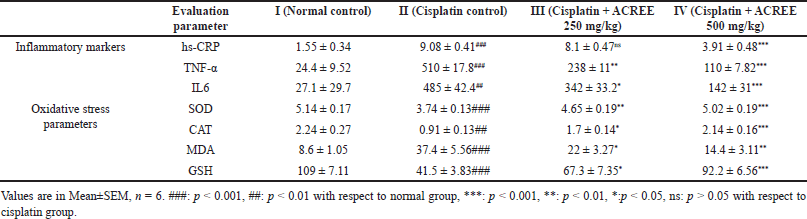 | Table 3. Effect of ACREE treatment on inflammatory markers and oxidative stress parameters. [Click here to view] |
CAT, an antioxidant enzyme, and reduced GSH, an antioxidant level, dropped to <40% from normal (p < 0.001); the same was confirmed by lowered oxidative stress markers SOD (p < 0.001) and elevated MDA (p < 0.001). All the markers mentioned above were improved with ACREE treatment (Table 3, Fig. 6). A study by Jiang et al. [47], suggested ROS generation elevation in cisplatin-exposed neurons. Another research [48,52] revealed cisplatin-induced oxidative stress due to mitochondrial dysfunction (caused by mitochondrial defects in dorsal root ganglion neurons) and increased ROS levels. Also, an inhibition of oxidative stress was associated with NCV improvement [53].
SOD, CAT, GSH, and MDA level alterations from groups I to II and then II to III and IV in this study showed cisplatin-induced oxidative stress and its reduction with ACREE treatments.
Histopathology study
Sciatic nerve histopathology (staining with H&E) was performed to confirm the effect of ACREE treatment on neuro-morphology. As shown in Figure 7A, the normal control group had typical myelinated nerve fibers with intact myelin sheath. In contrast, the cisplatin control group showed moderate nerve fiber degeneration (arrow) and demyelination (Fig. 7B). ACREE-treated groups had restored neuro-morphology with myelin sheath, appearing closer to normal, indicating the neuroprotective role of ACREE (Fig. 7C and D). These morphological observations align with NCV, oxidative stress, and inflammatory parameter evaluations. Also, the cisplatin-induced neuro-morphological defects observed are in line with cisplatin neurotoxicity histopathological results in another study by [54].
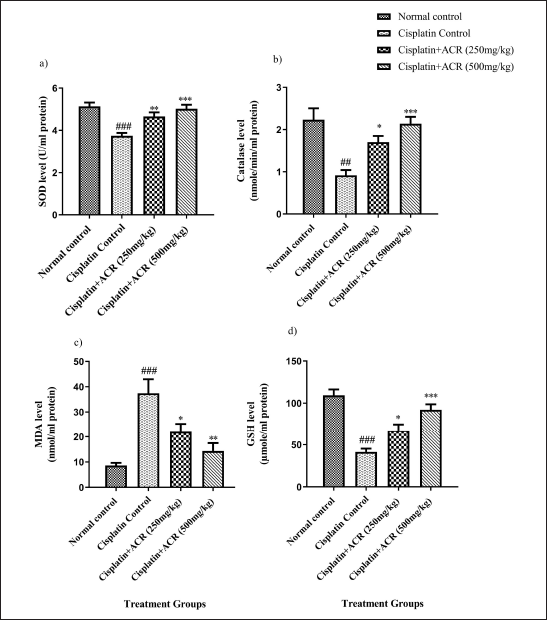 | Figure 6. ACREE treatment effect: a) SOD, b) CAT, c) MDA, and d) GSH. Values in Mean ± SEM, n = 6. ##: p < 0.01, ###: p < 0.001 with respect to normal group, *: p < 0.05, **: p < 0.01, ***: p < 0.001, with respect to cisplatin group. [Click here to view] |
Although the results in rat models are promising, extensive clinical trials are needed to transition from lab trials on rats to medicinal use in humans. Other challenges that any medicinal plant needs to address are flawless identification of ACR and type of extract, standardization, and repeatable and reliable extraction process. Without extensive study, it is challenging to say whether plant extraction method and source or origin affect drug concentration. This study gives hope for effectively managing CIPN clinically based on its effectiveness in rat models. However, extensive quantitative analysis would be needed to establish the appropriate human dosage and uncover any safety risks.
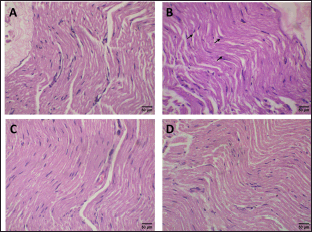 | Figure 7. Representative photos of sciatic nerve sections (Haematoxylin-eosin (H & E) stain with magnification 40x): (A) Normal control group showing normal myelinated nerve fibers; (B) Cisplatin control group showing focal areas of demyelination and moderate degeneration of nerve fibers (arrows); (C and D) A. calcarata (Haw.) Roscoe extract treated (250 mg/kg and 500 mg/kg) groups showing normal appearance of nerve fibers with a myelin sheath. [Click here to view] |
CONCLUSION
This study demonstrates ACREE’s neuroprotective effect in ameliorating CIPN’s detrimental impact in rat models. Through comprehensive observations like hot and cold hyperalgesia amelioration, oxidative stress and inflammation alleviation, improved motor coordination, and promoted nerve revival by limiting and reverting functional and structural (sciatic nerve morphology) damage, ACREE shows counteraction against cisplatin-induced neurotoxicity in rat models. detrimental impact in rat models. Through comprehensive observations like hot and cold hyperalgesia amelioration, oxidative stress and inflammation alleviation, improved motor coordination, and promoted nerve revival by limiting and reverting functional and structural (sciatic nerve morphology) damage, ACREE shows counteraction against cisplatin-induced neurotoxicity in rat models. This novel finding could improve cancer patient survival and life quality. Further research is recommended to elucidate the precise mechanisms of ACREE’s protective effects and explore its integration with existing cancer treatments to effectively neutralize the impact of CIPN.
ACKNOWLEDGMENTS
The authors thank the Organisation of Green Chem Bangalore (Karnataka, India) for providing services for the ethanolic extraction of A. calcarata rhizome.
LIST OF ABBREVIATIONS
ACREE, Alpinia calcarata (Haw.) Roscoe rhizome ethanolic extract; ACR, Alpinia calcarata (Haw.) Roscoe; CAT, Catalase; CDDP, cis-diamminedichloroplatinum (II); CIPN, cisplatin-induced peripheral neuropathy; GSH, Glutathione; hs-CRP, high sensitivity C reactive protein; IL6, Interleukin 6; i.p., intraperitoneal; MDA, Malondialdehyde; NCV, nerve conduction velocity; SOD, Superoxide dismutase; TNF-α, Tumor necrosis factor-α.
AUTHOR CONTRIBUTIONS
Apurwa Shyam Dhavale designed and conducted the experimental work and contributed to the writing and revision of this manuscript. Hariprasad M.G. supervised the entire experimental work and reviewed the written manuscript. Rajendran Ramaswamy guided on drug identification and the ethanolic extract. All authors have read and approved the final manuscript for publication and agree to be accountable for all aspects of the work, ensuring integrity and accuracy.
FINANCIAL SUPPORT
This research received no specific grant from any public or commercial funding agency.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by the Institutional Animal Ethics Committee (IAEC) of the KLE College of Pharmacy, Bengaluru, India with approval number 03/HP/2021.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Jayaweera DMA. Medicinal plants used in Sri Lanka. Colombo, Sri Lanka: National Science Council; 1982. Pp 70–1.
2. Arawwawala LD, Arambewelaa LS, Ratnasooriyab WD. Alpinia calcarata Roscoe: a rich source of phytopharmaceuticals in Sri Lanka. Nat Prod J. 2012;2:263–9. CrossRef
3. Arambewela LS, Arawwawala LD, Ratnasooriya WD. Antinociceptive activities of aqueous and ethanolic extracts of Alpinia calcarata rhizomes in rats. Ethnopharmacol. 2004;95(2-3):311–6. CrossRef
4. Arambewela LS, Arawwawala LD, Athauda N. Antioxidant and antifungal activities of essential oil of Alpinia calcarata Roscoe rhizomes. J Ayurveda Integr Med. 2010;1(3):199–202. CrossRef
5. Arawwawala LD, Arambewela LS, Ratnasooriya WD. Alpinia calcarata Roscoe: a potent antiinflammatory agent. J Ethnopharmacol. 2012;139(3):889–92. CrossRef
6. Ghosh S, Rangan L. Alpinia: the gold mine of future therapeutics. Biotech. 2013;3(3):173–85. CrossRef
7. Akbarzadeh F, Eslamzadeh M, Behravan G, Ebrahimi A, Emami SA, Gilan A, et al. Assessing the effect of Alpinia galanga extract on the treatment of SSRI-induced erectile dysfunction: a randomized triple-blind clinical trial. Front Psychiatry. 2023;14:1105828. CrossRef
8. Rajasekar R, Manokaran K, Rajasekaran N, Duraisamy G, Kanakasabapathi D. Effect of Alpinia calcarata on glucose uptake in diabetic rats-an in vitro and in vivo model. J Diabetes Metab Disord. 2014;13(1):33. CrossRef
9. Perveen R, Islam F, Khanum J, Yeasmin T. Preventive effect of ethanol extract of Alpinia calcarata Rosc on Ehrlich’s ascitic carcinoma cell induced malignant ascites in mice. Asian Pac J Trop Med. 2012 Feb;5(2):121–5. CrossRef
10. Ghosh S. Cisplatin: the first metal based anticancer drug. Bioorg Chem. 2019 Jul;88:102925. CrossRef
11. Argyriou AA, Kyritsis AP, Makatsoris T, Kalofonos HP. Chemotherapy-induced peripheral neuropathy in adults: a comprehensive update of the literature. Cancer Manag Res. 2014 Mar 19;6:135–47. CrossRef
12. Chiorazzi A, Semperboni S, Marmiroli P. Current view in platinum drug mechanisms of peripheral neurotoxicity. Toxics. 2015 Aug 7;3(3):304–21. CrossRef
13. Carozzi VA, Canta A, Chiorazzi A. Chemotherapy-induced peripheral neuropathy: What do we know about mechanisms. Neurosci Lett. 2015 Jun 2;596:90–107. CrossRef
14. Ohno N, Ikenaka K. Axonal and neuronal degeneration in myelin diseases. Neurosci Res. 2019 Feb;139:48–57. CrossRef
15. Akbar S, Subhan F, Shahid M, Wadood A, Shahbaz N, Farooq U, et al. 6 Methoxyflavanone abates cisplatin-induced neuropathic pain apropos anti-inflammatory mechanisms: a behavioral and molecular simulation study. Eur J Pharmacol. 2020 Apr 5;872:172972. CrossRef
16. Park HJ, Stokes JA, Pirie E, Skahen J, Shtaerman Y, Yaksh TL. Persistent hyperalgesia in the cisplatin-treated mouse as defined by threshold measures, the conditioned place preference paradigm, and changes in dorsal root ganglia activated transcription factor 3: the effects of gabapentin, ketorolac, and etanercept. Anesth Analg. 2013 Jan;116(1):224–31. CrossRef
17. Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer. 2008;44(11):1507–15. CrossRef
18. Park SB, Krishnan AV, Lin CS, Goldstein D, Friedlander M, Kiernan MC. Mechanisms underlying chemotherapy-induced neurotoxicity and the potential for neuroprotective strategies. Curr Med Chem. 2008;15(29):3081–94. CrossRef
19. Alberti P. Platinum-drugs induced peripheral neurotoxicity: clinical course and preclinical evidence. Expert Opin Drug Metab Toxicol. 2019 Jun;15(6):487–97. CrossRef
20. Vera G, Cabezos PA, Martín MI, Abalo R. Characterization of cannabinoid-induced relief of neuropathic pain in a rat model of cisplatin-induced neuropathy. Pharmacol Biochem Behav. 2013 Apr;105:205–12. CrossRef
21. Agthong S, Kaewsema A, Charoensub T. Curcumin ameliorates functional and structural abnormalities in cisplatin-induced neuropathy. Exp Neurobiol. 2015 Jun;24(2):139–45. CrossRef
22. Ostovar M, Akbari A, Anbardar MH, Iraji A, Salmanpour M, Hafez GS, et al. Effects of Citrullus colocynthis L. in a rat model of diabetic neuropathy. J Integr Med. 2020;18(1):59–67. CrossRef
23. Padgaonkar AV, Suryavanshi SV, Londhe VY, Kulkarni YA. Acute toxicity study and anti-nociceptive activity of Bauhinia acuminata Linn. leaf extracts in experimental animal models. Biomed Pharmacother. 2018;97:60–6. CrossRef
24. Nayak BP, Minaz N, Pasha K. Molsidomine ameliorates diabetic peripheral neuropathy complications in Wistar rats. Animal Model Exp Med. 2021 Mar 23;4(3):243–8. CrossRef
25. Addepalli V, Suryavanshi SV. Catechin attenuates diabetic autonomic neuropathy in streptozotocin induced diabetic rats. Biomed Pharmacother. 2018;108:1517–23. CrossRef
26. Xie J, Song W, Liang X, Zhang Q, Shi Y, Liu W, et al. Protective effect of quercetin on streptozotocin-induced diabetic peripheral neuropathy rats through modulating gut microbiota and reactive oxygen species level. Biomed Pharmacother. 2020;127(12):110147. CrossRef
27. Jia H, Qu M, Fan G, Wu H, Wang L. miR-499-5p suppresses C-reactive protein and provides neuroprotection in hypoxic-ischemic encephalopathy in neonatal rat. Neurosci Res. 2020;161:44–50. CrossRef
28. Meager A. Measurement of cytokines by bioassays: theory and application. Methods. 2006;38(4):237–52. CrossRef
29. Garud MS, Kulkarni YA. Gallic acid attenuates type I diabetic nephropathy in rats. Chem Biol Interact. 2018;282:69–76. CrossRef
30. Paoletti F, Aldinucci D, Mocali A, Caparrini A. A sensitive spectrophotometric method for the determination of superoxide dismutase activity in tissue extracts. Anal Biochem. 1986;154(2):536–41. CrossRef
31. Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. CrossRef
32. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–8. CrossRef
33. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–7. CrossRef
34. Tikoo K, Meena RL, Kabra DG, Gaikwad AB. Change in post-translational modifications of histone H3, heat-shock protein-27 and MAP kinase p38 expression by curcumin in streptozotocin-induced type I diabetic nephropathy. Br J Pharmacol. 2008;153(6):1225–31. CrossRef
35. Hariprasad MG, Razdan R, Yasha TC, Amrutanand ST. Thyroxine: a putative neuroprotectant in diabetes induced peripheral neuropathy in rats. J Diabetes Metab. 2015;6(9):595. CrossRef
36. Maurissen JP, Marable BR, Andrus AK, Stebbins KE. Factors affecting grip strength testing. Neurotoxicol Teratol. 2003 Sep-Oct;25(5):543–53. CrossRef
37. al M. A novel antioxidant alleviates heat hyperalgesia in rats with an experimental painful peripheral neuropathy. Neuroreport. 1996 May 31;7(8):1382–4. CrossRef
38. Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol. 2014 Sep;13(9):924–35. CrossRef
39. Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009 Oct 16;139(2):267–84. CrossRef
40. Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. Sodium channels in normal and pathological pain. Annu Rev Neurosci. 2010;33:325–47. CrossRef
41. Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009 Sep;10(9):895–926. CrossRef
42. Devor M. Sodium channels and mechanisms of neuropathic pain. J Pain. 2006 Jan;7(1 Suppl 1):S3–S12. CrossRef
43. Verdu E, Vilches JJ, Rodríguez FJ, Ceballos D, Valero A, Navarro X. Physiological and immunohistochemical characterization of cisplatin-induced neuropathy in mice. Muscle Nerve. 1999 Mar;22(3):329–40. CrossRef
44. Wongtawatchai T, Agthong S, Kaewsema A, Chentanez V. Altered phosphorylation of mitogen-activated protein kinases in dorsal root ganglia and sciatic nerve of rats with cisplatin-induced neuropathy. Asian Biomed. 2012;6:397–411. CrossRef
45. Wongtawatchai T, Agthong S, Kaewsema A, Chentanez V. Sex-related differences in cisplatin-induced neuropathy in rats. J Med Assoc Thai. 2009 Nov;92(11):1485–91.
46. Jirsova K, Mandys V, Gispen WH, Bar PR. Cisplatin-induced apoptosis in cultures of human Schwann cells. Neurosci Lett. 2006 Jan 9;392(1-2):22–6. CrossRef
47. Jiang Y, Guo C, Vasko MR, Kelley MR. Implications of apurinic/apyrimidinic endonuclease in reactive oxygen signaling response after cisplatin treatment of dorsal root ganglion neurons. Cancer Res. 2008 Aug 1;68(15):6425–34. CrossRef
48. Jaggi AS, Singh N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology. 2012 Jan 27;291(1-3):1–9. CrossRef
49. McDonald ES, Randon KR, Knight A, Windebank AJ. Cisplatin preferentially binds to DNA in dorsal root ganglion neurons in vitro and in vivo: a potential mechanism for neurotoxicity. Neurobiol Dis. 2005 Mar;18(2):305–13. CrossRef
50. Khan S, Shehzad O, Chun J, Kim YS. Mechanism underlying anti-hyperalgesic and anti-allodynic properties of anomalin in both acute and chronic inflammatory pain models in mice through inhibition of NF-κB, MAPKs and CREB signaling cascades. Eur J Pharmacol. 2013 Oct 15;718(1-3):448–58. CrossRef
51. Suryavanshi SV, Kulkarni YA. NF-κβ: a potential target in the management of vascular complications of diabetes. Front Pharmacol. 2017 Nov 7;8:798. CrossRef
52. Melli G, Taiana M, Camozzi F, Triolo D, Podini P, Quattrini A, et al. Alpha-lipoic acid prevents mitochondrial damage and neurotoxicity in experimental chemotherapy neuropathy. Exp Neurol. 2008 Dec;214(2):276–84. CrossRef
53. Carozzi VA, Marmiroli P, Cavaletti G. The role of oxidative stress and anti-oxidant treatment in platinum-induced peripheral neurotoxicity. Curr Cancer Drug Targets. 2010 Nov;10(7):670–82. CrossRef
54. Al Moundhri MS, Al-Salam S, Al Mahrouqee A, Beegam S, Ali BH. The effect of curcumin on oxaliplatin and cisplatin neurotoxicity in rats: some behavioral, biochemical, and histopathological studies. J Med Toxicol. 2013 Mar;9(1):25–33. CrossRef
yyy