INTRODUCTION
Alzheimer’s is a type of neurodegenerative disease that is characterized by progressive dementia [1,2]. Alzheimer’s disease (AD) mainly affects elderly people, as 50%–70% of the elderly population suffers from it [3]. The global burden of this disease is growing very fast, and it is estimated that the number of AD patients will double every 20 years, reaching over 66 million in 2030 and 100 million by 2050 [4]. The disease normally has a gradual onset followed by ongoing cognitive loss. Memory loss, confusion, and impairments of cognitive function are some of the first noticeable signs of AD that seriously impair the social or occupational performance of the patients [5–12]. On average, it takes about 8.5 years for a person to die after their first appearance of clinical symptoms [13]. The early symptoms of AD in its preclinical stages include hyperphosphorylated tau (p-tau) aggregation in neurofibrillary tangles, amyloid beta (Aβ) accumulation in senile plaques, and ultimately cell death. The development of Aβ plaques, which are an underlying neuropathology feature of AD, is thought to occur 15–20 years before the clinical presentation of the illness and is followed by the build-up of improperly phosphorylated tau in neurofibrillary tangles [14]. Other metabolic systems, such as neurotransmitter metabolism, lipid synthesis, inflammation, and mitochondrial function, are all disturbed in AD. Amyloid plaques are extracellular hydrophobic deposits of the Aβ peptide and are frequently categorized as diffuse or dense core depending on their morphology and whether they stain positively for dense core or negatively for diffuse core with congo-red or Thioflavin-S, both of which are specific dyes for the conformation of the β-pleated sheet [15]. Dominant mutations in one of the three disease genes (PSEN1, PSEN2, or Amyloid precursor protein (APP)), which are all connected to the production of Aβ, account for 10%–15% of all AD cases. Still, the great majority of sporadic cases have an etiology that is not understood. APP is cleaved by the α-secretase enzyme into α–APP and C–83 in healthy conditions. Additionally, APP can be broken down by the enzymes β and α-secretases to produce the peptides Aβ40 and Aβ42. The Aβ42 species are more likely to assume a beta-sheet shape and can therefore aggregate more easily to oligomers, bigger prefibrillary species, and insoluble plaques. The prefibrillar species are thought to possess neurotoxic qualities. Additionally, the presence of plaques can activate microglia, which in turn triggers the release of excessive amounts of proinflammatory cytokines, promoting the production of Aβ42 by the neurons and causing oxidative damage. The construction and stability of microtubules, a crucial part of the neuronal cytoskeleton, are dependent on the microtubule-associated protein, which has six primary isoforms. Neurofibrillary tangles are made of abnormally p-tau build-up in the brains of AD patients. The accumulation of defective Aβ and tau is assumed to cause the severe loss of neurons or synapses and inflammatory processes in the AD brain [4].
BIOMARKERS OF AD
Biomarkers are crucial for improving therapy development and diagnostics in the medical field [16–19]. Eventually, the use of biomarkers in AD could aid in the prediction of disease progression from the asymptomatic stages to full-blown AD [4]. Positron emission tomography (PET), structural magnetic resonance imaging (MRI), cerebrospinal fluid (CSF), and fluoro-deoxy-d-glucose (FDG)-PET measurements of Aβ and tau are the most often employed biomarkers in clinical trials for dementia. However, structural changes detected by MRI are probably present at relatively advanced stages of the illness. PET imaging is somewhat expensive and has restricted availability. In addition, FDG-PET and structural MRI are indirect measurements of the primary pathology indicators of AD (Aβ and tau), which may make them less specific for AD in some circumstances. Biomarkers used in the prognosis and diagnosis of AD have been summarized in Table 1.
Neuroimaging or brain imaging
This technique is used to detect AD in the early stages of progression. [20]. Structural imaging, functional imaging, and molecular imaging are the methods of neuroimaging [21–24].
Structural imaging
This method used MRI and CT scans to detect AD. It gives information about the structure of the brain such as its shape, size, volume, and position of brain tissues. In patients with AD shrinkage of the hippocampus can be seen as an early sign of Alzheimer’s.
Functional imaging
FDG-PET scans are used to check the changes in functions of the brain due to AD. The changes in blood circulation and cell metabolism are detected in this method. A person suffering from AD has a decrease in brain cell activity in some regions. In AD, FDG-PET imaging indicates a decrease in the consumption of glucose by the brain that is required for memory and problem solving
Molecular imaging
This method also uses PET scans to diagnose AD in its early stages to prevent its effect on memory, reasoning, learning, and thinking. FDA-approved four radiopharmaceutical medicinal products used in molecular imaging techniques. These agents are Neuraceq® injection containing Florabetaben, Amyvid® injection containing florbetapir, and Vizamyl® containing flutemetamol used with PET scan to detect beta-amyloid in the brain. Elli Lilly and company got FDA approval for Amyvid® [25] in 2012 as a diagnostic agent. Neuraceq® by Piramal Imaging got FDA approval on 19 March 2014 for PET imaging in the diagnosis of AD [26]. GE Healthcare received FDA approval [27] for Vizamyl® in October 2013 to detect amyloid neuritic plaque density for AD. Eli Lilly and company got FDA approval in 20020 to use Tauvid® containing Flortaucipir to detect tau neurofibrillary tangles in the brain of patients being evaluated for AD [28].
CSF tests
CSF fluid protects the brain and spinal cord from injuries by providing cushioning effects and also supplies nutrients. In the early stages of AD, the CSF level of tau and beta-amyloid changes. CSF tests are very helpful in the diagnosis and detection of AD. Most of the research on AD biomarkers has been done on biological fluids like blood or CSF. The best method for identifying AD biomarkers is using CSF since this fluid directly contacts brain interstitial fluid and more accurately reflects metabolic alterations associated with CNS functions. Aβ (Aβ-42), phosphorylated tau (P-tau), and total tau (T-tau) are CSF biomarkers that are crucial for the diagnosis of AD. An increase in tau and phospho-tau (pTau) and a decrease in Aβ in CSF of AD patients is the most well-known and widely accepted molecular-based tissue fluid diagnostic for AD [29]. FDA has approved Lumipulse G an automated immunoassay to measure the biomarkers in the CSF of Alzheimer patients. Fujirebio Diagnostics, Inc. got FDA approval in May 2022 for Lumipulse G, an in vitro diagnostic test to detect amyloid plaques in CSF for early detection of AD. [30,31]. Another FDA-approved Test of AD is Elecsys® AD CSF assays by Roche. This method includes three assays viz, Elecsys β-amyloid CSF II, Elecsys phospho-Tau CSF, and Elecsys total Tau CSF [32].
Blood tests
Blood tests are used as cheap, easy, and simple diagnostic tools to diagnose a disease. Blood tests are used only in patients with memory complaints. Blood tests may detect tau, beta-amyloid, or other biomarkers before and after the disease. However, in AD blood tests are not approved by FDA [17]. The most encouraging findings to be released so far have examined CSF samples that were taken via lumbar puncture. However, less invasive procedures that analyze proteins in blood or urine may be able to assist primary care doctors in providing their patients with long-term prognostic advice. Nonetheless, a lot of biomarker scientists believe that creating a diagnostic test that is sensitive and specific enough to be applied to urine or plasma samples will be extremely challenging, if not impossible [33].
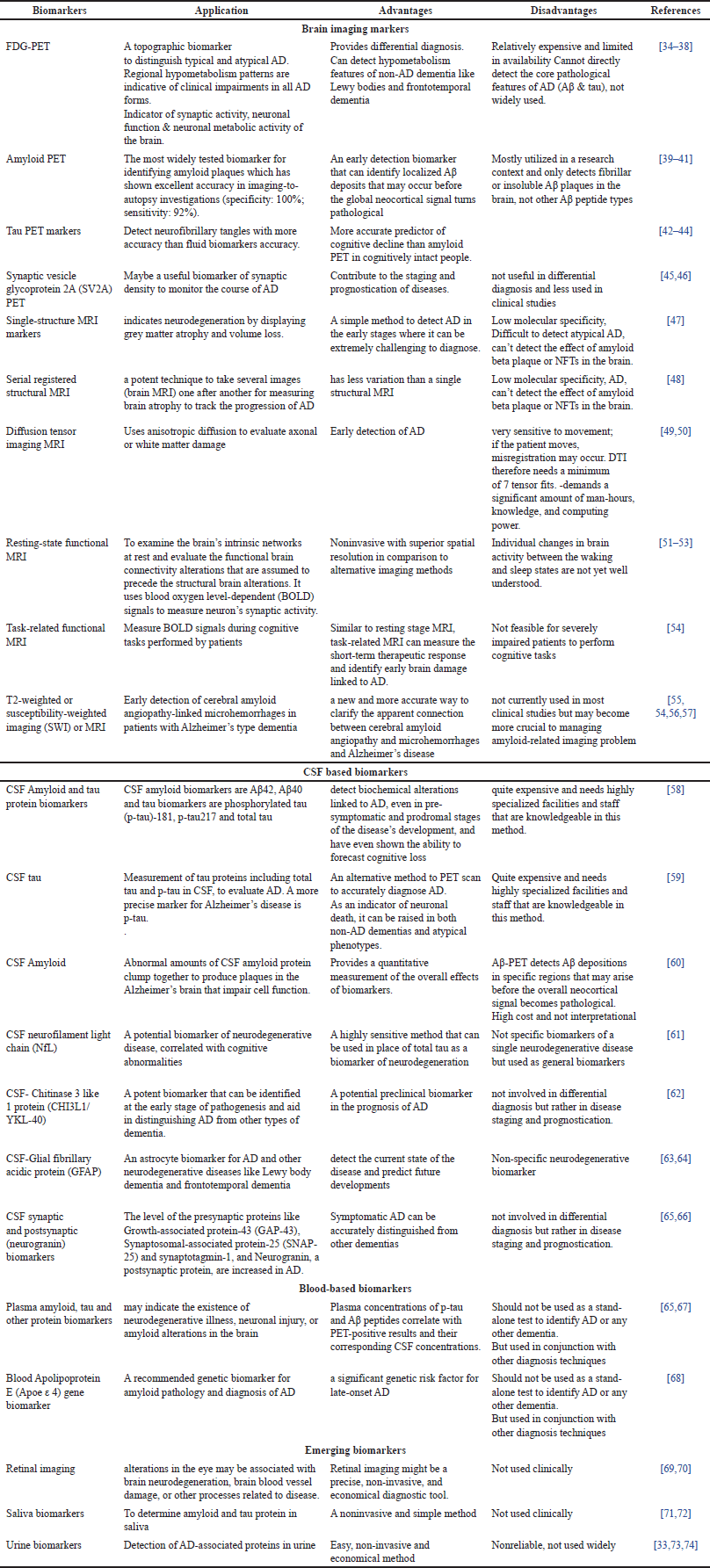 | Table 1. Biomarkers for AD. [Click here to view] |
VARIABILITY IN AD PATHOGENESIS
AD can have extremely diverse clinical presentations and pathological processes that vary greatly in severity, location, and composition. These variations include the amount and distribution of AB deposition and the spread of neurofibrillary tangles in different brain regions, which can lead to atypical clinical patterns and the emergence of unique AD variants. Variability in AD pathogenesis can adversely affect the diagnosis and treatment of AD. Variability in AD pathogenesis may be due to the presence of genetic, demographic, neuropsychiatric, and comorbidity-related factors [75]. APP processing and the significant amount of Aβ deposition brought on by individual mutations appear to be the primary initiators of the AD process, according to genetic studies of autosomal dominant types of AD. Demographic factors such as age at onset, sex, race, and ethnicity influence the prevalence of AD. 3% of persons between 65% and 74%, 17% of persons between 75% and 84%, and 32% of persons above 85 years of age have AD. A higher prevalence of AD and other dementias is seen in women due to their longer average lifespans than males. There are well-established ethnic and racial disparities in the likelihood of getting Alzheimer-related disorders. Older Black/African Americans are twice as likely to develop Alzheimer-related disorders as older White people and older Hispanic/Latinos are roughly 1.5 times more likely [76,77]. Comorbidities such as hypertension, diabetes mellitus, liver diseases, and so on, can increase the risk for AD and add to the heterogeneity of AD. The neuropsychiatric inventory is often used to quantify neuropsychiatric symptoms (NPSs), and it has been suggested that NPS influences both the phenotypic heterogeneity and the rate of progression of AD. There may be biological heterogeneity in the disease as seen by variability in biomarker profiles across persons with dementia and mild cognitive impairment as well as cognitively normal individuals. In AD, blood-based (plasma) and cerebrospinal biomarkers are examples of fluid biomarkers. It is evident from these biomarkers that the pathophysiology of AD is heterogeneous [78].
CHALLENGES OF BIOMARKER-BASED DIAGNOSIS
Despite our knowledge about the amyloid and tau pathology, the complete picture of AD pathophysiology remains elusive. Additionally, to diagnosis, the available biomarkers for AD are ineffective in predicting the course of the illness and cannot be utilized to track patients’ responses to immunotherapy using monoclonal antibodies against Aβ and tau or other currently being tested therapeutic modalities. Finding novel biomarkers that can also be used for these purposes is therefore extremely important. The limited therapeutic value of biomarkers, typically in elderly patients, is due to the extremely invasive (lumbar puncture) method of collecting CSF. This might make it impossible to use it for long-term investigations or clinical progression monitoring, both of which would require frequent CSF samples. The emphasis must be placed on standardizing the testing of these biomarkers due to the high inter-laboratory variation in the observed concentration of these biomarkers. Due to the heterogeneity of AD pathogenesis, potential AD CSF biomarkers should be looked at more thoroughly [13]. Blood-based biomarker assays are less invasive and more cost effective than alternative methods. These strategies are feasible to implement and offer repeated sampling in large cohorts, which makes them potentially superior to other biomarker modalities [13]. However, blood’s complex makeup makes it challenging to employ as a matrix for assessing biomarkers [13]. The enormous dynamic range of proteins in blood is the most difficult of many challenges to the development of blood-based biomarkers. It can be difficult to identify blood changes that are particular to AD since blood changes are frequently very small and represent a wide range of peripheral and central processes. As the brain is separated blood-brain barrier, it is difficult to relate the analytes found in blood and the changes the in brain. However, the BBB gets disrupted with age and increases the brain’s permeability. Therefore, the detection of protein-based biomarkers of AD in the blood is significant. However, blood levels of the most recognized possible biomarkers are far lower than those observed in CSF. For instance, the concentration of Aβ peptide in the blood is 100 times lower than that in CSF. Additionally, the presence of less abundant proteins that may act as potential biomarkers may be concealed by extremely abundant plasma proteins like albumin and IgG [13]. In addition to blood and CSF, other fluids, such as saliva, urine, and tear fluids, have also been studied in a few studies [3]. Analysis of the saliva of AD patients showed increased levels of proteins that are involved in homeostasis, ROS scavenging, neuroprotection, and antibacterial activities in comparison to control [79]. In contrast, proteins involved in gluconeogenesis, complement activation, and lipoprotein metabolism were changed in the urine of AD patients [80]. In the tear fluid, the Eukaryotic translation initiation factor 4E was present only in samples of AD individuals [81]. It has already been discovered that eukaryotic translation initiation factor 4E is elevated in the brain tissues of AD patients, and it may be involved in the mechanisms behind tau hyperphosphorylation [82].
CONCLUSION
Over the past few years, biomarker advancements have produced intriguing discoveries. Researchers can now monitor the beginning and course of AD, observe changes associated with the condition in living individuals, and assess the efficacy of promising medications and other possible treatments. Furthermore, new disease-modifying therapies for AD are currently being developed or authorized. Clinical trials are focused on individuals with early AD (mild cognitive impairment from AD or early AD dementia) making early AD diagnosis even more crucial. With the understanding of Aβ and tau pathologies and the subsequent discovery of CSF and neuroimaging biomarkers, new diagnostic, prognostic, and therapeutic options have become available leading to a better redefinition of AD. However, thorough characterization of the targeted biofluid or tissue samples is required for the identification, qualification, and validation of diagnostic and prognostic biomarkers, which demands the use of various approaches and instruments.
ACKNOWLEDGMENT
Authors are thankful to Uttaranchal University for the continuous support.
AUTHOR CONTRIBUTION
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Dhapola R, Beura SK, Sharma P, Singh SK, HariKrishnaReddy D. Oxidative stress in Alzheimer’s disease: current knowledge of signaling pathways and therapeutics. Mol Biol Rep. 2024 Jan 2;51(1):1–18.
2. Korczyn AD, Grinberg LT. Is Alzheimer disease a disease? Nat Rev Neurol. 2024;Feb 29;20(4):245–51.
3. Schumacher-Schuh A, Bieger A, Borelli W V, Portley MK, Awad PS, Bandres-Ciga S. Advances in proteomic and metabolomic profiling of neurodegenerative diseases. Front Neurol. 2022;12(January):1–14.
4. Khoonsari PE. Proteomics studies of subjects with Alzheimer ’s disease and chronic pain. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Medicine. Uppsala, Sweden: Department of Medical Sciences, Clinical Chemistry, Uppsala University; 2017.
5. Silva MVF, Loures CDMG, Alves LCV, De Souza LC, Borges KBG, Carvalho MDG. Alzheimer’s disease: risk factors and potentially protective measures. J Biomed Sci. 2019 May 9;26(1):1.
6. Aisen PS, Cummings J, Jack CR, Morris JC, Sperling R, Frölich L, et al. On the path to 2025: understanding the Alzheimer’s disease continuum. Alzheimers Res Ther. 2017 Aug 9;9(1):1.
7. Fan L, Mao C, Hu X, Zhang S, Yang Z, Hu Z, et al. New insights into the pathogenesis of Alzheimer’s Disease. Front Neurol. 2020 Jan 10;10:1312.
8. Deture MA, Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener. 2019 Aug 2;14(1):32.
9. Yiannopoulou KG, Papageorgiou SG. Current and future treatments in Alzheimer disease: an update. J Cent Nerv Syst Dis. 2020;12: 1–12.
10. Rabinovici GD. Controversy and progress in Alzheimer’s Disease — FDA approval of Aducanumab. New Engl J Med. 2021 Aug 26;385(9):771–4.
11. Romano JD, Truong V, Kumar R, Venkatesan M, Graham BE, Hao Y, et al. The Alzheimer’s knowledge base: a knowledge graph for Alzheimer disease research. J Med Internet Res. 2024 Jan 1;26(1):e46777.
12. Tsuji S, Hase T, Yachie-Kinoshita A, Nishino T, Ghosh S, Kikuchi M, et al. Artificial intelligence-based computational framework for drug-target prioritization and inference of novel repositionable drugs for Alzheimer’s disease. Alzheimers Res Ther. 2021 Dec 1;13(1):92.
13. Baird AL, Westwood S, Lovestone S. Blood-based proteomic biomarkers of Alzheimer’s disease pathology. Front Neurol. 2015;6:236.
14. Qiu Y, Cheng F. Artificial intelligence for drug discovery and development in Alzheimer’s disease. Curr Opin Struct Biol. 2024 Apr 1;85:102776.
15. Moya-Alvarado G, Gershoni-Emek N, Perlson E, Bronfman FC. Neurodegeneration and Alzheimer’s disease (AD). What can proteomics tell us about the Alzheimer’s brain? Mol Cell Proteomics. 2016;15(2):409–25.
16. Forte A, Lara S, Peña-Bautista C, Baquero M, Cháfer-Pericás C. New approach for early and specific Alzheimer disease diagnosis from different plasma biomarkers. Clinica Chimica Acta. 2024 Mar 15;556:117842.
17. Barthélemy NR, Salvadó G, Schindler SE, He Y, Janelidze S, Collij LE, et al. Highly accurate blood test for Alzheimer’s disease is similar or superior to clinical cerebrospinal fluid tests. Nat Med. 2024 Feb 21;30(4):1085–95. https://www.nature.com/articles/s41591-024-02869-z
18. Jia J, Ning Y, Chen M, Wang S, Yang H, Li F, et al. Biomarker changes during 20 years preceding Alzheimer’s disease. New Engl J Med. 2024 Feb 22;390(8):712–22. https://www.nejm.org/doi/full/10.1056/NEJMoa2310168
19. Therriault J, Schindler SE, Salvadó G, Pascoal TA, Benedet AL, Ashton NJ, et al. Biomarker-based staging of Alzheimer disease: rationale and clinical applications. Nat Rev Neurol. 2024 Mar 1;20(4):232–44. https://www.nature.com/articles/s41582-024-00942-2
20. Alzheimer, Association. Biomarkers and Alzheimer’s disease. Chicago, IL: An Official Publication of the Alzheimer’s Association; 2023.
21. Dubois B, von Arnim CAF, Burnie N, Bozeat S, Cummings J. Biomarkers in Alzheimer’s disease: role in early and differential diagnosis and recognition of atypical variants. Alzheimer’s Res Therap. 2023 Oct 13;15(1):175.
22. Askenazi M, Kavanagh T, Pires G, Ueberheide B, Wisniewski T, Drummond E. Compilation of reported protein changes in the brain in Alzheimer’s disease. Nat Commun. 2023 Dec 1;14(1):4466.
23. Blennow K, Zetterberg H. Biomarkers for Alzheimer’s disease: current status and prospects for the future. J Intern Med. 2018;284:643–63.
24. Haytural H, Benfeitas R, Schedin-Weiss S, Bereczki E, Rezeli M, Unwin RD, et al. Insights into the changes in the proteome of Alzheimer disease elucidated by a meta-analysis. Sci Data. 2021 Dec 1;8(1):312.
25. FDA Approves AmyvidTM (Florbetapir F 18 Injection) for use in patients being evaluated for Alzheimer’s disease and other causes of cognitive decline. Eli Lilly and Company [Internet]. https://investor.lilly.com/news-releases/news-release-details/fda-approves-amyvidtm-florbetapir-f-18-injection-use-patients
26. FDA Approves Piramal Imaging’s NeuraceqTM (florbetaben F18 injection) for PET Imaging of Beta-Amyloid Neuritic Plaques in the Brain [Internet]. PR Newswire; 2014. https://www.prnewswire.com/news-releases/fda-approves-piramal-imagings-neuraceq-florbetaben-f18-injection-for-pet-imaging-of-beta-amyloid-neuritic-plaques-in-the-brain-251216031.html
27. FDA Approves Vizamyl - Press Kits - News Center. GE HealthCare (United States) [Internet]. https://www.gehealthcare.com/news-center/fda-approves-vizamyl
28. Fleisher AS, Pontecorvo MJ, Devous MD, Lu M, Arora AK, Truocchio SP, et al. Positron emission tomography imaging with [18F] flortaucipir and postmortem assessment of Alzheimer disease neuropathologic changes. JAMA Neurol. 2020 Jul 1;77(7):829–39. https://investor.lilly.com/news-releases/news-release-details/lilly-receives-us-fda-approval-tauvidtm-flortaucipir-f-18
29. Pedrero-Prieto CM, García-Carpintero S, Frontiñán-Rubio J, Llanos-González E, Aguilera García C, Alcaín FJ, et al. A comprehensive systematic review of CSF proteins and peptides that define Alzheimer’s disease. Clin Proteomics. 2020;17: 1–24.
30. FDA Approves Fujirebio Diagnostics’ Lumipulse G ß-Amyloid Ratio Test for Alzheimer’s | 2022-05-12. FDAnews [Internet]. https://www.fdanews.com/articles/207784-fda-approves-fujirebio-diagnostics-lumipulse-g-%C3%9F-amyloid-ratio-test-for-alzheimers
31. DEN200072 FDA. Evaluation of automatic class Ill designation for C type of test: fully automated, chemiluminescent enzyme immunoassays (CLEIA). Malvern, PA: Fujirebio Diagnostics, Inc.
32. Ortner M, Lanz K, Goldhardt O, Müller-Sarnowski F, Diehl-Schmid J, Förstl H, et al. Elecsys cerebrospinal fluid immunoassays accurately detect Alzheimer’s disease regardless of concomitant small vessel disease. J Alzheimers Dis. 2023 Jun 13;93(4):1537–49. https://pubmed.ncbi.nlm.nih.gov/37212102/
33. Butcher J. Urine tests for Alzheimer’s disease-are they fool’s gold? Lancet Neurol. 2007 Feb 1;6(2):106–7. http://www.thelancet.com/article/S1474442207700157/fulltext
34. Minoshima S, Mosci K, Cross D, Thientunyakit T. Brain [F-18]FDG PET for clinical dementia workup: differential diagnosis of Alzheimer’s disease and other types of dementing disorders. Semin Nucl Med. 2021 May 1;51(3):230–40.
35. Na S, Kang DW, Kim GH, Kim KW, Kim Y, Kim H-J, et al. The usefulness of 18F-FDG PET to differentiate subtypes of dementia: the systematic review and meta-analysis. Dement Neurocogn Disord. 2024 Jan;23(1):54–66. http://www.ncbi.nlm.nih.gov/pubmed/38362056
36. Boccalini C, Caminiti SP, Chiti A, Frisoni GB, Garibotto V, Perani D. The diagnostic and prognostic value of tau-PET in amnestic MCI with different FDG-PET subtypes. Ann Clin Transl Neurol. 2024 May 1;11(5):1236–49.
37. Duignan JA, Haughey A, Kinsella JA, Killeen RP. Molecular and anatomical imaging of dementia with lewy bodies and frontotemporal lobar degeneration. Semin Nucl Med. 2021 May 1;51(3):264–74.
38. Minoshima S, Cross D, Thientunyakit T, Foster NL, Drzezga A. 18F-FDG PET Imaging in neurodegenerative dementing disorders: insights into subtype classification, emerging disease categories, and mixed dementia with copathologies. J Nucl Med. 2022 Jun 1;63(Suppl 1):2S–12S. https://pubmed.ncbi.nlm.nih.gov/35649653/
39. Teipel SJ, Spottke A, Boecker H, Daamen M, Graf E, Sahlmann J, et al. Patient-related benefits of amyloid PET imaging in dementia: rationale and design of the German randomized coverage with evidence development study ENABLE. Alzheimer’s & dementia. Transl Res Clin Interven. 2023 Jul 1;9(3):e12383. https://onlinelibrary.wiley.com/doi/full/10.1002/trc2.12383
40. Chapleau M, Iaccarino L, Soleimani-Meigooni D, Rabinovici GD. The role of amyloid PET in imaging neurodegenerative disorders: a review. J Nucl Med. 2022 Jun 1;63(Suppl 1):13S.
41. Palmqvist S, Zetterberg H, Mattsson N, Johansson P, Minthon L, Blennow K, et al. Detailed comparison of amyloid PET and CSF biomarkers for identifying early Alzheimer disease. Neurology. 2015 Oct 6;85(14):1240–9.
42. Tau-PET?: Future of Alzheimer’s patients. ScienceDaily [Internet]. https://www.sciencedaily.com/releases/2023/08/230809125914.htm
43. Boccalini C, Ribaldi F, Hristovska I, Arnone A, Peretti DE, Mu L, et al. The impact of tau deposition and hypometabolism on cognitive impairment and longitudinal cognitive decline. Alzheimer’s Dement. 2024 Jan 1;20(1):221–33.https://www.sciencedaily.com/releases/2023/08/230809125914.htm
44. Ossenkoppele R, Reimand J, Smith R, Leuzy A, Strandberg O, Palmqvist S, et al. Tau PET correlates with different Alzheimer’s disease-related features compared to CSF and plasma p-tau biomarkers. EMBO Mol Med. 2021 Aug 8;13(8):e14398.
45. Kong Y, Zhang S, Huang L, Zhang C, Xie F, Zhang Z, et al. Positron emission computed tomography imaging of synaptic vesicle glycoprotein 2A in Alzheimer’s disease. Front Aging Neurosci. 2021 Nov 2;13:731114.
46. Chang YY, King D, Holt K, Gladstein S, Horton WA, Bevis A, et al. A study to determine the mechanisms underlying changes in synaptic vesicle glycoprotein 2A density in Alzheimer’s disease. Alzheimer’s Dement. 2023 Dec;19(S24):e082561. https://onlinelibrary.wiley.com/doi/full/10.1002/alz.082561
47. Basaia S, Agosta F, Wagner L, Canu E, Magnani G, Santangelo R, et al. Automated classification of Alzheimer’s disease and mild cognitive impairment using a single MRI and deep neural networks. Neuroimage Clin. 2019 Jan 1;21:101645.
48. Fox NC, Cousens S, Scahill R, Harvey RJ, Rossor MN. Using serial registered brain magnetic resonance imaging to measure disease progression in Alzheimer disease: power calculations and estimates of sample size to detect treatment effects. Arch Neurol. 2000 Mar 1;57(3):339–44. https://jamanetwork.com/journals/jamaneurology/fullarticle/776187
49. Ranzenberger LR, Das JM, Snyder T. Diffusion tensor imaging. FL: StatPearls Publishing Florida; 2024 Jan. 147–51 pp. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537361/
50. Bell D, Abdrabou A. Diffusion tensor imaging and fibre tractography. Radiopaedia.org; 2013 Apr 17.
51. Mousa D, Zayed N, Yassine IA. Alzheimer disease stages identification based on correlation transfer function system using resting-state functional magnetic resonance imaging. PLoS One. 2022 Apr 1;17(4):e0264710. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0264710
52. Agosta F, Pievani M, Geroldi C, Copetti M, Frisoni GB, Filippi M. Resting state fMRI in Alzheimer’s disease: beyond the default mode network. Neurobiol Aging. 2012 Aug 1;33(8):1564–78.
53. Lv H, Wang Z, Tong E, Williams LM, Zaharchuk G, Zeineh M, et al. Resting-state functional MRI: everything that nonexperts have always wanted to know. AJNR. 2018 Aug 1;39(8):1390. /pmc/articles/PMC6051935/
54. Aramadaka S, Mannam R, Sankara Narayanan R, Bansal A, Yanamaladoddi VR, Suseel Sarvepalli S, et al. Neuroimaging in Alzheimer’s Disease for Early Diagnosis: a comprehensive review. Cureus. 2023 May 4;15(5):e38544.
55. Lee SN, Woo SH, Lee EJ, Kim KK, Kim HR. Association between T1w/T2w ratio in white matter and cognitive function in Alzheimer’s disease. Sci Rep. 2024 Mar 27;14(1):7228.
56. Park M, Moon WJ. Structural MR imaging in the diagnosis of Alzheimer’s disease and other neurodegenerative dementia: current imaging approach and future perspectives. Korean J Radiol. 2016;17:827–45.
57. Larsen JP, Britt W, Kido D, Olson BLB, Holshouser BA, Kirsch WM. Susceptibility-weighted magnetic resonance imaging in the evaluation of dementia. Radiol Case Rep. 2007;2(4):102.
58. Bouwman FH, Frisoni GB, Johnson SC, Chen X, Engelborghs S, Ikeuchi T, et al. Clinical application of CSF biomarkers for Alzheimer’s disease: from rationale to ratios. Alzheimer’s Dement Diagn Assess Dis Monit. 2022;14(1):e12314. https://alz-journals.onlinelibrary.wiley.com/doi/10.1002/dad2.12314.
59. Lantero-Rodriguez J, Montoliu-Gaya L, Benedet AL, Vrillon A, Dumurgier J, Cognat E, et al. CSF p-tau205: a biomarker of tau pathology in Alzheimer’s disease. Acta Neuropathol. 2024 Jun 1;147(1):12.
60. Hansson O, Lehmann S, Otto M, Zetterberg H, Lewczuk P. Advantages and disadvantages of the use of the CSF Amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer’s Disease. Alzheimer’s Res Therap. 2019;11:1–15.
61. Dhiman K, Gupta VB, Villemagne VL, Eratne D, Graham PL, Fowler C, et al. Cerebrospinal fluid neurofilament light concentration predicts brain atrophy and cognition in Alzheimer’s disease. Alzheimer’s Dement Diagn Assess Dis Monit. 2020 Jan 1;12(1):e12005.
62. Connolly K, Lehoux M, O’Rourke R, Assetta B, Erdemir GA, Elias JA, et al. Potential role of chitinase-3-like protein 1 (CHI3L1/YKL-40) in neurodegeneration and Alzheimer’s disease. Alzheimer’s Dement. 2023 Jan 1;19(1):9–24.
63. Yang Z, Wang KKW. Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015;38(6):364–74.
64. Wang X, Shi Z, Qiu Y, Sun D, Zhou H. Peripheral GFAP and NfL as early biomarkers for dementia: longitudinal insights from the UK Biobank. BMC Med. 2024 Dec 1;22(1):1–13. https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-024-03418-8
65. Liu W, Lin H, He X, Chen L, Dai Y, Jia W, et al. Neurogranin as a cognitive biomarker in cerebrospinal fluid and blood exosomes for Alzheimer’s disease and mild cognitive impairment. Transl Psychiatry. 2020 Apr 29;10(1):1–9. https://www.nature.com/articles/s41398-020-0801-2
66. Milà-Alomà M, Brinkmalm A, Ashton NJ, Kvartsberg H, Shekari M, Operto G, et al. CSF synaptic biomarkers in the preclinical stage of Alzheimer disease and their association with MRI and PET a cross-sectional study. Neurology. 2021 Nov 23;97(21):E2065–78.
67. Mattsson-Carlgren N, Collij LE, Stomrud E, Pichet Binette A, Ossenkoppele R, Smith R, et al. Plasma biomarker strategy for selecting patients with Alzheimer disease for antiamyloid immunotherapies. JAMA Neurol. 2024 Jan 8;81(1):69–78.
68. Ba M, Kong M, Li X, Pin Ng K, Rosa-Neto P, Gauthier S. Is ApoE ? 4 a good biomarker for amyloid pathology in late onset Alzheimer’s disease? Transl Neurodegener. 2016 Nov 16;5:20.
69. Cheung CY, Mok V, Foster PJ, Trucco E, Chen C, Wong TY. Retinal imaging in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2021 Sep 1;92(9):983–94. https://jnnp.bmj.com/content/92/9/983
70. Ashraf G, McGuinness M, Khan MA, Obtinalla C, Hadoux X, van Wijngaarden P. Retinal imaging biomarkers of Alzheimer’s disease: a systematic review and meta-analysis of studies using brain amyloid beta status for case definition. Alzheimer’s Dement Diagn Assess Dis Monit. 2023 Apr 1;15(2):e12421.
71. Nazir S. Salivary biomarkers: the early diagnosis of Alzheimer’s disease. Aging Med. 2024 Apr 1;7(2):202–13.
72. Bermejo-Pareja F, del Ser T, Valentí M, de la Fuente M, Bartolome F, Carro E. Salivary lactoferrin as biomarker for Alzheimer’s disease: brain-immunity interactions. Alzheimer’s Dement. 2020 Aug 1;16(8):1196.
73. Armenta-Castro A, Núñez-Soto MT, Rodriguez-Aguillón KO, Aguayo-Acosta A, Oyervides-Muñoz MA, Snyder SA, et al. Urine biomarkers for Alzheimer’s disease: a new opportunity for wastewater-based epidemiology? Environ Int. 2024 Feb 1;184:108462.
74. Wang Y, Wang Y, Zhu J, Guan Y, Xie F, Cai X, et al. Systematic evaluation of urinary formic acid as a new potential biomarker for Alzheimer’s disease. Front Aging Neurosci. 2022 Nov 30;14:1046066.
75. Duara R, Barker W. Heterogeneity in Alzheimer’s disease diagnosis and progression rates: implications for therapeutic trials. Neurotherapeutics. 2022;19:8–25.
76. Benzinger TLS, Blazey T, Jack CR, Koeppe RA, Su Y, Xiong C, et al. Regional variability of imaging biomarkers in autosomal dominant Alzheimer’s disease. Proc Natl Acad Sci U S A. 2013 Nov 19;110(47):E4502–9.
77. Gordon BA, Blazey T, Benzinger TLS. Regional variability in Alzheimer’s disease biomarkers. Future Neurol. 2014;9(2):131.
78. Bouteloup V, Pellegrin I, Dubois B, Chene G, Planche V, Dufouil C. Explaining the variability of Alzheimer disease fluid biomarker concentrations in memory clinic patients without dementia. Neurology. 2024 Apr 23;102(8):e209219.
79. Contini C, Olianas A, Serrao S, Deriu C, Iavarone F, Boroumand M, et al. Top-down proteomics of human saliva highlights anti-inflammatory, antioxidant, and antimicrobial defense responses in Alzheimer disease. Front Neurosci. 2021 May 26;15:668852.
80. Watanabe Y, Hirao Y, Kasuga K, Tokutake T, Semizu Y, Kitamura K, et al. Molecular network analysis of the urinary proteome of Alzheimer’s disease patients. Dement Geriatr Cogn Dis Extra. 2019 Jan 1;9(1):53–65.
81Kenny A, Jiménez-Mateos EM, Zea-Sevilla MA, Rábano A, Gili-Manzanaro P, Prehn JHM, et al. Proteins and microRNAs are differentially expressed in tear fluid from patients with Alzheimer’s disease. Sci Rep. 2019 Oct 28;9(1):1–14.
82. Li X, An WL, Alafuzoff I, Soininen H, Winblad B PJJ. Phosphorylated eukaryotic translation factor 4E is elevated. NeuroReport. 2004;15:2237.