INTRODUCTION
Healthcare-associated infections, caused by superbugs such as Staphylococcus aureus, Pseudomonas aeruginosa, and Klebsiella pneumoniae, have become primary mortality causes in significant populations. Classified as ESKAPE bugs under the World Health Organization’s Priority I, these microorganisms pose a significant challenge due to their heightened antimicrobial resistance [1–3]. The rise of multidrug resistance has emphasized the inadequacy of current antibiotics, with high doses leading to potential toxic effects. This situation has intensified the research for alternate treatment strategies.
Nanoparticulate drug delivery technology emerges as a promising alternative, particularly against multidrug-resistant infections. Their distinct chemical and physical properties have demonstrated beneficial outcomes. Various nanoparticles, including liposomes, niosomes, nanotubes, silver nanoparticles, solid lipid nanoparticles, and polymeric nanoparticles, have been explored for targeted drug delivery and controlled drug release at infection sites [4,5]. Such nanoparticles offer the advantage of delivering multiple therapeutics simultaneously, potentially mitigating resistance development. Furthermore, they can enhance the pharmacokinetic profiles of drugs, reduce required doses, and thus decrease potential toxic effects and dosing frequency.
Of the nanoparticles studied, mesoporous silica nanoparticles (MSNs) stand out due to their design versatility, tunable pore size, extensive surface area, optimal pore volume, and ease of functionalization. This makes them particularly valuable in combating bacterial infections [6–8]. The most widely explored type of mesoporous material is Mobil Composition of Matter No. 41 (MCM-41), characterized by its 2.5–6 nm pore diameter and hexagonal arrangement [6]. In addition, MSNs are recognized for their biocompatibility and substantial drug-loading capacity.
Understanding that bacteria interact with host cell glycoconjugates using various cell wall proteins is crucial. Carbohydrate-binding proteins, such as lectins, primarily facilitate bacterium-host adhesion [9,10]. This interaction has paved the way for glycomimetics, molecules that mimic carbohydrate activity [11]. Various multivalent neo-glycoconjugates, like glycoclusters using multivalent scaffolds such as di-, tri-, tetravalent sugars, cyclodextrin core, porphyrins, calixarenes, and cyclic peptides have been explored. Mannoside clusters used as ligands for targeting type-1 fimbrial lectin found in Escherichia coli (FimH), extended tetramannosylated clusters for increasing multivalency, FimH binding, glyconanotubes, glycodendrimers involving PPI dendrimers, carbosilane, glycopeptide, and hyperbranched boltorn dendrimer have been developed for effectively addressing the problem of antimicrobial resistance [12,13]. Incorporating sugar moieties in carrier molecules augments interaction points between bacterial sugar-binding proteins (like lectins) and carbohydrates, enhancing binding and efficient antimicrobial agent delivery [14,15].
Based on this proof-of-concept, in the present study, we have designed glycosylated MSNs (GLY MSNs) loaded with levofloxacin (LVF) for its plausible use in combating bacterial resistance observed in urinary tract infections (UTIs). LVF, a third-generation fluoroquinolone, has a 6–8 hours half-life and acts by inhibiting the bacterial DNA gyrase enzyme, crucial for bacterial DNA replication (topoisomerase II inhibition) [16]. Despite its efficacy in treating complicated UTIs [17], resistance development remains a concern [18] due to various factors like mutations at the topoisomerase gene and upregulated drug efflux [19,20]. While LVF delivery via MSNs and chitosan nanoparticles has been explored [21], our study is a pioneering effort to harness glycosylation for targeted LVF delivery using MSNs in UTI treatments. This approach seeks to reduce both dosages and the potential for bacterial resistance.
MATERIALS AND METHODS
Materials
LVF was procured from Yarrow Chemicals, Mumbai, India. From Loba Chemie, India, we procured N-cetyl-N, N, N, trimethyl ammonium bromide (98%, CTAB). Tetraethylorthosilicate (TEOS), 3-aminopropyltriethoxysilane (APTES), and D-glucuronic acid were all obtained from TCI Chemicals, India. N(3-dimethylaminopropyl)N’-ethylcarbodiimide hydrochloride (EDC.HCl) and N-hydroxysuccinimide were obtained from Spectrochem Pvt. Ltd., Mumbai. All other reagents utilized in the study were of analytical grade.
Methodology
Preparation of MSNs
MSNs were synthesized using a method previously described by Narayan et al. 2021 [22,23]. Briefly, CTAB (1.37 mmol) was dissolved in distilled water containing a 2.5 M sodium hydroxide solution and stirred at 80°C for 30 minutes. TEOS (11.28 mmol) was added to the above solution under vigorous stirring at 950 rpm. After allowing the mixture to react overnight, ethanol was added. The resultant product was filtered and dried. The dried MSNs were subjected to calcination at 500°C for 6 hours.
Preparation of amine functionalized MSNs (MSN-NH2)
To prepare amine-functionalized MSNs, a post-grafting method was utilized. MSNs were dispersed in dry toluene, and APTES (2.6 mmol) was added dropwise with continuous stirring. This mixture was subjected to reflux at 120°C for 10 hours. The product was washed with toluene and residual toluene was removed with the help of rotavapor (Buchi, Labortechnik AG, Flawil, Switzerland). The resultant product was dried under vacuum. Figure 1 illustrates the synthesis of MSNs followed by their amino functionalization.
Glycosylation of amine-functionalized MSNs
The amine-functionalized MSNs were glycosylated by coupling them with a sugar acid moiety, D-glucuronic acid, in aqueous media using the EDAC-NHS coupling chemistry [24]. Initially, an equimolar amount of EDAC.HCl, NHS, and D-glucuronic acid (0.16 mmol each) were dissolved in water. The pH was adjusted to 7.5–8, and the solution was heated to 50°C while stirring for 2 hours. Subsequently, 100 mg of MSN-NH2 was added, and the reaction was allowed to proceed for 24 hours. The resulting product, designated as GLY-MSN, was filtered, washed with ethanol and water, and dried under a vacuum.
Drug loading into MSNs
MSN-NH2 and GLY-MSN particles were loaded with LVF using the wet-impregnation method, as described in prior literature [24]. In brief, MSNs were dispersed in an aqueous solution of LVF and stirred at room temperature for 18 hours. The drug-loaded MSNs were then separated by centrifugation at 18,000 rpm for 15 minutes. The supernatant was analyzed using UV-visible spectrophotometry (Shimadzu, Kyoto, Japan) at 288 nm, allowing the analysis of unentrapped LVF. Different batches were tested with varying drug-to-MSN ratios: 1:2, 1:5, and 1:8. The batch demonstrating optimal LVF loading was chosen for GLY-MSN loading. The loading capacity was determined using the following formula:
Characterization of the prepared formulation
The nanoparticles were characterized using various techniques. Field emission scanning electron microscopy (FESEM) was carried out using a GEMINI SEM300. Transmission mode FTIR spectra were recorded using an FTIR–8,300 spectrometer (Shimadzu Corporation, Japan). Differential Scanning Calorimetry (DSC) analyzed the LVF’s physical state within the MSNs. Using nitrogen adsorption, the Barrett–Joyner–Halenda (BJH) method assessed the MSNs’ pore volume and size. The Brunauer–Emmett–Teller (BET) method determined the specific surface area. Dynamic light scattering measured particle size distribution and zeta potential with a Malvern Zetasizer (Malvern Nano Series NanoZS, UK).
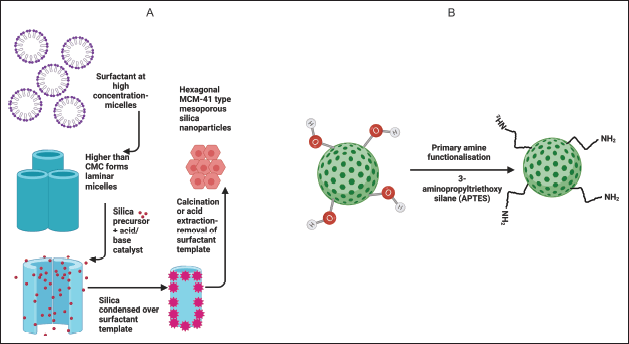 | Figure 1. Illustration of A) Preparation of plain mesoporous silica nanoparticle by liquid template crystal mechanism; B) Amine functionalization of MCM-41 by post grafting. [Click here to view] |
In vitro drug release profile
In vitro LVF release was gauged using the dialysis bag method [12,13]. Drug-loaded MSNs were dispersed in 2 ml of phosphate buffer (pH 7.4) and placed into a dialysis bag (MWCO 12 kDa). After sealing both ends, the bag was submerged in pH 7.4 phosphate buffer and agitated in a shaking incubator at 100 rpm and 37°C. At predefined intervals, samples were collected and replenished with fresh media. The LVF content was determined spectrophotometrically at 288 nm.
In vitro antimicrobial efficacy study
Inoculum preparation for the broth dilution test
The antimicrobial efficacy was evaluated using the reference strain E. coli (ATCC 25922). For pure culture isolation, the bacteria were cultured on 5% sheep blood agar with incubation at 37°C for 24 hours. Subsequently, 2–3 isolated colonies were introduced into Mueller–Hinton broth and incubated for 4 hours to achieve the logarithmic phase. The broth’s turbidity was adjusted to match the 0.5 McFarland standard.
Minimum inhibitory concentration (MIC)
The MIC was determined using the broth microdilution method and sterile microtiter plates. Fresh stock solutions of antimicrobial agents, including LVF, MSN LVF, and LVF-GLY-MSN, were prepared with a final concentration of 5,120 µg/ml. These solutions underwent a two-fold dilution. 50 µl of the bacterial inoculum (adjusted to 0.5 McFarland standard) was added to each well of the microtiter plates. The plates were incubated at 37°C for 16–20 hours. A growth control well was included for each antimicrobial agent, containing the broth and bacterial inoculum without the antibiotic. The MIC endpoint was identified as the lowest concentration, preventing visible bacterial growth.
Minimum bactericidal concentration (MBC)
Post-MIC determination, broth from the microtiter plate wells with no visible microbial growth was subcultured onto sterile Mueller–Hinton agar plates. These plates were incubated at 37°C for 24 hours. The MBC was defined as the minimum concentration required to kill 99.9% of the bacteria after the 24-hour incubation [25–27].
RESULTS AND DISCUSSION
Characterization of MSNs
FTIR analysis of MSNs displayed distinct peaks of Si-O-Si stretching at 1,085.92 cm−1, Si-OH stretching and bending at 3,450.65 cm−1 and 1,629.85 cm−1, respectively. Evidence of amine functionalization on MSNs was evident from the N-H stretching at 3,113.11 cm−1. The aminopropyl chain is attached to the MSNs by aliphatic C-H stretching between 2,922.16 and 2,858.51 cm−1, N-H bending at 1,678.07 cm−1, and C-N stretching at 1,089.78 cm−1. GLY-MSN showed the presence of two distinct peaks at 1,651.07 cm−1 and 1,548.84 cm−1 corresponding respectively to C = O str (amide-I) and N-H bending vibration (amide-II) of amide. The presence of O-H and secondary amine was ascertained from the bands between 3,506.59 and 3,109.25 cm1, and C-H of alkane at 2,953.02 cm−1. The FTIR spectra are presented in Fig. 2. The FTIR spectrum of LVF showed characteristic peaks at 3,269.34 cm−1 (OH), 2,931.80 cm−1 (C–H), 1,722.43 cm−1 (C = O), 1,300.02 cm−1 (C–N), and 1,083.99 cm−1 (C-F) [28], which are presented in Fig. 2. Notably, MSNs loaded with LVF did not showcase characteristic peaks of LVF, suggesting the complete adsorption of LVF within the MSN pores. These observations align with previous studies [29]. The DSC thermograms for LVF and LVF-MSN are shown in Fig. 3. DSC thermograms of LVF displayed a pronounced endotherm at 234.70°C, signifying its crystalline character. Upon its incorporation into MSNs, a transformation to the amorphous form was observed, as indicated by the lack of peaks near the melting point of LVF. FESEM imagery, illustrated in Fig. 4, portrayed plain MSNs as spherical, while GLY-MSN depicted a rougher surface, likely due to glucuronic acid attachment. The particle size and zeta potential of the MSNs, MSN-NH2, and GLY-MSN were ascertained via a Malvern zeta sizer (Malvern Instruments, Malvern, UK). GLY-MSN demonstrated an enlarged size relative to plain MSNs (Table 1). The positive zeta potential in amine-functionalized MSN is likely attributed to protonated amine groups. Metrics from nitrogen adsorption highlighted an average pore size of 3.81 nm, a pore volume of 0.678 cm3/g, and a BET surface area of 993.8811 m²/g [30].
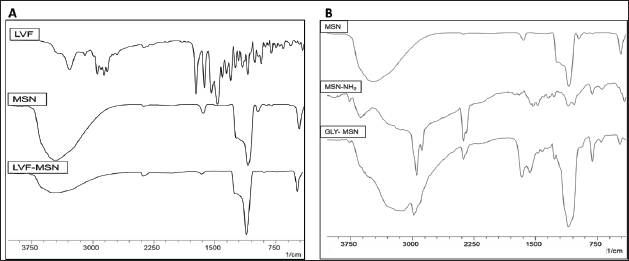 | Figure 2. FTIR spectra for A) Levofloxacin (LVF), synthesized Mesoporous silica nanoparticles (MSNs) and LVF loaded MSNs; B) Mesoporous silica nanoparticles, Amine-functionalized MSNs (MSN-NH2), and Glycosylated MSNs. [Click here to view] |
 | Figure 3. DSC thermograms for (a) Pure levofloxacin, (b) levofloxacin-loaded mesoporous silica nanoparticles, and (c) mesoporous silica nanoparticles. [Click here to view] |
 | Figure 4. FESEM images of (a) plain mesoporous silica nanoparticles and (b) glycosylated mesoporous silica nanoparticles. [Click here to view] |
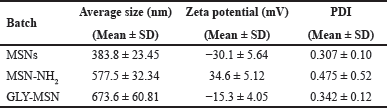 | Table 1. Particle size and zeta potential of MSNs. [Click here to view] |
Drug loading and entrapment efficiency
The amount of LVF entrapped in the MSNs was quantified using an indirect method. Exploring various LVF: MSN ratios revealed an increased carrier proportion relative to LVF, amplifying entrapment efficiency. Interestingly, GLY-MSN and plain MSNs presented comparable loadings. This may result from the sugar acid entities near MSN pores hindering drug molecule penetration. Associated data are tabulated in Table 2.
In vitro drug release study
The release of LVF from MSNs in phosphate buffer saline (pH 7.4) yielded a sustained release from GLY-MSN of 21.02% ± 3.38 % over 48 hours. In contrast, the plain LVF-MSN showed a release of just 7.50% ± 1.31% in the same duration. This enhanced release from GLY-MSN may be due to the presence of LVF on the surface and at the pore openings. Release dynamics are graphically represented in Fig. 5.
 | Table 2. Data for drug loading efficiency. [Click here to view] |
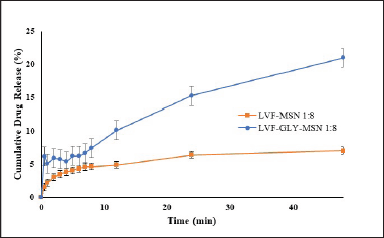 | Figure 5. In vitro release profile of levofloxacin loaded MSN (1:8 of LVF and MSN), and levofloxacin loaded glycosylated MSN (1:8 of LVF and glycosylated MSN) in phosphate buffer saline pH 7.4. [Click here to view] |
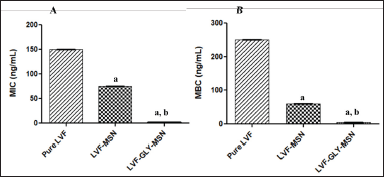 | Figure 6. (A) Minimum inhibitory concentration (MIC) and (B) Minimum bactericidal concentration (MBC) of pure LVF, LVF-loaded MSNs, and LVF-loaded glycosylated MSNs. All values are expressed as mean ± SD, ap < 0.05 in comparison to pure LVF, bp < 0.05 in comparison to LVF-MSN. [Click here to view] |
In vitro antibacterial efficacy study
The LVF-loaded MSNs showed a noticeable decline in MIC and MBC levels compared to pure LVF, indicating enhanced antimicrobial potency when incorporated into MSNs. Remarkably, the glycosylated MSNs (GLY-MSN) containing LVF exhibited even lower MIC and MBC levels, significantly outperforming both the pure LVF and the LVF loaded into MSNs (p < 0.05). This enhanced efficacy is likely due to the enhanced uptake of the GLY MSNs. The presence of the glucuronic acid moiety enhances interactions with lectin receptors, promoting improved adhesion between the bacterial cell and MSNs. The attached sugar units increase their multivalent nature, thus facilitating more effective interactions with bacterial sugar-binding proteins [31,32]. The precise MIC and MBC data can be referenced in Fig. 6.
CONCLUSION
MSNs have emerged as a promising platform for delivering antimicrobial agents, offering a potential solution to bacterial resistance. Their appeal lies in their porous structure, flexibility for surface modifications, and inherent biocompatibility, making them especially conducive to antimicrobial drug delivery.
In our study, the GLY MSNs loaded with LVF showcased markedly lower MIC and MBC values than the pure LVF and the LVF encapsulated in MSNs. This highlights the superior efficacy of the glycosylated formulation against E. coli. This enhanced performance can be attributed to amplified sugar-lectin interactions, which likely boost the bacterial cellular uptake of the drug. Our findings suggest that glycosylated LVF-loaded MSNs could present a viable strategy for addressing drug resistance in UTIs. However, to further validate these promising results, it is essential to conduct studies on multidrug-resistant strains. In addition, adhesion studies are necessary to understand the underlying mechanisms by which glycosylation counteracts antimicrobial resistance.
ACKNOWLEDGMENTS
The authors would like to express their sincere gratitude to the Manipal Academy of Higher Education, Manipal, for providing facilities for the literature search. The authors are also thankful to the Indian Council of Medical Research (ICMR), New Delhi, Ref. No 35/13/2020-/Nano/BMS and Ref. No. AMR/Fellowship/25/2022-ECD-11 for the fund support.
AUTHOR CONTRIBUTIONS
Songhita Mukhopadhyay—Data Acquisition, Data Analysis/Interpretation, Drafting Manuscript, Critical Revision of Manuscript
Reema Narayan—Data Analysis/Interpretation, Critical Revision of Manuscript, Statistical Analysis
Shivaprasad Gadag—Data Analysis/Interpretation, Critical Revision of Manuscript
Padmaja A Shenoy—Data Analysis/Interpretation, Critical Revision of Manuscript
Sanjay Garg- Data Analysis/Interpretation, Critical Revision of Manuscript
Ashwini T—Data Analysis/Interpretation, Funding
Usha Y Nayak—Concept and Design, Data Analysis/Interpretation, Critical Revision of Manuscript, Funding, Statistical Analysis, Admin, Technical or Material Support, Supervision, Final Approval
CONFLICTS OF INTEREST
All authors declare that they have no conflict of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Vestby LK, Grønseth T, Simm R, Nesse LL. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics. 2020;9:59. CrossRef
2. Reygaert WC. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018;4:482–501. CrossRef
3. Aslam B, Wang W, Arshad MI, Khurshid M, Muzammil S, Rasool MH, et al. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist. 2018;11:1645–58. CrossRef
4. Gao P, Nie X, Zou M, Shi Y, Cheng G. Recent advances in materials for extended-release antibiotic delivery system. J Antibiot. 2011;64:625–34. CrossRef
5. Forier K, Raemdonck K, De Smedt SC, Demeester J, Coenye T, Braeckmans K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J Control Release. 2014;190:607–23. CrossRef
6. Narayan R, Nayak U, Raichur A, Garg S. Mesoporous silica nanoparticles: a comprehensive review on synthesis and recent advances. Pharmaceutics. 2018;10:118. CrossRef
7. ?en Karaman D, Manner S, Rosenholm JM. Mesoporous silica nanoparticles as diagnostic and therapeutic tools: how can they combat bacterial infection? Ther Deliv. 2018;9:241–4. CrossRef
8. Selvarajan V, Obuobi S, Ee PLR. Silica nanoparticles-a versatile tool for the treatment of bacterial infections. Front Chem. 2020;8:602. CrossRef
9. Sharon N. Carbohydrates as future anti-adhesion drugs for infectious diseases. Biochim Biophys Acta. 2006;1760:527–37. CrossRef
10. Cecioni S, Imberty A, Vidal S. Glycomimetics versus multivalent glycoconjugates for the design of high affinity lectin ligands. Chem Rev. 2015;115:525–61. CrossRef
11. Imberty A, Chabre YM, Roy R. Glycomimetics and glycodendrimers as high affinity microbial anti-adhesins. Chemistry. 2008;14:7490–9. CrossRef
12. Zambito Y, Pedreschi E, Di Colo G. Is dialysis a reliable method for studying drug release from nanoparticulate systems?—A case study. Int J Pharm. 2012;434:28–34. CrossRef
13. Weng J, Tong HHY, Chow SF. In vitro release study of the polymeric drug nanoparticles: development and validation of a novel method. Pharmaceutics. 2020;12:1–18. CrossRef
14. Pieters RJ. Maximising multivalency effects in protein-carbohydrate interactions. Org Biomol Chem. 2009;7:2013–25. CrossRef
15. Fasting C, Schalley CA, Weber M, Seitz O, Hecht S, Koksch B, et al. Multivalency as a chemical organization and action principle. Angew Chem Int Ed. 2012;51:10472–98. CrossRef
16. Niemelä E, Desai D, Nkizinkiko Y, Eriksson JE, Rosenholm JM. Sugar-decorated mesoporous silica nanoparticles as delivery vehicles for the poorly soluble drug celastrol enables targeted induction of apoptosis in cancer cells. Eur J Pharm Biopharm. 2015;96:11–21. CrossRef
17. Rafat C, Debrix I, Hertig A. Levofloxacin for the treatment of pyelonephritis. Expert Opin Pharmacother. 2013;14:1241–53. CrossRef
18. Bouchillon SK, Badal RE, Hoban DJ, Hawser SP. Antimicrobial susceptibility of inpatient urinary tract isolates of gram-negative bacilli in the United States: results from the study for monitoring antimicrobial resistance trends (SMART) program: 2009−2011. Clin Ther. 2013;35:872–7. CrossRef
19. Hooper DC. Mechanisms of action and resistance of older and newer fluoroquinolones. Clin Infect Dis. 2000;31:S24–8. CrossRef
20. Tran JH, Jacoby GA. Mechanism of plasmid-mediated quinolone resistance. Proc Natl Acad Sci U S A. 2002 Apr 16;99(8):5638–42. doi: 10.1073/pnas.082092899 CrossRef
21. Pedraza D, Díez J, Barba II, Colilla M, Vallet-Regi M. Amine-functionalized mesoporous silica nanoparticles: a new nanoantibiotic for bone infection treatment. Biomed Glas. 2018;4:1–12. CrossRef
22. Zhang Y, Zhi Z, Jiang T, Zhang J, Wang Z, Wang S. Spherical mesoporous silica nanoparticles for loading and release of the poorly water-soluble drug telmisartan. J Control Release. 2010;145:257–63. CrossRef
23. Narayan R, Gadag S, Cheruku SP, Raichur AM, Day CM, Garg S, et al. Chitosan-glucuronic acid conjugate coated mesoporous silica nanoparticles: a smart pH-responsive and receptor-targeted system for colorectal cancer therapy. Carbohydr Polym. 2021;261:117893. CrossRef
24. Cheng K, Blumen SR, MacPherson MB, Steinbacher JL, Mossman BT, Landry CC. Enhanced uptake of porous silica microparticles by bifunctional surface modification with a targeting antibody and a biocompatible polymer. ACS Appl Mat Interfaces. 2010;2:2489–95. CrossRef
25. Determination of minimum inhibitory concentrations (MICs) of antibacrial agents by broth dilution. Clin Microbiol Infect. 2003;9:ix–xv. CrossRef
26. Minimum Inhibitory (MIC) and Minimum Bactericidal Concentration (MBC) Evaluations as R&D Tools [Internet]. Welcome to Q Laboratories. 2017 [cited 2022 Apr 9]. Available from: https://www.qlaboratories.com/minimum-inhibitory-mic-and-minimum-bactericidal-concentration-mbc-evaluations-as-rd-tools/
27. Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001 Jul;48 Suppl 1:5–16. doi: 10.1093/jac/48.suppl_1.5 CrossRef
28. Baby B, Harsha NS, Jayaveera KN, Abraham A. Formulation and evaluation of levofloxacin nanoparticles by ionic gelation method. Res rev?: J Pharm Pharm Sci. 2012;1:7–15.
29. Shah PV, Rajput SJ. Facile synthesis of chitosan capped mesoporous silica nanoparticles: a pH responsive smart delivery platform for raloxifene hydrochloride. AAPS PharmSciTech. 2018;19:1344–57. CrossRef
30. Narayan R, Gadag S, Mudakavi RJ, Garg S, Raichur AM, Nayak Y, et al. Mesoporous silica nanoparticles capped with chitosan-glucuronic acid conjugate for pH-responsive targeted delivery of 5-fluorouracil. J Drug Deliv Sci Technol. 2021;63:102472. CrossRef
31. Ramström O, Yan M. Glyconanomaterials for combating bacterial infections. Chemistry— Eur J. 2015;21:16310–7. CrossRef
32. Sattin S, Bernardi A. Glycoconjugates and glycomimetics as microbial anti-adhesives. Trends Biotechnol. 2016;34:483–95. CrossRef