INTRODUCTION
Riceberry (RB) (Oryza sativa L.) is a popular colored rice variety in Thailand, known for its dark-purple appearance. It is a hybrid resulting from the crossbreeding of two Thai strains, namely Khao Dawk Mali 105 (Thai jasmine rice) and Hom-Nil (Thai non-glutinous purple rice). Since its introduction in 2002, RB has gained significant popularity and is widely consumed in Thailand [1]. The aleurone layer of dark purple rice has a significantly higher concentration of anthocyanin (a subclass of flavonoids) compared to unpigmented rice. This chemical demonstrates potent antioxidant activity [2,3]. Several studies have identified an abundance of compounds in RB with beneficial effects on human health, including aminobutyric acid (GABA), phenolic acids, flavonoids, phytosterols, and antioxidant compounds [4,5]. It has been reported to exhibit a number of potential health benefits, such as hypoglycemic, antioxidant, anti-hyperlipidemic, anti-inflammatory, and anti-cancer properties [6,7]. Moreover, previous research has shown that RB has the potential to effectively reduce cognitive impairment and hippocampal neurodegeneration in a rat model of Alzheimer’s disease (AD) [8].
Cerebral ischemia, which involves reduced blood flow to the brain or cerebral hypoperfusion, is considered a primary cause of vascular cognitive impairment [9] and is also seen as a pathogenic process in AD [10]. Cerebral ischemia-reperfusion (IR), a condition where the brain experiences a temporary lack of blood flow followed by its restoration, initiates a series of harmful processes. During ischemia, the brain cells release excess glutamate, an excitatory neurotransmitter, leading to excitotoxicity—a phenomenon where nerve cells suffer damage due to excessive stimulation [11–13]. Upon reperfusion, the reintroduction of oxygen results in the generation of reactive oxygen species (ROS), causing oxidative stress and cellular harm [12,14,13]. These events interplay and contribute to cognitive impairment.
GABA acts as the principal inhibitory neurotransmitter in the mammalian brain, exerting an important impact on the regulation of the excitatory-inhibitory balance. A study of the mechanism by which GABA is sensitive to cerebral IR was also described, along with information on how GABAergic drugs could prevent neuronal death [15]. Despite some evidence, the provided evidence that RB contains various bioactive compounds that contribute to its antioxidant properties [4,5] and that the germination of RB increases its phytochemical composition, particularly increasing GABA levels [16], the specific effect of both RB and germinated riceberry (GRB) on vascular cognitive impairment has not been thoroughly investigated. Therefore, the objective of this study is to investigate the neuroprotective effects of RB and GRB extract, with a specific focus on their effects on oxidative stress and cognitive function in the situation of cerebral IR injury. The effects of these rice cultivars provide insight into their potential therapeutic applications for reducing the cognitive impairment of cerebral IR injury.
MATERIALS AND METHODS
Preparation of GRB
The RB used in this study was collected from Phayao province, Thailand. The germination process was modified according to what was reported by Komatsuzaki et al. [17] The paddy RB (100 g) was rinsed with tap water once and soaked in deionized water. Soaked RB was allowed to germinate for 18 hours in the dark at 35°C. After germination, GRB was dried and kept at −20°C. For a comparative study, the ungerminated RB was also prepared and used as the control.
Preparation of crude extracts
The grinding germinated and ungerminated RB was extracted with an ethanol–water mixture (v/v) (70:30). All extractions were continuously shaken at room temperature for 12 hours. Subsequently, the ethanol extract was filtered with a Whatman No. 1 filter paper. The filtrates were concentrated using a vacuum rotary evaporator (Marshall Scientific, Hampton, VA, USA) and freeze-dried using a lyophilizer (Thermo Fisher Scientific, Waltham, MA, USA). The crude extract was stored at −20°C until use.
Determination of phytochemical content and antioxidant activity
GABA content was detected by gas chromatography-mass spectrometry (GC: Agilent model 6890, Germany, and MS: Agilent model 5973, USA) with a Phenomenex Zebron ZB-AAA 10 m × 0.25 mm 0.25 film thickness. The method was applied based on previous reports [18,19]. The GABA content was expressed in mg/kg of extract. Total phenolic content (TPC) was analyzed by the Folin–Ciocalteu assay, as described previously [20]. TPC was expressed as mg of gallic acid equivalent (GAE)/g of extract. Total flavonoid content (TFC) was estimated using the aluminum chloride colorimetric method according to Khanaree et al. [20] with some modifications. The result was demonstrated in terms of mg catechin equivalent (CE)/g of extract. Total anthocyanin content (TAC) was quantified using the pH difference method of Tantipaiboonwong et al. [21]. TAC value was expressed as mg cyanidin-3-glucoside equivalents per/g of extract. The free radical scavenging activity of the extracts was carried out using the 2,2′-Azino-bis (3-Ethylbenzothiazoline-6-Sulfonic Acid) (ABTS) and Diphenyl-1-Picrylhydrazyl (DPPH) assays as described by Punfa et al [22] with some modifications. The results of the antioxidant activity were expressed as the 50% of inhibition (IC50) value.
Animal
The Ethics Committee of the Laboratory Animal Research Center, University of Phayao, Thailand, granted approval (approval No. 61 01 04 027) for the experimental procedures with the aim of reducing animal suffering. Male ICR mice, aged 4–5 weeks and weighing 25–30 g, were procured from Nomura Siam International Co., Ltd., located in Bangkok, Thailand. Before beginning the experiments, the mice were accommodated for a duration of 1 week under regulated conditions. The environmental conditions were maintained at a consistent temperature of 25 ± 2°C, a relative humidity of 60% ± 10%, and a daily light exposure of 12 hours (from 6:00 to 18:00). The animals were provided free access to the standard diet, specifically Mouse Feed Food No. 082, manufactured by C.P. Company in Bangkok, Thailand.
Treatment and experimental schedule
After a 7-day acclimation period, the mice were randomly divided into five groups (N = 8 per group), including the sham-operated group (Sham), the transient bilateral common carotid artery occlusion (tBCCAO) group (cerebral IR), the tBCCAO+RB500 group receiving RB 500 mg/kg BW, the tBCCAO+GRB250 group receiving GRB 250 mg/kg BW, and the tBCCAO+ GRB500 group receiving GRB 500 mg/kg BW. The animals were orally administered either vehicle (0.1% carboxymethyl cellulose), RB, or GRB extract once daily for 28 days. The cerebral IR process induced by tBCCAO was performed on day 21. The Morris water maze (MWM) training, probe trial, and Novel object recognition (NOR) were used to test the memory and learning abilities of mice in each group from days 22–26, on days 27 and 28, respectively, before being sacrificed. A diagram of an animal experimental design is shown in Figure 1.
Surgical procedure
The experimental procedure for inducing cerebral IR in mice was conducted in accordance with the methodology previously described by Kangwan et al. [23], though with slight modifications. In brief, the mice were subjected to anesthesia via intraperitoneal injection of zoletil at a dose of 40 mg/kg BW. A ventral cervical incision of the neck skin of mice was carried out under deep anesthesia. The right and left common carotid arteries were carefully isolated from surrounding veins and vagus nerves and subsequently subjected to a 20-minute ligation procedure using surgical silk. Sham-operated animals were subjected to the same surgical procedure, except that the arteries were not ligated. The surgical procedures were conducted in a sterile environment. It is standard practice to ensure that animals are fully recovered from anesthesia before their return to their cages. The maintenance of the mouse’s body temperature during the surgical procedure and postoperative phase was achieved through the utilization of a heating lamp.
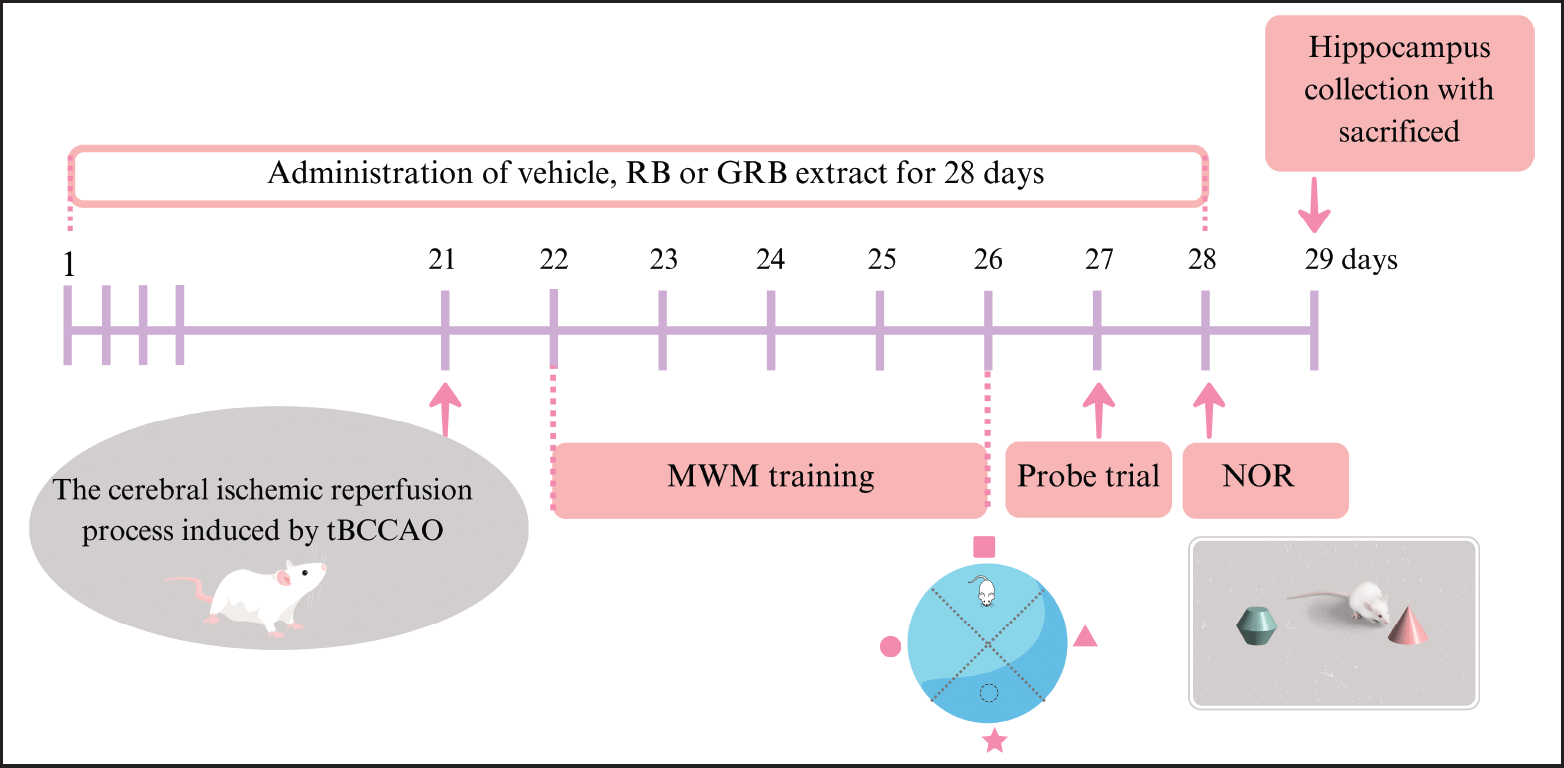 | Figure 1. Schematic representation of an animal experimental design; RB = riceberry; GRB = germinated riceberry; tBCCAO = transient bilateral common carotid artery occlusion; MWM = Morris water maze; NOR = novel object recognition. [Click here to view] |
Assessment of cognitive function
Morris water maze (MWM) test
The MWM test was used to evaluate the spatial learning and memory of all animals 24 hours. after surgery. The procedure was modified slightly from the previous study [23]. The maze consisted of a 23-cm-deep, 70-cm-diameter black circular pool filled with 25–27°C water. The pool was divided conceptually into four equal-sized quadrants. Bright visual signals of various shapes (star, triangle, circle, and square) were placed on the tank walls of each quadrant that could be seen from within the pool. A circular platform made of transparent acrylic, measuring 10 cm in diameter, was positioned at the center of one quadrant and submerged 1 cm below the water level. The addition of starch to the water was employed to render the platform within the pool invisible. The mice performed three trials per day for five consecutive days (training days). Each individual mouse was randomly released into the water from three different quadrants, except the target (star) quadrant. If the mouse found the platform within 60 seconds and remained on it for at least 10 seconds, the latency (the time taken to climb onto the platform) was recorded. If the animal was unable to locate the platform, it was led to it and permitted to remain on it for 10 seconds. On day 27 of the probe trial, the platform was removed. For 60 seconds, the mouse was allowed to freely swim in the pool, and the time spent in the target quadrant was measured. Swimming speed and distance were recorded as measures of motor function. All movements were recorded by a CCTV camera positioned above the swimming pool and analyzed by ANY-Maze video tracking software.
The novel object recognition (NOR) test
NOR is commonly used to evaluate recognition memory in rodents. The test was conducted in a 37 × 51 × 20 cm plastic chamber. This examination consisted of two phases: a familiarization phase and a testing phase. Before the experiment, the mouse underwent a habituation period of 5 minutes in an empty apparatus. During the familiarization phase, two sample objects (designated as objects A and A) were positioned in separate locations within the arena. Each mouse received a 5-minute period to explore the objects before being returned to their home cage for a 5-minute interval. To eliminate olfactory signals, the objects and the arena floor were cleansed with 50% ethanol. In the subsequent testing phase, the same mouse was returned to the arena with two objects: one familiar object (FO) was replaced with a novel object (NO) (object B). Object exploration was defined as touching the object or sniffing it with their nose within approximately 1 cm. During a 5-minute period, the duration of exploration by mice of both a NO and a FO was recorded. The discrimination index (DI) was formulated as (NO−FO)/(NO+FO).
Determination of malondialdehyde (MDA) concentration in the hippocampus
On day 29, all experimental animals were euthanized by neck dislocation following a fasting period of one night. The hippocampus from both hemispheres was subjected to dissection and homogenization in ice-cold phosphate buffer (0.1 M, pH 7.4). The homogenate was centrifuged for 60 minutes at 13,000 rpm and 4°C to obtain the supernatant. The supernatant was stored at −80°C until the MDA concentration was determined.
Combine 0.1 ml of supernatant from hippocampal tissues with 1.5 ml of 20% acetic acid (pH 3.5), 0.2 ml of 8.1% sodium dodecyl sulfate, and 1.5 ml of 0.8% thiobarbituric acid. Heat the mixture at 95°C for 60 minutes. After cooling, the mixture was centrifuged at 10,000 g for 5 minutes. The concentration of MDA was determined at a wavelength of 540 nm using a microplate reader. The level of lipid peroxidation was assessed by comparing it to an MDA standard curve and reported as MDA equivalent in nmol/mg protein. Finally, the MDA level was normalized based on the protein content of the supernatant from hippocampal tissues using the Pierce® BCA Protein Assay Reagent Kit (Thermo Fisher Scientific, Rockford, IL, USA).
Statistical analysis
The statistical analysis for this study utilized GraphPad Prism version 9.2.0. Phytochemical content and antioxidant activity data were presented as mean ± standard deviation and analyzed utilizing a paired t-test. Cognitive performance and hippocampal MDA levels affected by RB and GRB extract were expressed as the mean value ± standard error of the mean (SEM). Statistical significance was assessed through one-way analysis of variance, followed by Dunnett’s test for multiple comparisons. A p-value of less than 0.05 was considered statistically significant.
RESULTS
Phytochemical content and antioxidant activity of RB and GRB extracts
GRB extract had GABA levels of 205.20 mg/kg, which were 11.55 times higher than RB extract’s (17.76 mg/kg) levels. The results regarding the TPC, TFC, TAC, and antioxidant capacity (measured using DPPH and ABTS assays) of both RB and GRB ethanol extracts are presented in Table 1. The GRB extract showed a slight reduction in TPC and a significant reduction in TAC. In contrast, the TFC content exhibited a minor increase. In addition, the analysis of the GRB extract revealed a significant decrease in antioxidant activity, as indicated by the increased IC50 values for DPPH and ABTS.
Effects of RB and GRB extract on spatial learning and memory performance
The MWM test assessing spatial learning and memory was performed after cerebral IR surgery in mice. Mice in all groups underwent a five-day training regimen. Initially, there were no significant differences in escape latency during the first 2 days, indicating comparable baseline abilities. However, from the third to fifth days, untreated tBCCAO mice (26.44 ± 7.07, 20.06 ± 5.86, and 10.54 ± 1.68 seconds, respectively) took significantly longer to locate the platform compared to the sham group (6.93 ± 1.41, 6.79 ± 0.83, and 5.31 ± 0.67 seconds, respectively), indicating impaired spatial learning and memory (p < 0.01). Interestingly, mice treated with RB (500 mg/kg BW) and GRB (250 and 500 mg/kg BW) showed marked improvements. Specifically, on the fourth to fifth days, escape latency significantly decreased in the tBCCAO mice treated with RB 500 mg/kg BW (7.35 ± 1.34 and 5.56 ± 0.87 seconds, respectively) (p < 0.05), GRB 250 mg/kg BW (7.18 ± 1.77 and 5.44 ± 1.17 seconds, respectively) (p < 0.05 and p < 0.01, respectively), and GRB 500 mg/kg BW (6.32 ± 1.36, and 5.73 ± 0.81 seconds, respectively) (p < 0.01 and p < 0.05, respectively). Remarkably, the tBCCAO+GRB500 group exhibited a significant decrease in escape latency even on the third day of training (8.55 ± 2.00 seconds) compared to the IR model group (p < 0.05) (Fig. 2A).
Moreover, to evaluate the retention of spatial memory, we conducted probe trials on day 27 of the study. The results of the study indicated that mice in the untreated tBCCAO group (12.89 ± 0.93 seconds) exhibited impaired memory function and spent less time in the platform quadrant compared to the Sham group (21.71 ± 1.46 seconds) (p < 0.001). However, the administration of RB (500 mg/kg BW) and GRB (250 and 500 mg/kg BW) before the experiment resulted in a significant improvement in the time spent by mice in the target quadrant (18.19 ± 1.62, 17.82 ± 0.97, and 20.41 ± 0.74 seconds, respectively) (p < 0.05, p < 0.05, and p < 0.001, respectively) (Fig. 2). We observed that cerebral IR impaired spatial memory, resulting in longer escape latency times during training and reduced time spent in the target quadrant during the probe trial of the IR group. Notably, motor performance, assessed through swimming speed and distance, remained consistent across groups (Fig. 2C and D).
Effects of RB and GRB extract on object recognition memory performance
Recognition memory was measured in all mice using the NOR test. As shown in Figure 3A and B, the untreated tBCCAO group exhibited a significant decline in DI compared to the Sham group (0.33 ± 0.05 vs. −0.126 ± 0.11, p < 0.001). This decline indicated an impaired capacity to differentiate between a NO and a previously encountered one due to cerebral ischemia-induced memory impairment. Remarkably, intervention with RB extract and GRB extract demonstrated significant improvements in recognition memory. In the tBCCAO+RB500, tBCCAO+GRB250, and tBCCAO+GRB500 groups, the DI increased to 0.22 ± 0.08 (p < 0.05), 0.25 ± 0.07 (p < 0.01), and 0.28 ± 0.05 (p < 0.01), respectively, compared to the untreated tBCCAO group. This enhancement in DI indicated the restoration of recognition memory abilities in mice subjected to cerebral IR.
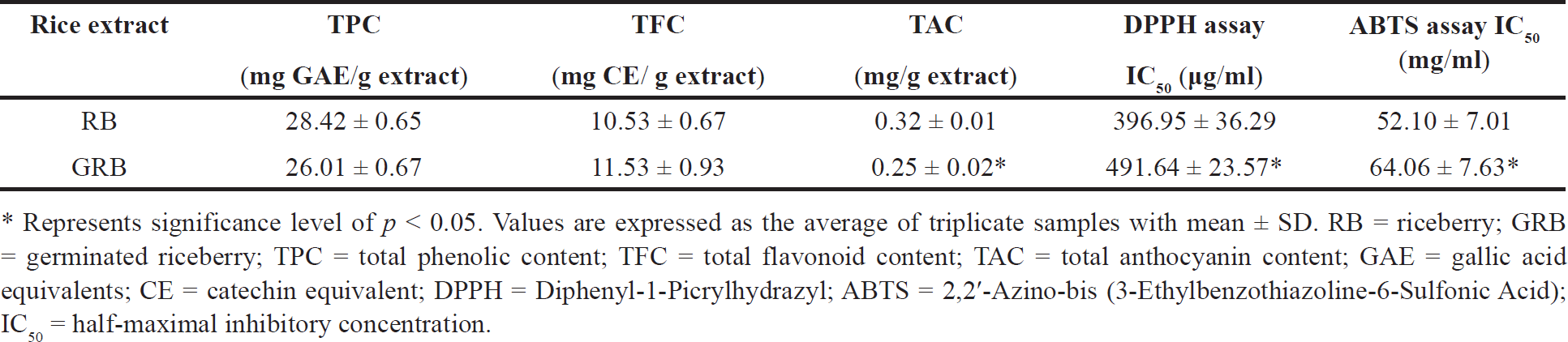 | Table 1. Phytochemical content and antioxidant activity of RB and GRB extracts. [Click here to view] |
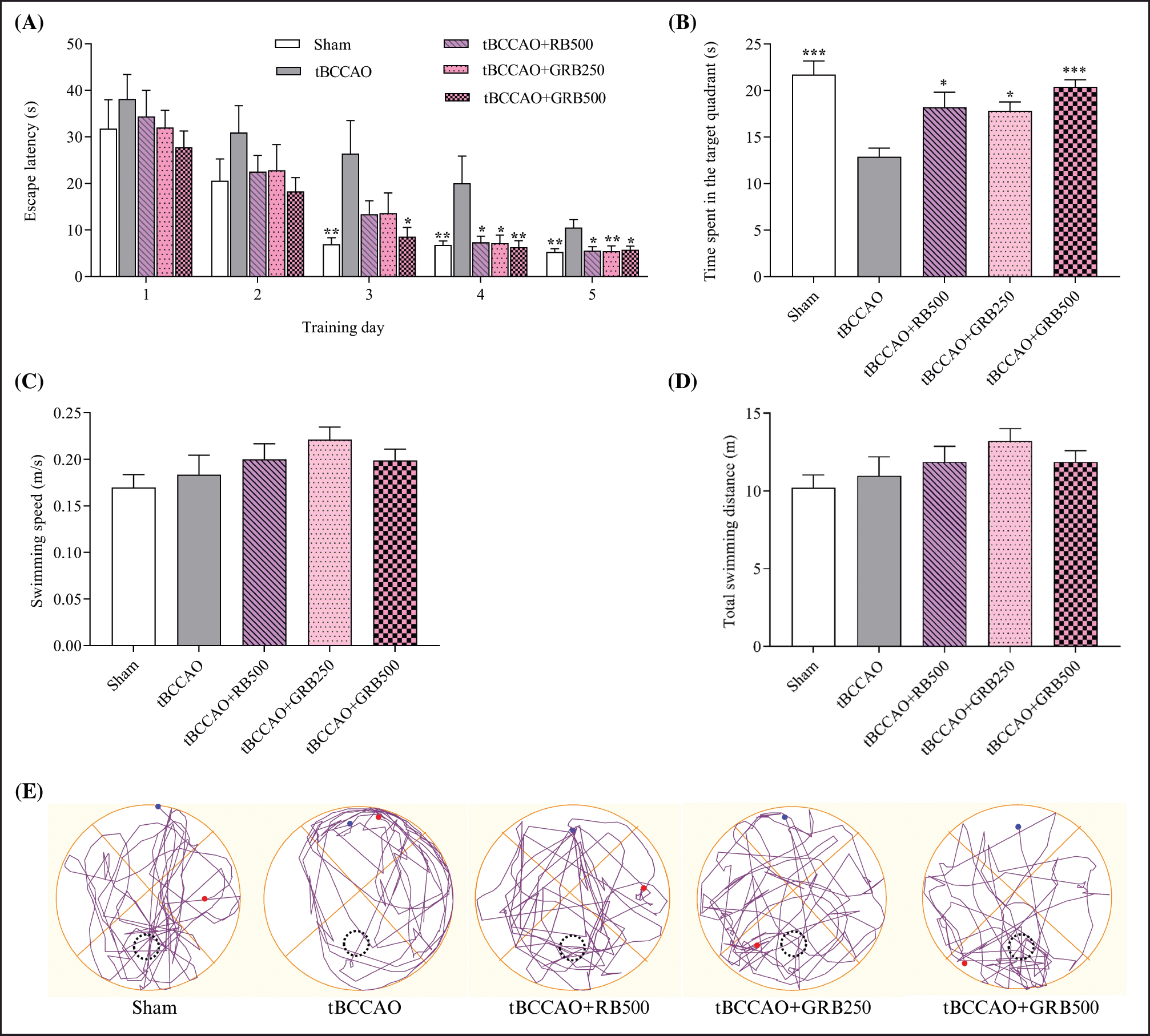 | Figure 2. Effect of RB and GRB extracts on spatial learning and memory performance in tBCCAO mice using a MWM test (A) Escape latency (time to find the hidden platform) during trials over 5 consecutive days; (B) time spent in the target quadrant; (C) swimming speed; (D) total swimming distance; and (E) tracings of the typical swim patterns during the probe trial. Data are expressed as the mean ± SEM, N = 8. *p < 0.05, ** p < 0.01, and *** p < 0.001 responses are significantly different compared with the tBCCAO group. RB = riceberry; GRB = germinated riceberry; tBCCAO = transient bilateral common carotid artery occlusion. [Click here to view] |
Effects of RB and GRB extract on hippocampal MDA level
To determine the antioxidation properties of RB and GRB extracts, the hippocampal MDA level of tBCCAO mice was determined. As represented in Figure 4, a statistically significant increase in hippocampal MDA level was observed in the tBCCAO group (75.62 ± 4.84 nmol/mg proteins) compared to the Sham group (47.51 ± 3.73 nmol/mg proteins) (p < 0.001). Our study revealed that tBCCAO resulted in significant oxidative stress, as shown by increased levels of lipid peroxidation, which indicates the occurrence of oxidative damage in the hippocampus.
Administration of RB (250 mg/kg BW) and GRB extract (250 and 500 mg/kg BW) significantly reduced oxidative stress, demonstrated by notable decreases in hippocampal MDA levels (57.36 ± 3.95, 50.07 ± 6.12, and 50.71 ± 2.33 nmol/mg proteins, respectively) (p < 0.05, p < 0.001, and p < 0.01, respectively).
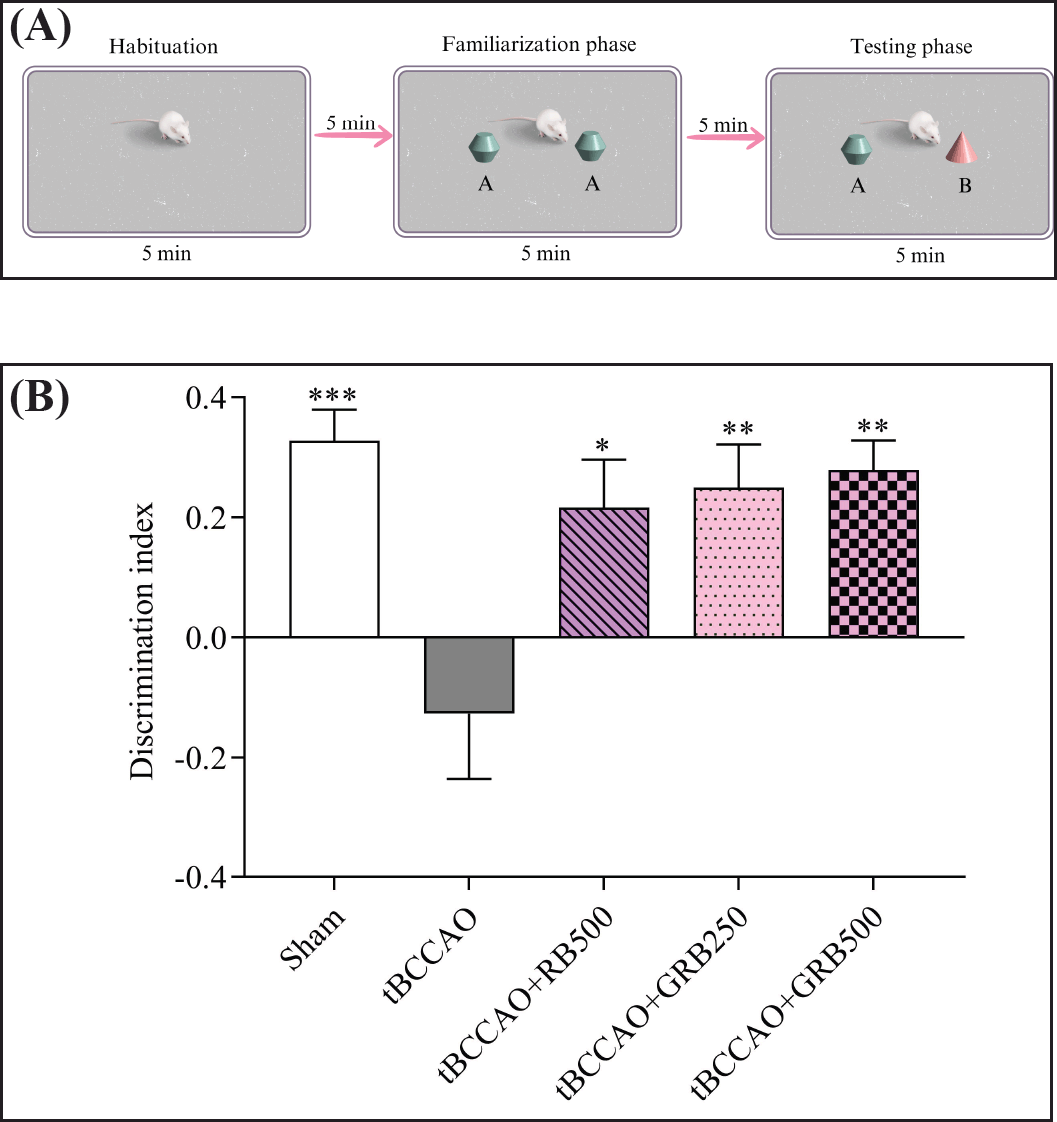 | Figure 3. Effects of RB and GBR extracts on NOR index in a NOR task represents recognition memory performance (A) experimental design of a NOR task; (B) DI. Data are expressed as the mean ± SEM, N = 8. *p < 0.05, ** p < 0.01, and *** p < 0.001 responses are significantly different compared with the tBCCAO group. RB = riceberry; GRB = germinated riceberry; tBCCAO = transient bilateral common carotid artery occlusion. [Click here to view] |
DISCUSSION
The rising trend in the consumption of RB in recent years is driven by the perception of its positive health benefits. RB is notably abundant in a variety of antioxidant bioactive compounds, as previously emphasized in studies such as polyphenol, flavonoids, anthocyanin [4,5,8]. The process of germination plays a pivotal role in enhancing and augmenting the nutritional profile of rice and various cereals. During germination, there is a notable increase in essential components such as proteins, amino acids, sugars, and vitamins. In addition, bioactive compounds such as phenolics, γ-oryzanol, antioxidants, and GABA experience a significant rise in their levels [24,25]. In this study, various parameters, including TPC, TFC, TAC, and levels of GABA, were evaluated, along with the antioxidant activity measured through DPPH and ABTS assays. However, our investigation revealed that GRB extracts displayed a slight reduction in TPC and significantly in TAC, while the TFC content exhibited a minor increase. In addition, the decrease in antioxidant activity, reflected in higher IC50 values in the DPPH and ABTS assays for GRB, correlates with the observed decline in TAC. These results emphasize the complex biochemical alterations that occur during germination, which influence the phenolic and flavonoid compositions as well as the antioxidant capacity of rice extracts. This finding resonates with Nach et al.’s [16] discovery, suggesting that the soaking and rinsing of rice grains during germination reduced phenolic compounds and anthocyanins such as cyanidin and peonidin. Furthermore, our study revealed a significant increase in GABA content, with the GRB extract showing an 11.55-fold greater concentration compared to the RB extract. The rise in GABA levels can be attributable to the stimulation of enzymes during germination, specifically the glutamate decarboxylase enzyme, which stimulates the conversion of L-glutamic acid into GABA [26]. The GRB extract exhibited a significant increase in GABA, a neurotransmitter that functions as an inhibitory neurotransmitter and a naturally occurring bioactive component in mammalian brains [27,28]. Several studies [29–31] have demonstrated the cognitive protective effects of germinated rice. The purpose of this study was to determine the effect of RB and GRB extracts on cerebral IR rodents following tBCCAO, with a focus on oxidative stress and cognitive decline reduction.
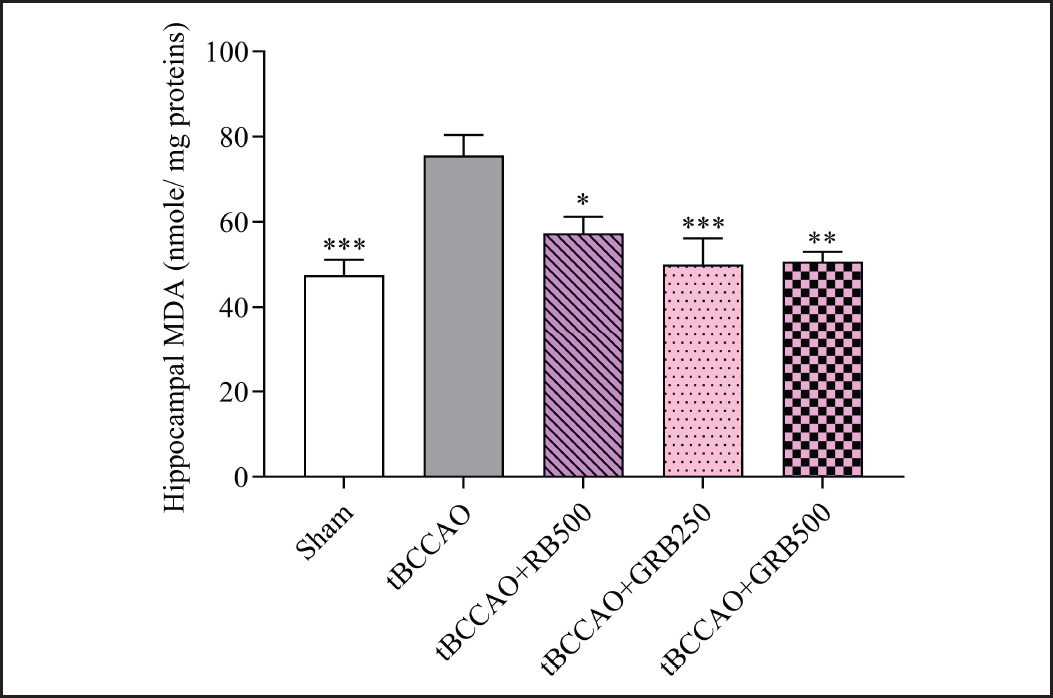 | Figure 4. Effect of RB and GRB extracts on oxidative stress marker MDA in hippocampal tissue. Data are expressed as mean ± SEM; N = 8; *p < 0.05, **p < 0.01, and ***p < 0.001 response significantly different compared with the tBCCAO group. RB = riceberry; GRB = germinated riceberry; tBCCAO = transient bilateral common carotid artery occlusion; MDA = Malondialdehyde. [Click here to view] |
The tBCCAO-induced cerebral IR damage approach is commonly used as a global stroke model in rodents [32]. According to a prior investigation, tBBCAO induces an excess of free radicals, resulting in the death of neurons and subsequent decline in memory in rodents [33]. Similarly, the present study demonstrated that cerebral IR caused a significant decline in cognitive function in cerebral IR mice, which paralleled an increase in hippocampal MDA levels. Cerebral IR injury constitutes a multifaceted process marked by the temporary disruption and subsequent restoration of cerebral blood flow. This intricate phenomenon initiates a series of deleterious events, notably the excessive release of glutamate, a pivotal excitatory neurotransmitter. The hyperactivation of glutamate receptors culminates in excitotoxicity, triggering neuronal damage and cell death [11–13]. Concurrently, the reintroduction of oxygen during reperfusion instigates the generation of ROS, giving rise to oxidative stress and thereby exacerbating neuronal injury [14,13]. These intricate molecular and cellular mechanisms collectively underlie cognitive impairment, a prevalent consequence of cerebral IR injury, characterized by compromised learning, memory deficits, and impaired cognitive functions.
The selection of the doses (250 and 500 mg/kg BW) in our study was informed by preliminary investigations and existing literature. Given the limited research on the cognitive effects of RB and GRB extracts, we referred to relevant studies to establish appropriate dosage levels. Notably, Pannangrong et al. [8] conducted a study using Wistar rats, administering RB at doses of 180, 360, and 720 mg/kg BW. Their research demonstrated the potential of RB in mitigating cognitive impairment and hippocampal neurodegeneration in a rat model of AD. In addition, we drew insights from a study by Kangwan et al. [23], which explored the effects of an ethanolic extract from black rice, known for its antioxidant compounds, especially anthocyanins, on learning and memory in mice with cerebral ischemia induction. In their investigation, daily administration of black rice extracts (125, 250, and 500 mg/kg BW) led to a significant reduction in escape latency and increased crossings in the former platform quadrant during the probe trial. Moreover, a decrease in lipid oxidation was observed in the brains of mice subjected to cerebral ischemia and treated with black rice extract at doses of 250 and 500 mg/kg BW. Furthermore, data from a previous study indicated a significant increase in GABA concentration in black rice during germination. The administration of germinated black rice at doses of 500 and 1,000 mg/kg BW demonstrated an enhancement in both total antioxidant capacity and antioxidant enzyme levels in diabetic rats [24].
In this study, cognitive function was assessed through the MWM and NOR tasks. The MWM is a widely used model for studying learning and memory behavior in mice, specifically designed to assess spatial learning and memory abilities [34]. The findings of this study are consistent with prior research [35,36], demonstrating that cerebral IR impaired spatial memory in mice. Interestingly, our findings indicate a significant improvement in spatial learning and memory in mice subjected to cerebral IR and treated with RB (500 mg/kg BW) and GRB (250 and 500 mg/kg BW) extracts. Remarkably, the GRB-treated mice at a dosage of 500 mg/kg BW displayed a significant decrease in escape latency even as early as the third day of training, suggesting an accelerated learning response compared to the IR model group. In addition to evaluating spatial memory, we assessed the impact of RB and GRB extracts on recognition memory using the NOR test. Our results are consistent with prior research [37], confirming that cerebral IR leads to a decline in recognition memory, as indicated by a reduced DI in the NOR test. Interestingly, our findings demonstrated a significant improvement in recognition memory following cerebral IR in mice treated with RB (500 mg/kg BW) and GRB (250 and 500 mg/kg BW) extracts. These findings demonstrated that RB and GRB extracts improved cognitive performance in both the MWM and NOR tests.
MDA is one of the products of lipid peroxidation that can reflect the degree of lipid peroxidation in cases of cerebral IR oxidative stress injury [12,38]. In this investigation, mice with tBCCAO-induced cerebral IR were used. The hippocampus, which is in the temporal lobe of the brain, is a complex and multifaceted neural structure. It is known for its essential involvement in cognitive processes, especially learning and memory [39]. To determine the antioxidation properties of RB and GRB extracts, the hippocampal MDA level cerebral IR mice were determined. Our study revealed that cerebral IR has shown increased levels of lipid peroxidation, and MDA level, which indicates the occurrence of oxidative damage in the hippocampus. This result accords with previous studies [35,36,38]. During cerebral ischemic reperfusion, there is an upsurge in oxidative stress, leading to lipid peroxidation and subsequent MDA production. Elevated MDA levels indicate substantial oxidative damage within the hippocampus, underscoring the severity of oxidative stress in this region during ischemic events. This oxidative damage can disrupt cellular integrity and impair hippocampal function, contributing to cognitive deficits commonly observed after ischemic events [40]. Administration of RB and GRB extract significantly reduced oxidative stress, demonstrated by notable decreases in hippocampal MDA levels. These effects are related to the presence of high amounts of phenolic and flavonoid compounds, particularly anthocyanins such as cyadinin and peonidin. Evidence suggests that anthocyanin-rich foods can enhance cognitive function. For instance, Kangwan et al. [23] observed that mice with cerebral IR exhibited improved memory and learning, along with reduced brain damage from lipid peroxidation when administered anthocyanin-rich black rice extract. Furthermore, Zhang and Jing [41] demonstrated that a red cabbage anthocyanin-rich extract alleviated age-related cognitive dysfunction in mice by reducing lipid peroxidation and increasing antioxidant levels in the hippocampus. In addition, RB contains active compounds such as phenolic acids, flavonoids, gamma-oryzanol, alpha-tocopherol, and GABA, which function as antioxidants [4,5,7]. While the process of RB germination leads to a reduction in TPC and TAC as well as a decline in antioxidant activity, the results of this study did not show a significant difference in hippocampal MDA levels among the cerebral IR groups treated with RB (500 mg/kg BW) or GRB (250 and 500 mg/kg BW).
IR has a profound impact on brain tissues, specifically involving glutamate, a key excitatory neurotransmitter within the CNS [42]. This neurotransmitter plays a pivotal role in driving excitotoxicity induced by ischemia [12,13,43]. The excessive release of glutamate from both neurons and glial cells into the synaptic cleft leads to hyperactivation of postsynaptic NMDA receptors (glutamate receptors). This hyperactivation initiates cellular processes that ultimately lead to cell death. In addition, this overexcitation leads to elevated intracellular Ca2+ levels, exacerbating the damage and impairing synaptic plasticity [12,13], consequently impacting cognitive function [43]. Moreover, cerebral IR can cause damage to the GABAergic system in the hippocampus, which is associated with cognitive decline. For example, studies have shown that both GABA transporters-1, which are responsible for removing GABA from the synaptic cleft, and GABA transaminase, the primary enzyme involved in breaking down GABA, were increased in the CA1 region of the hippocampus 24 hours after ischemia [44]. Our data showed that the GABA concentration in the GRB extract was 11.55 times greater than the RB extract. The limitation of this study was the absence of GABA content measurement in both serum and brain, which would have provided precise information about the GABA levels in RB and GRB treatment. Existing evidence suggests that GABA can cross the blood-brain barrier and regulate neurochemical processes in the brain. This claim is supported by the documented increase in hippocampal GABA concentrations following prolonged GABA supplementation [45]. In studies exploring the neuroprotective properties of GABA, it was observed that the GABA agonist enhanced the survival of pyramidal cells in the CA1 hippocampal region. Reducing phosphorylated c-Jun had this effect by preventing apoptotic cell death [46–48]. Furthermore, the administration of pregabalin, a GABA agonist, resulted in a reduction of lipid peroxidation and an increase in antioxidant enzymes such as glutathione peroxidase and catalase in the hippocampal region of cerebral IR rats [49]. Pregabalin, a structural analog of GABA, has a high affinity for the CaVα2δ-1 subunit of voltage-gated calcium channels. This binding decreases Ca2+ influx at presynaptic nerve ends and inhibits the release of numerous neurotransmitters, including glutamate and noradrenaline [50,51]. It is possible that the large amount of GABA in the GRB extract might be more effective than those in the RB extract at restoring the balance between excitatory and inhibitory neurotransmitters and improving cognitive performance in mice with cerebral IR.
CONCLUSION
This study illuminates the potential neuroprotective effects of RB and GRB extracts in mitigating cognitive impairment induced by cerebral IR injury. Both RB and GRB extracts exhibited enhancements in spatial and recognition memory and were associated with reduced oxidative stress, as indicated by decreased hippocampal MDA levels. Significantly, GRB showed better cognitive performance enhancement at the same dose than RB extract due to its higher GABA content, suggesting that it has a promising future in treating cerebral IR injury-induced cognitive impairment. However, further research is imperative to elucidate its underlying molecular mechanisms, establish optimal dosages, assess long-term effects, and determine safety profiles. These efforts are crucial for transformative advancements in neurology and functional foods.
ACKNOWLEDGMENTS
The authors express their gratitude to the School of Medical Sciences and the Laboratory Animal Research Center, Innovation and Technology Transfer Institute, University of Phayao, Thailand, for providing essential resources and facilities vital to the successful execution of this study.
AUTHOR CONTRIBUTIONS
Watcharaporn Preedapirom Jeefoo: contributed to literature search, conceptualization, methodology development, investigation, data collection, statistical analysis, writing of the original draft, review, and editing of the manuscript. Payungsak Tantipaiboonwong: contributed to literature search, experimental studies, data collection, statistical analysis, and investigation. Komsak Pintha: contributed to literature search, conceptualization, and investigation. Napapan Kangwan: contributed to literature search, experimental studies, data collection, and investigation. Dej Mann and Prathakphong Riyamongkhol: contributed to experimental studies. All authors approved the final version for publication and agreed to submit it to this journal.
FINANCIAL SUPPORT
The generous financial support from the School of Medical Sciences, University of Phayao, through capital contract number 631006, Unit of Excellence grant number FF66-UoE020, and Talent Mobility is greatly appreciated. Their contributions played a crucial role in the completion of this research.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest related to this research study.
ETHICAL APPROVAL
The study protocol was approved by the Research Ethics Committee of the Laboratory Animal Research Center Innovation and Technology Transfer Institute, University of Phayao, Thailand with approval No. 61-01-04-027.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Settapramote N, Laokuldilok T, Boonyawan D, Utama-ang N. Physiochemical, antioxidant activities and anthocyanin of riceberry rice from different locations in Thailand. Food Appl Biosci J. 2018;6(Special):84–94.
2. Yamuangmorn S, Prom-U-Thai C. The potential of high-anthocyanin purple rice as a functional ingredient in human health. Antioxidants (Basel). 2021 May 24;10(6):833. CrossRef.
3. Yodmanee S, Karrila TT, Pakdeechanuan P. Physical, chemical and antioxidant properties of pigmented rice grown in Southern Thailand. Int Food Res J. 2011;18(3):901–6.
4. Peanparkdee M, Iwamoto S. Bioactive compounds from by-products of rice cultivation and rice processing: extraction and application in the food and pharmaceutical industries. Trends Food Sci Technol. 2019;86:109–17.
5. Poosri S, Thilavech T, Pasukamonset P, Suparppromand C, AdisakwattanaS. Studies on riceberry rice (Oryza sativa L.) extract on the key steps related to carbohydrate and lipid digestion and absorption: a new source of natural bioactive substances. NFS J. 2019;17:17–23. CrossRef
6. Arjinajarn P, Chueakula N, Pongchaidecha A, Jaikumkao K, Chatsudthipong V, Mahatheeranont S, et al. Anthocyanin-rich riceberry bran extract attenuates gentamicin-induced hepatotoxicity by reducing oxidative stress, inflammation and apoptosis in rats. Biomed Pharmacother.2017 Aug;92:412–20. CrossRef. Epub 2017 May 27.
7. Leardkamolkarn V, Thongthep W, Suttiarporn P, Kongkachuichai R, Wongpornchai S, Wanavijitr A. Chemopreventive properties of the bran extracted from a newly-developed Thai rice: The Riceberry. Food Chem. 2011;125(3):978–85. CrossRef
8. Pannangrong W, Wattanathorn J, Muchimapura S, Tiamkao S, Tong-Un T. Purple rice berry is neuroprotective and enhances cognition in a rat model of Alzheimer’s disease. J Med Food. 2011 Jul-Aug;14(7-8):688–94. CrossRef. Epub 2011 Apr 21.
9. Wolters FJ, Zonneveld HI, Hofman A, van der Lugt A, Koudstaal PJ, Vernooij MW, et al. A; heart-brain connection collaborative research group. Cerebral perfusion and the risk of Dementia: a population-based study. Circulation. 2017 Aug 22;136(8):719–28. CrossRef. Epub 2017 Jun 6.
10. Park JH, Hong JH, Lee SW, Ji HD, Jung JA, Yoon KW, et al. The effect of chronic cerebral hypoperfusion on the pathology of Alzheimer’s disease: a positron emission tomography study in rats. Sci Rep. 2019 Oct 1;9(1):14102. CrossRef
11. Bonventre JV, Huang Z, Taheri MR, O’Leary E, Li E, Moskowitz MA, et al. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997 Dec 11;390(6660):622–5. CrossRef.
12. Belov Kirdajova D, Kriska J, Tureckova J, Anderova M. Ischemia-triggered glutamate excitotoxicity from the perspective of glial cells. Front Cell Neurosci. 2020 Mar 19;14:51 CrossRef
13. Puzio M, Moreton N, O’Connor JJ. Neuroprotective strategies for acute ischemic stroke: targeting oxidative stress and prolyl hydroxylase domain inhibition in synaptic signaling. Brain Disorders. 2022;5(2022):100030. CrossRef
14. Dugan LL, Sensi SL, Canzoniero LM, Handran SD, Rothman SM, Lin TS, et al. Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-D-aspartate. J Neurosci. 1995 Oct;15(10):6377–88. CrossRef.
15. Schwartz-Bloom RD, Sah R. gamma-Aminobutyric acid(A) neurotransmission and cerebral ischemia. J Neurochem. 2001 Apr;77(2):353–71. CrossRef.
16. Nacha J, Soodpakdee K, Chamyuang S. Nutritional improvement of germinated riceberry rice (Oryza sativa) cultivated with pleurotus ostreatus mycelium. Trends Sci. 2023;20(9):5574. CrossRef
17. Komatsuzaki N, Tsukahara K, Toyoshima H, Suzuki T, Shimizu N, Kimura T. Effect of soaking and gaseous treatment on GABA content in germinated brown rice, J Food Eng. 2007;789(2):556–60. CrossRef
18. Iwaki K, and Kitada Y. Availability of partially milled rice as a daily source of γ-aminobutyric acid. Food Sci Technol Res. 2007;13(1):41–4. CrossRef
19. Herbert P, Barros P, Ratola N and Alves A. HPLC determination of amino acids in musts and port wine using OPA/FMOC derivatives. J Food Sci. 2000;65(7):1130–33. CrossRef
20. Khanaree C, Punfa W, Tantipaiboonwong P, Nuntaboon P, Suttajit M, Topanurak S, et al. In vitro anti-metastasis of Perilla frutescens leaf water extract on aggressive human breast cancer cells. J Assoc Med Sci. 2022;55(3):51–9. CrossRef
21. Tantipaiboonwong P, Pintha K, Chaiwangyen W, Chewonarin T, Pangjit K, Chumphukam O, et al. Anti-hyperglycaemic and anti-hyperlipidaemic effects of black and red rice in streptozotocin-induced diabetic rats. Sci Asia. 2017;43(2017):281–8. CrossRef
22. Punfa W, Khanaree C, Pintha K, Chumphukam O, Suttajit M, Tantipaiboonwong P. Protective effect of Perilla leaf extract against ROS formation and inflammation induced by TNF-α in A549 human lung carcinoma cell line. Songklanakarin J Sci Technol. 2022;44(2):361–9.
23. Kangwan N, Pintha K, Preedapirom W, Tantipaiboonwong P, Chumphukam O, Suttajit M. Learning and memory enhancing effects of anthocyanin in black rice extract on cerebral ischaemia in mice. Sci Asia. 2015;41(2015):315–21. CrossRef
24. Chaiyasut C, Sivamaruthi BS, Pengkumsri N, Keapai W, Kesika P, Saelee M, et al. Germinated Thai black rice extract protects experimental diabetic rats from oxidative stress and other diabetes-related consequences. Pharmaceuticals (Basel). 2016 Dec 28;10(1):3. CrossRef.
25. Kim H, Kim OW, Ahn JH, Kim BM, Oh J, Kim HJ. Metabolomic analysis of germinated brown rice at different germination stages. Foods. 2020 Aug 17;9(8):1130. CrossRef.
26. Jannoey P, Niamsup H, Lumyong S, Tajima S, Nomur M, Chairote G. γ-aminobutyric acid (GABA) accumulations in rice during germination. Chiang Mai J Sci. 2010;37(1):124–33.
27. Boonstra E, de Kleijn R, Colzato LS, Alkemade A, Forstmann BU, Nieuwenhuis S. Neurotransmitters as food supplements: the effects of GABA on brain and behavior. Front Psychol. 2015 Oct 6;6:1520. CrossRef.
28. McCormick DA. GABA as an inhibitory neurotransmitter in human cerebral cortex. J Neurophysiol. 1989 Nov;62(5):1018–27. CrossRef.
29. Oo EM, Ruamyod K, Khowawisetsut L, Turbpaiboon C, Chaisuksunt V, Uawithya P, et al. Germinated brown rice attenuates cell death in vascular cognitive impaired mice and glutamate-induced toxicity In HT22 Cells. J Agric Food Chem. 2020 May 6;68(18):5093–106. CrossRef. Epub 2020 Apr 24.
30. Zhang R, Lu H, Tian S, Yin J, Chen Q, Ma L, et al. Protective effects of pre-germinated brown rice diet on low levels of Pb-induced learning and memory deficits in developing rat. Chem Biol Interact. 2010 Mar 30;184(3):484–91. CrossRef. Epub 2010 Feb 6.
31. Mamiya T, Asanuma T, Kise M, Ito Y, Mizukuchi A, Aoto H, et al. Effects of pre-germinated brown rice on beta-amyloid protein-induced learning and memory deficits in mice. Biol Pharm Bull. 2004 Jul;27(7):1041–5. CrossRef.
32. Handayani ES, Susilowati R, Setyopranoto I, Partadiredja G. Transient bilateral common carotid artery occlusion (tBCCAO) of rats as a model of global cerebral ischemia. Bangladesh J Med Sci. 2019;18(3):491–500.
33. Naderi Y, Sabetkasaei M, Parvardeh S, Moini Zanjani T. Neuroprotective effects of pretreatment with minocycline on memory impairment following cerebral ischemia in rats. Behav Pharmacol. 2017 Apr;28(2 and 3-Spec Issue):214–22. CrossRef.
34. D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001 Aug;36(1):60–90. CrossRef.
35. Xu L, Gao Y, Hu M, Dong Y, Xu J, Zhang J, et al. Edaravone dexborneol protects cerebral ischemia reperfusion injury through activating Nrf2/HO-1 signaling pathway in mice. Fundam Clin Pharmacol. 2022 Oct;36(5):790–800. CrossRef. Epub 2022 May 4.
36. Yang CJ, Li X, Feng XQ, Chen Y, Feng JG, Jia J, et al. Activation of LRP1 ameliorates cerebral ischemia/reperfusion injury and cognitive decline by suppressing neuroinflammation and oxidative stress through TXNIP/NLRP3 signaling pathway in mice. Oxid Med Cell Longev. 2022 Aug 18;2022:8729398. CrossRef.
37. Choi S, Jang DC, Chung G, Kim SK. Transcutaneous auricular vagus nerve stimulation enhances cerebrospinal fluid circulation and restores cognitive function in the rodent model of vascular cognitive impairment. Cells. 2022 Sep 27;11(19):3019. CrossRef.
38. Thong-Asa W, Puenpha K, Lairaksa T, Saengjinda S. Neuroprotective effects of betanin in mice with cerebral ischemia-reperfusion injury. Exp Anim. 2023 Aug 7;72(3):336–45. CrossRef. Epub 2023 Feb 8.
39. O’Reilly RC, Rudy JW. Conjunctive representations in learning and memory: principles of cortical and hippocampal function. Psychol Rev. 2001 Apr;108(2):311–45. CrossRef.
40. Shang J, Jiao J, Yan M, Wang J, Li Q, Shabuerjiang L, et al. Chrysin protects against cerebral ischemia-reperfusion injury in hippocampus via restraining oxidative stress and transition elements. Biomed Pharmacother. 2023 May;161:114534. CrossRef. Epub 2023 Mar 16.
41. Zhang N, and Jing P. Red cabbage anthocyanins attenuate cognitive impairment by attenuating neuroinflammation and regulating Gut Microbiota in Aging Mice. J Agric Food Chem. 2023;71(41):15064–72. CrossRef
42. Pinky NF, Wilkie CM, Barnes JR, Parsons MP. Region- and activity-dependent regulation of extracellular glutamate. J Neurosci. 2018 Jun 6;38(23):5351–66. CrossRef. Epub 2018 May 14.
43. Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, et al. Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal. 2011 Apr 15;14(8):1505–17. CrossRef. Epub 2011 Jan 9.
44. Kang TC, Park SK, Hwang IK, An SJ, Choi SY, Cho SW, et al. Spatial and temporal alterations in the GABA shunt in the gerbil hippocampus following transient ischemia. Brain Res. 2002;944(1-2):10–8. CrossRef
45. Tabassum S, Ahmad S, Madiha S, Khaliq S, Shahzad S, Batool Z, et al. Impact of oral supplementation of Glutamate and GABA on memory performance and neurochemical profile in hippocampus of rats. Pak J Pharm Sci. 2017May;30(3(Suppl.):1013–21.
46. Han D, Zhang QG, Li C, Zong YY, Yu CZ, Wang W, et al. Co-activation of GABA receptors inhibits the JNK3 apoptotic pathway via the disassembly of the GluR6-PSD95-MLK3 signaling module in cerebral ischemic-reperfusion. FEBS Lett. 2008;582(9):1298–306. CrossRef
47. Qi SH, Liu Y, Wang WW, Wang M, and Zhang GY. Neuroprotection of ethanol against cerebral ischemia/reperfusion induced brain injury through GABA receptor activation. Brain Res. 2009;1276:151–8. CrossRef
48. Xu J, Li C, Yin XH and Zhang GY. Additive neuroprotection of GABA A and GABA B receptor agonists in cerebral ischemic injury via PI-3K/Akt pathway inhibiting the ASK1-JNK cascade. Neuropharmacology. 2008;54(7):1029–40. CrossRef
49. Sanem ASÇI, Demirci S, Halil ASÇI, Doguç DK, and Onaran I. Neuroprotective effects of pregabalin on cerebral ischemia and reperfusion. Balkan Med J. 2016;33(2):221–7.
50. Fink K, Dooley DJ, Meder WP, Suman-Chauhan N, Duffy S, Clusmann H, et al. Inhibition of neuronal Ca2+ influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology. 2002;42(2):229–36. CrossRef
51. Kammerer M, Brawek B, Freiman TM, Jackisch R and Feuerstein TJ. Effects of antiepileptic drugs on glutamate release from rat and human neocortical synaptosomes. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:531–42. CrossRef