INTRODUCTION
A stroke is characterized by a sudden blockage in the blood supply to brain tissues, leading to brain ischemia; and stroke-related brain damage, and has become one of the leading causes of death worldwide [1,2]. Despite advancements in medical facilities, the incidence of stroke continues to rise, resulting in a growing global burden on both stroke victims and survivors [2,3]. More than half of those who survive a stroke end up with some form of disability, with cognitive decline being a prominent complication among them [4–6]. Additionally, stroke survivors often experience physical disabilities, anxiety, depression, and even convulsions [7]. Tissue plasminogen activator, a thrombolytic agent, stands as the sole FDA-approved option to mitigate obstruction of blood flow responsible for stroke. the mortality linked with stroke incidence. However, its efficacy is contingent upon administration within 4.5 hours of the stroke’s onset [8]. The restoration of blood supply engenders oxidative stress, exacerbating neuronal injury a phenomenon known as reperfusion injury [9].
Post-stroke complications constitute a multifactorial pathology: oxidative stress, inflammation, energy deprivation, mitochondrial dysfunction, and excitotoxicity [10–12]. These pathological mechanisms collectively lead to neuronal injury and death. It impacts specific brain regions and consequently affects vital functions [6,12]. Hippocampal cellular injury following a stroke is correlated with long-term cognitive impairment, thereby affecting the quality of life of stroke survivors [13,14]. It leads to and results in a substantial socioeconomic burden [3,15]. Addressing multiple pathological mechanisms of stroke holds promise in enhancing the quality of life for stroke survivors [1,16]. Hence, employing a combination of two or more therapeutic agents that target distinct pathways could prove beneficial in mitigating stroke and its associated complications.
Metformin (MET), an oral hypoglycemic agent and an activator of AMP-activated protein kinase (AMPK) [17], is recognized for its neuropharmacological actions [18,19]. Previous reports suggest that MET can diminish the incidence and severity of stroke [19,20]. AMPK activation is linked to the maintenance of energy homeostasis [21], reduction of inflammation [22], and mitigation of oxidative stress [23], thereby promoting cell survival [24]. This AMPK-mediated neuroprotective potential underscores the neuroprotective action of MET [24,25]. Furthermore, earlier studies also suggest a potential role for MET and AMPK activation in enhancing learning and memory function [26,27]. However, some reports also present inconsistent findings regarding the effects of MET on stroke [19], potentially due to differences in dosage regimens and the intensity of AMPK activation [18]. Moreover, limited data are available regarding its effects on post-stroke conditions.
CoQ10 is an endogenous antioxidant involved in energy production and mitochondrial stabilization, extensively investigated for its cardioprotective effects against myocardial ischemia-reperfusion injury [28,29]. Additionally, the neuroprotective role of CoQ10 in neurological complications such as Alzheimer’s and Parkinson’s diseases, could be associated with its antioxidant and mitochondrial stabilization action [30,31]. CoQ10, also known as ubiquinone, plays a crucial role in cellular energy production, particularly in mitochondria where ATP is generated. In neurons, ATP is primarily generated through oxidative phosphorylation, within the inner mitochondrial membrane. CoQ10 regulates energy production via con-tributing to the electron transport chain in mitochondria; and serves as an electron carrier between Complexes I and II (which donate electrons) and Complex III (which accept electrons) [32,33]. These critical mechanisms of CoQ10 are essential for energy-dependent neuronal functions, including neurotransmission, maintaining membrane potentials, and overall cellular homeostasis in the nervous system [33,34]. Similarly, CoQ10 has been studied for its neuroprotective properties in stroke models. It acts as an antioxidant; scavenges free radicals and reduces oxidative stress, thereby contributing to restricting neuronal damage during ischemic stroke [35,36]. Ischemic stroke triggers inflammatory responses that exacerbate brain injury; CoQ10 attenuates inflammation by modulating cytokine, i.e., TNF-α, IL-6 production, and blocking inflammatory pathways activation cascade. Thus, CoQ10 plays a vital role in reducing secondary damage in post-stroke injury [37].
Combining MET and CoQ10 will target different pathological mechanisms of stroke injury, MET will maintain energy homeostasis by acting through AMPK, while CoQ10 will prevent oxidative stress. This could result in better outcomes in stroke injury as compared to individual treatment. Earlier, simultaneous administration of MET and CoQ10 has been reported to enhance anti-inflammatory, antidiabetic action [38-40], indicating CoQ10 contributes to MET action. However, data on their combined effects on cerebral ischemia-reperfusion injury are lacking. Therefore, the aim of the present study is to evaluate the effects of post-injury treatment with MET and CoQ10 on experimentally induced post-stroke injury and associated cognitive complications.
MATERIAL AND METHODS
Materials
MET was obtained as a gift sample from Emcure Pharmaceutical, Pune, Maharashtra, whereas CoQ10 was purchased by SV Agro Mumbai, Maharashtra. All other chemicals and reagents used in this work were of analytical grade.
Animals
Adult Wistar rats (200–250 g) of either sex were obtained from the central animal house facility of the institute. The study protocol was approved by the Institutional Animal Ethics Committee (IAEC), VNS Institute of Pharmacy, Bhopal and according to the guidelines of CPCSEA (Approval No.: PH/IAEC/VNS/2K22/01, Date:19 January 2021). The animals were maintained at a controlled temperature of 24°C and provided standard food and water ad libitum under a 12-hour light/dark cycle.
Induction of IR injury using bilateral common carotid artery occlusion (BCCAO)
The ischemia-reperfusion injury was induced by the BCCAO model described in Iwasaki et al. [41] with slight modification. The rats were anesthetized by administration of Ketamine (60 mg/kg i.p) and Xylazine (10 mg/kg i.p.). The rats were kept in the supine position, and body temperature was maintained at 37°C throughout the surgical intervention. The neck area was shaved, and the midline incision was done to expose both common carotid arteries (CCAs). Both CCAs were isolated carefully from adjacent tissue and vagus nerve. Furthermore, both CCAs were ligated by a thread for 15 minutes in all animals, except Sham operated group. After 15 minutes of occlusion reperfusion began by removing the thread and allowed reperfusion for the next 3 days.
Experimental design
The rats were randomly allocated into five groups, each group containing 24 rats, and further subdivided into four subsets (n = 6). Sham; vehicle-treated and both CCAs were isolated by not ligated; IR: Both CCAs ligated for 15 minutes followed by reperfusion for 3 days; MET: Metformin treated; (200 mg/kg; i.m. for 3 days), CoQ10: Coenzyme Q10 (200 mg/kg; i.m. for 3 days) and MET+CoQ10: MET and CoQ10 coadministration. All the treatments were administered for post injury 03 days. Each subset will be used for evaluating different parameters. The details of each subset are as follows:
Set I: Neurological score, Behavioral, Infarct area
Set II: Oxidative stress (GSH and LPO)
Set III: Blood–brain barrier permeability
Set IV: Histopathology.
Neurological scoring
Neurologic examinations were performed on the third day of post injury using modified neurological scores described by Li et al. [42] and Akhoundzadeh et al. [43] (Table 1). Neurological score was graded on a scale of 0–14. One point is given for the inability to perform the tasks or for the lack of a tested reflex. A score between 10 and 14 would be severe, 5–9 moderate and 1–4 would be a mild injury. The maximum point score would be 14 [42,43].
Novel objective recognition test (NORT)
NORT was performed to assess the ability to recognize two different objects. This test was performed as described in Leger et al. [44] and Panta et al. [45]. The animals were trained for 5 days before BCCAO surgery. Day 1 (Familiarization session); the rat was placed into an activity box and acclimatized for 5 minutes. Day 2 to 4 (Familiar object); the rat was exposed to the two same objects (A and B) for 10 minutes and spent time with both objects was recorded. The cut off time of 20 seconds with both objects indicates maximum exploratory behavior. On day 5 (Novel object); the rat was exposed to one familiar object A and the novel object (N) instead of object B. After completion of the training on the fifth day the rat underwent for BCCAO surgery and on post stroke third day the rat was again exposed to NORT with familiar (A) and novel (N) objects. Time explored with each object was recorded and the discrimination ratio was calculated using the following formula [46].
 | Table 1. Modified neurological severity score. [Click here to view] |
Discrimination ratio: [Time spent with novel object]-Time spent with familiar object] / [total time spent with bot object].
% Spontaneous alteration on Y-maze
Spontaneous alteration behavior was analyzed using the method described in Wahl et al. [47] using Y-maze [47]. Y-maze comprises three wooden arms (40 × 15 × 35) at 120° to each other. During 5-day training the rat was exposed to the central area of Y-maze and allowed to free move and record the alteration behavior for 8 minutes. The correct alteration was considered when the rat alternatively moved in each arm, i.e., ABC, BAC, CAB, and so on. Total correct alterations during 8 minutes were recorded and % alteration was calculated using formula:
% alteration: [Total number of alterations]/[number of arms entered]*100
The rat was trained on Y-maze for 5 days before BCCAO surgery, and on post injury third day again exposed to assess the % alteration response.
Transfer latency (LT) on elevated plus maze
LT was assessed using the Elevated plus maze (EPM) test, as described previously [48–50]. All rats were trained on EPM for 5-days before the BCCAO. Each rat was placed next to the open arm of the EPM, facing away to the closed arm of the EPM. LT was recorded as the time taken by the animal to reached open arm to the closed arm. The cut off time for LT is 90 seconds. After the third day of BCCAO animal was again exposed to EPM and LT was recorded.
Brain infarction analysis
Infarct analysis was conducted on coronal brain slices (2 mm thick) immediately after isolation, which were then stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC) for 30 minutes at room temperature [51]. Subsequently, a 10% formalin solution was employed to fix the slices overnight, followed by the assessment of the infarct area using ImageJ software version 1.30V (http://www.rsb.into.nih/ij). The total infarct area was determined by summing the individual areas across all sections. This total infarct area was then multiplied by the thickness of the brain slice to compute the infarct volume per brain in cubic millimeters (mm3).
Oxidative stress
Brain tissue weighing 1 g was homogenized in ice-cold 10% trichloroacetic acid (TCA) using a tissue homogenizer. Malondialdehyde levels were measured as an indicator of lipid peroxidation by observing the generation of thiobarbituric acid-reactive substances at 532 nm and, expressed as millimoles per milligram of tissue protein [52]. Reduced glutathione (GSH) levels were determined using 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) reagent at 412 nm and expressed as micrograms of GSH per milligram of protein [53]. The protein content of each sample was quantified using the method described by Lowry et al. [54].
Estimation of blood–brain barrier (BBB) permeability
The investigation into BBB integrity involved assessing Evans blue (EB) extravasation as indicative of brain leakage. BBB permeability was quantified as micrograms of EB per hemisphere, serving as a measure of vascular permeability. On the third day following reperfusion, a 0.1 ml solution of 4% EB was administered via the tail vein of rats. Subsequently, rats were anesthetized and underwent transcardial perfusion with 100 ml of heparinized saline solution (10 IU/ml) post-reperfusion. Following sacrifice, the brains were extracted and homogenized in 1 ml of 0.1 mol/l PBS, followed by centrifugation at 1,000 g for 15 minutes. To the resulting 0.7 ml supernatant, 0.7 ml of a 100% TCA solution was added. The mixture was incubated at 14°C for 18 hours and then centrifuged at 1,000 g for 30 minutes. The spectrophotometric determination of EB concentration in the supernatant at 610 nm was conducted by comparison against readings obtained from standard solutions [55].
Histological analysis
Following the decapitation of the rats, the brain was promptly removed, rinsed with saline, and then fixed in a solution of 10% buffered formalin. Subsequently, the brain, preserved in 10% formalin, was embedded in paraffin, and sections measuring 5 mm were prepared. These sections were then stained with a 1% cresyl violet solution. Examination of the brain sections under a microscope was conducted to detect any histological alterations.
Statistical analysis
All data were summarized as mean ± SEM, analyzed with One Way Analysis of Variance (ANOVA) followed by Tukey’s test. However, the neurological score was analyzed with the Kruskal Wallis test and reported as median values with the quartile range (25%–75%). Statistical difference was considered significant with the values p < 0.05 using GraphPad Prism 5 (GraphPad Software, USA).
RESULTS
Neurological improvement
The neurological score was significantly higher in IR group (p < 0.05) as compared to Sham, indicating neurological impairment due to IR injury (Fig. 1A). Three-day post stroke treatment with coadministration of MET and CoQ10 significantly (p < 0.05) reduces the score as compared to IR group. The median (25%–75% quartile range) for the coadministration group was 2.00 (1.75–3.00) compared with 10.00 (9.75–11.00) in the IR group. Alone treatment of MET 2.50 (1.75–3.25) and CoQ10 3.00 (2.00–4.00) also reduces the score as compared to the IR group. However, alone treatment does not show a significant reduction.
Improving discrimination ratio
IR injury significantly reduces the discrimination ratio in the IR group (0.27 ± 0.16) as compared to the Sham group (0.90 ± 0.04), indicating the impact of IR injury on recognition memory (Fig. 3C). Three-day treatment of coadministration of MET and CoQ10 improve this recognition power (0.49 ± 0.03). This coadministration also shows a better discrimination ratio as compared to alone MET (0.52 ± 0.15) and CoQ10 (0.46 ± 0.18).
Amelioration of spontaneous alteration behavior
Coadministration of MET and CoQ10 showed higher % alteration behavior on Y-maze (31.17 ± 4.90) as compared to IR group (17.10 ± 6.01) indicating improvement in spatial memory upon treatment; however, this improvement does not achieve a significant level (Fig. 3A). Similarly, no significant difference was observed in alone MET (36.41 ± 14.65) and CoQ10 (29.72 ± 7.15) as compared to coadministration group. Although alone treatment also showed better alteration response as compared to IR.
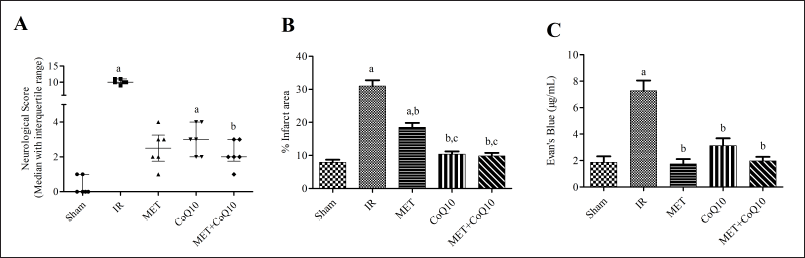 | Figure 1. (A) Neurological score; (B): Brain infarct volume; (C): Blood brain barrier permeability. [Click here to view] |
Minimizing LT
IR injury significantly (p < 0.05) enhances the LT on EPM indicating impairment in cognition. LT of the IR group was (49.33 ± 7.81) as compared to the Sham group (15.33 ± 1.69). Significant reduction in LT was found with post stroke 3-day treatment with MET (19.67 ± 1.43), CoQ10 (21.17 ± 2.72), and coadministration (23.50 ± 10.37) as compared to the IR group (Fig. 3B).
Limiting brain infarction
BCCAO-induce IR injury showed significantly (p < 0.05) higher brain infarcted area in TTC stained coronal brain slice (Fig. 2) in the IR group (30.99 ± 1.74) as compared to the Sham group (7.92 ± 0.79). Whereas, 3-day post injury coadministration of MET and CoQ10 significantly minimizing the infarcted area (9.83 ± 0.95) as compared to IR as well as to the alone MET-treated group (18.53 ± 1.33). While alone CoQ10 treated group (10.39 ± 0.85) also significantly (p < 0.05) reduced the infarcted area as compared to the IR group (Fig. 1B).
Restoring BBB integrity
Three-day post stroke set up significantly (p < 0.05) enhances the BBB permeability in the IR group (7.28 ± 0.76) as compared to the Sham group (1.86 ± 0.45). Treatment groups significantly attenuate the EB level in MET (1.74 ± 0.37), CoQ10 (3.12 ± 0.56), and Coadministration (1.97 ± 0.31) as compared to the IR group. However, coadministration does not show a significant (p < 0.05) difference as compared to alone MET and CoQ10 treated groups (Fig. 1C).
Attenuating oxidative stress
IR injury markedly generated oxidative stress, which has been estimated as a significantly (p < 0.05) reduction in GSH level in the IR group brain (3.54 ± 0.79) as compared to the Sham group (17.46 ± 1.17) (Fig. 4A). Three-day post stroke coadministration of MET and CoQ10 significantly ameliorates the GSH level (14.92 ± 2.03) as compared to IR group. Coadministration also shows better improvement in GSH level as compared to alone MET (12.08 ± 1.56) and CoQ10 (8.51 ± 1.61). While, LPO levels were significantly (p < 0.05) augmented in the IR group (13.63 ± 1.15) as compared to the Sham group (4.92 ± 0.64) (Fig. 4B). Coadministration significantly reduces the LPO level (6.01 ± 0.55) as compared to IR and alone MET group (12.76 ± 1.05), indicating coadministration having a better effect over alone treatment.
Improving cellular injury
Histopathology of the brain of the Sham group indicates normal pyramidal neuron cells in hippocampus CA1, CA2, and CA3 areas. IR group indicated pyknosis, constraint nuclei, and vacuolation in CA1, CA2, and CA3 areas of the brain indicating cellular injury. Whereas, all treatment groups demonstrated less sign of pyknosis, constrain nuclei, and vacuolation. The coadministration group showed better improvement as compared to alone MET and C0Q10 treated animals (Fig. 5).
 | Figure 2. Infarcted area in brain slice after IR injury. [Click here to view] |
DISCUSSION
The present study demonstrated the post injury acute treatment effect of alone MET, CoQ10, and coadministration on BCCAO-induced ischemic reperfusion (IR) injury in rat brains. The coadministration of MET and CoQ10 show reduced brain infarction, neurological deficit, oxidative stress, improved cognitive ability, and cellular signs of tissue injury. The outcome of the study demonstrated the MET effect are potentiated with coadministration of potent antioxidant CoQ10.
Targeting multiple pathological mechanisms using a combination of drugs is stroke shows better outcomes in earlier studies. Atif et al. [56] demonstrated combining vitamin-D hormone enhances the neuroprotective potential of progesterone in experimentally induced ischemic stroke in rats. The combination of vitamin-D hormone and progesterone activated brain-derived neurotrophic factor and enhances the anti-apoptotic protein Bcl-2 leading to functional recovery and reduction in brain infarcted area [56]. Similarly, a combination of progesterone and chloroquine also reduces the infarcted area as compared to alone treated. This combination also enhances the Bcl-2 protein level and suppressed Bax expression resulting neuroprotective action of this combination [57]. These earlier reports highlighted the beneficial outcomes of the combination of drugs over the alone treatment. Thereby, we combined CoQ10 with MET, to check whether this combination shows a beneficial effect in cerebral IR injury. MET would show neuroprotective potential by activating AMPK, while CoQ10 can augment MET-mediated neuroprotection by exerting potent antioxidant defense.
AMPK activation has been widely reported for its beneficial role in various clinical complications [58–60]60]. However, earlier reports of MET-mediated AMPK activation role in stroke remain conflicting [25,61]. Li et al. [18] demonstrated the acute 3-day treatment prior to stroke induction exacerbated the infarct size, whereas chronic treatment for 3 weeks showed neuroprotective action in the middle cerebral artery occlusion model in mice [18]. Moreover, another study indicates that pre-treatment MET at a dose of 200 mg/kg (7 days) in rats had significantly attenuated the infarct size, LPO, MPO, and leukocyte infiltration. However, post treatment at the same dose and duration only reduces infarct size and doesn’t affect the LPO, MPO, and leukocyte infiltration, indicating the beneficial effect of pretreatment [62]. On contrary, Zemgulyte et al. [63] indicated the neuroprotective action of post stroke MET treatment, but the dose and model used are different. This divergent effect of MET treatment explains the duration and extent of AMPK activation as an important factor for MET neuroprotective effect in cerebral IR injury. Three-day treatment in post-stroke setup (MET and CoQ10) significantly reduces the brain infarct area. AMPK is an energy sensor and activates the catabolic pathway such as glycolysis and fatty acid oxidation to produce energy for cell survival. AMPK activation is known to salvage mitochondrial damage [64–66] and would have of vital importance in restricting post-stroke brain damage [11,67]. The generation of oxidative stress is one of the key pathological mechanisms in stroke pathology leading to cellular injury. CoQ10, a potent antioxidant attenuates this free radical generation and associated neuronal cell death. Coadministration of MET and CoQ10 shows a greater reduction in infarcted brain area as compared to the IR group as well as to the MET individual treated group.
IR injury results in the generation of free radicals, the reactive oxygen species that cause damage and disruption of the lipid membrane. Lipid peroxidation further contributes to inflammation, cell apoptosis in IR injury [65]. Furthermore, GSH depletion is another input to oxidative stress, resulting in cellular damage. The present study demonstrated coadministration of MET and CoQ10 significantly reduces the LPO as compared to IR and alone MET treatment, indicating coadministration has better antioxidant defense in IR injury. Similarly, GSH levels were significantly restored in the coadministration group as compared to IR and also indicated better results as compared to the alone treated group, exhibiting coadministration shows augmented effect as compared to alone treatment. MET treatment has been reported to enhance antioxidant defense by AMPK/Nrf2 (Nuclear factor-like 2) pathway [22]. Similarly, CoQ10 is also reported for enhancing the level of antioxidant enzyme [69] as well as activating the transcription factor Nrf-2 [69]. Furthermore, CoQ10 can also regenerate Vitamin-E which can protect the cells from oxidative damage [69,70].
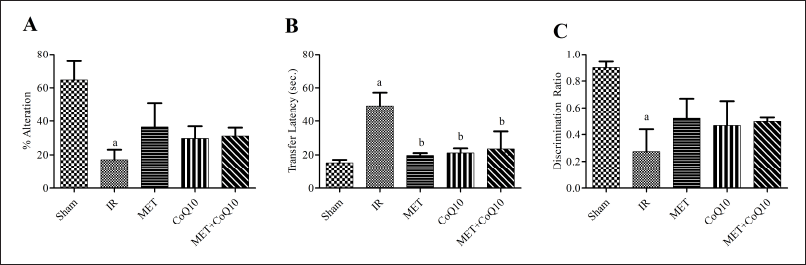 | Figure 3. Cognitive parameters (A) % Alteration on Y-maze; (B): Transfer latency on EPM; (C): Discrimination Ratio on NORT. [Click here to view] |
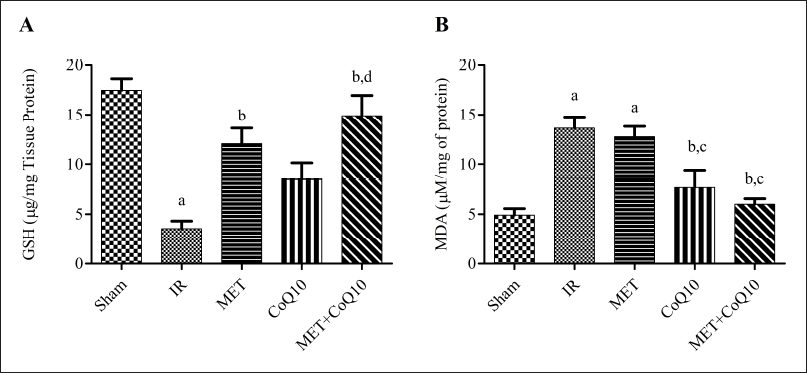 | Figure 4. Oxidative stress (A): GSH; (B) LPO. [Click here to view] |
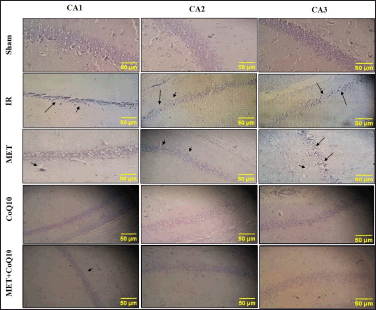 | Figure 5. Histopathology of hippocampus area of brain (400x). [Click here to view] |
Furthermore, the generation of oxidative stress also causes damage to BBB, leading to enhanced permeability. BBB limits the passage of large molecules and toxins, enhanced permeability results in inflammation, and cellular injury. The MET treatment can improves the BBB integrity by enhancing the level of tight junction proteins like occluding, zonula occludens-1 (ZO-1), and claudin-5 [71,72]. Matrix metalloproteinases (MMPs) which are activated by increased levels of ROS result in degrading the tight junction proteins, thus contributing to BBB disruption. Earlier reports indicate that CoQ10 reduces the MMPs in various pathological conditions through its potent antioxidant mechanism [73]. The present study also coincides with the earlier reports and both MET and CoQ10 significantly improve the BBB integrity [36,71]. Coadministration also significantly restores the BBB permeability as compared to the IR group and the alone MET group.
The depletion of GSH in IR injury leads to oxidative damage in hippocampus [74], which is responsible for learning and memory function and the most vulnerable brain region to ischemia [[75–77]. The current study also underscores the cellular injury in brain histology as pyknosis and vacuolation in the CA1, CA2, and CA3 areas of the hippocampus. All treatment groups show marked reduction of pyknosis and vacuolation in the hippocampus area. It could be the result of the biogenesis of mitochondrial proteins like Peroxisome proliferator-activated receptor gamma co-activator 1? (PGC-1?) & Transcription factor A, mitochondrial (TFAM), which are correlated with a decrease in cell death of hippocampus neurons [17,78]. This hippocampus injury is related with learning and memory impairment, which can be assessed by using Y-maze. Ahmadi-Soleimani et al. [79] demonstrated that CoQ10 exerts anticholinesterase activity in hippocampus of the treated rats. Furthermore, the author correlated this activity with reducing oxidative stress in the hippocampus of rats, suggesting a role of CoQ10 to protect cholinergic neurons from damage and preserve acetylcholine levels. This preservation of acetylcholine would help maintain normal neurotransmission in the hippocampus, thereby improving memory and cognitive function.
Y maze is commonly used to assess spatial working memory [80]. Herein we demonstrated less spontaneous alteration behavior of IR group rats on Y-maze. On the other hand, the treatment groups show higher spontaneous alteration response, indicating improvement in cognitive ability. Similarly, on NORT, treatment groups exhibited a higher discrimination ratio as compared to IR groups, indicating the ability to discriminate familiar and novel objects. In addition, LT of EPM was significantly reduced in all treatment groups. This improvement in spontaneous alteration, discrimination ratio, and less LT leading to mitigation of learning and memory impairment associated with post-stroke injury. However, in both spontaneous alteration on Y-maze and discrimination ratio on the NORT test significant levels were not obtained in any of the treatments as well as in LT on EPM.
This outcome could be the result of only a 3-day post-injury treatment, which suggests the need for more extensive treatment in post-stroke subjects. Similarly, the neurological score was marked reduced in coadministration groups as compared to IR and alone-treated groups. Furthermore, the limitation of this study is that it does not evaluate the effect of this coadministration on musculoskeletal problems, which is one of the common disabilities in stroke survivors. In addition, this study exhibits cognitive improvement after coadministration treatment, but the estimation of acetylcholinesterase as the biomarker of cognition could add more value to the outcome.
CONCLUSION
The results of this study indicate acute post stroke treatment of MET and CoQ10 prevents neuronal cell injury and improves associated cognitive impairments. Coadministration also exhibits better neuroprotective potential in post-stroke injury as compared to the individual-treated group. However, coadministration does not attain a significance level as compared to individual treatment groups. This indicates that 3-day treatment of post stroke injury is not adequate to achieve synergistic or additive effects. Further studies are required to evaluate the long-term treatment effect of this combination on post stroke injury, Moreover, the outcome of musculoskeletal impairment & estimation of cognitive biomarkers would be interesting.
ACKNOWLEDGMENTS
Extensive support from the institutions is highly acknowledged by all authors.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Ethical approval given in the 'Material and Method section'.
DATA AVAILABILITY
All data generated and analyzed are included with this article (additional files).
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Kuriakose D, Xiao Z. Pathophysiology and treatment of stroke: present status and future perspectives. Int J Mol Sci. 2020;21(20):1–24. CrossRef
2. Donkor ES. Stroke in the 21st Century: a snapshot of the burden, epidemiology, and quality of life. Stroke Res Treat. 2018;2018:3238165. CrossRef
3. Kalita J, Bharadwaz MP, Aditi A. Prevalence, contributing factors, and economic implications of strokes among older adults: a study of North-East India. Sci Rep [Internet]. 2023;13(1):1–12. CrossRef
4. Huang Y, Wang Q, Zou P, He G, Zeng Y, Yang J. Prevalence and factors influencing cognitive impairment among the older adult stroke survivors: a cross-sectional study. Front Public Heal. 2023;11:1254126. CrossRef
5. Sun JH, Tan L, Yu JT. Post-stroke cognitive impairment: epidemiology, mechanisms and management. Ann Transl Med. 2014;2(8);80. CrossRef
6. Chohan SA, Venkatesh PK, How CH. Long-term complications of stroke and secondary prevention: an overview for primary care physicians. Singapore Med J. 2019;60(12):616–20. CrossRef
7. Choi HL, Yang K, Han K, Kim B, Chang WH, Kwon S, et al. Increased risk of developing depression in disability after stroke: a Korean Nationwide Study. Int J Environ Res Public Health. 2023;20(1):842. CrossRef
8. Lansberg MG, Bluhmki E, Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: a meta-analysis. Stroke [Internet]. 2009;40(7):2438–41. CrossRef
9. Zhang M, Liu Q, Meng H, Duan H, Liu X, Wu J, et al. Ischemia-reperfusion injury: molecular mechanisms and therapeutic targets. Signal Transduct Target Ther. 2024;9(1):12. CrossRef
10. Feng S, Yang M, Liu S, He Y, Deng S, Gong Y. Oxidative stress as a bridge between age and stroke: a narrative review. J Intensive Med. 2023;3(4):313–9. CrossRef
11. Zhou X, Chen H, Wang L, Lenahan C, Lian L, Ou Y, et al. Mitochondrial dynamics: a potential therapeutic target for ischemic stroke. Front Aging Neurosci. 2021;13:721428. CrossRef
12. Sekerdag E, Solaroglu I, Gursoy-Ozdemir Y. Cell death mechanisms in stroke and novel molecular and cellular treatment options. Curr Neuropharmacol. 2018;16(9):1396–415. CrossRef
13. Torres-López C, Cuartero MI, García-Culebras A, De La Parra J, Fernández-Valle ME, Benito M, et al. Ipsilesional hippocampal GABA is elevated and correlates with cognitive impairment and maladaptive neurogenesis after cortical stroke in mice. Stroke. 2023;54(10):2652–65. CrossRef
14. Pluta R, Januszewski S, Czuczwar SJ. Post-ischemic neurodegeneration of the hippocampus resembling alzheimer’s disease proteinopathy. Int J Mol Sci. 2022;23(1):306. CrossRef
15. Tian J, Wang Y, Guo L, Li S. Association of income with post-stroke cognition and the underlying neuroanatomical mechanism. Brain Sci. 2023;13(2).363. CrossRef
16. Jiang Y, Liu Z, Liao Y, Sun S, Dai Y, Tang Y. Ischemic stroke: from pathological mechanisms to neuroprotective strategies. Front Neurol. 2022;13(2):1013083. CrossRef
17. Ashabi G, Khodagholi F, Khalaj L, Goudarzvand M, Nasiri M. Activation of AMP-activated protein kinase by metformin protects against global cerebral ischemia in male rats: interference of AMPK/PGC-1α pathway. Metab Brain Dis. 2014;29(1):47–58. CrossRef
18. Li J, Benashski SE, Venna VR, McCullough LD. Effects of metformin in experimental stroke. Stroke. 2010;41(11):2645–52. CrossRef
19. Jia J, Cheng J, Ni J, Zhen X. Neuropharmacological actions of metformin in stroke. 2015;389–94. CrossRef
20. Cheng YY, Leu HB, Chen TJ, Chen CL, Kuo CH, Lee S Da, et al. Metformin-inclusive therapy reduces the risk of stroke in patients with diabetes: a 4-year follow-up study. J Stroke Cerebrovasc Dis. 2014;23(2):e99–105. CrossRef
21. Amato S, Man H ye. Bioenergy sensing in the brain the role of AMP-activated protein kinase in neuronal metabolism, development and neurological diseases. Cell Cycle. 2011;10(20):3452–60. CrossRef
22. Ashabi G, Khalaj L, Khodagholi F, Goudarzvand M, Sarkaki A. Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab Brain Dis. 2015;30(3):747–54. CrossRef
23. Choi IY, Ju C, Anthony Jalin AMA, Lee DI, Prather PL, Kim WK. Activation of cannabinoid CB2 receptor-mediated AMPK/CREB pathway reduces cerebral ischemic injury. Am J Pathol. 2013;182(3):928–39. CrossRef
24. Li J, McCullough LD. Effects of AMP-activated protein kinase in cerebral ischemia. J Cereb Blood Flow Metab. 2010;30(3):480–92. CrossRef
25. Jiang S, Li T, Ji T, Yi W, Yang Z, Wang S, et al. AMPK: Potential therapeutic target for ischemic stroke. Theranostics. 2018;8(16):4535–51. CrossRef
26. Cho SY, Kim EW, Park SJ, Phillips BU, Jeong J, Kim H, et al. Reconsidering repurposing: long-term metformin treatment impairs cognition in Alzheimer’s model mice. Transl Psychiatry. 2024;14(1):1–10. CrossRef
27. Khandelwal M, Manglani K, Upadhyay P, Azad M, Gupta S. AdipoRon induces AMPK activation and ameliorates Alzheimer’s like pathologies and associated cognitive impairment in APP/PS1 mice. Neurobiol Dis [Internet]. 2022;174:105876. CrossRef
28. Awad K, Sayed A, Banach M. Coenzyme Q10 reduces infarct size in animal models of myocardial ischemia-reperfusion injury: a meta-analysis and summary of underlying mechanisms. Front Cardiovasc Med. 2022;9:857364. CrossRef
29. Khan N, Abid M, Ahmad A, Abuzinadah M, Basheikh M, Kishore K. Cardioprotective effect of coenzyme Q10on apoptotic myocardial cell death by regulation of bcl-2 gene expression. J Pharmacol Pharmacother. 2017;8(3):122–7. CrossRef
30. Ishrat T, Khan MB, Hoda MN, Yousuf S, Ahmad M, Ansari MA, et al. Coenzyme Q10 modulates cognitive impairment against intracerebroventricular injection of streptozotocin in rats. Behav Brain Res. 2006;171(1):9–16. CrossRef
31. Attia HN, Maklad YA. Neuroprotective effects of coenzyme Q10 on paraquat-induced Parkinson’s disease in experimental animals. Behav Pharmacol. 2018;29(1):79–86. CrossRef
32. Hidalgo-Gutiérrez A, González-García P, Díaz-Casado ME, Barriocanal-Casado E, López-Herrador S, Quinzii CM, et al. Metabolic targets of coenzyme q10 in mitochondria. Antioxidants. 2021;10(4):1–15. CrossRef
33. Rauchová H. Coenzyme Q10 effects in neurological diseases. Physiol Res. 2021;70:683–714. CrossRef
34. Bagheri S, Haddadi R, Saki S, Kourosh-Arami M, Rashno M, Mojaver A, et al. Neuroprotective effects of coenzyme Q10 on neurological diseases: a review article. Front Neurosci. 2023;17:1188839. CrossRef
35. Abd El-Aal SAA, El-Fattah MAA, El-Abhar HS. CoQ10 augments rosuvastatin neuroprotective effect in a model of global ischemia via inhibition of NF-κB/JNK3/Bax and activation of Akt/FOXO3A/Bim cues. Front Pharmacol. 2017;8:1–15. CrossRef
36. Fatemi I, Askari PS, Hakimizadeh E, Kaeidi A, Moghaddam SE, Pak-Hashem M, et al. Chronic treatment with coenzyme Q10 mitigates the behavioral dysfunction of global cerebral ischemia/reperfusion injury in rats. Iran J Basic Med Sci. 2022;25(1):39–45. CrossRef
37. Nasoohi S, Simani L, Khodagholi F, Nikseresht S, Faizi M, Naderi N. Coenzyme Q10 supplementation improves acute outcomes of stroke in rats pretreated with atorvastatin. Nutr Neurosci. 2019;22(4):264–72. CrossRef
38. Jhun JY, Lee SH, Kim SY, Na HS, Kim EK, Kim JK, et al. Combination therapy with metformin and coenzyme Q10 in murine experimental autoimmune arthritis. Immunopharmacol Immunotoxicol. 2016;38(2):103–12. CrossRef
39. Al-Kuraishy HM, Al-Gareeb AI, Shams HA, Al-Mamorri F. Endothelial dysfunction and inflammatory biomarkers as a response factor of concurrent coenzyme Q10 add-on metformin in patients with type 2 diabetes mellitus. J Lab Physicians. 2019;11(04):317–22. CrossRef
40. Sun IO, Jin L, Jin J, Lim SW, Chung BH, Yang CW. The effects of addition of coenzyme q10 to metformin on sirolimus-induced diabetes mellitus. Korean J Intern Med. 2019;34(2):365–74. CrossRef
41. Iwasaki Y, Ito S, Suzuki M, Nagahori T, Yamamoto T, Konno H. Forebrain ischemia induced by temporary bilateral common carotid occlusion in normotensive rats. J Neurol Sci. 1989;90(2):155–65. CrossRef
42. Li Y, Chopp M, Chen J, Wang L, Gautam SC, Xu YX, et al. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab. 2000;20(9):1311–9. CrossRef
43. Akhoundzadeh K, Vakili A, Shadnoush M, Sadeghzadeh J. Effects of the oral ingestion of probiotics on brain damage in a transient model of focal cerebral ischemia in mice. Iran J Med Sci. 2018;43(1):32–40.
44. Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, et al. Object recognition test in mice. Nat Protoc. 2013;8(12):2531–7. CrossRef
45. Panta A, Montgomery K, Nicolas M, Mani KK, Sampath D, Sohrabji F. Mir363-3p treatment attenuates long-term cognitive deficits precipitated by an ischemic stroke in middle-aged female rats. Front Aging Neurosci. 2020;12:1–12. CrossRef
46. Sivakumaran MH, Mackenzie AK, Callan IR, Ainge JA, O’Connor AR. The discrimination ratio derived from novel object recognition tasks as a measure of recognition memory sensitivity, not bias. Sci Rep. 2018;8(1):1–12. CrossRef
47. Wahl F, Allix M, Plotkine M, Boulu RG. Neurological and behavioral outcomes of focal cerebral ischemia in rats. Stroke. 1992;23(2):267–72. CrossRef
48. Nade VS, Kawale LA, Dwivedi S, Yadav AV. Neuroprotective effect of Hibiscus rosa sinensis in an oxidative stress model of cerebral post-ischemic reperfusion injury in rats. Pharm Biol. 2010;48(7):822–7. CrossRef
49. Morales-Delgado N, Popovi? N, De la Cruz-Sánchez E, Caballero Bleda M, Popovi? M. Time-of-day and age impact on memory in elevated plus-maze test in rats. Front Behav Neurosci. 2018;12:10–4. CrossRef
50. Sharma AC, Kulkarni SK. Evaluation of learning and memory mechanisms employing elevated plus-maze in rats and mice. Prog Neuropsychopharmacol Biol Psychiatry. 1992;16(1):117–25. CrossRef
51. Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2, 3, 5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17(6):1304–8. CrossRef
52. Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186(C):421–31. CrossRef
53. Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. BBA—Gen Subj. 1979;582(1):67–78. CrossRef
54. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem [Internet]. 1951;193(1):265–75. CrossRef
55. Gürsoy-Özdemir Y, Bolay H, Sariba? O, Dalkara T. Role of endothelial nitric oxide generation and peroxynitrite formation in reperfusion injury after focal cerebral ischemia. Stroke. 2000;31(8):1974–81. CrossRef
56. Atif F, Seema Y, Iqbal S, Ishrat T, Hua F, Stein DG. Combination treatment with progesterone and vitamin D hormone is more effective than monotherapy in ischemic stroke: the role of BDNF/TrkB/Erk1/2 signaling in neuroprotection. Neuropharmacology. 2013;67:78–87. CrossRef
57. Qin A, Zhang Q, Wang J, Sayeed I, Stein DG. Is a combination of progesterone and chloroquine more effective than either alone in the treatment of cerebral ischemic injury? Restor Neurol Neurosci. 2019;37(1):1–10. CrossRef
58. Pei CZ, Zhang Y, Wang P, Zhang BJ, Fang L, Liu B, et al. Berberine alleviates oxidized low-density lipoprotein-induced macrophage activation by downregulating galectin-3 via the NF-κB and AMPK signaling pathways. Phyther Res. 2019;33(2):294–308. CrossRef
59. Abedimanesh N, Motlagh B, Abedimanesh S, Bathaie SZ, Separham A, Ostadrahimi A. Effects of crocin and saffron aqueous extract on gene expression of SIRT1, AMPK, LOX1, NF-κB, and MCP-1 in patients with coronary artery disease: a randomized placebo-controlled clinical trial. Phyther Res. 2020;34(5):1114–22. CrossRef
60. Dengler F. Activation of ampk under hypoxia: many roads leading to rome. Int J Mol Sci. 2020;21(7):1–15. CrossRef
61. Arbeláez-Quintero I, Palacios M. To use or not to use metformin in cerebral ischemia: a review of the application of metformin in stroke rodents. Stroke Res Treat. 2017;2017:9756429. CrossRef
62. Karimipour M, Shojaei Zarghani S, Mohajer Milani M, Soraya H. Pre-treatment with metformin in comparison with post-treatment reduces cerebral ischemia reperfusion induced injuries in rats. Bull Emerg Trauma. 2018;6(2):115–21. CrossRef
63. Zemgulyte G, Tanaka S, Hide I, Sakai N, Pampuscenko K, Borutaite V, et al. Evaluation of the effectiveness of post-stroke metformin treatment using permanent middle cerebral artery occlusion in rats. Pharmaceuticals. 2021;14(4):312. CrossRef
64. Zhang J, Wang Y, Liu X, Dagda RK, Zhang Y. How AMPK and PKA interplay to regulate mitochondrial function and survival in models of ischemia and diabetes. Oxid Med Cell Longev. 2017;2017(1):4353510. CrossRef
65. Dugan LL, You YH, Ali SS, Diamond-Stanic M, Miyamoto S, DeCleves AE, et al. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Invest. 2013;123(11):4888–99. CrossRef
66. Szewczuk M, Boguszewska K, Ka?mierczak-Bara?ska J, Karwowski BT. The role of AMPK in metabolism and its influence on DNA damage repair. Mol Biol Rep [Internet]. 2020;47(11):9075–86. CrossRef
67. Monsour M, Gordon J, Lockard G, Alayli A, Borlongan V. Mitochondria in cell-based therapy for stroke. Antioxidants. 2023;12(1):1–13. CrossRef
68. Zhang Y, Huang X, Liu N, Liu M, Sun C, Qi B, et al. Discovering the potential value of coenzyme Q10 in oxidative stress: enlightenment from a synthesis of clinical evidence based on various population. Front Pharmacol. 2022;13:936233. CrossRef
69. Dai S, Tian Z, Zhao D, Liang Y, Liu M, Liu Z, et al. Effects of coenzyme Q10 supplementation on biomarkers of oxidative stress in adults: a GRADE-assessed systematic review and updated meta-analysis of randomized controlled trials. Antioxidants. 2022;11(7):1360. CrossRef
70. Shetty RA, Ikonne US, Forster MJ, Sumien N. Coenzyme Q10 and α-tocopherol reversed age-associated functional impairments in mice. Exp Gerontol. 2014;58:208–18. CrossRef
71. Liu Y, Tang G, Li Y, Wang Y, Chen X, Gu X, et al. Metformin attenuates blood-brain barrier disruption in mice following middle cerebral artery occlusion. J Neuroinflammation. 2014;11:177. CrossRef
72. Kadry H, Noorani B, Bickel U, Abbruscato TJ, Cucullo L. Comparative assessment of in vitro BBB tight junction integrity following exposure to cigarette smoke and e-cigarette vapor: a quantitative evaluation of the protective effects of metformin using small-molecular-weight paracellular markers. Fluids Barriers CNS [Internet]. 2021;18(1):1–15. CrossRef
73. Ayengin K, Alp HH, Huyut Z, Y?ld?r?m S, Alt?ndag F, Avci V. The effects of CoQ10 supplement on matrix metalloproteinases, oxidative DNA damage and pro-inflammatory cytokines in testicular ischaemia/reperfusion injury in rats. Andrologia. 2021;53(2):1–14. CrossRef
74. Higashi Y, Aratake T, Shimizu T, Shimizu S, Saito M. Protective role of glutathione in the hippocampus after brain ischemia. Int J Mol Sci. 2021;22(15):7765. CrossRef
75. Block F, Schwarz M. Correlation between hippocampal neuronal damage and spatial learning deficit due to global ischemia. Pharmacol Biochem Behav. 1997;56(4):755–61. CrossRef
76. Wang X, Michaelis EK. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010;2(MAR):1–13. CrossRef
77. Hayashi Y. Molecular mechanism of hippocampal long-term potentiation—towards multiscale understanding of learning and memory. Neurosci Res. 2022;175(xxxx):3–15. CrossRef
78. You W, Knoops K, Berendschot TTJM, Benedikter BJ, Webers CAB, Reutelingsperger CPM, et al. PGC-1a mediated mitochondrial biogenesis promotes recovery and survival of neuronal cells from cellular degeneration. Cell Death Discov. 2024;10(1):1–15. CrossRef
79. Ahmadi-Soleimani SM, Ghasemi S, Rahmani MA, Gharaei M, Mohammadi Bezanaj M, Beheshti F. Oral administration of coenzyme Q10 ameliorates memory impairment induced by nicotine-ethanol abstinence through restoration of biochemical changes in male rat hippocampal tissues. Sci Rep [Internet]. 2024;14(1):1–12. CrossRef
80. Kim J, Kang H, Lee YB, Lee B, Lee D. A quantitative analysis of spontaneous alternation behaviors on a Y-maze reveals adverse effects of acute social isolation on spatial working memory. Sci Rep [Internet]. 2023;13(1):1–13. CrossRef