Introduction
Angiogenesis is a natural process of growing blood vessels from preexisting vasculature by the interaction of endothelial cells with cytokines, cellular matrix, and proteolytic enzymes. It occurs throughout the life of an individual to maintain the active state of tissues. It is intricately regulated by a balance of positive and negative factors, ensuring precise control over the formation of blood capillaries in healthy tissues [1]. Certain pathogenic conditions such as ischemic heart disease, peripheral arterial disease, ophthalmic conditions, and rheumatoid arthritis trigger abnormalities in the angiogenesis process [2].
Angiogenesis is also responsible for promoting the growth of solid tumors in the body. The growing mass of cells in a neoplasm creates a hypoxic microenvironment that leads to the release of proangiogenic factors namely; hypoxia-inducible factor 1 α (HIF 1 α), cyclooxygenase 2 (COX2), vascular endothelial growth factor (VEGF), and vascular endothelial growth factor receptors (VEGFRs). They promote the proliferation of endothelial cells and the formation of vascular networks ensuring the supply of blood to growing tumors [3]. It is closely associated with growth, metastasis, recurrence, and overall cancer prognosis. Anti-angiogenesis is therefore a desirable strategy used for targeting tumor suppression. Various drugs targeting the pathways of tumor angiogenesis have been developed. To date over 19 monoclonal antibodies and VEGF/VEGFR tyrosine kinase inhibitors are available for the treatment of metastatic colorectal and various other cancers [4]. However, less stability, resistance, and high cost pose limitations to their clinical efficacy. Moreover, the toxic side effects elicited by these drugs also restrict their applications. An increasing number of studies are being undertaken for the development of novel compounds to increase the effectiveness of anti-angiogenic therapies and overcome side effects [5]. Natural products offer an advantage in targeting specific angiogenesis pathways and enhancing the efficacy of VEGF/ VEGFR inhibitors for improvement in the outcome of cancer [6].
The marine environment is a focus of many novel natural products. Marine plants, animals, and microbes produce compounds that have pharmaceutical potential [7]. Many of these plants and animals live in densely populated habitats. They synthesize evolved chemical compounds that help to protect themselves and defend against predators. These naturally occurring chemical compounds have been identified to inhibit cell division which is a primary target for many anticancer drugs. The drugs Ara-C and Ara-A used in the treatment of acute myelocytic leukemia and herpes infection, respectively, are derived from the nucleosides of shallow water marine sponge [8].
The cephalopods represent an advanced and diverse group of marine animals. An Indian squid, Loligo duvauceli is a valuable cephalopod species characterized by a short life span and high culinary value. It is prevalent in the Indian Ocean, Red Sea, Arabian, Chinese, and Philippines seas, occurring at depths between 30 and 170 meters [9]. It contains ink glands that release ink as a defense to escape from predators. These ink sacs are discarded during their processing for consumption. The ink is a dark, cloudy liquid containing amino acids, carbohydrates, phenols, flavonoids, alkaloids, and saponins in addition to tannins and glycosides. It also shows the presence of proteins, minerals, taurin, hydroxyproline, dopamine, and enzymes [10]. Nisha [11] and Gajendra et. al. [12] have reported prominent antioxidant properties of squid ink which can be used for the retardation of lipid peroxidation and enhancement of food shelf-life [11,12]. Girija et al. [13] have fractionated the black ink and demonstrated antibacterial activity against pathogens and antifungal effect against Candida albicans [13]. Zhong et al. [14] investigated the effects of squid ink on the protection of the hematopoietic system against chemotherapeutic injury. Hermelin et al. [15] and Senan et al. [16] determined the anticarcinogenic activity of L. duvauceli ink on the HepG2 cancer cell line.
The presence of natural bioactive compounds namely; polyphenols, alkaloids, saponins, flavonoids, sulfated polysaccharides, and the pharmacological effects of squid ink are suggestive of its high medicinal value. Its potential for inhibiting angiogenesis has however not been investigated. In the present study, the angiogenesis inhibitory effect of L. duvauceli ink was assessed using a human umbilical cord endothelial cell line in vitro and, on a chick chorioallantoic membrane (CAM) model in vivo. Its effect on the expression of key angiogenic effectors HIF 1 α, VEGF, VEGFR 2, and COX 2 genes was investigated for assessment of the likely mechanism of action used in the inhibition of neovascularization. The study aimed to explore the pharmaceutical potential of this cephalopod ink as a natural angiogenesis inhibitor.
MATERIAL AND METHODS
Collection of L. duvauceli squid and isolation of ink
The use of L. duvauceli squid for The study protocol was approved by the Bharati Vidyapeeth Deemed to be University’s Institutional Animal Ethics Committee, Pune and the experiments were performed following CPCSEA guidelines. (Approval Number BVDUMC/1887/2018/002/016 and Date: 22.11.2018) The L. duvauceli squid was procured from the coastal regions of the state (Colaba, Maharashtra) and authenticated by Central Marine Fisheries Research Institute, Mumbai. Freshly obtained Squid were dissected and Ink glands were removed by cutting it from the siphon. The ink was collected in a sterile 15 ml centrifuge tube using the milking method. It was diluted with PBS in a ratio of 1:4. Diluted ink was centrifuged at 10,000 rpm for 20 minutes to separate the melanin pigment. The supernatant was collected and stored at −20°C. It was referred to as melanin-free ink (MFI) and diluted with sterile PBS to achieve 50%, 25%, 12.5%, 6.25%, 3.125%, and 1.56% concentrations for use in subsequent assays.
Cell culture
The cancer cell lines SiHa (Squamous cell carcinoma; Cervix) and MCF-7 (Adenocarcinoma, Breast; Mammary gland) were procured from the Animal Cell Repository at the National Centre for Cell Science, Pune, India. The Human Umbilical Cord Vein Endothelial Cell line (Normal, Umbilical vein; Vascular endothelium) (HUVEC) was procured from ATCC Cell Bio, Hi-Media Laboratories, India. The two cancer cell lines were maintained in DMEM (Hi-Media Laboratories) supplemented with 10% fetal bovine serum (FBS) (Invitrogen), 2mM L-glutamine, 50 Units/ml penicillin, and 50 µg/ml streptomycin (Hi-Media Laboratories). The HUVEC were grown in endothelial cell growth medium (Basal Medium, Hi Media) supplemented with 150 µg/ml of crude endothelial cell growth factor (ECGF), 5 U/ml of heparin, 100 IU/ml penicillin, 100 µg/ml streptomycin, and 20% FBS. The cell lines were grown at 37°C in a humidified 5% CO2 atmosphere.
Cytotoxicity assay (MTT)
Cytotoxic effect of various concentrations of MFI on two selected cancer and HUVEC cell lines were determined using colorimetric microculture assay with MTT (3-(4,5-dimethylthiazo-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) dye (Sigma-Aldrich). Cells were seeded at a density of 1 × 104 cells/well in 96 well microplates and allowed to adhere for 24 hours at 37°C in a CO2 incubator. The cells were then treated with various concentrations (1.56%–100%) of MFI for 48 hours. 10µl of MTT (5 mg/ml, Sigma-Aldrich) solution was added to each well and the plates were incubated for 4 hours in the dark in a CO2 incubator. The medium was then aspirated and the formazan crystals formed were solubilized by adding 100 µl of DMSO followed by 25 µl of glycine buffer per well. The optical density was measured at 570 nm (Bio-Rad, PR 4,100 plate reader). The experiment was performed in triplicate.
HUVEC cell tube formation assay
Tube formation assay was performed as described previously [17]. Briefly, 150 µl of growth factor reduced matrigel (BD bioscience) was layered in each well of 24 well plates. The plates were incubated at 37°C for 30 minutes to facilitate the polymerization. 5 x 104 HUVEC cells were seeded in each well and treated with four selected concentrations of (12.5%, 25%, 50%, and 100%) of MFI diluted in endothelial growth media in triplicates. The plates were incubated overnight at 37°C in a CO2 incubator. After 18 hours of incubation, the medium was removed and cells were fixed using methanol and stained with Giemsa. The plates were observed on a Zeiss light microscope for the formation of tubes and photographed.
Chick chorio-allantoic membrane (CAM) assay
The Fertilized white leghorn chicken eggs were obtained from a certified farm (Venkateshwara Hatcheries Pvt. Ltd. Pune-India) and incubated at 37°C having 60%–65% humidity in a position with tapering end below. After 3 days of incubation, 2 ml of albumin from the top end of each egg was aspirated with the help of a 20 G syringe needle. This is required to detach the developing CAM from the upper part of the shell. On the seventh day of incubation, a window of around 1.5–2 cm was opened on the blunt end. A sterile gel foam (Abcam) piece (3 mm x 3 mm x 1 mm) was positioned carefully on the CAM surface and 30 µl of the test sample (6.25, 12.5, 25, 50, and 100 % of MFI) was placed on it. In the control group, an equivalent quantity of sterile PBS was loaded on the gel foam. The positive control group received 2.5, 5, and 10 ng of vascular endothelial growth factor (VEGF, Himedia). Various concentrations of anti-angiogenic Itraconazole drug were used as a standard anti-angiogenic drug. The eggs were returned to the incubator and incubated undisturbed till the 12th day.
On the 12th day, the eggshell was removed and the CAM was separated for fixing in 10% formalin. The formalin-fixed CAM was analyzed by histopathology involving sectioning and hematoxylin & eosin (H&E) staining. The procedure described by Mathur et al. [18] was used for counting mean microvessel density. Blood vessels at the boundary between the sponge and surrounding CAM mesenchyme were counted in 5 consecutive fields of sections using the 40X objective of the microscope (Carl Zeiss Ltd., Germany). The number of blood vessels/unit area (mean microvessel density, MVD) for each group was defined using an objective having the least count of 0.01 mm (Carl Zeiss Ltd.) [18]. The experiment was conducted in triplicate, with each concentration tested in duplicate for increased reliability of the results.
Quantitative real-time PCR
1 x 106 cells of the HUVEC cell line were plated in each well of 24 well plates and incubated for 24 hours. The cells were then treated with 6.25%, 12.5%, 25%, and 50% MFI concentrations in duplicate and incubated for 48 hours. Total RNA was extracted from endothelial cells using Pure Link RNA Mini Kit by Ambion, Life Technologies (Thermo Fisher Scientific India Pvt. Ltd.) following the manufacturer’s instructions. The concentration of RNA was measured using a Nanodrop Biophotometer (Biophotometer Plus, Eppendorf, Germany). 2 μg of each RNA sample was reverse transcribed with the Super Script VILO cDNA Synthesis Kit (Invitrogen) according to the manufacturer’s protocol (25°C for 10 minutes, 42°C for 60 minutes, and termination at 85°C, 5 minutes). RT-qPCR was set up using the SYBR Green PCR Master Mix (Thermo Fisher Scientific), on the 7,500 Real-time PCR system (Thermo Fisher Scientific), along with cDNA and commercial primers (Sigma). The primers (Table 1) were designed for human VEGF, COX 2, HIF1-α and VEGFR2. GAPDH was used as an internal reference control. Each reaction was performed in duplicate. A negative control without a cDNA template was run simultaneously with every assay. Standard reactions (20 µl) consisted of 10 µl SYBR Green master mix (Applied Biosystems by Thermo Fisher Scientific), 1µl forward primer (10 µM), 1µl reverse primer (10 µM), 2 µl cDNA template (10 ng), and 6 µl of nuclease-free water. The quantitative real-time PCR was carried out using conditions given in Table 1. The results of gene expression were normalized to reference gene expression and the fold change was determined in comparison with untreated cell control. The quantification of relative mRNA expression was calculated using the 2-ΔΔct method [19]. Graphs were plotted based on log 2-fold change.
Statistical analysis
The statistical significance was calculated by ANOVA with Graph Pad Prism 6 software. The experiments were performed at three different times. The results were expressed as mean ± standard deviation (S.D.) and *, **, and *** indicated significance < 0.1, < 0.01, <0.001, respectively.
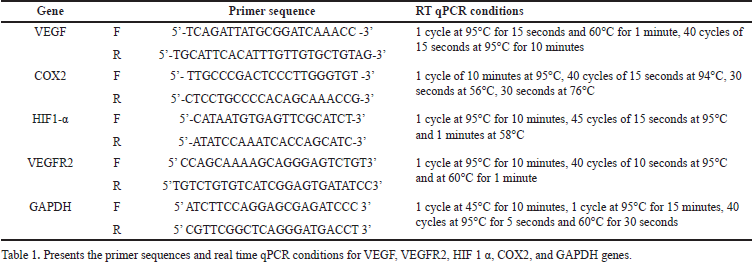 | Table 1. Primer sequences and operation conditions of real time qPCR. [Click here to view] |
RESULT AND DISCUSSION
Angiogenesis is a primary step involved in the growth of cancer tumors. At the early stage of neoplasm, a slight increase in tumor volume creates hypoxic conditions causing the release of proangiogenic factors. It promotes the formation of disorganized and immature vascular networks that nurture the subsequent growth of tumors. Treatment strategies focusing on endothelial cells and angiogenic promoters play a crucial role in addressing various types of solid tumors. The marine-derived products serve as a valuable reservoir of unique bioactive compounds, offering high efficacy and minimal toxicity. They are increasingly being explored for the development of novel therapeutic drugs.
The squid ink, a dark liquid ejected by cuttlefish to threaten predators has been demonstrated to be rich in functional bioactive compounds. The current investigation centered on examining its anti-angiogenic properties through viability, chick CAM, and HUVEC-based tube formation assays. The impact on the expression of key angiogenic mediator genes, namely VEGF, VEGFR, COX-2, and HIF1-α, was assessed using quantitative real-time PCR.
Preparation of squid ink extract
The ink from freshly collected L. duvauceli squid was collected by milking of the ink sacs. About 4 ml of ink was collected from a single squid and 3 squids were used to collect the milk. The ink was diluted 1:4 with PBS and centrifuged at 10,000 rpm for 20 minutes. to precipitate the melanin pigment. 10 ml of supernatant was stored at −20°C to serve as a melanin-free extract (MFI).
Cytotoxic effect of MFI on cancer cell lines
The antiproliferative effect ofMFI on MCF7 (human breast adenocarcinoma) and SiHa (human cervical squamous cell carcinoma) cell lines was evaluated through an MTT assay. In Fig 1A and B, the influence of various concentrations (1.56%–100%) of MFI extract on the percent cell viability of MCF-7 and SiHa cell lines is depicted. A dose-dependent decrease in the viability of cancer cells was observed, with IC50 values of 34% ± 2.2% for MCF7 and 38% ± 3.2% for SiHa cell lines. Notably, the 25% and 50% concentrations of MFI exhibited significant cytotoxic effects on cancer cells, surpassing the efficacy demonstrated by the ink of cuttlefish Sepia officinalis on the MCF7 cell line [20]. These findings align with the results reported by Hermelin et al. [15] and Moovendhan et al. [21] in studies of Loligo duveuceli squid ink involving HepG2 and A549 lung cancer cell lines, respectively [15,21]. The present study for the first time reported the influence of MFI on breast and cervical cancer cell lines, signalling its potential as a therapeutic agent against cancer. The findings highlight the need for additional investigations and validation to further elucidate its efficacy in cancer treatment.
Effect of MFI and standard drug Itraconazole on the viability of HUVEC cell line
The inhibitory effect of MFI on endothelial cells was assessed using an MTT assay. It exhibited a dose-dependent inhibitory effect on the HUVEC cell line, with an IC50 value of 13% ± 1.5% (Fig. 2A). The substantial cytotoxic effect could be observed from a concentration of 12.5% onwards. This effect was comparable with that demonstrated by the standard drug Itraconazole (Fig. 2B), with an IC50 value of 26 ± 4.2 µg/ml on the HUVEC cell line. Although direct comparison of the two values was not feasible due to differences in units (percent values for MFI and µg/ml for Itraconazole), the IC50 values suggest a significant potential of MFI to inhibit HUVEC cells, comparable to the effect exhibited by the standard drug. The results also indicate a higher ability of MFI to inhibit the HUVEC cell line than the cancer cell lines used in the present study.
Effect of MFI on differentiation of HUVEC cells using tube formation assay
HUVEC cells demonstrate a remarkable ability to generate capillary-like structures when cultured on Matrigel-coated surfaces [22]. Microscopic images presented in Figure 3 A–F were captured after an 18-hour incubation of Matrigel-layered HUVEC cells treated with MFI. The image of an untreated control well illustrates the complete formation of a tube network by the endothelial cells within 18 hours (Fig. 3A). Positive control VEGF (VEGF)-treated cells showed a pronounced formation of a tube network within the same incubation period (Fig. 3B). However, the formation of the tube network was altered in MFI-treated wells. Short and discontinuous branches of the tubes were observed at 12.5 % and 25 % concentrations (Fig. 3 C and D), while complete inhibition of tube formation was noted at 50 % and 100 % MFI concentrations respectively (Fig. 3 E and F). The standard drug Itraconazole (Fig. 4 A–D) exhibited total inhibition of tube formation from concentrations of 125 µg/ml onward.
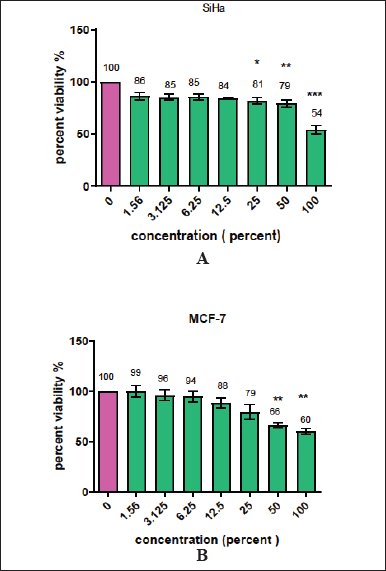 | Figure 1A and B. Show the cytotoxic effect of various concentrations (1.56%–100%) of MFI extract on SiHa and MCF-7 cell lines. The percent viability values at various concentrations of extract were calculated by comparing them with the control group. *, **, and *** stars indicate significance <0.1, <0.01, and <0.001, respectively. [Click here to view] |
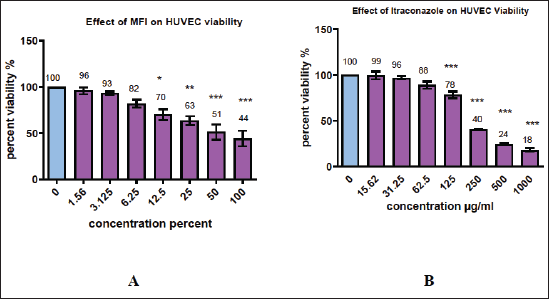 | Figure 2 A and B. Effect of various MFI and Itraconazole concentrations on HUVEC cell line. The percent viability values at various concentrations of extracts were calculated by comparing with the control group. Significant (30% ± 4.03%, 37% ± 3.2%, 49% ± 2.4%, and 56% ± 2.7%) reduction in viability of HUVEC cells was demonstrated by 12.5%, 25%, 50%, and 100% of MFI, respectively, and 22% ± 2.5%, 60% ± 1.1%, 76% ± 1.9%, and 82% ± 2.9% reduction in viability of HUVEC cells was demonstrated by 125, 250, 500, and 1000 µg/ml of Itraconazole. *, **, and *** indicate significance <0.1, <0.01 and <0.001, respectively. [Click here to view] |
A positive marker, VEGF used in this study resulted in prominent tube network formation whereas, significant dose-dependent inhibition of tube formation was noted in MFI-treated wells with a complete absence of tube formation at 50% and 100 % concentrations. The findings indicate a significant potential of MFI to impede the differentiation of endothelial cells. A similar inhibition has earlier been displayed by marine organisms Ianthella basta, Pseudoceratina Arabica, Rhabdastrella globostellata, and Agelas nakamurai during the elucidation of their antiangiogenic properties. These marine organisms are of considerable interest as prospective candidates for anticancer therapy [23,24].
Anti-angiogenic effect of MFI using CAM assay
The CAM serves as a highly sensitive in vivo model for the investigation of anti-angiogenic properties, with mean vascular density (MVD) acting as a marker for evaluating the extent of angiogenesis. The impact of MFI on the reduction of MVD was examined in the CAM model. In this study, various concentrations of MFI ranging from 6.25% to 100% were administered in duplicate on the CAM layer of seventh-day embryonated eggs. The evaluation of its effect on the reduction of blood vessels was observed on the twelfth day. The CAM tissues, fixed in formalin were analyzed by histopathology, and the number of blood vessels per unit area (MVD) was assessed. The normal control group (Fig. 5 A) displayed typical angiogenesis with a normal branching pattern in blood vessel formation. Figure 5A–D illustrates the effects of different concentrations (2.5, 5, and 10 ng) of VEGF, demonstrating an increase in MVD to 145% ± 4.32% and 175% ± 3.2%, respectively. This effect is characterized by the growth of numerous smaller vessels, indicated by arrows, signifying the pro-angiogenic influence of VEGF.
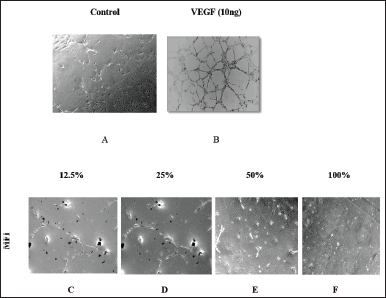 | Figure 3 A to F: A shows normal tube formation in control wells. B shows the image of VEGF treated well depicting an increase in tube formation. C, D, E, and F show the effects of various concentrations of MFI on the inhibition of tube formation. Complete absence of tube formation is seen in 50 % and 100 % concentration. [Click here to view] |
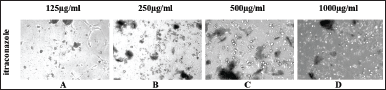 | Figure 4 A-D. Shows inhibition of tube formation from 125 µg/ml to 1,000 µg/ml concentration of standard drug Itraconazole. [Click here to view] |
 | Figure 5. Photograph showing histopathology images of CAM, (A) control group treated with phosphate buffer saline and (B, C, and D) treated with 2.5, 5, and 10ng concentrations of VEGF, respectively. [Click here to view] |
Figures 6 and 7 depict the outcomes of the standard drug itraconazole and the test drug MFI, respectively. In the case of 62.5 and 125 µg/ml Itraconazole-treated CAM (Fig. 6 B and C), there is a noticeable reduction in microvessel formation. However, concentrations of 250, 500, and 1,000 µg/ml resulted in necrotic CAM tissue with an absence of blood vessels (Fig. 6 D–F). The decrease in MVD ranged from 17% ± 3.2 % to 96% ± 1.2 % at the highest concentrations.
An equally prominent antiangiogenic effect was demonstrated by MFI in the CAM assay. Treatments with 6.25 %, 12.5 %, and 25 % of MFI resulted in the presence of few small blood vessels, indicating a decrease in MVD by 30% ± 3.73 %, 38% ± 1.5 %, and 63% ± 2.05 %, respectively (Fig. 7 B–D). In contrast, CAM treated with 50 % and 100 % of MFI displayed necrotic tissue and the absence of blood vessels (Fig 7 E and F), indicating a dose-dependent anti-angiogenic effect. The mean vessel density (MVD) of the VEGF, Itraconazole, and MFI-treated CAM groups is summarized in Fig 8 and Table 2. MFI demonstrated effectiveness in inducing a significant decrease in MVD at a concentration of 6.25%, with a remarkably prominent 94% ± 3.1% decrease in MVD observed at higher concentrations. This effect was comparable to the antiangiogenic drug itraconazole. These results highlight the significant antiangiogenic potential of MFI, aligning with findings reported by Subramanian et al. [25] and Luay et al. [26] for marine algae, specifically Bifurcaria bifurcate and Dictyota dichotoma, as well as for the Horn snail Telescopium telescopium, using the CAM assay [25,26].
Effect of MFI on the expression of angiogenesis effector genes
The study investigated the influence of MFI on key angiogenesis effector genes in the HUVEC cell line using quantitative real-time PCR. The genes assessed included HIF 1α, VEGF, VEGFR2, and COX-2, known to be associated with tumor angiogenesis [27]. The fold change in the expression level of target genes is presented in Fig 9. The results revealed a substantial downregulation in the expression of VEGF, VEGFR2, and HIF 1α genes, starting from a 6.25% concentration of MFI. A significant inhibition, with 1.3, 1.7, and 3.6-fold downregulation of VEGF, and 2.8, 3.1, and 4.3-fold downregulation of VEGFR2 were observed at 6.25, 12.5, 25 and 50 % MFI concentrations respectively. Additionally, the HIF 1α gene exhibited a 2.1, 3.6, and 2.8-fold downregulation. A similar effect on the COX 2 gene expression was however not observed. These findings suggest the MFI to be a potent inhibitor of the critical angiogenic regulatory HIF1-α, VEGF, and VEGFR2 genes.
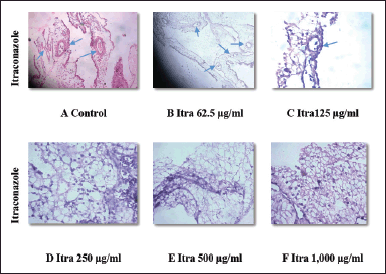 | Figure 6 A-F. Denotes histopathology images of CAM, (A) after treatment with phosphate buffer saline (control) and different concentrations (62.5, 125, 250, 500, and 1,000µg/ml) of standard drug itraconazole. B and C show a decrease in micro vessel formation whereas D, E, and F show necrotic tissues after treatment with itraconazole. [Click here to view] |
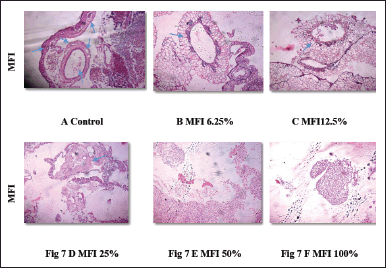 | Figure 7 A-F. denotes histopathology images of CAM; (A) after treatment with phosphate buffer saline (control) and (B, C, and D) with different concentrations of MFI treatment. B, C, and D show decrease in micro vessel formation, while E and F show necrotic tissues and no micro vessel formation. [Click here to view] |
An increasing number of studies have shown that the restricted oxygen supply to the tumor tissues significantly contributes to the proliferation and metastasis of cancer. It activates hypoxia–inducible factor (HIF1α) which is a heterodimeric protein comprising of HIF 1 α and HIF 1 β subunits. The presence of oxygen promotes the binding of HIF1α to a Von Hipper-Lindau tumor suppressor leading to its proteasomal degradation. In the hypoxic conditions caused by an increase in tumor size, HIF1α binds to the HIF1β subunit and inactivates enzyme proline hydroxylase to prevent normal HIF 1 α protein degradation. It translocates to the nucleus and promotes efficient transcription of proangiogenic VEGF, erythropoietin, GLUT-1, TGF β, PDGF β, and plasminogen activator genes. It results in neo-vascularization, cell proliferation, survival, and metastasis of cancer. Therefore, upregulation of HIF 1 α is strongly associated with poor prognosis in patients with different solid tumors [28]. The pharmacological targeting of HIF1 α is considered as a promising cancer therapeutic strategy in recent years. Moreover, HIF1α inhibition in neuroblastoma tumors has shown a correlation with an increase in sensitivity to antiangiogenic drugs in mice [29]. The MFI in the present study has displayed a remarkable potential to inhibit HIF-1α, a master regulator of tumor angiogenesis, and hence presents itself as a novel natural inhibitor of angiogenesis having potential for further exploration.
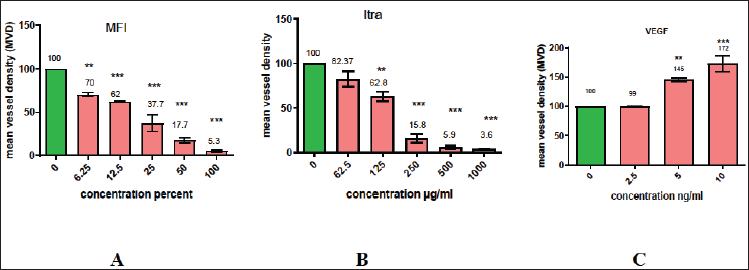 | Figure 8 A–C. Graphically represents the effect of MFI, Itraconazole, and VEGF on mean vessel density (MVD) of chick CAM. Significant 30% ± 3.73%, 38% ± 1.5%, 63% ± 2.05%, 83% ± 2.5%, and 95% ± 2.5% reduction is shown by 6.25%, 12.5%, 25%, 50%, and 100% of MFI and 17% ± 3.2%, 37% ± 2.2%, 84% ± 3.3%, 94% ± 3.1%, and 96% ± 1.2% by 62.5, 125, 250,500, and 1,000 µg/ml of Itraconazole respectively. Significant increase (45% ± 3.2% and 72% ± 2.2%) in MVD is observed in VEGF treated CAM layer. *, **, and *** indicate significance <0.1, <0.01, and <0.001, respectively. [Click here to view] |
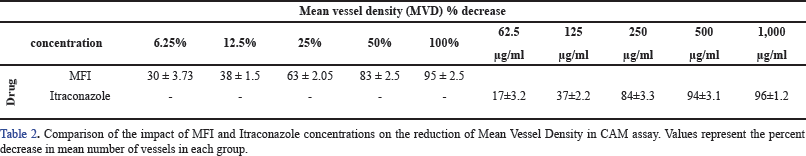 | Table 2. Comparison of the impact of MFI and itraconazole on mean vessel density. [Click here to view] |
VEGF and its receptor VEGFR are the major transcriptional targets of HIF-1 α. Expression of VEGF activates the pathway leading to tumor angiogenesis. The VEGF ligands bind to three types of endothelial receptor tyrosine kinases namely, VEGFR 1, VEGFR 2, and VEGFR 3 with different affinities. Activation of the VEGF-receptor pathway is mediated by its binding to VEGFR 2 which promotes endothelial cell growth and migration [30]. The tumor angiogenesis process results in the formation of unusual vessels that are disorganized, irregular, leaky, and hemorrhagic. This causes suboptimal blood flow resulting in hypoxia which in turn again triggers HIF and VEGF pathways. Hu et al. [31] reported an increase in the effectiveness of anti-VEGF antibody bevacizumab when used in combination with chemotherapy in patients with metastatic colorectal cancer and thus validated VEGF pathway inhibitors as an important treatment modality in cancer therapy [31]. The present study has shown MFI to be a potent inhibitor of VEGF and VEGFR2 highlighting its important role in the exploration of drugs for targeting this pathway.
In the present study, the test drug MFI displayed no effect on COX-2 gene expression. Cyclooxygenase-2 (COX-2) is another factor upregulated under hypoxic conditions. It is overexpressed in some malignancies and associated with the release of VEGF in HIF 1 α independent manner mediating angiogenesis. Elevated COX-2 expression correlated with a decreased overall survival [32]. Our present results suggest that MFI is a selective inhibitor of the HIF 1α mediated VEGF angiogenesis process and may not have an influence on HIF 2α which is another protein of hypoxic stress response. HIF 1 α and HIF 2 α regulate a set of overlapping and distinct target genes in both physiologic and pathologic conditions [33]. The COX2 expression is activated by HIF 2 α and is not affected by cephalopod ink tested in this study suggesting its selectivity for HIF 1 α. This natural product is thus of further interest to investigate the mechanism for inhibition of angiogenesis and specific targeting of HIF 1 α mediated pathways.
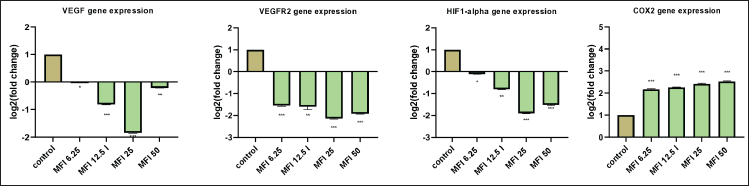 | Figure 9. Bar graph showing the effect of MFI on VEGF and VEGFR2, HIF1-α, and COX 2 gene expression evaluated by real-time qPCR. 1., 1.7, 3.6, and 1.16-fold downregulation of VEGF; 2.8, 3.1, 4.3, and 3.7-fold of VEGFR2; 2.1, 3.6, and 2.8 fold of HIF 1-α; and 0.16, 0.17, 0.19, 0.2 fold increase in expression of COX2 gene at the treatment concentrations of 6.25 %, 12.5 %, 25 %, and 50 % of MFI, respectively. [Click here to view] |
Overall, the outcomes from in vitro HUVEC tube formation assay, in vivo chick CAM assay, and the evaluation of proangiogenic gene expression collectively highlighted a substantial antiangiogenic activity associated with the MFI derived from L. duvauceli squid ink.
SUMMARY
To summarize, the present study has evaluated L. duvauceli squid ink for effect on the angiogenesis process using in vitro HUVEC-based viability and tube formation assays as well as in vivo CAM assay. Its potential to arrest the expression of angiogenesis promoter genes HIF 1α, COX2, VEGF, and VEGFR was also measured. A definitive inhibition of tube formation with a complete absence of tubes at 50 % and 100 % MFI concentrations was noted. Up to 95% decline in MVD was observed in the CAM assay which was very similar to the effect shown by standard drug Itraconazole. Furthermore, a very significant downregulation of proangiogenic factors HIF-1 α, VEGF, and VEGFR was displayed by this natural compound. Its neutrality to another hypoxia-induced proangiogenic factor COX 2 is indicative of the utilization of selective molecular mechanism for exerting an antiangiogenic effect. The study pointed out a very significant pharmaceutical role of this seafood processing industry waste product.
CONCLUSION
The present study aimed to investigate the Loligo duveuceli squid ink (MFI) for its ability to influence angiogenesis. While it had a moderate impact on the viability of cancer MCF 7 and SiHa cell lines, it displayed a prominent ability to inhibit angiogenesis in HUVEC cell-based tube formation assay, CAM assay, and gene expression studies. The study pointed out the profound potential of MFI to inhibit angiogenesis which could be explored for the presence of bioactive compounds, the mechanism of angiogenesis inhibition, and the development of novel drugs.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by the Bharati Vidyapeeth Deemed to be University’s Institutional Animal Ethics Committee, Pune and the experiments were performed following CPCSEA guidelines. (Approval Number BVDUMC/1887/2018/002/016 and Date: 22.11.2018).
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Otrock ZK, Mahfouz RA, Makarem JA, Shamseddine AI. Understanding the biology of angiogenesis: review of the most important molecular mechanisms. Blood Cells Mol Dis. 2007;39(2):212–20. CrossRef
2. Yoo SY, Kwon SM. Angiogenesis and its therapeutic opportunities. Mediators Inflamm. 2013;2013:127170. CrossRef
3. Teleanu RI, Chircov C, Grumezescu AM, Teleanu DM. Tumor angiogenesis and anti-angiogenic strategies for cancer treatment. J Clin Med. 2019 Dec 29;9(1):84. CrossRef
4. Oguntade AS, Al-Amodi F, Alrumayh A, Alobaida M, Bwalya M. Anti-angiogenesis in cancer therapeutics: the magic bullet. J Egypt Natl Canc Inst. 2021 Jul 2;33(1):15. CrossRef
5. Lopes-Coelho F, Martins F, Pereira SA, Serpa J. Anti-angiogenic therapy: current challenges and future perspectives. Int J Mol Sci. 2021 Apr 5;22(7):3765. CrossRef
6. Li R, Song X, Guo Y, Song P, Duan D, Chen ZS. Natural products: a promising therapeutics for targeting tumor angiogenesis. Front Oncol. 2021 Oct 22;11:772915. CrossRef
7. Karthikeyan A, Joseph A, Nair BG. Promising bioactive compounds from the marine environment and their potential effects on various diseases. J Genet Eng Biotechnol. 2022 Jan 26;20(1):14. CrossRef
8. Barreca M, Spanò V, Montalbano A, Cueto M, Díaz Marrero AR, Deniz I, et al. Marine anticancer agents: an overview with a particular focus on their chemical classes. Mar Drugs. 2020 Dec 4;18(12):619. CrossRef
9. Sabrah MM, Aly Y. El-Sayed, Azza A. El-Ganiny. Fishery and population characteristics of the Indian squids Loligo duvauceli Orbigny, 1848 from trawl survey along the north-west Red Sea. Egypt J Aquat Res. 2015;41(3):279–85. CrossRef
10. Nadarajah SK, Vijayaraj R, Mani J. Therapeutic significance of Loligo vulgaris (Lamarck, 1798) ink extract: a biomedical approach. Pharmacogn Res. 2017 Dec;9 (Suppl 1):S105–9. CrossRef
11. Nisha N, Suja S. Phytochemical evaluation and antioxidant activity of methanol extract of Loligo duvauceli ink. J Pharmacogn Phytochem. 2018;7(1):1764–67.
12. Gajendra Raju CV, Sarojini A Amitha, Lakshmisha IP, Arun Kumar P. Antioxidant activity of melanin free ink (MFI) extract from the ink sac of Loligo duvauceli.J Entomol Zool Stud. 2020;8(4):1388–92.
13. Girija S, Duraipandiyan V, Kuppusamy PS, Gajendran H, Rajagopal R. Chromatographic characterization and GC-MS evaluation of the bioactive constituents with antimicrobial potential from the pigmented ink of Loligo duvauceli. Int Sch Res Notices. 2014 Nov 10;2014:820745. CrossRef
14. Zhong JP, Wang G, Shang JH, Pan JQ, Li K, Huang Y, et al. Protective effects of squid ink extract towards hemopoietic injuries induced by cyclophosphamine. Mar Drugs. 2009;7(1):9–18. CrossRef
15. Hermelin J, Diaz JHJ, Thilaga RD, Sivakumar V. Cytotoxic activity of crude and partially purified ink of L. duvauceli towards HepG2 cell line. Int J Pharm Res Rev. 2014;3(6):19–23. CrossRef
16. Senan VP, Sherief PM, Nair JR. Cytotoxic effect of ink extracts of cuttlefish and squid on chick embryo fibroblasts. Int J Pharm Sci Res. 2013;4(5):1893–96. CrossRef
17. Varinská L, Fáber L, Kello M, Petrovová E, Balážová ?, Solár P, et al. β-Escin effectively modulates HUVECS proliferation and tube formation. Molecules. 2018 Jan 17;23(1):197. CrossRef
18. Mathur R, Gupta, SK, Singh N, Mathur S, Kochupillai V, Velpandian T. Evaluation of the effect of Withania somnifera root extracts on cell cycle and angiogenesis. J Ethnopharmacol.2006;105(3):336–41. CrossRef
19. Ganger MT, Dietz GD, Ewing SJ. A common base method for analysis of qPCR data and the application of simple blocking in qPCR experiments. BMC Bioinformatics. 2017 Dec 1;18(1):534. CrossRef
20. Khudhair IH, Suker DK, Hanna BA, Khudair IH, Marina B. Cytotoxic effect of ink extracted from cephalopoda on cancer cell line corresponding author. Sci J Med Res. 2019;03:139–45. CrossRef
21. Meivelu Moovendhan, Seedevi P, Vairamani S, Shanmugam A. Exploring the chemical composition and anticancer potential of oil from squid (Loligo duvauceli) liver waste from fish processing industry. Waste Biomass Valor. 2019;10:2967–73. CrossRef
22. Gentile MT, Pastorino O, Bifulco M, Colucci-D’Amato L. HUVEC tube-formation assay to evaluate the impact of natural products on angiogenesis. J Vis Exp. 2019;24(148):e58591. CrossRef
23. Gupta P, Arumugam M, Azad RV, Saxena R, Ghose S, Biswas NR, et al. Screening of antiangiogenic potential of twenty-two marine invertebrate extracts of phylum Mollusca from South East Coast of India. Asian Pac J Trop Biomed. 2014 May;4(Suppl 1):S129–38. CrossRef
24. Senthilkumar K, Jayachandran Venkatesan, Panchanathan Manivasagan, Se-Kwon Kim. Antiangiogenic effects of marine sponge derived compounds on cancer. Environ Toxicol Pharmacol. 2013;36(3):1097–108. CrossRef
25. Subramanian U, Kishorekumar MS, Muthuraman S, Munusamy AP, Sundaram R. Marine algal secondary metabolites promising anti-angiogenesis factor against retinal neovascularization in CAM model. Res Rev J Life Sci. 2018;8:19–25.
26. Luay MAM, Gonzaga MFR, Po SKD, Arollado EC. Determination of the antiangiogenic activity of telescopium telescopium (horn snail) extract using in ovo chorioallantoic membrane (CAM) assay. Acta Medica Philippina. 2018;52(4):366–73. CrossRef
27. Lee SH, Jeong D, Han YS, Baek MJ. Pivotal role of vascular endothelial growth factor pathway in tumor angiogenesis. Ann Surg Treat Res. 2015 Jul;89(1):1–8. CrossRef
28. Zimna A, Kurpisz M. Hypoxia-Inducible factor-1 in physiological and pathophysiological angiogenesis: applications and therapies. Biomed Res Int. 2015;2015:549412. CrossRef
29. Hartwich J, Orr WS, Ng CY, Spence Y, Morton C, Davidoff AM. HIF-1α activation mediates resistance to anti-angiogenic therapy in neuroblastoma xenografts. J Pediatr Surg. 2013 Jan;48(1):39–46. CrossRef
30. Liu ZL, Chen HH, Zheng LL, Sun LP, Shi L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct Target Ther. 2023 May 11;8(1):198. CrossRef
31. Hu W, Xu WS, Liao XF, He HJ. Bevacizumab in combination with first-line chemotherapy in patients with metastatic colorectal cancer: a meta-analysis. Minerva Chirurgica. 2015;70(6):451–58.
32. Lin PC, Lin YJ, Lee CT, Liu HS, Lee JC. Cyclooxygenase-2 expression in the tumor environment is associated with poor prognosis in colorectal cancer patients. Oncol Lett. 2013 Sep;6(3):733–9. CrossRef
33. Xue X, Shah YM. Hypoxia-inducible factor-2α is essential in activating the COX2/mPGES-1/PGE2 signaling axis in colon cancer. Carcinogenesis. 2013 Jan;34(1):163–9. CrossRef