INTRODUCTION
Chronic kidney disease (CKD) is highly prevalent globally, with consistent global prevalence estimates ranging from 11% to 13% (Hill et al., 2016). The progression of CKD to its terminal form, which prompts renal replacement therapy (RRT), also known as end-stage renal disease (ESRD), continues to be a significant cause of diminished quality of life and premature mortality. In several nations, continuous ambulatory peritoneal dialysis (CAPD) and hemodialysis (HD) are the most prevalent RRT. In Hong Kong, 80% of patients with ESRD undergo CAPD; many patients also undergo CAPD in New Zealand, Korea, and Singapore. Meanwhile, HD was utilized by 90% of dialysis patients in China, Japan, and Taiwan. In Indonesia, the estimated incidence was 251 per million individuals, and the prevalence was 499 per million healthy individuals (Indonesian Association of Nephrology, 2018). In the past 12 years, the number of peritoneal kidney patients in developing nations reached 24.9 per million compared to 21.8 per million in developed nations (Yulianti et al., 2015).
CKD is an independent risk factor for coronary heart disease (CHD) and CHD complications cause the majority of CKD-related deaths (Hill et al., 2016). CKD patients have 1.5 to 3 times the general population’s CHD incidence. More than 50% of CKD deaths are associated with CHD. Atherosclerosis and chronic inflammation are components of the malnutrition-inflammatory-atherosclerosis syndrome, which underlies the pathogenesis of CHD in patients with ESRD. As kidney function declines, the risk of cardiovascular disease rises (Dastani et al., 2015; Gansevoort et al., 2013; Kasliwal et al., 2014; Kaya et al., 2019).
The carotid intima-media thickness (CIMT) examination is a noninvasive diagnostic tool widely used to determine the presence of carotid plaque in CHD. In healthy middle-aged adults, a CIMT value between 0.6 and 0.7 mm is considered normal. In contrast, a CIMT value of 1 mm or greater is associated with a significantly increased absolute risk of CHD. CIMT has been the subject of extensive research and is linked to cardiovascular events and subclinical atherosclerotic disease (Gary et al., 2013; Kaligis et al., 2016; Muljadi et al., 2014; Sharma et al., 2017).
The neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are commonly used as markers of systemic inflammation in a variety of disease states, including limb ischemic and arterial stiffness (Taymez et al., 2016; Yu et al., 2015). NLR and PLR are also associated with chronic inflammation, mortality risk, and erythropoietin resistance in CKD (Guasti et al., 2011). These findings suggest that NLR and PLR may be used to determine individuals at high risk for CHD characterized by elevated CIMT. This study will investigate whether NLR and PLR can predict CIMT in ESRD patients undergoing dialysis.
MATERIAL AND METHODS
Setting
Moewardi General Hospital was founded in 1977 due to the merging of three separate general hospitals in the city of Surakarta. Besides its mission to improve the quality of healthcare in surrounding cities, it also operates as a university hospital to accommodate the education of medical doctors at Sebelas Maret University. Since its founding, the Department of Nephrology and Hypertension has become responsible for managing patients with various types of kidney disease. Currently, the Moewardi General Hospital is the only tertiary hospital located in the city of Surakarta. Patients with renal disease receive outpatient service and inpatient services if necessary. HD and CAPD are performed as indicated. Most patients receive healthcare coverage from a national health insurance legal entity.
Study design and population
This observational analytic study uses a cross-sectional approach conducted at the Department of Renal-Hypertension and Hemodialysis Unit in Moewardi General Hospital in Surakarta, Indonesia, from January 1 to July 1, 2022. All patients aged 18 years or older with a diagnosis of ESRD and on at least 3-month HD or CAPD-based RRTs were included in the study. The presence of cardiovascular, neoplastic, chronic inflammatory, or infectious disease and the usage of immunosuppressants or antiplatelet drugs preclude the patient from participating in the study.
Outcomes and predictors
The main outcome of this study is the presence of a high risk of CHD characterized by CIMT ≥1 mm (Kaligis et al., 2016; Muljadi et al., 2014). The risk of CHD will be operated as a categorical variable: CIMT <1 mm and CIMT ≥1 mm. The main predictors used in this study are NLR and PLR. The absolute values of neutrophils and platelets were divided by the absolute values of lymphocytes to get the NLR and PLR values, respectively. Several confounding variables were also considered, including age, gender, nutrition status, CKD duration, presence of diabetes mellitus, and other hematologic parameters. These hematologic parameters include hemoglobin, leukocyte count, platelet count, neutrophils, and lymphocytes. These predictors will be operated as numerical or categorical variables, accordingly.
Measurement
Patients who met the inclusion and exclusion criteria will be asked to sign informed consent followed by history-taking and blood sampling. The blood samples were then ordered for a routine hematology examination using a hematology analyzer in the clinical pathology department. The measurement of CIMT will be carried out by a cardiologist in the cardiovascular department using B-mode ultrasound. The cardiologist will measure both carotid arteries and a higher measurement of CIMT will be recorded (Fig. 1).
Data extraction and statistical analysis
The data were first exported to Microsoft Excel and then imported into Statistical Package for the Social Sciences (version 22) for quantitative statistical analysis software. All variables were subjected to descriptive statistics to describe the characteristics of the subject’s frequency, percentage, means, and SDs before analysis. A comparative analysis was also performed to identify the factor of the confounding variable that affected the primary outcome. Separate logistic regression models were performed to analyze the relationship between the significant and the main outcome. The predictive values of the main predictors were also evaluated by constructing receiver operating characteristics (ROCs) curves and identifying the most discriminatory cutoff values. A level of significance was accepted when the two-tailed p-value was less than or equal to 0.05.
Ethical clearance and clinical registry
The study was conducted in accordance with the Declaration of Helsinki. Before the commencement of the study, a proposal was submitted to the local ethics committee. This study was approved by the Health Research Ethics Committee at Moewardi General Hospital with the number 1.338/I/HREC/2021. We also obtained informed consent from all the eligible patients. The study protocol was registered on ClinicalTrials.gov with ID NCT05472805.
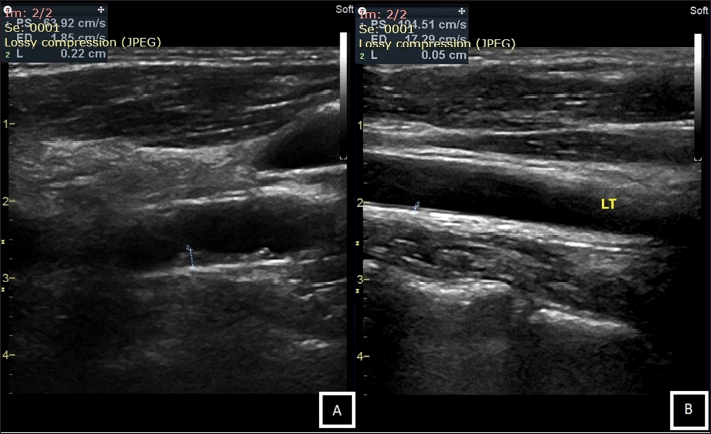 | Figure 1. CIMT measurement using B-mode ultrasound; (A). Example of an individual with increased thickness of CIMT (CIMT = 2.2 mm). (B). Another example of an individual with a normal thickness of CIMT (CIMT = 0.5 mm). [Click here to view] |
RESULTS
The present study includes 72 patients with ESRD in the Department of Renal-Hypertension and Hemodialysis Unit at Moewardi General Hospital. Most of the patients were males (68.1%), with a mean age of 45.06 ± 11.30, with a mean CKD duration of 4 years. Most ESRD patients were on HD-based RRT (HD, n = 44 vs. CAPD, n = 28). Table 1 shows that most of these individuals (78.6%) had CIMT ≥1 mm and were subjected to a higher risk of CHD.
The comparison analysis of the effect of confounding variables, namely, age, gender, nutrition status, CKD duration, RRT method, and other routine hematologic parameters, was carried out and is shown in Table 2. The analysis showed that the effect of confounding variables was insignificant (p > 0.05), excluding neutrophils and lymphocytes, which also influence the NLR and PLR.
Using logistic regression analysis, the strength of the predictive utility of NLR and PLR in determining CIMT status was determined and is presented in Table 3. The analysis showed that only NLR has a significant predictive utility in determining CIMT status, collectively [OR 1.474 (95% CI: 1.031–2.106); p = 0.033] and on CAPD group only [OR 5.957 (95% CI: 1.189–29.847); p = 0.030].
Further ROC analysis was also performed to determine the area under the curve (AUC) and the predictors’ cutoff value with the best sensitivity and specificity values. Previous analysis (Table 2) that showed the significance of the RRT method used prompted the ROC analysis to be performed separately according to the method used. As shown in Table 4, NLR (AUC: −0.909; p = <0.001; 95% CI: 0.567–0.983) and PLR (AUC-0.969; p = < 0.001; 95% CI; 0.757–0.996) in CAPD patients showed the highest AUC, and lower NLR and PLR value of AUC was observed in HD patients. Taking these RRTs together, only NLR was found to have a significant (AUC-0.663; p = 0.010; 95% CI; 0.501–0.781), albeit lower than in the CAPD group.
Based on the ROC curve, an optimal cutoff with high sensitivity and specificity for the risk of CHD was established for the CAPD patients (Table 5), with NLR ≥2.72 (sensitivity = 95.45%; specificity = 83.33%) and PLR ≥93.09 (sensitivity = 90.91%; specificity = 83.33%). Our ROC analysis also determined the cutoff of NLR and PLR on both RRT and HD-only patients, as shown in Table 5, albeit with a lower specificity value.
DISCUSSION
To the best of our knowledge, this is the first study to propose the utility of NLR and PLR, as well as their clinical cutoff point, for determining the risk of CHD in ESRD patients, with respect to the RRT method used, with significant high sensitivity and specificity. It was hypothesized that NLR and PLR, identified as inflammatory biomarkers, would be significantly related to increased CIMT in CKD patients undergoing dialysis. We present evidence that ESRD patients with CIMT ≥1 at risk for CHD had significantly greater NLR and PLR than patients with lower values of these markers.
Our analysis showed that the NLR and PLR cutoff points of 2.72 and 93.09 on CAPD patients, respectively, had an optimal sensitivity and specificity in determining the risk of CHD, as assessed by another noninvasive measurement of CHD risk, namely, CIMT. Previous studies have consistently shown that higher NLR and PLR were associated with worse cardiovascular outcomes (Demir et al., 2014; Li et al., 2020; Yüksel et al., 2016; Yulianti et al., 2015). These studies showed that both of these easily available markers always had prognostic utility in managing cardiovascular disease. Despite that, evidence regarding the utility of these biomarkers as CHD risk assessment on individuals at risk, especially in the ESRD population, was lacking and therefore was urgently needed. Regarding NLR, available evidence shows that even at a low level of 1.53, it was significantly associated with coronary microvascular dysfunction (Chen et al., 2021). Our NLR cutoff point of 2.72 was a bit higher than other studies on non-ESRD patients, which invariably set the cutoff at 1.96–2.26, albeit ours had higher sensitivity and specificity (Fernando et al., 2015; Kizilarslano?lu et al., 2017). Our PLR cutoff point of 93.09 was considerably lower than any available evidence of cutoff point in other studies of non-ESRD patients, which was invariably determined at 137–150, although these studies ultimately observe the prognostic implication of the cutoff point (Lee et al., 2018; Li et al., 2017). Our high sensitivity and specificity of these cutoff values in CAPD patients should be noted with caution because these values were determined based on CIMT status, which did not conclude the presence of CHD but rather determined the higher risk of CHD. This should also apply to the cutoff in our HD groups and collectively, albeit our results showed lower specificity in these groups.
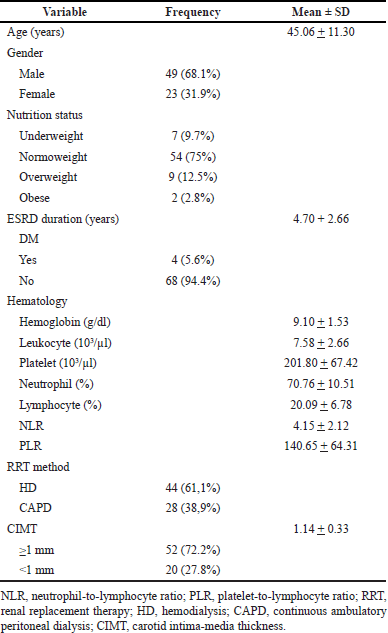 | Table 1. Clinical and laboratory parameters characteristic of the subjects. [Click here to view] |
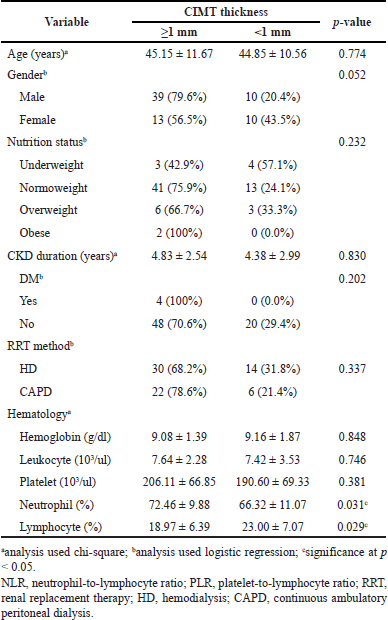 | Table 2. Comparison of the effect of confounding variables on the status of CIMT. [Click here to view] |
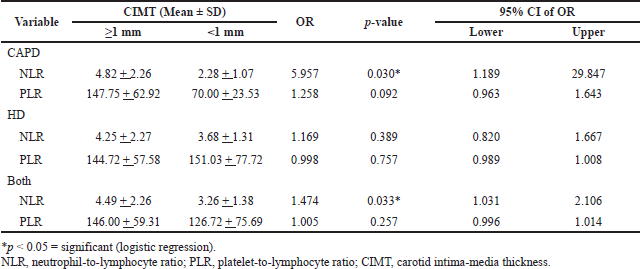 | Table 3. Association between NLR and PLR to the status of CIMT between RRT methods and collectively. [Click here to view] |
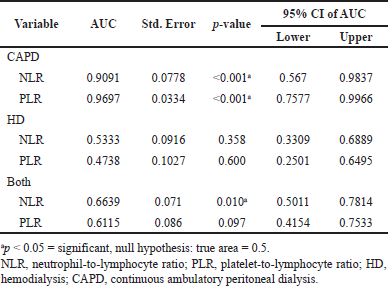 | Table 4. Receiver operative curve analysis between NLR and PLR to the status of CIMT between RRT methods and collectively. [Click here to view] |
The underlying role of neutrophils, platelets, and lymphocytess in the pathophysiology of CHD and related disorders has been extensively researched worldwide. It was thought to be related to their nature in immune-inflammatory activity. These laboratory tests provide a low-cost and broadly accessible screening technique for at-risk groups, particularly CKD patients. Increased inflammatory activity in CKD patients was mainly attributed to elevated systemic concentrations of proinflammatory cytokines due to impaired renal clearance and increased synthesis (Uysal et al., 2022). These conditions will further increase the process of atherosclerosis, increasing the risk of an injury to the tunica intima of arteries and the blood vessels’ endothelium, which causes neutrophil adhesion, aggregation, and platelet activation. Neutrophil extracellular traps (NETs) are the most well-known initiation mechanism for thrombus formation in individuals at risk. DNA and histones constitute the matrix of active NETs (Kapoor et al., 2018). This matrix serves as a scaffold and binds erythrocytes and platelets (Fuchs et al., 2007, 2010). Through platelet binding to DNA and subsequent activation by histones, they increase platelet activation, platelet aggregation, and thrombosis (Fuchs et al., 2010, 2011). Activated platelets resulting from the interaction between NETs and platelets connect to neutrophils via glycoprotein Ib and stimulate further NET recruitment; this vicious cycle propagates thrombus development (Kapoor et al., 2018; von Brühl et al., 2012). This platelet activation will further propagate atherosclerosis formation (Yulianti et al., 2015). The role of lymphocytes in the development of atherosclerosis remained contentious. The T-helper (Th1), a subpopulation of CD4+ T cells, which generates IFN-γ and TNF-α preferentially (Frostegård et al., 1999), possesses strong proatherogenic activity (Hedrick, 2015; Li and Ley, 2015). Th2 cells, in contrast to Th1 cells, are antiatherogenic because switching the T-cell response from Th1 to Th2 is related to a reduction in atherogenicity, although this remains controversial (Huber et al., 2001; Schulte et al., 2008). Regulatory T cells (Tregs) and B cells have been proposed as antiatherogenic, despite the small number of studies in this area (Hedrick, 2015; Li and Ley, 2015). Furthermore, in acute coronary syndrome (ACS) patients, NLR and PLR have also been established to be related to the severity of the ACS (Goutham et al., 2015). The increase in NLR will increase the risk of death by 2.1 times (Tatar et al., 2016), and especially in CAPD patients, poses a higher risk of arterial stiffness and a greater risk of cardiovascular death (Lu et al., 2018).
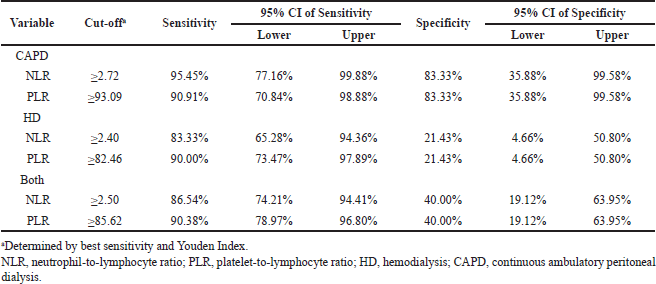 | Table 5. Receiver operative curve analysis of sensitivity and specificity of NLR and PLR cutoff to the status of CIMT between RRT methods and collectively. [Click here to view] |
Dialysis-related cardiac complications in CKD patients were not uncommon. It was long debated whether CAPD or HD patients had a higher risk of cardiovascular disease or mortality. Among these, HD was associated with a higher prevalence of de novo congestive heart failure (Wang et al., 2016) and more cardiovascular events, including atrial fibrillation and peripheral artery disease, compared to CAPD patients, despite considerably lower cardiovascular mortality (Ng et al., 2020). This is in favors of our study result, which showed that all patients in HD groups are at risk of CHD, marked by elevated CIMT. Evidence of aberrant metabolic marker alterations, mainly albumin, apolipoprotein-B, homocysteine, and C-reactive protein, between HD and CAPD was available, yet unremarkable, rendering the pathophysiology behind this trend mostly unclear (Helal et al., 2010). It was long believed that the dialysis procedure in CAPD and HD produces inflammation and oxidative stress related to its dialysate, regardless of the technique. Bioincompatibility and other dialysis-related factors can result in the release of proinflammatory cytokines and endothelial dysfunction. These elevated proinflammatory cytokines will increase the number of circulating neutrophils, which will, in turn, promote platelet activation in impaired endothelium locations and initiate the vicious cycle of neutrophil-platelet activation previously described (Kapoor et al., 2018; von Brühl et al., 2012). These will increase the neutrophil and platelet, thus relatively increasing NLR and PLR, consecutively. These vicious cycles of recruitment of neutrophils and platelet would increase inflammatory markers, such as TNF-α and CRP, thereby accelerating atherosclerosis as renal function decreases. Several other factors, including oxidative stress and chronic infection, may also be involved in the inflammatory process (Mehrotra et al., 2016; Nurhayatun, 2017).
The chronic inflammatory conditions experienced by CKD patients undergoing dialysis contribute to the incidence of atherosclerosis and its complications (Nusair et al., 2012). Cardiovascular mortality is two times higher in stage III CKD patients and three times higher in stage IV CKD patients compared to individuals with normal kidney function (Kasliwal et al., 2014). Other studies support the relationship between kidney damage and cardiac cell death by reporting that in CAPD patients, CIMT showed an association with cardiac troponin T levels and concentric left ventricular hypertrophy (Asicioglu et al., 2021; Benedetto et al., 2001).
In this study, the results of the CIMT measurement obtained a mean value of 1.14 ± 0.33 mm and more subjects in CIMT ≥1 mm groups. CIMT in this study was measured in both the right and left carotid. Then the maximum CIMT value is used in the statistical analysis. The maximal CIMT has been shown as an independent predictor of CHD and has a positive relationship with anterior and posterior coronary wall thickness (Ruscica et al., 2019). Across several studies, the cutoff ≥1 mm gave 31%–66% sensitivity and 79%–90% specificity in the prediction of CHD (Lu et al., 2018; Zhang et al., 2014).
As a cross-sectional study, the primary limitation of the current investigation is that its nature cannot establish a causal relationship between exposure and disease. In determining the risk of cardiovascular disease, the study also did not compare NLR and PLR with other established inflammatory biomarkers, such as TNF-α, IL-1, IL-6, and CRP. This is justified in our study because we aim to determine alternative cost-effective, and widely available CHD markers, especially in lower setting centers, wherein those biomarkers are typically unaffordable.
The primary diagnosis of CHD by coronary arteriography and other noninvasive cardiovascular imaging modalities, including CIMT, is expensive and time-consuming and exposes the patient to radiation. On the other hand, we showed that more affordable and widely available hematology analysis parameters, namely, NLR and PLR, could also be used as an initial screening to determine the risk of CHD. These parameters could be developed further as a preliminary CHD risk assessment or routine examination of individuals with ESRD, particularly in lower setting centers, before referral to advanced imaging modalities available in more advanced centers.
CONCLUSION
The present study demonstrated a new option in assessing, predicting, and evaluating the occurrence of atherosclerosis in ESRD, both HD or CAPD, by using one of the most commonly available and simple laboratory analyses, the NLR and PLR. We advocate the use of these laboratory data in a novel strategy for early diagnosis, prediction, and assessment of atherosclerosis that is less expensive and less complicated, especially in limited-resource settings. Furthermore, these findings could promote several similar studies to further develop better choices in the early detection of cardiovascular complications to better minimize the already heavy burden of ESRD patients needing RRTs.
AUTHORS’ CONTRIBUTIONS
WP, DNB, and AW were involved in planning and supervising the work; AS, HP, NAP, and YSP performed the measurements; WP, DNB, AW, AS, HP, NAP, and YSP processed the experimental data, performed the analysis, and designed the figures. WP, DNB, AW, AS, HP, NAP, and YSP performed the data calculation and statistical analysis. All authors equally contributed to interpreting the results and worked on the draft of the manuscript. All authors discussed the results and approved the manuscript.
ACKNOWLEDGMENTS
The authors sincerely send our gratitude to our patients involved in this study. They also warmly appreciate Moewardi General Hospital and Sebelas Maret University for providing treatment to our patients and providing us with the necessary data regarding our study.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study was approved by the Health Research Ethics Committee at Moewardi General Hospital with the number 1.338/I/HREC/2021. We also obtained informed consent from all the eligible patients. The study protocol was registered on ClinicalTrials.gov with ID NCT05472805.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Asicioglu E, Velioglu A, Arikan H, Koc M, Tuglular S, Ozener C. Baseline carotid intima-media thickness is associated with cardiovascular morbidity and mortality in peritoneal dialysis patients. Ther Apher Dial, 2021; 25(6):962–9. CrossRef
Benedetto FA, Mallamaci F, Tripepi G, Zoccali C. Prognostic value of ultrasonographic measurement of carotid intima media thickness in dialysis patients. J Am Soc Nephrol, 2001; 12(11):2458–64. CrossRef
Chen Y, Chai Q, Wang Q, Zhang Z, Shan Y, Lu D, Liu M, Wu W. Neutrophil-to-lymphocyte ratio is associated with coronary microvascular dysfunction in type 2 diabetes mellitus patients. Diabetes Res Clin Pract, 2021; 178:108983. CrossRef
Dastani M. Coronary artery disease in patients with chronic kidney disease: a brief literature review. Rev Clin Med, 2015; 2(4):182–6.
Demir K, Avci A, Altunkeser BB, Yilmaz A, Keles F, Ersecgin A. The relation between neutrophil-to-lymphocyte ratio and coronary chronic total occlusions. BMC Cardiovasc Disord, 2014; 14(1):1–6. CrossRef
Fernando ML, Silambanan S, Malar J. Neutrophil to lymphocyte ratio as an indicator of presence of coronary artery disease in diabetic patients. Int J Clin Biochem Res, 2015; 2(3):143–7.
Frostegård J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, Hansson GK. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis, 1999; 145(1):33–43. CrossRef
Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol, 2007; 176(2):231. CrossRef
Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood, 2011; 118(13):3708. CrossRef
Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A, 2010; 107(36):15880–5. CrossRef
Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJL, Mann JF, Matsushita K, Wen CP. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet, 2013; 382(9889):339–52. CrossRef
Gary T, Pichler M, Belaj K, Hafner F, Gerger A, Froehlich H, Eller P, Rief P, Hackl G, Pilger E, Brodmann M. Platelet-to-lymphocyte ratio: a novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. PLoS One, 2013; 8(7):e67688. CrossRef
Goutham Reddy K, Jagadesh M, Ranjan Shetty K, Devasia T. Association of serum platelet-lymphocyte ratio (PLR), neutrophil-lymphocyte ratio (NLR), non-HDL cholesterol levels with severity of acute coronary syndrome. Indian Heart J, 2015; 67:S17–8. CrossRef
Guasti L, Dentali F, Castiglioni L, Maroni L, Marino F, Squizzato A, Ageno W, Gianni M, Gaudio G, Grandi AM, Cosentino M, Venco A. Neutrophils and clinical outcomes in patients with acute coronary syndromes and/or cardiac revascularisation. A systematic review on more than 34,000 subjects. Thromb Haemost, 2011; 106(4):591–9. CrossRef
Hedrick CC. Lymphocytes in atherosclerosis. Arterioscler Thromb Vasc Biol, 2015; 35(2):253. CrossRef
Helal I, Smaoui W, Hamida F, Ouniss M, Aderrahim E, Hedri H, ELyounsi F, Maiz HB, Abdallah TB, Kheder A. Cardiovascular risk factors in hemodialysis and peritoneal dialysis patients. Saudi J Kidney Dis Transpl, 2010; 21(1):59.
Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, Hobbs FDR. Global prevalence of chronic kidney disease—a systematic review and meta-analysis. PLoS One, 2016; 11(7):e0158765. CrossRef
Huber SA, Sakkinen P, David C, Newell MK, Tracy RP. T Helper–cell phenotype regulates atherosclerosis in mice under conditions of mild hypercholesterolemia. Circulation, 2001; 103(21):2610–6. CrossRef
Indonesian Association of Nephrology. 11th Report of Indonesian renal registry. Indonesian Association of Nephrology, Jakarta, Indonesia, 2018.
Kaligis RWM, Adiarto S, Erwinanto, Nugroho J, Pradnyana BA, Lefi A, Rifqi S. Carotid intima-media thickness in Indonesian subjects with cardiovascular disease risk factors who were not receiving lipid-lowering agents. Int J Angiol, 2016; 25(3):174. CrossRef
Kapoor S, Opneja A, Nayak L. The role of neutrophils in thrombosis. Thromb Res, 2018; 170:87. CrossRef
Kasliwal R, Bansal M, Desai D, Sharma M. Carotid intima-media thickness: current evidence, practices, and Indian experience. Indian J Endocrinol Metab, 2014; 18(1):13–22. CrossRef
Kaya Y, Yaman M, Karata? A, Gül T, Kaygisiz S, Alta? H, Yilmaz A, Kaya A. Platelet lymphocyte ratio is associated with carotid atherosclerosis in hemodialysis patients. Middle Black Sea J Health Sci, 2019; 5(3):199–205. CrossRef
Kizilarslano?lu MC, Kuyumcu MC, Kiliç MK, Çinar E, Kara O, Arik G, Kizilarslano B, Halil M, Ye Y, Yavuz BB, Cankurtaran M, Ario S. Neutrophil to lymphocyte ratio may predict coronary artery disease in geriatric patients. Acta Med, 2017; 46(1):58–63.
Lee YSG, Baradi A, Peverelle M, Sultani R, Adams H, Garlick J, Wilson AM. Usefulness of platelet-to-lymphocyte ratio to predict long-term all-cause mortality in patients at high risk of coronary artery disease who underwent coronary angiography. Am J Cardiol, 2018; 121(9):1021–6.
Li XT, Fang H, Li D, Xu FQ, Yang B, Zhang R, An Y. Association of platelet to lymphocyte ratio with in-hospital major adverse cardiovascular events and the severity of coronary artery disease assessed by the Gensini score in patients with acute myocardial infarction. Chin Med J, 2020; 133(4):415. CrossRef
Li J, Ley K. Lymphocyte migration into atherosclerotic plaque. Arterioscler Thromb Vasc Biol, 2015; 35(1):40–9. CrossRef
Li H, Zhou Y, Ma Y, Han S, Zhou L. The prognostic value of the platelet-to-lymphocyte ratio in acute coronary syndrome: a systematic review and meta-analysis. Kardiol Pol, 2017; 75(7):666–73. CrossRef
Lu X, Wang S, Zhang G, Xiong R, Li H. High neutrophil-to-lymphocyte ratio is a significant predictor of cardiovascular and all-cause mortality in patients undergoing peritoneal dialysis. Kidney Blood Press Res, 2018; 43(2):490–9. CrossRef
Mehrotra R, Devuyst O, Davies SJ, Johnson DW. The current state of peritoneal dialysis. J Am Soc Nephrol, 2016; 27(11):3238–52. CrossRef
Muljadi R, Murtala B, Kabo P, Suhadi FB. Comparison between carotid intima-media thickness and coronary artery calcification in the prediction of atherosclerosis in diabetic patients. Indones Biomed J, 2014; 6(1):45–50. CrossRef
Ng CH, Ong ZH, Sran HK, Wee TB. Comparison of cardiovascular mortality in hemodialysis versus peritoneal dialysis. Int Urol Nephrol, 2020; 53(7):1363–71. CrossRef
Nurhayatun E. Oral N-Acetyl cysteine lowered IL 6 level among stage V chronic kidney disease patients on continuous ambulatory peritoneal dialysis (CAPD). J Kedokt Kesehat Indones, 2017; 8(3):183–90. CrossRef
Nusair MB, Rajpurohit N, Alpert MA. Chronic inflammation and coronary atherosclerosis in patients with end-stage renal disease. Cardiorenal Med, 2012; 2(2):117–24. CrossRef
Ruscica M, Castelnuovo S, Macchi C, Gandini S, Mombelli G, Ferri N, Labombarda F, Sirtori CR. Left main coronary wall thickness correlates with the carotid intima media thickness and may provide a new marker of cardiovascular risk. Eur J Prev Cardiol, 2019; 26(9):1001–4. CrossRef
Schulte S, Sukhova GK, Libby P. Genetically programmed biases in Th1 and Th2 immune responses modulate atherogenesis. Am J Pathol, 2008; 172(6):1500–8. CrossRef
Sharma K, Patel AK, Shah KH, Konat A. Is neutrophil-to-lymphocyte ratio a predictor of coronary artery disease in Western Indians? Int J Inflam, 2017; 2017:4136126. CrossRef
Tatar E, Mirili C, Isikyakar T, Yaprak M, Guvercin G, Ozay E, Asci G. The association of neutrophil/lymphocyte ratio and platelet/lymphocyte ratio with clinical outcomes in geriatric patients with stage 3-5 chronic kidney disease. Acta Clin Belg, 2016; 71(4):221–6. CrossRef
Taymez DG, Ucar E, Turkmen K, Ucar R, Afsar B, Gaipov A, Turk S. The predictive value of platelet/lymphocyte ratio in hemodialysis patients with erythropoietin resistance. Ther Apher Dial, 2016; 20(2):118–21.
Uysal S, Toker A, Türkmen K, Keskin S. The role of decoy receptor 3 in inflammation and atherosclerosis in patients with chronic kidney disease and renal transplant patients. Nefrología, 2022; https://doi.org/10.1016/j.nefro.2021.12.007 CrossRef
von Brühl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Tirniceriu A, Coletti R, Köllnberger M, Byrne RA, Laitinen I, Walch A, Brill A, Pfeiler S, Manukyan D, Braun S, Lange P, Riegger J, Ware J, Eckart A, Haidari S, Rudelius M, Schulz C, Echtler K, Brinkmann V, Schwaiger M, Preissner KT, Wagner DD, Mackman N, Engelmann B, Massberg S. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med, 2012; 209(4):819. CrossRef
Wang IK, Lu CY, Lin CL, Liang CC, Yen TH, Liu YL, Sung FC. Comparison of the risk of de novo cardiovascular disease between hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Int J Cardiol Heart Vasc, 2016; 218:219–24. CrossRef
Yu X, Li X, Li Y, Liu T, Wang R. Neutrophil-lymphocyte ratio is associated with arterial stiffness in postmenopausal women with osteoporosis. Arch Gerontol Geriatr, 2015; 61(1):76–80. CrossRef
Yüksel M, Y?ld?z A, Oylumlu M, Akyüz A, Ayd?n M, Kaya H, Acet H, Polat N, Bilik MZ, Alan S. The association between platelet/lymphocyte ratio and coronary artery disease severity. Anatol J Cardiol, 2016; 15(8):640. CrossRef
Yulianti M, Suhardjono S, Kresnawan T, Harimurti K. Factors correlating with nutritional status in continuous ambulatory peritoneal dialysis patients (CAPD). J Penyakit Dalam Indones, 2015; 2(1):2–8. CrossRef
Zhang Y, Guallar E, Qiao Y, Wasserman BA. Is carotid intima-media thickness as predictive as other noninvasive techniques for the detection of coronary artery disease? Arterioscler Thromb Vasc Biol, 2014; 34(7):1341–5. CrossRef