INTRODUCTION
Following a tooth extraction, the alveolar bone may undergo resorption, leading to an ongoing alteration in its shape and a decrease in size. The alterations happening in the alveolar bone are commonly known as residual ridge resorption. Dentists face further challenges with implants due to the excessive resorption in the alveolar bone and the positioning and longevity of the implants. Chrcanovic's study investigated the implant failure rate in type E resorption bone, according to the Lekholm and Zarb classification, which pertains to severe resorption of the alveolar bone; from the 295 implants placed, 56 (18.98%) eventually failed [1]. Therefore, grafts were necessary to stimulate the formation of new bone. Several alternative materials can be used to achieve this: allogeneic grafts, artificial bone grafts, polymers, growth factors, and biocompatible tissue engineering products (scaffold). Recent advancements in biomaterials have increased interest in the final two procedures [2]. The porous scaffold is a template for bone regeneration and new bone formation. The scaffold biomaterial replaces the biological and mechanical functions of the extracellular matrix of tissues in the body by acting as an artificial extracellular matrix. Therefore, the synthetic scaffold must have osteoinductive and osteoconductive properties, high mechanical integrity, biodegradability, biocompatibility (readily accepted by the body's immune system), and porosity, leading to bone tissue growth. In addition, the scaffold must be degraded when the damaged tissue has been regenerated [3].
The reported quantity of Rajungan shells from Indonesia reached as high as 302,107.5 tons, posing a threat of human disturbance. To mitigate this waste, the Rajungan shells, which have a high content of calcium carbonate (40%–70%), were transformed into calcium hydroxyapatite [Ca10(PO4)6(OH)2]. Calcium hydroxyapatite is a calcium phosphate ceramic that is highly biocompatible and non-toxic, and it plays a crucial role in supporting the survival of bone and tooth tissue. Calcium hydroxyapatite has shown significant effectiveness in bone formation, as its components are similar to the inorganic components of bone tissue [2,4]. This material is expected to be the alternative source of hydroxyapatite while reducing crab shell waste. Little is known about this material being used as a scaffold. In this study, we observed the calcium hydroxyapatite (HA) scaffold use derived from Portunus pelagicus shells towards the expression of osteonectin and VEGF-B in the tooth extraction socket.
Physiologically, bone turnover is categorized into two phases: modeling, which takes place during development, and remodeling, an ongoing process that involves repairing bone tissue. Remodeling begins with osteoclasts removing the bone matrix in a process called resorption, followed by the recruitment of osteoblasts to other areas of the resorption to produce and mineralize a new matrix. Osteoblasts are a type of mesenchyme cell whose differentiation process is crucial for the ossification process because of their ability to produce organic substances within cells or the matrix that undergo calcification [4]. Osteonectin, a bone-specific protein secreted by osteoblasts during bone formation, selectively binds to HA and collagen and has a molecular weight of 32,000 daltons [5].
One factor with an essential role in bone healing is angiogenesis. The two primary regulatory pathways that regulate angiogenesis are the angiopoietin route and the Vascular Endothelial Growth Factor (VEGF) [6]. The homodimeric protein family, which includes at least six more members in addition to VEGF, has VEGF-A (VEGF), VEGF-B, VEGF-C, VEGF-D, VEGF-E, and placental growth factor. VEGF also increases the expression of growth factors and endothelial cell cytokines, increasing the number of nodules and alkaline phosphatase activity, culminating in osteoblasts' stimulation, migration, and proliferation. VEGF also helps inhibit the apoptosis process of osteoblasts, resulting in the mineralization of the osteoid matrix, which triggers bone regeneration [7]. Because of specific bindings to HA from a scaffold and its essential role in the mineralization of the osteoid matrix, osteonectin, and VEGF-B expression were chosen to be observed. The primary objective of this research was to investigate the effects of the HA scaffold derived from crab shells (Portunus pelagicus) on bone tissue regeneration in Cavia cobaya subjects. The novelty of this research lies in the utilization of waste crab shells to create a scaffold for bone regeneration, showcasing an innovative and sustainable approach to biomaterial development. Another novel aspect is the examination of VEGF-B and osteonectin expressions in response to the scaffold treatment, providing valuable insights into the molecular mechanism involved in bone healing.
MATERIAL AND METHODS
This research has received ethical clearance number 557/HRECC.FODM/VIII/2019, using Portunus pelagicus crab shells to be processed as a scaffold and Cavia cobaya as an experimental animal. Portunus pelagicus crab shells were used according to the ethical aspect of the experiment. The crab shells were obtained from export commodity waste; therefore, the researchers did not harm the animal. For tooth extraction in Cavia cobaya, the tooth extraction and termination process was done carefully and minimized the pain [8].
This research adopted an experimental laboratory methodology incorporating a post-test-only control group design. The design included a control group containing untreated Cavia cobaya and a Cavia cobaya group to which HA scaffold from crab shells (Portunus pelagicus) was applied.
Sample calculation
This research involved four groups (although, according to Lemeshow’s formula, there should be at least five) with 77 available samples. Each study group contained seven healthy and active male Cavia cobaya subjects, aged 3–3, 5 months, 300–350 g in weight, with a normal appetite, no injured limbs, skin defects, deformities, or a limp. They were acclimatized for a week before the study began; each cage consisted of 7 Cavia cobaya. They were kept at ambient temperature and fed regular pellets with unlimited access to water during a 12-hour light/dark cycle. Their senses, gait, and body temperature were all average. The research subjects were managed at the Department of Biochemistry, Faculty of Medicine Universitas Airlangga, where they were prepared and treated for 7 or 14 days.
Sample preparation
We obtained shell waste (Portunus pelagicus) from the local market at Semedu Sari, Grati, Pasuruan, East Java, cleaned of soft tissue using distilled water, soaked in a solution of 0,6% chlorine, washed in 3% H2O2 for 24 hours and dried in a warm environment. Afterward, by heating a furnace, the shell calcination procedure was performed at a temperature of 1,000°C (Perkin Elmer lambda 25 no.101n3070801). The initial heating temperature of approximately 50°C was increased gradually at a rate of 5°C/minute, with the temperature kept constant at 1,000°C for 2 hours. The temperature then automatically dropped to approximately 100°C. The HA compound was characterized using SEM-EDX (Phenom, Pro-X), and then, we obtained crab shell HA powder with an approximate size of 150–350 μm by sieving through a mesh (Tyler Co., Cleveland, Ohio).
Scaffold preparation
Five grams of gelatin (Merck, Germany) were added to distilled water and stirred at 40°C for 1 hour. The HA-gelatin composite was prepared by adding 1.5 g of hydroxyapatite powder to the gelatin solution and stirring for 6 hours. The mixture was then centrifuged for 10 minutes to separate the water and gel. The gel solution was poured into pre-prepared molds with a diameter of 5 mm and a height of 2 mm, then frozen at −80°C for 24 hours. Subsequently, freeze-drying was performed for 24 hours to obtain the scaffold in a sponge-like form.
Application of HA scaffold
Cavia cobaya specimens were administered anesthesia using a combination of ketamine (Kepro, ZA, Denmark) and xylazine (Castran, Venray, Holland) at a dosage of 0.1 ml per 10 g of body weight [9]. Following the acquisition of the HA scaffold, the subjects were prepared for dental extraction and subsequently treated with a gelatin scaffold that incorporated HA, measuring 150 microns, which was implanted into the extraction socket of Cavia cobaya. The mandibular left incisor was extracted, and the wound site received specific treatment tailored to each experimental group. Post-extraction, all Cavia cobaya in both the control and treatment groups had their wound sites sutured with sterile polyamide monofilament sewing thread from the Braun Aesculap brand, characterized as DS 12 3/8 c, 12 mm, 6/10 met, and 0.7. measuring DS 12 3/8 c, 12 mm, 6/10 met, and 0.7.
Collection of bone tissue samples
Termination of Cavia cobaya in group I and III members was conducted on the seventh day after tooth extraction. On the 14th day following a tooth extraction, the Cavia cobaya in groups II and IV were euthanized with a 0.2cc dose of ketamine administered from 100 mg/cc packs (Pfizer®). The mandible was then removed. The preparation consisted of hard mandibular bones that required prior decalcification with EDTA over approximately 2 months. At room temperature, mandibular fragments were placed in a 10% formalin buffer for 24 hours. After softening the mandibular bone tissue, the mandible surrounding the socket of the left lower incisor was cut into a small, roughly rectangular shape. The biopsy material was sliced into pieces measuring 1 × 1 × 1 / 2 cm and then dehydrated by successive 15-minute immersion in alcohol at graded concentrations of 70%, 80%, 90%, 95%, and 100%. The clearing was performed by inserting the dehydrated material into the xylol solution for 2 × 30 minutes. Embedding was executed for 2 × 30 minutes with a paraffin solution at 56°C. The paraffin block was placed on a glass slide after being cut with a microtome rotary to a thickness of approximately 4 microns. The terminated Cavia cobaya were then buried.
Immunohistochemistry staining
Slide preparation was carried out at the Airlangga University's Research Center. Deparaffinization was carried out by dissolving in xylol for 2 × 3 minutes. The remaining xylol was washed with absolute alcohol at concentrations of 99%, 95%, 90%, 80%, and 70%, each for 2 × 1 minutes. The residual alcohol was removed using running water before the preparations were put in a glass box with citrate buffer and autoclaved for 15 minutes to maximize its antigenicity. After drying, the preparation was delineated with a pap pen after cooling at room temperature for an hour. Before being incubated with 0.3% hydrogen peroxide for 15 minutes, the preparation was rinsed with H2O for 5 minutes and PBS for 5 minutes. The preparation was then immersed in a blocking solution for 30 minutes to halt the avidin in the tissue after the endogenous peroxidase had been inhibited. The preparation was incubated overnight at a temperature of −4°C with Osteonectin antibody primers (Santacruz biotech, Cat#73472, USA) and VEGF-B (Bioss, Cat#bs-10072R, USA) diluted to a ratio of 1:100.
Before being incubated with the secondary antibody and streptavidin for 30 minutes each, the preparation was rinsed three more times with H2O. The experiment was then dehydrated using alcohol, which was gradually increased in concentration in four stages for 2 minutes each (70%, 80%, 90%, and 100%) before being submerged in xylene for 5 minutes. Finally, the preparation was added to Entellan (Merck, German - Gmbh) before being enclosed with a glass cover. The results of the immunohistochemical examination were then grouped according to the number of cells colored by the stain.
Measurement of VEGF-B and osteonectin expression
Immunohistochemical examination and calculation of VEGF-B and osteonectin expression were conducted at the Faculty of Medicine, Universitas Brawijaya. Observation of preparations and analysis of the number of osteonectin and VEGF-B expressions were undertaken using a light microscope at 1,000x magnification. Observing 20 fields of view at the base of the extraction socket proved necessary to ensure accurate representation and reduce potential errors in the results. Those Cavia cobaya osteoblasts express osteonectin with a brown oval circle attached to osteoclasts. FGF2 induces VEGF-B expression with a brown appearance.
Statistical analysis
Data calculation was completed using the R Core Team (R Core Team, Vienna, Austria). A Shapiro-Wilk normality test and a Levene's homogeneity test were carried out based on the analysis of the number of osteonectin and VEGF-B expressions. A Multivariate ANOVA and Pearson tests were conducted to establish the correlation between VEGF-B and Osteonectin.
RESULTS AND DISCUSSION
Osteonectin
The mean level of osteonectin results showed that the lowest occurred in the control group without providing an HA scaffold on the seventh day, while the highest was found in the treatment group with the condition of HA scaffold on the 14th day (Fig. 1).
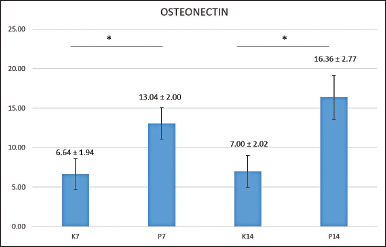 | Figure 1. Mean of osteonectin expressions found in post-extraction sockets of control groups observed after 7 (K7) and 14 days (K14); and the treatment groups observed after 7 (P7) and 14 days (P14). *Statistical difference from control group (p < 0.05) by Tukey HSD analysis. [Click here to view] |
The research data were subjected to a Shapiro-Wilk normality test, which revealed that the data for the entire sample were normally distributed (p > 0.05). A Levene's homogeneity test was subsequently conducted. All research groups produced normal data; the distribution was homogeneous with p = 0.545 (p > 0.05). The data were subjected to a further parametric test, a one-way ANOVA test, which produced a value of p = 0.000 (p < 0.05), indicating a significant difference in the amount of osteonectin between the control and treatment groups. A subsequent Tukey HSD multiple comparison tests performed to identify the differences between groups' locations produced the results in Figure 1.
Figure 1 above shows the significance comparisons between the seventh-day control group and the seventh-day treatment group, and between the 14th-day control group and the 14th-day treatment group, with a symbol denoting significance (*).
The following are images of osteonectin expression in the tooth extraction socket (Cavia cobaya) produced by immunohistochemical examination using a 1,000x magnification light microscope (Fig. 2).
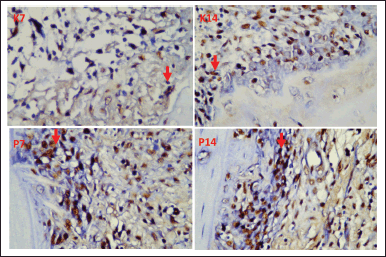 | Figure 2. Red arrows show an overview of osteonectin expression in the tooth extraction sockets of Cavia cobaya in K7 (day seven control group), K14 (day 14 control group), P7 (day seven treatment group), P14 (day 14 treatment group). [Click here to view] |
VEGF-B
A mean calculation of VEGF-B was carried out, which produced the following results: the lowest level of VEGF-B was found on day 14 in the control group, which had not received the HA scaffold and the highest occurred in the treatment group on day 14 following administering the HA scaffold ( Fig. 3). Shapiro-Wilk normality testing was first performed on the research data, revealing that all samples had normal distributions (p > 0.05) and Levene's homogeneity test. All research groups produced normal data; the distribution was homogeneous with p = 0.369 (p > 0.05).
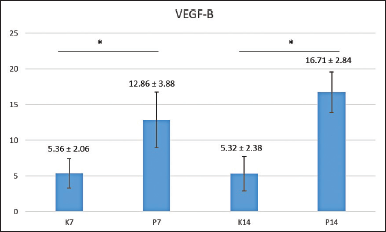 | Figure 3. The mean of VEGF-B expressions found in the post-extraction sockets of control groups observed after 7 (K7) and 14 days (K14); and the treatment groups observed after 7 (P7) and 14 days (P14). *Statistical difference from control group (p < 0.05) by Tukey HSD analysis. [Click here to view] |
The data were then put through more parametric testing, namely the one-way ANOVA test, which returned a value of p = 0.000 (p < 0.05), suggesting a significant difference between the amount of osteonectin in the control group and that in the treatment group. A Tukey HSD, multiple comparison tests, was then performed to identify the location of the differences between groups.
Figure 3 shows the significant difference (*) of VEGF-B between the seventh-day control group and the seventh-day treatment group, and also between the 14th-day control group and 14th-day treatment groups, with a Tukey HSD statistic analysis. The following were images of VEGF-B expression in the tooth extraction sockets of Cavia cobaya taken during immunohistochemical examination using a 1,000x magnification light microscope (Fig. 4). After calculating the levels of VEGF-B and osteonectin, a correlation test between the two markers was carried out using a Pearson diagram (Fig. 5). The result, 0.87, indicated that VEGF-B and osteonectin are incredibly synergistic.
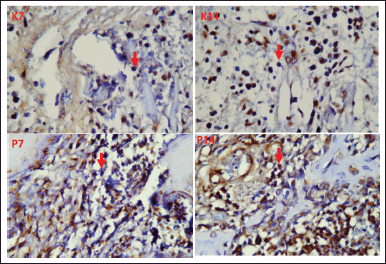 | Figure 4. Red arrows indicate VEGF-B expression in the tooth extraction sockets of Cavia cobaya in K7 (day seven control group), K14 (day 14 control group), P7 (day seven treatment group), and P14 (day 14 treatment group). [Click here to view] |
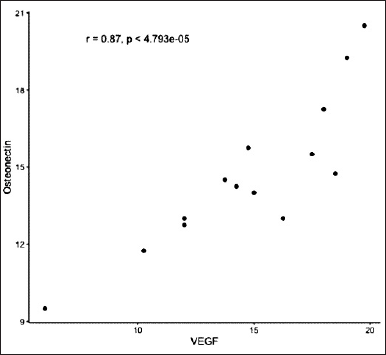 | Figure 5. Pearson product-moment correlation graphic test involving osteonectin and VEGF-B. [Click here to view] |
Horizontal alveolar bone resorption was more extensive than vertical resorption 6 months after extraction [10]. Several alternative materials can stimulate the formation of new bone, including allogenous graft, synthetic bone graft, polymer, growth factor, and biocompatible products from tissue engineering (scaffold) [6,11,12]. The use of a mixture of blood cockle shell (A. granosa) granules and Lemuru fish oil (Sardinella longiceps) gel significantly speeds up the healing of sockets, as indicated by higher numbers of osteoblast cells and greater collagen density [13]. Previous studies revealed that the ideal combination of HA and TCP content, pore dimensions, and porous nature could be a crucial element for the growth of osteoblasts [4].
This research involved using Cavia cobaya experimental subjects whose lower left incisor was extracted. This particular species was selected due to its human-like tooth and bone anatomy and the similarity of its metabolism and immunological response to humans [14]. The members of each Cavia cobaya group underwent a socket preservation procedure. The resulting socket was filled with a hydroxyapatite scaffold derived from a crab shell (Portunus pelagicus).
The study results of the treatment group indicated that the number of osteonectin expressions in the hydroxyapatite scaffold was higher than that in the control group on both day 7 and day 14. The function of osteonectin in bone is related to the differentiation of bone cells, regulation of bone mass, and control of remodeling. Increased expression in blood vessels during physiological and pathological angiogenesis and the modulation activity this protein exerts on growth factors involved in angiogenesis processes, including VEGF, FGF2, and TGF- provide evidence for osteonectin's regulatory involvement in angiogenesis [15].
Hydroxyapatite, a bioceramic employed in the medical field due to its similarity to the mineral in bones and teeth, possesses bioactive biocompatibility properties that allow the surrounding tissue to grow into the implant. Moreover, the presence of porosity promotes superior bonding with the tissue. Based on the research data collated on day 7 and day 14, the number of osteonectin expressions ranging from the lowest to highest was as follows: (1) control group on day 7, (2) control group on day 14, (3) treatment group on day 7, and (4) treatment group on day 14.
Increased expression of osteonectin is associated with reduced angiogenesis and scar tissue formation, with the highest expression of osteonectin occurring on day 7 [15]. This finding followed that of research conducted by Widyaningrum in 2019, where an increase in osteonectin expression was reported 3 days after an incisional skin injury, and peak expression was recorded on day seven during skin wound healing[16]. In this study, significant differences between the results of the day seven control group and day seven treatment group were identified, as well as between those of the day 14 control group and day 14 treatment group.
Calculating the expression of VEGF-B led to significantly different results between the control and treatment groups. In this study, VEGF-B expression in the control group decreased on day 14. However, after administering the Hydroxyapatite Scaffold, the expression in the treatment group did not decrease on day 14 but increased significantly. As stated by Senger, VEGF expression increased substantially on days 2 and 3. However, it had decreased slightly by day seven but remained higher than the average basal level [17].
The selection of material used in the tooth extraction socket preservation method plays an essential role in bone formation. Hydroxyapatite was used because it has osteoconductive and osteoinductive properties, which can stimulate ontogenesis. According to the definition of osteoconduction, in its role as a scaffold, a bone graft can act as a medium for stem cells and osteoblasts to adhere to, exist in, and develop fully in bone defects. From the results of the study, it was proved that the osteoconductive and osteoinductive properties significantly increased the number of osteonectin and VEGF-B expressions between the control group and the treatment group after the administering of hydroxyapatite scaffold derived from crab shells (Portunus pelagicus) in the tooth extraction sockets of Cavia cobaya. Previous research also proved that the combination of BMP2 and VEGF resulted in superior in vivo osteogenesis compared to the individual administration of BMP2 or VEGF at the same time frame [18]. These results significantly enhance the prospects of successful post-revocation socket preservation.
However, the study did not observe VEGF-B expression on the second and third days, which could provide valuable insights into the early stages of bone healing. The research focused on a specific animal model (Cavia cobaya), limiting the generalizability of the findings to other species or human applications. The study did not investigate the hydroxyapatite scaffold's long-term effects on bone regeneration, warranting further research to assess its sustainability and efficacy over extended periods. In addition, it is necessary to research and analyze other bone markers that have yet to be studied to obtain meaningful results regarding the effectiveness of administering hydroxyapatite scaffolding. Long-term follow-up studies are essential to evaluate the scaffold's durability and long-lasting effects on bone regeneration, ensuring its viability for clinical use in dental and orthopedic applications.
CONCLUSION
Providing a hydroxyapatite scaffold derived from crab shells (Portunus pelagicus) can increase the degree of expression of osteonectin and VEGF-B in the tooth extraction socket of Cavia cobaya. Additionally, the combination of gelatin scaffold containing HA implanted into tooth extraction sites of Cavia cobaya demonstrates a unique method for delivering and studying the effects of the scaffold on bone tissue regeneration.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Ethical approval details given in the 'Material and Methods' section.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Chrcanovic BR. Bone quality and quantity and dental implant failure: a systematic review and meta-analysis. 2017;30(3):219–37. CrossRef
2. O’Keefe JH, Bergman N, Carrera-Bastos P, Fontes-Villalba M, DiNicolantonio JJ, Cordain L. Nutritional strategies for skeletal and cardiovascular health: hard bones, soft arteries, rather than vice versa. Open Hear. 2016;3(1):e000325. CrossRef
3. Shafiu Kamba A, Zakaria ZAB. Osteoblasts growth behaviour on bio-based calcium carbonate aragonite nanocrystal. Biomed Res Int. 2014;2014:1–9. CrossRef
4. Prananingrum W, Sularsih, Ashrin MN, Revianti S, Sari RP. Osteoblast on porous HA-TCP sscaffold derived from blood cockle shells synthesis: in vivo study. J Int Dent Med Res [Internet]. 2021;14(2):613–7. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85067013194&partnerID=40&md5=3ecc16194d87f2064958f5a3c125c939
5. Chen JE, Glover GH. SPARC/osteonectin in mineralized tissue. HHS Public Access. 2016;25(3):289–313.
6. Ozturk BY, Inci I, Egri S, Ozturk AM, Yetkin H, Goktas G, et al. The treatment of segmental bone defects in rabbit tibiae with vascular endothelial growth factor (VEGF)-loaded gelatin/hydroxyapatite “cryogel” scaffold. Eur J Orthop Surg Traumatol. 2013;23(7):767–74. CrossRef
7. Kai H, Bjorn RO. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone. 2016;October(91):30–8. CrossRef
8. Ghasemi M, Dehpour AR. Ethical considerations in animal studies. J Med Ethics Hist Med. 2009;2(12)1–3.
9. Khoswanto C. A new technique for research on wound healing through extraction of mandibular lower incisors in Wistar rats. Eur J Dent. 2019;13(2):235–7. CrossRef
10. Pagni G, Pellegrini G, Giannobile WV, Rasperini G. Postextraction alveolar ridge preservation: biological basis and treatments. Int J Dent. 2012;2012:151030. CrossRef
11. Sari RP, Revianti S, Andriani D, Prananingrum W, Rahayu RP, Sudjarwo SA. The effect of Anadara granosa Shell’s- Stichopus hermanni Scaffold on CD44 and IL-10 expression to decrease osteoclasts in socket healing. Eur J Dent. 2021 May 1;15(2):228–35. CrossRef
12. Ashrin MN, Prananingrum W, Rahmitasari F, Lirungan TT, Anindita RDC, Sari RP. Mechanical evaluation of Anadara Granosa scaffold with various Gelatin concentrations for bone regeneration. J Int Dent Med Res [Internet]. 2021;14(2):514–8. Available from: http://www.jidmr.com/journal/wp-content/uploads/2021/07/9-D20_1245_Meinar_Nur_Ashrin_Indonesia.pdf
13. Hermanto E, Sari RP, Ariestania V, Sari ME, Kamadjaja DB, Narmada IB, et al. Enhanced osteoblasts and collagen production using a blood cockle (Anadara granosa) and lemuru fish oil (Sardinella longiceps) granule combination for tooth socket healing. J Int Dent Med Res. 2024;17(1):58–63.
14. Turner LM, Chuong EB, Hoekstra HE. Comparative analysis of testis protein evolution in rodents. Genetics. 2008;179(4):2075–89. CrossRef
15. Ciceri P, Elli F, Cappelletti L, Tosi D, Savi F, Bulfamante G, et al. Osteonectin (SPARC) expression in vascular calcification: in vitro and ex vivo studies. Calcif Tissue Int. 2016;99(5):472–80. CrossRef
16. Widyaningrum D, Soeroso Y, Lessang R, Bachtiar BM. Expression level of osteonectin mRNA as periodontal healing response after scaling and root planing on periodontitis patients. J Int Dent Med Res. 2019;12(3):1106–11.
17. Senger DR, Van De Water L. VEGF expression by epithelial and stromal cell compartments. Am J Pathol. 2000;157(1):1–3. CrossRef
18. Guo T, Yuan X, Li X, Liu Y, Zhou J. Bone regeneration of mouse critical-sized calvarial defects with human mesenchymal stem cell sheets co-expressing BMP2 and VEGF. J Dent Sci [Internet]. 2023;18(1):135–44. Available from: https://doi.org/10.1016/j.jds.2022.06.020 CrossRef