INTRODUCTION
Cognition and diabetes mellitus are complex and multifaceted conditions in the current scenario that have been subject to extensive research. The prevalence of diabetes mellitus is escalating every year. As per the report of the Indian Council of Medical Research published in 2023, the prevalence of diabetes mellitus is 10.1 crore. It is predicted that by 2045, the prevalence of T2DM in India will increase to 134 million [1]. It devastates many organs of the human body. Among the diverse complications of diabetes, an underdiagnosed and less addressed complication is cognitive impairment. Various studies conducted in animal models and humans have suggested that there is a strong connection between diabetes and dementia. Learning and memory skills seem to be controlled by insulin and glucose. The abnormal glucose metabolism and impaired insulin function noticed in diabetic patients are intensely connected with cognitive dysfunction. A constant rise or drop in the blood sugar level leads to brain damage and cognitive decline. Throughout the world, over 55 million people were living with dementia in 2020, and it doubles every 20 years; by 2050, it will reach 139 million [2,3]. There are more women with T2DM compared to men. The association between women and T2DM is stronger, especially in overweight, physically inactive women, and women undergoing menopausal transition [4]. The connection between menopause and diabetes remains a query and is often reported to be the most common chronic disease among women. The notable decrease in the production of endogenous estradiol after menopause results in a relative increment in androgenic effects and body composition changes, which are believed to influence pancreatic β-cell functioning, hepatic glucose output, and glucose transport [5,6]. The induction of glycolytic enzymes and glycogen synthetase by estrogen has been thought to regulate the capacity of pancreatic islet cells to produce insulin [7]. The estrogen signaling pathway has been demonstrated to be important in regulating glucose homeostasis. Lack of estrogen receptor-α causes both insulin insensitivity and glucose intolerance in mice, rather than estrogen receptor-β (Erβ) [8].
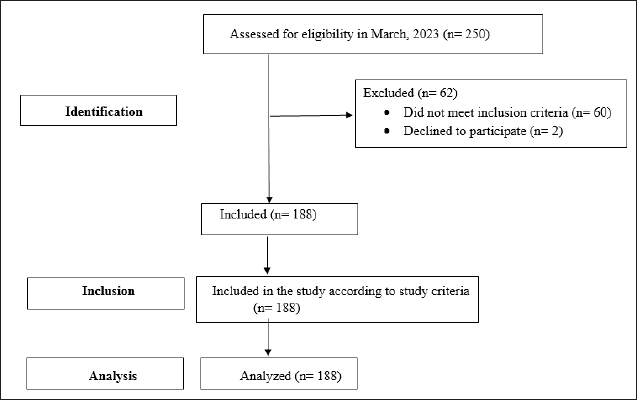 | Figure 1. STROBE flow chart. [Click here to view] |
The occurrence of earlier menopause is found to predispose a woman to develop diabetes, cardiovascular diseases, and osteoporosis. Contrary to the above statement, a delay in menopause elevates the likelihood of developing endometrial and breast cancer [9]. Generally, the incidence of diabetes and glucose intolerance increases after 40 years of age. Women aged 50 years exhibited a lower prevalence of diabetes than women in their 60s and 70s, who had a greater risk of developing the condition [10]. A study conducted in female Japanese individuals without diabetes revealed that hyperglycemia developed independently in postmenopausal women with a significant association, irrespective of demographic, age, and metabolic factors. Menopause and advanced age might have a linear influence on the increased likelihood of dysglycemia in Japanese women [11].
T2DM in elderly women increases the risk of brain atrophy that causes problems with memory, and thinking that eventually lead to vascular dementia. Females diagnosed with T2DM have a higher likelihood of experiencing rapid cognitive decline than males. The duration of diabetes has a significant effect on the severity of cognitive impairment [12,13]. Cognitive impairment is influenced by both T2DM and the reduction in estrogen levels during perimenopause. Regular physical activity and estrogen replacement therapy seem to possess beneficial hypoglycaemic effects by enhancing the ability of β-cells to secrete insulin [14]. There is a vital demand for propitious health resources centered on dementia related to T2DM. Furthermore, a recent study carried out in Mysuru entrenched that there is a higher prevalence of cognitive impairment among T2DM patients but the association was not concluded due to the inaccessibility of HbA1c data [15]. On this burden, this study has been designed to explore a better understanding of the significant association between cognitive performance and T2DM by measuring HbA1c, especially among post-menopausal women in the community setting.
MATERIALS AND METHODS
Study design
The present cross-sectional study was carried out in the community setting in and around Anaicut, Vellore District, for a period of 6 months, from July 2023 to December 2023. The study protocol was approved by the Institutional Ethics Committee of the PM Medical Centre, Vellore, India (Approval No.: EC/2023/01), and the research was carried out in compliance with the Helsinki Declaration. Written informed consent was acquired from the study subjects before they were included in the study. The STROBE guideline has been followed for this study design, and the flow chart is shown in Figure 1.
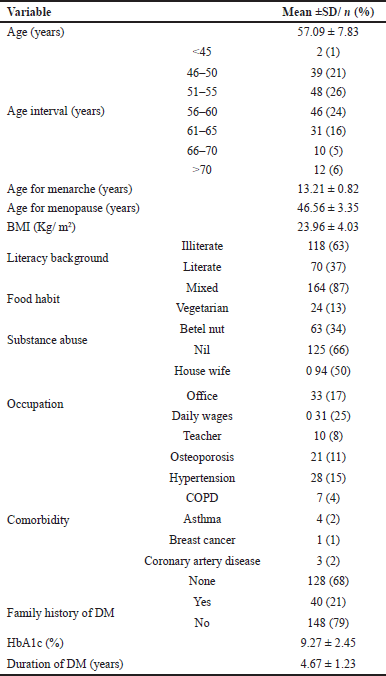 | Table 1. Baseline characteristics. [Click here to view] |
Study population
This study included post-menopausal women with T2DM above 35 years of age and those who could read and write English or Tamil languages. This study excluded post-menopausal women without T2DM, those who are physically incapable of reading, hearing, or comprehending written or explained instructions properly, or who may have a motor impairment that impairs their writing and drawing skills, and those who are not willing to participate.
Sample size calculation
The sample size was estimated using the n = Z2P (1−P)/d2 formula, resulting in a sample size of 188. Based on a pilot study, the standard normal variate (Z) at 5% is 1.96, the estimated population proportion (P) is 0.143, and the absolute error (d) is 5%.
 | Table 2. Cognitive function using MoCA. [Click here to view] |
Montreal cognitive assessment (MoCA) scale
A standard and validated MoCA scale has been adapted in English and Tamil languages to assess the cognitive function of the study subjects. The scale was used after obtaining proper permission. It is a highly sensitive tool for discerning mild cognitive impairment (MCI). MoCA examines language, memory, visual thinking, orientation, and reasoning skills. The highest achievable score on the MoCA assessment is 30. A score of 26–30 signifies the absence of cognitive impairment, while a score between 18 and 25 indicates MCI. A score ranging from 10 to 17 suggests moderate cognitive impairment, whereas a score below 10 indicates severe cognitive impairment [16].
Estimation of HbA1c
A blood sample of 2 ml was obtained from the study subjects by venous puncture and transferred into a vacutainer. After that, the aliquots were transferred into Eppendorf tubes and preserved at a temperature of −20°C until further analysis. The quantification of HbA1c was performed by the high-performance liquid chromatography method.
Statistical analysis
Data analysis was done using IBM SPSS version 23. Descriptive statistics were reported as mean (SD) for continuous variables and frequency (percentage) for categorical variables. Pearson’s correlation coefficients were employed to evaluate the association between the MoCA score and the duration of T2DM and HbA1c. A statistical significance was indicated by a p-value of less than 0.05.
RESULTS
Socio-demographic and clinical details
After screening, 188 subjects were included in this study. A significant proportion of the study subjects were in the age bracket of 51–55 years, accounting for 26% of the population. Subsequently, the age range of 56–60 years constituted 24% of the study population. The most common comorbidities observed in this study subjects were hypertension (28%) and osteoporosis (21%). Only 1% of the subjects had breast cancer as shown in Table 1.
Assessment of cognitive function
The cognitive function of post-menopausal women was assessed using the MoCA scale as shown in Table 2. The majority of the women were with MCI (67%) followed by normal cognitive function (25%). The mean and SD of the MoCA score and duration of T2DM were 20.91 ± 3.99 and 4.67 ± 1.23. The performance score in each cognitive subdomain and the total MoCA score are outlined in Table 3.
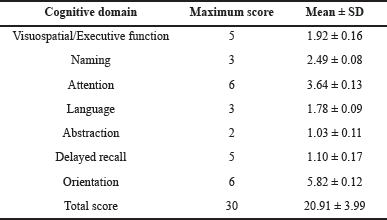 | Table 3. Mean of subdomain and total MoCA score. [Click here to view] |
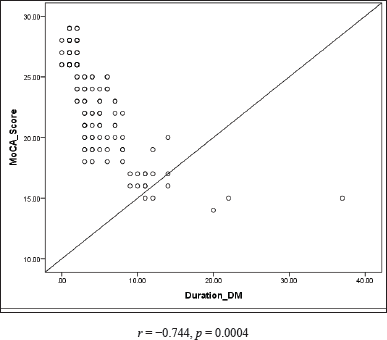 | Figure 2. Correlation of MoCA score with duration of T2DM. [Click here to view] |
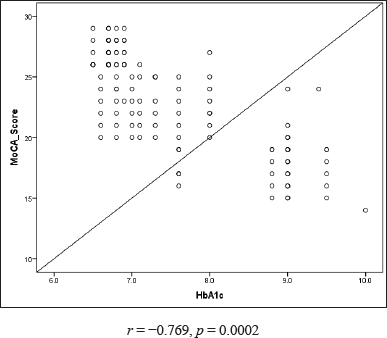 | Figure 3. Correlation of MoCA score with HbA1c. [Click here to view] |
Correlation of MoCA with duration of DM and HbA1c
An association between the MoCA score and duration of T2DM as well as between the MoCA score and HbA1c was established to correlate the influence of hyperglycemia on cognitive impairment as depicted in Figures 2 and 3.
DISCUSSION
The current study was designed to discern the connection between cognitive function and T2DM in post-menopausal women. Women with T2DM have a higher chance of developing diabetes-associated complications than males. The majority of the study population lies within an age range of 51–55 years (26%), and the average age was 57 years. This study depicted the average age for menarche and menopause to be 13 years and 46 years, respectively. With advancing age, cognitive function tends to decline naturally. This decline is worsened and accelerated in individuals with diabetes when compared to those without diabetes. In a study performed by Bjelland et al. [17] the average age for menarche was 13 years, whereas menopause was in the range of 49–54 years. The average age of menopause seems to vary depending on geographic and racial differences.
The mean BMI of subjects was found to be 23.96, which falls under the normal BMI category. Some studies have shown that women with higher BMI are related to impaired glucose tolerance independent of menopause, and changes in adiposity in postmenopausal women with higher BMI predispose to elevated fasting blood glucose levels [18]. Women with early menopausal age have a higher likelihood of being affected by hypertension, chronic obstructive pulmonary disease, osteoporosis, asthma, peptic ulcer, and breast cancer which abides with our study as shown in Table 1. The common comorbidities noticed in our study were hypertension and osteoporosis [19,20].
Cognitive function was assessed using the MoCA scale, which is a comprehensively accepted tool with high sensitivity and specificity. In addition, we measured the level of HbA1c to evaluate its association with cognitive function. The current study population evinced a mean MoCA score of 20.91 ± 3.99 and a mean HbA1c level of 9.27 ± 2.45. The range of cognitive impairment can vary from mild to severe dementia. Early recognition of cognitive decline could improve the management of the condition. In this study, 67% of women had MCI, and 8% had moderate cognitive impairment. Only 25% of the women had normal cognitive function as shown in Table 2. These outcomes are in line with the previous study performed by Lalithambika et al. [21]. The performance score of the diabetic population in each cognitive subdomain was assessed. The diminished score was seen in domains including delayed recall, attention, and visuospatial/executive function. However, modest scores in the domains including naming, language, abstraction, and orientation were observed as exhibited in Table 3. These results are congruent with the earlier investigation conducted by Kinattingal et al. [15].
The complex nature of diabetes, specifically oxidative stress, vascular changes, and chronic inflammation, makes hyperglycemia a vulnerable component for developing Alzheimer’s disease and vascular dementia [22]. A noticeable inverse relationship was found between the MoCA score and duration of T2DM (r = −0.744), the MoCA score and HbA1c (r = −0.769), suggesting a strong link between cognitive dysfunction and hyperglycemia, as shown in Figures 2 and 3. High blood sugar changes cerebral structure and function. The occurrence of cognitive impairment and dementia is 1.5 times greater in individuals with hyperglycemia when compared to healthy individuals. The longer duration of DM has also been shown to be one of the risk factors for cognitive dysfunction [23]. Besides that, estrogen exerts neurocognitive effects through its action on brain regions such as the hippocampus and prefrontal cortex. Estrogen counteracts the negative influence of cortisol on cognitive function. A noticeable surge in follicle-stimulating hormone and a simultaneous decline in estradiol are noticed in the menopausal transition [24]. Hence, a fall in estrogen levels in addition to T2DM affects the cognitive function of post-menopausal women. However, estrogen level was not measured in our study. Hence, further research is imperative to establish a correlation between cognitive function and T2DM among post-menopausal women by considering the estrogen level and other reproductive factors along with the HbA1c level which may act as independent factors in developing cognitive dysfunction.
Our study highlights that the escalation of HbA1c diminishes the cognition of hyperglycemic patients. This would be the first community-based study in South India to enlighten the association between cognition and T2DM in post-menopausal women based on real-world data. The limitations of the study were a single-center study and a smaller sample size.
CONCLUSION
Our study emphasizes the significance of a strong correlation between cognitive function and T2DM. Three-fourths of the present study population had mildly declined cognitive function. This study will be the first step to shed light on the impact of diabetes-associated cognitive dysfunction among post-menopausal women in South India. On the basis of this obtained evidence, this study drags attention to healthcare professionals in demonstrating effective prevention strategies to mandate the importance of screening cognitive performance routinely and early. This study also opens new avenues of future research to probe into the depth of knowledge on cognition and diabetes among post-menopausal women.
ACKNOWLEDGEMENT
The authors would like to extend their gratitude to the management of SRM College of Pharmacy, SRMIST for their encouragement, support, guidance, and provision of research facilities.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by the Institutional Ethics Committee of the PM Medical Centre, Vellore, India (Approval No.: EC/2023/01), and the research was carried out in compliance with the Helsinki Declaration. Written informed consent was acquired from the study subjects before they were included in the study. The STROBE guideline has been followed for this study.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Pradeepa R, Mohan V. Epidemiology of type 2 diabetes in India. Indian J Ophthalmol. 2021;69:2932–8. CrossRef
2. Cholerton B, Baker LD, Montine TJ, Craft S. Type 2 diabetes, cognition, and dementia in older adults: toward a precision health approach. Diabetes Spectr. 2016;29:210–9. CrossRef
3. Shin JH. Dementia epidemiology fact sheet 2022. Ann Rehabil Med. 2022;46:53–9. CrossRef
4. Ciarambino T, Crispino P, Leto G, Mastrolorenzo E, Para O, Giordano M. Influence of gender in diabetes mellitus and its complication. Int J Mol Sci. 2022;23:8850. CrossRef
5. Wang M, Gan W, Kartsonaki C, Guo Y, Lv J, Chen Z, et al. Menopausal status, age at natural menopause and risk of diabetes in China: a 10-year prospective study of 300,000 women. Nutr Metab (Lnd). 2022;19:7. CrossRef
6. Mauvais-Jarvis F, Manson JE, Stevenson JC, Fonseca VA. Menopausal hormone therapy and type 2 diabetes prevention: evidence, mechanisms, and clinical implications. Endocr Rev. 2017;38:173–88. CrossRef
7. Vogel H, Mirhashemi F, Liehl B, Taugner F, Kluth O, Kluge R et al. Estrogen deficiency aggravates insulin resistance and induces β-cell loss and diabetes in female New Zealand obese mice. Horm Metab Res 2013;45:430–5. CrossRef
8. Matic M, Bryzgalova G, Gao H, Antonson P, Humire P, Omoto Y et al. Estrogen signalling and the metabolic syndrome: targeting the hepatic estrogen receptor alpha action. PLoS One 2013;8:e57458. CrossRef
9. Surakasula A, Nagarjunapu GC, Raghavaiah KV. A comparative study of pre- and post-menopausal breast cancer: risk factors, presentation, characteristics and management. J Res Pharm Pract. 2014;3:12–8. CrossRef
10. Valadares AL, Machado VS, Costa-Paiva LS, de Sousa MH, Pinto-Neto AM. Factors associated with the age of the onset of diabetes in women aged 50?years or more: a population-based study. BMJ Open. 2014;4:e004838. CrossRef
11. Heianza Y, Arase Y, Kodama S, Hsieh SD, Tsuji H, Saito K et al. Effect of postmenopausal status and age at menopause on type 2 diabetes and prediabetes in Japanese individuals: Toranomon Hospital Health Management Center Study 17 (TOPICS 17). Diabetes Care. 2013;36:4007–14. CrossRef
12. Zilliox LA, Chadrasekaran K, Kwan JY, Russell JW. Diabetes and cognitive impairment. Curr Diab Rep. 2016 Sep;16(9):87. CrossRef
13. Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol. 2018;14:591–604. CrossRef
14. Sochocka M, Karska J, Pszczolowska M, Ochnik M, Fulek M, Fulek K, et al. Cognitive decline in early and premature menopause. Int J Mol Sci. 2023;24:6566. CrossRef
15. Kinattingal N, Mehdi S, Undela K, Wani SUD, Almuqbil M, Alshehri S, et al. Prevalence of cognitive decline in type 2 diabetes mellitus patients: a real-world cross-sectional study in Mysuru, India. J Pers Med. 2023;13:524. CrossRef
16. Yeung PY, Wong LLL, Chan CC, Yung CY, Leung LMJ, Tam YY et al. Montreal cognitive assessment—single cutoff achieves screening purpose. Neuropsychiatr Dis Treat. 2020;16:2681–7. CrossRef
17. Bjelland EK, Hofvind S, Byberg L, Eskild A. The relation of age at menarche with age at natural menopause: a population study of 336 788 women in Norway. Hum Reprod. 2018;33:1149–57. CrossRef
18. Cho GJ, Lee JH, Park HT, Shin JH, Hong SC, Kim T et al. Postmenopausal status according to years since menopause as an independent risk factor for the metabolic syndrome. Menopause. 2008;15:524–9. CrossRef
19. Dalal PK, Agarwal M. Postmenopausal syndrome. Indian J Psychiatry. 2015;57:S222–32. CrossRef
20. Chai H, Ge J, Li L, Li J, Ye Y. Hypertension is associated with osteoporosis: a case-control study in Chinese postmenopausal women. BMC Musculoskelet Disord. 2021;22:253
21. Lalithambika CV, Arun CS, Saraswathy LA, Bhaskaran R. Cognitive impairment and its association with glycemic control in type 2 diabetes mellitus patients. Indian J Endocrinol Metab. 2019;23:353–6. CrossRef
22. Agrawal M, Agrawal AK. Pathophysiological association between diabetes mellitus and alzheimer’s disease. Cureus. 2022;14:e29120. CrossRef
23. Jin CY, Yu SW, Yin JT, Yuan XY, Wang XG. Corresponding risk factors between cognitive impairment and type 1 diabetes mellitus: a narrative review. Heliyon. 2022 Aug 3;8(8):e10073. CrossRef
24. Bortz J, Klatt KC, Wallace TC. Perspective: estrogen and the risk of cognitive decline: a missing choline(rgic) link? Adv Nutr. 2022;13:376–87. CrossRef