INTRODUCTION
Cissus cornifolia (Baker) Planch. (Fig. 1) is a wild plant commonly used in tropical Africa as food and traditional medicine. Cissus cornifolia, commonly known as “black wild grape”, “ivy grape”, or “wild grape” belongs to the Vitaceae or the grape family. The Vitaceae family consists of about 14 genera and 910 species distributed in tropical Africa, America, Asia, and Australia [1,2]. The Vitaceae family is well-known for edible grape vines (several species of Vitis L. genus), this is one of the most valuable fruit species in the world in terms of its ecological, economic, and social importance [3], with the berries often fermented to produce wine. Plant species belonging to the Vitaceae family are also ecologically important as climbers or lianas in the montane, temperate and tropical forests [4]. The largest genus of the Vitaceae family is Cissus L. with approximately 350 species recorded in the tropical areas or regions such as east, south, west, and central Africa with about 135 species, approximately 85 species in southern Asia, the Americas with about 77 species and roughly 12 species in Australia [5]. Cissus species are herbaceous to woody climbers or small shrubs, occasionally succulent and fleshy with tuberous roots, corky, finely striate, often angulate stems that are constricted at nodes, and tendrils that are usually opposite the leaves [6]. Recent molecular phylogenetic research showed that the genus Cissus is polyphyletic, and characterized by enormous morphological diversity [5,7]. Species within the genus Cissus are commonly used in African traditional medicine, Ayurveda, Medieval Islamic medicine, and European and Chinese traditional medicine systems [8]. Cissus species have also been reported to contain numerous phytochemical compounds such as ascorbic acid, alkaloids, terpenoids, saponins, flavonoids, sterols, quinones, phenolics, lignins, and tannins which display various pharmacological properties such as anti-inflammatory, antimicrobial, anti-diabetic, anti-ulcer, antiviral, anti-arthritic, anticancer, and antioxidant [8]. Cissus species widely used as sources of traditional medicines in different parts of the world include C. aralioides (Welw. ex Baker) Planch., C. assamica (M.A.Lawson) Craib, C. populnea Guill. & Perr., C. pteroclada Hayata, C. quadrangularis L., C. repens Lam., C. rotundifolia Lam., C. verticillata (L.) Nicolson & C.E.Jarvis, and C. vitiginea L. [8–10]. Similarly, C. cornifolia has been incorporated into the traditional materia medica in central, southern, western, and eastern Africa, characterized by a wide range of medicinal applications throughout its distributional range [11]. It is, therefore, within this background and context that this study was conducted aimed at evaluating the ethnomedicinal, chemical, and biological activities of C. cornifolia.
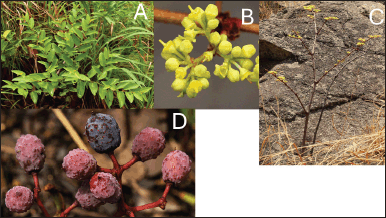 | Figure 1. Cissus cornifolia: A: general habit of the species, B: a branch showing flowers, C: plant specimen in flower, and D: a branch showing ripe fruits) (photos: B Wursten). [Click here to view] |
MATERIALS AND METHODS
Multiple literature searches on ethnomedicinal uses, chemical and biological activities of C. cornifolia were conducted from July 2023 to April 2024. This information on these aspects was obtained using online databases such as PubMed® (https://pubmed.ncbi.nlm.nih.gov/), Web of Science (https://www.webofknowledge.com), Google Scholar (https://scholar.google.com/), Scopus® (http://www.scopus.com/), SpringerLink® (https://link.springer.com/), SciELO (https://search.scielo.org/) and ScienceDirect® (https://www.sciencedirect.com/search). Additional information on the medicinal uses, and chemical and pharmacological properties of Cissus cornifolia was also obtained by a systematic search of various resources that are not covered by electronic databases, and these included journal papers, books, book chapters, dissertations, heses, and other scientific articles secured from the University library. The keywords used incorporated into the search included “C. cornifolia”, the synonyms of the species “Cissus cornifolia (Baker) Planch.”, English common names “black wild grape”, “ivy grape” and “wild grape”. An additional search was also conducted using the keywords “biological activities of Cissus cornifolia”, “pharmacological properties of Cissus cornifolia”, “ethnobotany of Cissus cornifolia”, “medicinal uses of Cissus cornifolia”, “phytochemistry of Cissus cornifolia” and “traditional uses of Cissus cornifolia”. The literature sources that have been included in the current review are those that evaluated the botany, taxonomy, ethnomedicinal uses, and chemical and pharmacological properties of C. cornifolia (Fig. 2). The literature sources excluded from the current review are those scientific publications that are partially accessible, that is, accessible only as abstracts, or published as ethnopharmacological surveys with limited information on botany, taxonomy, ethnomedicinal uses, chemical and pharmacological properties of C. cornifolia.
RESULTS AND DISCUSSION
Morphological description and taxonomy of C. cornifolia
The generic name “Cissus” is based on the Greek word “kissos”, meaning “ivy”, describing the climbing habit of most Cissus species that is also a key characteristic of “ivy”, a common name for climbing Hedera L. species [12]. The specific name “cornifolia” comes from two Latin words “cornu” meaning “horn” and “folium” meaning “leaf” [13]. The synonyms associated with the name C. cornifolia (Baker) Planch. include C. volkensii Gilg, C. lonicerifolia C.A.Sm., and Vitis cornifolia Baker [14–21]. The English common names of C. cornifolia include “black wild grape”, “ivy grape”, and “wild grape” [22,23].
Cissus cornifolia is a scandent shrub, sometimes even a small tree, reaching 3 meters in height [24]. The plant grows from a large, tuberous, fire-resistant, and sometimes watery rootstock. The stems are woody at the base, thick, rusty, hairy, tomentose, cylindrical with swollen nodes, and only occasionally with tendrils. The leaves are alternate, simple, elliptic to ovate in shape, appearing after the flowers. The leaves of C. cornifolia are hairless with serrated margins, gives off a strong smell when crushed, midrib has prominent veins, apex tapering to a point, base tapering to rounded, and sometimes lobed. The flowers of C. cornifolia are small, bisexual, on leaf-opposed cymes on a common stalk. The fruit is ovoid, purple to black in color with a single seed [22]. Cissus cornifolia has been recorded in Uganda, Malawi, Benin, Sudan, Burkina Faso, Ghana, Botswana, Nigeria, Cameroon, Zambia, Chad, Central African Republic, Ethiopia, the Democratic Republic of Congo, Gabon, Ivory Coast, Guinea, Kenya, Mozambique, South Sudan, South Africa, Togo, Tanzania, and Zimbabwe [14–21] (Fig. 3). The species has been recorded in thickets, open woodland, and grassland, often on black soil and granite outcrops, sometimes in cultivated land, fallow agricultural land, and conspicuous growth observed after bush fire. Cissus cornifolia has been recorded from 300 m in altitude to 1,800 m above sea level [14,22,24,25].
Ethnomedicinal and traditional uses of C. cornifolia
Throughout tropical Africa, C. cornifolia have been used as food, ornamental plant, traditional medicine, fodder, and for various cultural applications. In Tanzania, C. cornifolia is widely used as a garden and ornamental plant and a valuable source of bee forage in local communities [25]. In Ethiopia, Malawi, Tanzania, and Zimbabwe, the ripe fruits that resemble commercial grapes (Vitis species) are eaten raw [25–31]. In Ethiopia, South Sudan, and Sudan, C. cornifolia is used as fodder [32]. Current reports show that C. cornifolia is mainly collected from the wild and not threatened with extinction is common in woodland, thickets, and grassland. For example, in South Africa, C. cornifolia is widespread, recorded in a wide range of habitats, characterized by a large population size, and categorized as of Least Concern on IUCN Red List Categories and Criteria [33].
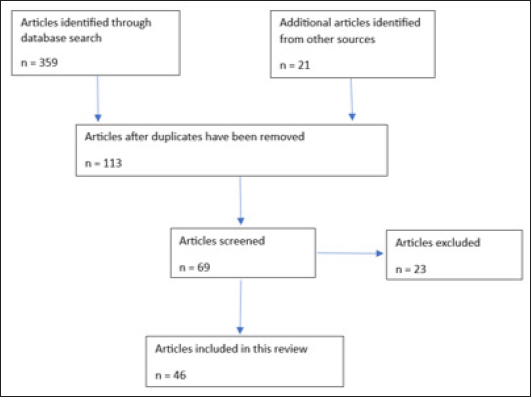 | Figure 2. Flow diagram showing the identification and screening of the articles used in this review. [Click here to view] |
In traditional medicine, the bark, fruits, leaf sap, leaves, root bark, roots, rootstock, stem bark, and twigs of C. cornifolia are used against 33 human and animal diseases and ailments (Tables 1 and 2). The ethnomedicinal uses of C. cornifolia have been documented in Botswana, Ghana, Mozambique, Nigeria, South Africa, Malawi, Tanzania, Zimbabwe, and Uganda, representing 37.5% of the countries in tropical Africa where C. cornifolia is indigenous. The main ailments and diseases treated by C. cornifolia crude extracts include its use to induce labor, and traditional medicine against diabetes, convulsions, gastro-intestinal problems, gonorrhea, hernia, malaria, respiratory problems, skin infections, and wounds (Fig. 4). This long list of medicinal uses and overlap in medicinal applications of the bark, fruits, leaf sap, leaves, root bark, roots, rootstock, stem bark, and twigs of C. cornifolia in Botswana, Ghana, Mozambique, Malawi, Nigeria, South Africa, Tanzania, Uganda, and Zimbabwe and this is an indication that local communities in these countries in tropical Africa have an interest in C. cornifolia as a source of traditional medicines. While there are still some gaps in ethnomedicinal knowledge in 62.5% of the countries were C. cornifolia is indigenous, it is clear that this widespread species has tremendous potential as a source of traditional medicines.
In Tanzania, the leaves of C. cornifolia are mixed with those of Senna singueana (Delile) Lock as remedy for convulsions [34,35] while in South Africa, the rootstock is mixed with roots of Pollichia campestris Aiton and Ipomoea bolusiana Schinz tubers as a remedy for foot ache [36]. In Malawi, the leaves and roots of C. cornifolia are mixed with those of Cissus integrifolia Planch., Cissus quadrangularis, Cissus rotundifolia, Diospyros zombensis (B.L.Burtt) F.White and Piliostigma thonningii (Schumach.) Milne-Redh. as remedy to induce labour [37]. In South Africa, the rootstock of C. cornifolia is mixed with the roots of Harpagophythum procumbens (Burch.) DC. ex Meisn., Ipomoea albivenia (Lindl.) Sweet, Waltheria indica L. and Senna italica Mill., and stem bark of Peltophorum africanum Sond. as traditional medicine for infertility [36]. Cissus cornifolia is a popular medicinal southern African, and its bark and roots are sold in informal herbal medicine markets in Malawi [38]. Similarly, the species is also listed in the monograph “Medicinal and magical plants of southern Africa: An annotated checklist” [39].
Phytochemistry and pharmacological properties of C. cornifolia
Qualitative chemical analyses of C. cornifolia root bark and leaves revealed that the species has alkaloids, steroids, triterpenoids, flavonoids, cardiac glycosides, coumarins, saponins, tannins, and terpenoids [42,44,49,51,61–65]. Similarly, various reports on the phytochemical screening of leaves, rootstock, and roots of C. cornifolia revealed the presence of alkanes, methyl esters, organoheterosilane, prenylated benzo-lactone, catechol, fatty acids, phenolics, and dicarboxylic acid (Table 3) which are characteristic of the root bark, leaves, rootstock and roots of C. cornifolia. Some of the phytochemical compounds isolated from C. cornifolia and its crude extracts exhibited antibacterial, antifungal, anticonvulsant, anti-diabetic, antidiarrhoeal, anti-inflammatory, antioxidant, antiproliferative, central nervous system depressant, nematicidal, and neuropharmacological activities.
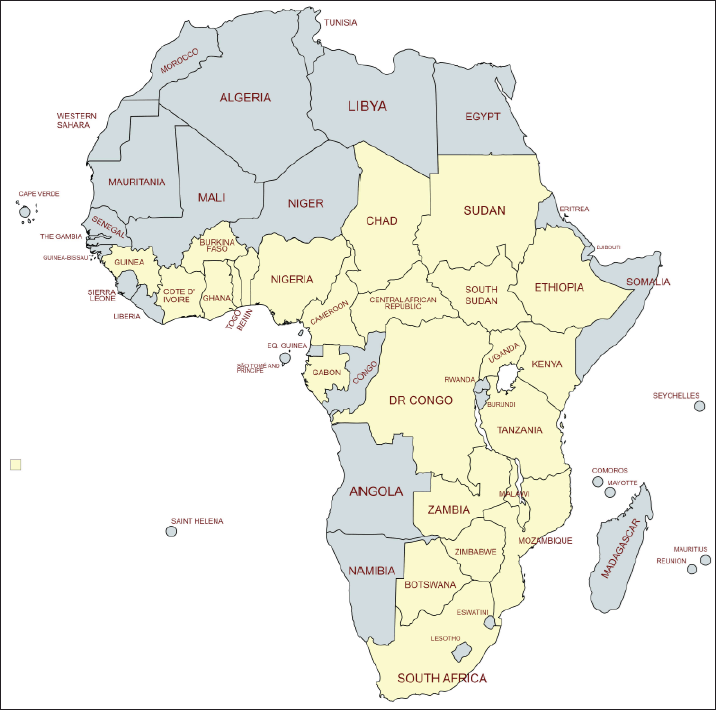 | Figure 3. Distribution of C. cornifolia in tropical Africa (map drawn using mapchart.net). [Click here to view] |
Antibacterial activities
Atiku et al. [64] assessed antibacterial activities of the crude methanolic extracts of C. cornifolia leaves against Salmonella typhi, Streptococcus pneumoniae, Staphylococcus aureus and Proteus vulgaris using microdilution assay. The extract demonstrated antibacterial activities against tested pathogens with a minimum inhibitory concentration (MIC) value of 5.0 mg/ml [64]. Musa et al. [60] assessed antibacterial activities of the phytochemical compound 4, 6-dihydroxy-5-methoxy-3-(1,2,3,4,5-pentahydroxypentyl)-2-benzofuran-1(3H)-one identified from the rootstock of C. cornifolia against S. aureus, S. typhi, Streptococcus pyogenes, Bacillus subtilis, and Shigella dysentriae using agar well diffusion method with sparfloxacin and fluconazole as the positive controls. The compound demonstrated antibacterial activities against the tested pathogens exhibiting a zone of inhibition of 17.0–25.0 mm [60]. Mongalo et al. [59] assessed antibacterial activities of acetone, methanol, dichloromethane, ethyl acetate and hexane extracts of C. cornifolia rootstock against Mycoplasma hominis, Pseudomonas aeruginosa, Escherichia coli, P. vulgaris, Enterococcus faecalis, Bacillus cereus, S. aureus, Streptococcus agalactiae and Moraxella catarrhalis using microdilution assay with neomycin as the positive control. The extracts demonstrated antibacterial activities against tested pathogens with MIC values of 0.2–≥12.5 mg/ml [59].
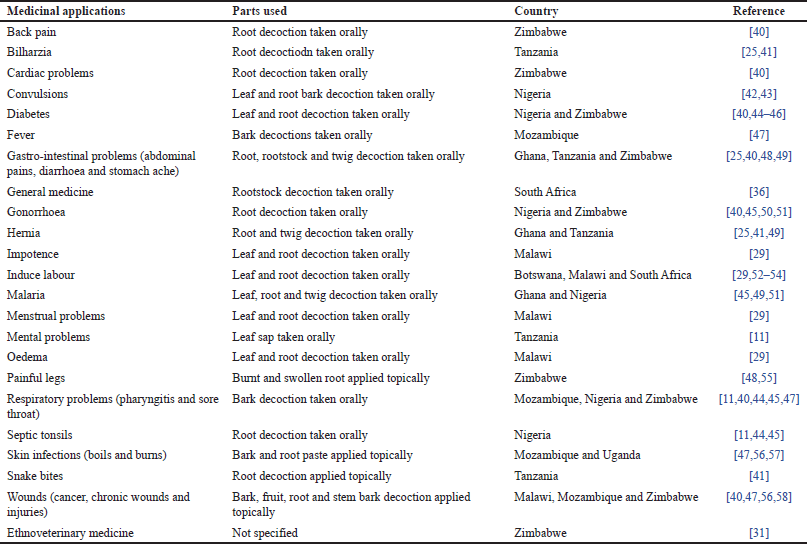 | Table 1. Mono-therapeutic applications of C. cornifolia. [Click here to view] |
 | Table 2. Use of C. cornifolia as traditional medicine in combination with other plant species. [Click here to view] |
Antifungal activities
Musa et al. [60] assessed antifungal activities of the phytochemical compound 4,6-dihydroxy-5-methoxy-3-(1,2,3,4,5-pentahydroxypentyl)-2-benzofuran-1(3H)-one identified from the rootstock of C. cornifolia against Candida albicans using agar well diffusion with fluconazole as the positive control. The compound demonstrated activities against the tested pathogen showing a zone of inhibition of 17.0 mm [60]. Mongalo et al. [59] assessed antifungal activities of acetone, methanol, dichloromethane, hexane, and ethyl acetate extracts of C. cornifolia rootstock against Candida parapsilosis, C. albicans, and Cryptococcus neoformans using the microdilution assay with amphotericin B as a positive control. The extracts demonstrated antifungal activities against the tested pathogens with MIC values of 0.2–≥12.5 mg/ml [59].
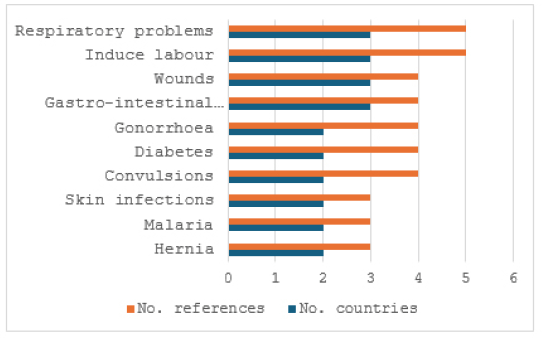 | Figure 4. Main ethnomedicinal applications of C. cornifolia in tropical Africa. [Click here to view] |
Anticonvulsant activities
Yaro et al. [42] assessed anticonvulsant activities of the methanol extract of C. cornifolia root bark using maximal electroshock test, pentylenetetrazole, strychnine and 4-aminopyridine-induced seizure models. The extracts exhibited anticonvulsant activities [42]. Yaro et al. [43] evaluated anticonvulsant activities of methanol extracts of C. cornifolia leaves in chicks using the maximal electroshock assay, and in male mice by making use of 4-aminopyridine, strychnine, pentylenetetrazole, and picrotoxin induced seizure assays. The extracts exhibited activities [43].
Anti-diabetic activities
Jimoh et al. [44] evaluated the anti-diabetic activities of the methanol extracts of C. cornifolia leaves on alloxan-induced hyperglycemic in male Wistar rats. The effect of the extract on glucose levels, concentration, and histopathology of the liver and pancreas were evaluated. The methanolic extract exhibited activities by lowering the glucose level in alloxan-induced diabetic male rats [44]. Jimoh et al. [51] assessed the hypoglycemic properties of the methanolic extract of C. cornifolia leaves on blood glucose levels of normoglycemic Wistar rats. The extracts exhibited hypoglycemic activities [51]. Chipiti et al. [46] assessed the antidiabetic properties of ethanol and aqueous extract of C. cornifolia leaves and roots using α?glucosidase and α?amylase inhibitory assays with acarbose as the positive drug. The extracts demonstrated properties with the half maximal inhibitory concentrations (IC50) value of 2.8–33.7 μg/ml [46].
Antidiarrhoeal activities
Tanko et al. [62] assessd the antidiarrhoeal properties of methanol extract of C. cornifolia leaves using castor oil-induced diarrhoeal in mice. The extract exhibited dose-dependent activities [62]. Although more tests are required, but these preliminary findings corroborate the use of root, rootstock, and twig decoction against gastro-intestinal problems in countries such as Ghana, Tanzania, and Zimbabwe [25,40,48,49].
Anti-inflammatory activities
Borquaye et al. [49] assessed anti-inflammatory properties of ethanol extract of C. cornifolia leaves by making use of the carrageenan-induced edema model of inflammation in 7-day-old chicks with diclofenac and dexamethasone as the positive controls. The extract demonstrated weak anti-inflammatory activities with half-maximal reduction in edema (ED50) value of 79.4 mg/kg in comparison with 0.6 mg/kg and 10.6 mg/kg demonstrated by positive controls [49]. Mongalo et al. [59] assessed anti-inflammatory properties of dichloromethane, methanol, and ethyl acetate extract of C. cornifolia bulb using the cyclooxygenase (COX-1 and COX-2) and soybean lipoxygenase (15-LOX) assays with celecoxib and quercetin as positive controls. The extracts exhibited activities against 15-LOX) and COX-2 with IC50 value of 15.6–68.9 μg/ml [59]. These findings somehow corroborate the traditional applications of C. cornifolia crude extracts against various inflammatory diseases and ailments ranging from injury to microbial infections that lead to cell injury and death.
Antioxidant activities
Chipiti et al. [40] assessed the antioxidant properties of the ethanol and aqueous extract of leaves and roots of C. cornifolia making use of 2,2-diphenyl-1-picrylhydrazyl, ferric reducing power, hydroxyl radical scavenging and nitric oxide (NO) radical scavenging assays. The extracts exhibited activities using all four in vitro experimental models [40]. Borquaye et al. [49] assessed antioxidant properties of ethanol extract of C. cornifolia leaves by making use of the NO scavenging method with the ascorbic acid as a positive control. The extract demonstrated weak activities with IC50 value of 1,381.0 μg/ml in comparison to 23.3 μg/ml demonstrated by positive control [49].
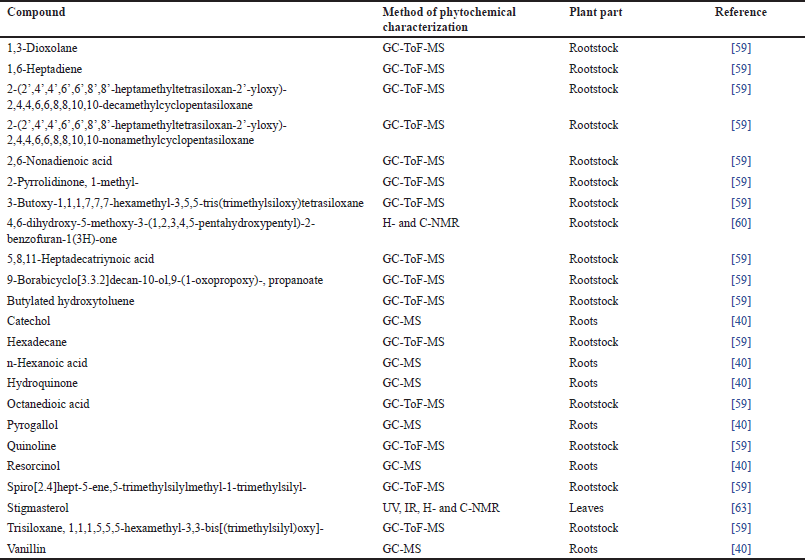 | Table 3. Phytochemical composition of C. cornifolia. [Click here to view] |
Antiproliferative activities
Chipiti et al. [46] assessed the cytotoxicity properties of ethanol extracts of C. cornifolia leaf and roots against the HEK 293 cell lines using the tetrazolium-based colorimetric (MTT) assay. The leaf and root extracts demonstrated cytotoxicity properties with IC50 values of 1.6 μg/ml and 2.7 μg/ml, respectively [46]. Mongalo et al. [59] assessed the antiproliferative properties of methanol and ethyl acetate extracts of C. cornifolia bulb against the breast cancer (MCF7-21) cells using the MTT assay with doxorubicin as the positive control. The methanol and ethyl acetate extracts demonstrated cytotoxicity properties against MCF7-21 cells with IC50 values of 10.8 μg/ml and 24.1 μg/ml in comparison with 1.3 μg/ml demonstrated by a positive control [59].
Central nervous system depressant activities
Yaro et al. [65] evaluated the central nervous system depressant activities of aqueous, chloroform, ethyl acetate, and methanol fractions C. cornifolia leaves in male mice by making use of the head-dip, diazepam-induced sleep, and motor-coordination assays. The fractions exhibited the central nervous system depressant activities [65].
Nematicidal activities
Nyoni et al. [66] assessed nematicidal properties of aqueous and acetone extracts of C. cornifolia root against Meloidogyne javanica in in vivo screening. The extracts exhibited moderate and weak activities causing 53.0% and 18.0% reduction in gall formation in aqueous and acetone extracts, respectively [66]. Therefore, C. cornifolia has potential to be used as a biocontrol agent against insect pests.
Neuropharmacological activities
Musa et al. [61] assessed neuropharmacological properties of methanol extracts of C. cornifolia leaves in male mice by making use of diazepam sleeping time, motor coordination and exploratory behaviour models. The extract exhibited activities [61]. Yaro et al. [67] assessed the neuropharmacological properties of methanol extract of C. cornifolia roots and leaves in male mice employing diazepam-induced sleep, motor coordination, and exploratory behavior. The extracts exhibited activities [67]. Yaro et al. [68] evaluated the neuropharmacological activities of the butanol-soluble extracts generated from the methanol extracts of C. cornifolia leaves by making use of the head-dip, diazepam-induced sleep, and motor coordination assays in male mice. The extracts exhibited sedative and central nervous depressant activities [68].
Toxicity activities
Jimoh et al. [44] and Yaro et al. [65] independently conducted toxicological studies of aqueous, chloroform, methanol, and ethyl acetate extracts of C. cornifolia leaves and roots by using specific doses of 1,600.0 mg/kg bw, 2,900.0 mg/kg bw and 5,000.0 mg/kg bw which were administered in Wistar rats using the intraperitoneal route and the male mice were monitored for any signs of toxicity which also included death. Results obtained from the oral acute toxicity studies showed that the median lethal dose (LD50) values of all the extracts were in excess of 5,000.0 mg/kg body weight [44,65]. In addition to this, the histopathological assessment of the diabetic Wistar male rats treated with methanolic extract of C. cornifolia leaves exhibited the restoration of pancreatic disturbance and damage caused by the alloxan [44]. These findings seem to suggest that the leaves and roots of C. cornifolia to be safe and nontoxic, and therefore, an excellent candidate for clinical studies as traditional medicine [45].
CONCLUSION
The current study provides a summary of medicinal, phytochemical, and biological properties of C. cornifolia. Such evaluations are needed considering that C. cornifolia is widely used as a traditional medicine throughout tropical Africa, and it is clear that the therapeutic potential of the species is not fully realized. Literature studies show that there is a growing demand for medicinal plants such as C. cornifolia used as traditional medicines, nutraceuticals, and sources of complementary treatments. This is usually the case with medicinal plants that are characterized by bioactive components such as flavonoids, phenolics, methyl esters, and terpenoids which are beneficial to human health. However, the lack of standardized quality control procedures in the usage of C. cornifolia as traditional medicine throughout its distributional range is a major concern for regulatory authorities. Therefore, future studies should focus on detailed ethnopharmacological evaluations of the species, emphasizing phytochemical, pharmacological, toxicological, in vivo, and clinical research aimed at corroborating the traditional medical and food applications of the species.
ACKNOWLEDGMENTS
The author appreciates the University of Fort Hare, South Africa, for funding(Grant number R188) this research.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The author declares that there are no conflicts of interest associated with this research work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Christenhusz MJM, Byng JW. The number of known plants species in the world and its annual increase. Phytotaxa. 2016;261:201–17. CrossRef
2. Wen J, Lu LM, Nie ZL, Liu XQ, Zhang N, Ickert-Bond S, et al. A new phylogenetic tribal classification of the grape family (Vitaceae). J Syst Evol. 2028;56:262–72. CrossRef
3. Bates J. Vitis sp., Vitaceae and viticulture in the Indus Civilization, South Asia ca. 3200–1500 BC: a critical review. Veg Hist Archaeobot. 2022;31:205–20. CrossRef
4. Wen J, Lu LM, Boggan JK. Diversity and evolution of Vitaceae in the Philippines. Philippine J Sci. 2013;142:223–44.
5. Rodrigues JG, Lombardi JA, Lovato MB. Phylogeny of Cissus (Vitaceae) focusing on South American species. Taxon. 2014;63(2):287–98. CrossRef
6. Latiff A. Studies in Malesian Vitaceae XV1II: revision of Cissus L. Malayan Nature J. 2022;74(1):89–107.
7. Jackes BR, Trias-Blasi A. Apocissus Jackes & Trias-Blasi, a new genus in the Vitaceae. Austrobail. 2023;13:94–104.
8. Ansarali S, Manikandan S, Lakshmanan GG. Review on phytochemical and pharmacological activities of the genus Cissus Linn. Int J Pharmaceut Res. 2016;8(4):1–7.
9. Fernandes G, Banu J. Medicinal properties of plants from the genus Cissus: a review. J Med Plants Res. 2012;6(16):3080–6. CrossRef
10. Joshi R, Saini P, Nathawat RS, Patni V. Micropropagation studies on genus Cissus: a review. In: Pullaiah T, editor. Micropropagation of medicinal plants 2. Karachi, India: Bentham Science Publishers; 2024. pp. 1–25. CrossRef
11. Burkill HM. The useful plants of West tropical Africa. Richmond, UK: Royal Botanical Garden, Kew; 2000.
12. Mothogoane MS. Cissus quadrangularis L.; 2022 [cited 2024 Jan 21]. Available from: https://pza.sanbi.org/cissus-quadrangularis#
13. Brown RW. Composition of scientific words. Washington, DC: Smithsonian Institution Press; 1956.
14. Wild H, Drummond RB. Vitaceae. In: Exell AW, Fernandes A, Wild H, editors. Flora Zambesiaca volume 2 part 2. Richmond, UK: Royal Botanic Gardens, Kew; 1966. pp. 353–653.
15. Hedberg I, Edwards S. Flora of Ethiopia and Eritrea. Addis Ababa, Ethiopia: The National Herbarium, Addis Ababa University; 1990. Vol. 3.
16. Verdcourt B. Vitaceae: flora of tropical East Africa. Rotterdam, The Netherlands: AA Balkema; 1993.
17. Akoègninou A, Van der Burg WJ, Van der Maesen LJG. Flore analytique du Bénin. Wageningen, The Netherlands: Backhuys Publishers; 2006.
18. Brundu G, Camarda I. The Flora of Chad: a checklist and brief analysis. PhytoKeys. 2023;23:1–18. CrossRef
19. Darbyshire I, Kordofani M, Farag I, Candiga R, Pickering H. The plants of Sudan and South Sudan. Richmond, UK: Kew publishing, Royal Botanic Gardens, Kew; 2015.
20. Burrows JE, Burrows SM, Lötter MC, Schmidt E. Trees and shrubs Mozambique. Cape Town, South Africa: Publishing Print Matters (Pty); 2018.
21. Gosline G, Bidault E, Van der Burgt X, Cahen D, Challen G, Condé N, et al. A taxonomically-verified and vouchered checklist of the vascular plants of the Republic of Guinea. Sci Data. 2023;10:327. CrossRef
22. Palgrave MC. Keith Coates Palgrave trees of southern Africa. Cape Town, South Africa: Struik Publishers; 2002.
23. Hyde MA, Wursten BT, Ballings P, Palgrave MC. Flora of Zimbabwe: species information: Cissus cornifolia; 2024. [cited 2024 June 6]. Available from: https://www.zimbabweflora.co.zw/speciesdata/species.php?species_id=137870
24. Germishuizen G, Meyer NL. Plants of southern Africa: an annotated checklist. Pretoria, South Africa: National Biodiversity Institute, Strelitzia; 2003.
25. Ruffo CK, Birnie A, Tengnäs B. Edible wild plants of Tanzania. Nairobi, Kenya: Regional Land Management Unit (RELMA), Technical Handbook Series 27; 2002.
26. Wild H, Mavi S, Biegel SM. A southern Rhodesia botanical dictionary of native and English plant names. Salisbury, Zimbabwe: Government Printer; 1972.
27. Williamson J. Useful plants of Malawi. Zomba, Malawi: University of Malawi; 1974.
28. Asfaw Z, Tadesse M. Prospects for sustainable use and development of wild food plants in Ethiopia. Econ Bot. 2001;55(1):47–62. CrossRef
29. Morris B. Wildlife and landscapes in Malawi: selected essays on natural history. Victoria, British Columbia: Trafford Publishing; 2009
30. Lulekal E, Asfaw Z, Kelbessa E, Van Damme P. Wild edible plants in Ethiopia: a review on their potential to combat food insecurity. Afr Focus. 2011;24(2):71–121. CrossRef
31. Dowo GM, Kativu S, Garine-Witchatitsky MD. Assessing plant utilisation by communities bordering a protected area in Zimbabwe using utilitarian diversity metrics. Trans Royal Soc South Afr. 2024;79(1):87–99. CrossRef
32. Awas T, Asfaw Z, Nordal I, Demissew S. Ethnobotany of Berta and Gumuz people in western Ethiopia. Biodiv. 2010;11(3):45–53. CrossRef
33. Raimondo D, Von Staden L, Foden W, Victor JE, Helme NA, Turner RC, et al. Red list of South African plants. Pretoria, South Africa: Strelitzia 25. South African National Biodiversity Institute; 2009.
34. Chhabra SC, Mahunnah RLA, Mshiu EN. Plants used in traditional medicine in Eastern Tanzania. VI. Angiosperms (Sapotaceae to Zingiberaceae). J Ethnopharmacol. 1993;39:83–103. CrossRef
35. Moshi MJ, Mbwambo ZH, Nondo RSO, Masimba PJ, Kamuhabwa A, Kapingu MC, et al. Evaluation of ethnomedical claims and brine shrimp toxicity of some plants used in Tanzania as traditional medicines. Afr J Trad Compl Alt Med. 2006;3(3):48–58. CrossRef
36. Mongalo NI, Makhafola TJ. Ethnobotanical knowledge of the lay people of Blouberg area (Pedi tribe), Limpopo province, South Africa. J Ethnobiol Ethnomed. 2018;14:46. CrossRef
37. Maliwichi Nyirenda CP, Maliwichi LL. Medicinal plants used to induce labour and traditional techniques used in determination of onset of labour in pregnant women in Malawi: a case study of Mulanje district. J Med Plants Res. 2010;4(24):2609–14.
38. Meke GS, Mumba RFE, Bwanali RJ, Williams VL. The trade and marketing of traditional medicines in southern and central Malawi. Int J Sustain Develop World Ecol. 2017;24(1):73–87. CrossRef
39. Arnold TH, Prentice CA, Hawker LC, Snyman EE, Tomalin M, Crouch NR, et al. Medicinal and magical plants of southern Africa: an annotated checklist. Pretoria, South Africa: National Botanical Institute, Strelitzia 13; 2002.
40. Chipiti T, Ibrahim MA, Koorbanally NA, Islam S. In vitro antioxidant activity and GC-MS analysis of the ethanol and aqueous extracts of Cissus cornifolia (Baker) Splanch (Vitaceae) parts. Acta Poloniae Pharmaceut Drug Res. 2025;72(1):119–27.
41. Ruffo CK. A survey of medicinal plants in Tabora region, Tanzania. In: Proceedings of the International Conference on Traditional Medicinal Plants, Arusha, Tanzania, 18–23 February 1990. Arusha, Tanzania: pp. 155–68.
42. Yaro AH, Musa AM, Ya’u J, Nazifi AB. Anticonvulsant properties of methanol root bark extract of Cissus cornifolia Planch (Vitaceae) in mice and chicks. Biol Environ Sci J Trop. 2015;12(1):634–9.
43. Yaro AH, Musa AM, Magaji MG, Nazifi AB. Anticonvulsant potentials of methanol leaf extract of Cissus cornifolia Planch (Vitaceae) in mice and chicks. Int J Herbs Pharmacol Res. 2015;4(2):25–32.
44. Jimoh A, Tanko Y, Mohammed A. Anti-diabetic effect of methanolic leaf extract of Cissus cornifolia on alloxan-induced hyperglycemic in wistar rats. Annals Biol Res. 2013;4(3):46–54.
45. Okoye TC, Uzor PF, Onyeto CA, Okereke EK. Safe African medicinal plants for clinical studies. In: Kuete V, editor. Toxicological survey of African medicinal plants. London, UK: Elsevier; 2014. pp. 535–55.
46. Chipiti T, Ibrahim MA, Singh M, Islam MS. In vitro α-amylase and α-glucosidase inhibitory and cytotoxic activities of extracts from Cissus cornifolia Planch parts. Pharmacog Mag. 2017;13:329–33. CrossRef
47. Conde P, Figueiro R, Saraiva S, Catarino L, Romeiros M, Duarte MC. The botanic mission to Mozambique (1942–1948): contributions to knowledge of the medicinal flora of Mozambique: The medicinal flora of Mozambique. Hist Ciênc Saúde Manguinhos Rio Janeiro. 2014;21(2):1–49. CrossRef
48. Gelfand M, Drummond RB, Mavi S, Ndemera B. The traditional medical practitioner in Zimbabwe: His principles of practice and pharmacopoeia. Gweru, Zimbabwe: Mambo Press; 1985.
49. Borquaye LS, Laryea MK, Gasu EN, Boateng MA, Baffour PK, Kyeremateng A, et al. Anti-inflammatory and antioxidant activities of extracts of Reissantia indica, Cissus cornifolia and Grosseria vignei. Cogent Biol. 2020;6(1):1785755. CrossRef
50. Dalziel JM. The useful plants of west tropical Africa. London, UK: The Crown Agents for the Colonies; 1948.
51. Jimoh A, Tanko Y, Mohammed A. Modulatory role of methanolic leaf extract of Cissus cornifolia on blood glucose levels of normoglycemic wistar rats. European J Exp Biol. 2013;3(1):22–7.
52. Hedberg I, Staugård F. Traditional medicine in Botswana: traditional medicinal plants. Gaborone, Botswana: Ipeleng Publishers; 1989
53. Mwafongo E, I Nordal, Z Magombo, B Stedje. Ethnobotanical study of Hyacinthaceae and non-hyacinthaceous geophytes in selected districts of Malawi. Ethnobot Res Appl. 2010;8:75–93. CrossRef
54. Van Wyk BE, Gericke N. People’s plants: a guide to useful plants of southern Africa. Pretoria, South Africa: Briza Publications; 2018.
55. Wild H, Gelfand M. Some native herbal remedies at present in use in Mashonaland. Central Afr J Med. 1959;5:292–305.
56. Ribeiro A, Romeiras MM, Tavares J, Faria MT. Ethnobotanical survey in Canhane village, district of Massingir, Mozambique: medicinal plants and traditional knowledge. J Ethnobiol Ethnomed. 2010;6:33. CrossRef
57. Masters ET. Medicinal plants of the upper Aswa River catchment of northern Uganda: a cultural crossroads. J Ethnobiol Ethnomed. 2023;19:48. CrossRef
58. Masumbu FFF, Mwamatope B, Tembo D, Mwakikunga A, Kamanula J. Ethnobotanical survey of medicinal plants claimed by traditional herbal practitioners to manage cancers in Malawi. J Herbal Med. 2023;42:100796. CrossRef
59. Mongalo NI, Raletsena MV, Munyai R. In vitro pharmacological activity, and comparison GC-ToF-MS profiling of extracts from Cissus cornifolia (Baker) Planch. Life. 2023;13:728. CrossRef
60. Musa AM, Tajuddeen N, Idris AY, Rafindadi AY, Abdullahi MI, Aliyu AB, et al. A new antimicrobial prenylated benzo-lactone from the rhizome of Cissus cornifolia. Phcog Res 2015;7:363–6. CrossRef
61. Musa AM, Yaro AH, Usman H, Magaji MG, Habu M. Phytochemical and some neuropharmacological studies on the methanolic leaf extracts of Cissus cornifolia [Vitaceae] in mice. Int J Pharmacol. 2008;4(2):145–8. CrossRef
62. Tanko Y, Kadiri OT, Mohammed A, Mahdi MA, Musa KY. Preliminary antidiarrhoeal activity of methanol extract of Cissus cornifolia (Bak.) Planch on experimental animals. Ann Biol Res. 2011;2(4):229–37.
63. Atiku I, Musa AM, Sule MI. Isolation of stigmasterol from methanolic extract of Cissus cornifolia Baker (Planch). Nigerian J Pharmaceut Sci. 2013;12(1):1–4.
64. Atiku I, Musa AM, Sani YM, Hanwa UA, Abdullahi SM, Sule MI. Phytochemical and antimicrobial studies of the crude methanolic extract of the leaf of Cissus cornifolia Baker (Planch) (family: Vitaceae). Asian J Pharmaceut Res Develop. 2013;1(6):79–83.
65. Yaro AH, Musa AM, Ya’u J, Nazifi AB, Garba K. Central nervous system depressant effects of fractions of methanol leaf extract of Cissus cornifolia (Baker) Planch. Pak J Pharm. Sci. 2019;32(2):563–8.
66. Nyoni M, Muzemu S, Chinheya C, Mushayabasa T, Ncube B. Screening indigenous nematicidal plants in Zimbabwe against Meloidogyne javanica. Paper Presented at a Conference Held on the 14th-16th July 2015, Elephant Hills Resort, Victoria Fallls, Zimbabwe.
67. Yaro AH, Anuka JA, Salawu OA, Hussaini IM, Usman H, Musa AM. Comparative neuropharmacological activities methanolic extracts of leaves and roots of Cissus cornifolia in mice. Afr J Biomed Res. 2009;12(3):219–23.
68. Yaro AH, Muhammad MA, Nazifi AB, Garba MM. Butanol soluble fractions of Cissus cornifolia methanolic leaf extract and behavioural effects in mice. J Phytopharm. 2015;4(4):202–6. CrossRef