INTRODUCTION
Chronic myelogenous leukemia (CML) is one of the most lethal myeloproliferative disorders if left untreated. It is characterized by three major phases. It starts with a chronic phase that later shifts to an accelerated phase and ends with a blastic phase. Blastic phase mimics acute leukemia and is managed with mutual regimens [1]. A hallmark for diagnosis of CML is the detection of the expression of the signature gene, Philadelphia chromosome, or Breakpoint Cluster Region—abelson fusion oncogene (BCR-ABL) [2,3]. Treatment of CML progressed through multiple phases and was culminated with the design of targeted therapy to BCR-ABL tyrosine kinase product, imatinib [4]. Resistance to imatinib is encountered during treatment. There are two major types of resistance to imatinib: BCR-ABL-dependent and BCR-ABL-independent. The former is mostly mediated through changes in the structure of BCR-ABL product through point mutations. This form of resistance requires switching therapy to other alternatives, such as nilotinib, dasatinib, or bosutinib. In some mutations, such as T315I, those alternatives are still inactive, and treatment requires the use of other therapies, such as ponatinib or omacetaxine [5–7]. The BCR-ABL-independent mechanisms depend on the activation of pathways alternative to BCR-ABL [4].
Multitargeted kinase use is another approach to overcome resistance. Sorafenib is a multitargeted kinase that is approved for renal cell carcinoma and hepatocellular carcinoma [8,9]. Sorafenib has only been used experimentally in imatinib-resistant K-562 CML cells and in combination with axitinib [10].
Combination therapy is an approach used to overcome or reduce the development of resistance [11]. In this research, we tested the effect of combining sorafenib and imatinib on the K-562 CML cell line.
MATERIALS AND METHODS
Cell line and tissue culture
The cell line K-562 (ATCC CCL-243™) was purchased from The American Type Culture Collection (ATCC). Before the cell line activation, cells were washed with 9 ml of media to get rid of any quantity of DMSO that may affect cell growth. K562 cells were cultured in Iscove’s Modified Dulbecco’s Medium (Euroclone, ECB2072), supplemented with 1% penicillin/streptomycin and 10% fetal bovine serum. The culture flasks were incubated at 37°C and 5% CO2. Trypan blue viability and media replacement were performed every two days.
Drug preparation
For imatinib (Santa Cruz Biotechnology, sc-267106), the content of a 10 mg vial was dissolved in sterile distilled water to prepare a 1 mM stock solution and was stored at −20ºC. For sorafenib (Santa Cruz Biotechnology, sc-220125A), the content of a 50 mg vial was dissolved in dimethyl sulfoxide (DMSO) cell culture reagent (SC-358801) as a 10 mM stock solution and was stored at −20ºC. For both drugs, working dilutions were prepared in Iscove’s Modified Dulbecco’s Medium.
Cell viability assay
Briefly, 100 µl of 6 × 104 cells/ml of K-562 cell suspension was treated with 100 µl of varying concentrations of imatinib (Final concentrations of 0.1, 0.2, 0.5, 1, 2, 5, and 10 µM), and 100 µl of sorafenib with varying concentrations (Final concentration of 1, 2, 5, 10, 20, and 50 µM). Then, 200 µl of each suspension was plated in each well of a 96-well plate. Cells were cultured for 48 hours; then 20 µL of 5 mg/mL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide was added to each well, and plates were incubated at 37ºC for 4 hours. Plates were then centrifuged at 400 g for 10 min. Supernatants were removed carefully from the wells, and the formazan crystals in each well were solubilized with 200 µl of DMSO. Absorbance was measured using Biochrom™ EZ Read 400 at 570 nm.
Flow cytometric analysis of cell cycle and apoptosis
The apoptosis assay was conducted using the Annexin V-FITC Apoptosis detection kit (Cat#: BMS500FI/100CE). The experiments were conducted in triplicate. A total of 1 × 106 cells/2 ml of a K562 cell suspension were treated with 1 µM of imatinib,10 µM of sorafenib, and a combination of both. Then, 2 ml of each suspension was plated on 6 well plates and incubated for 48 hours with 5% CO2 at 37°C. After that, the cells were collected by centrifugation at 130 g for 10 minutes, and then washed with 1 ml of cold phosphate-buffered saline and resuspended into 1x binding buffer. Then, 195 µl of resuspended cells were added to 5 µl of Annexin V-Fluorescein Isothiocyanate (FITC). After 10 minutes of incubation at room temperature, cells were washed with 1x binding buffer, 10 µl of Propidium iodide was added to each one, and flow cytometry was performed by using BD FACSCanto™ II Clinical Flow Cytometry System (BD Biosciences). The fraction of cells in the Annexin V quadrant was reported in the results.
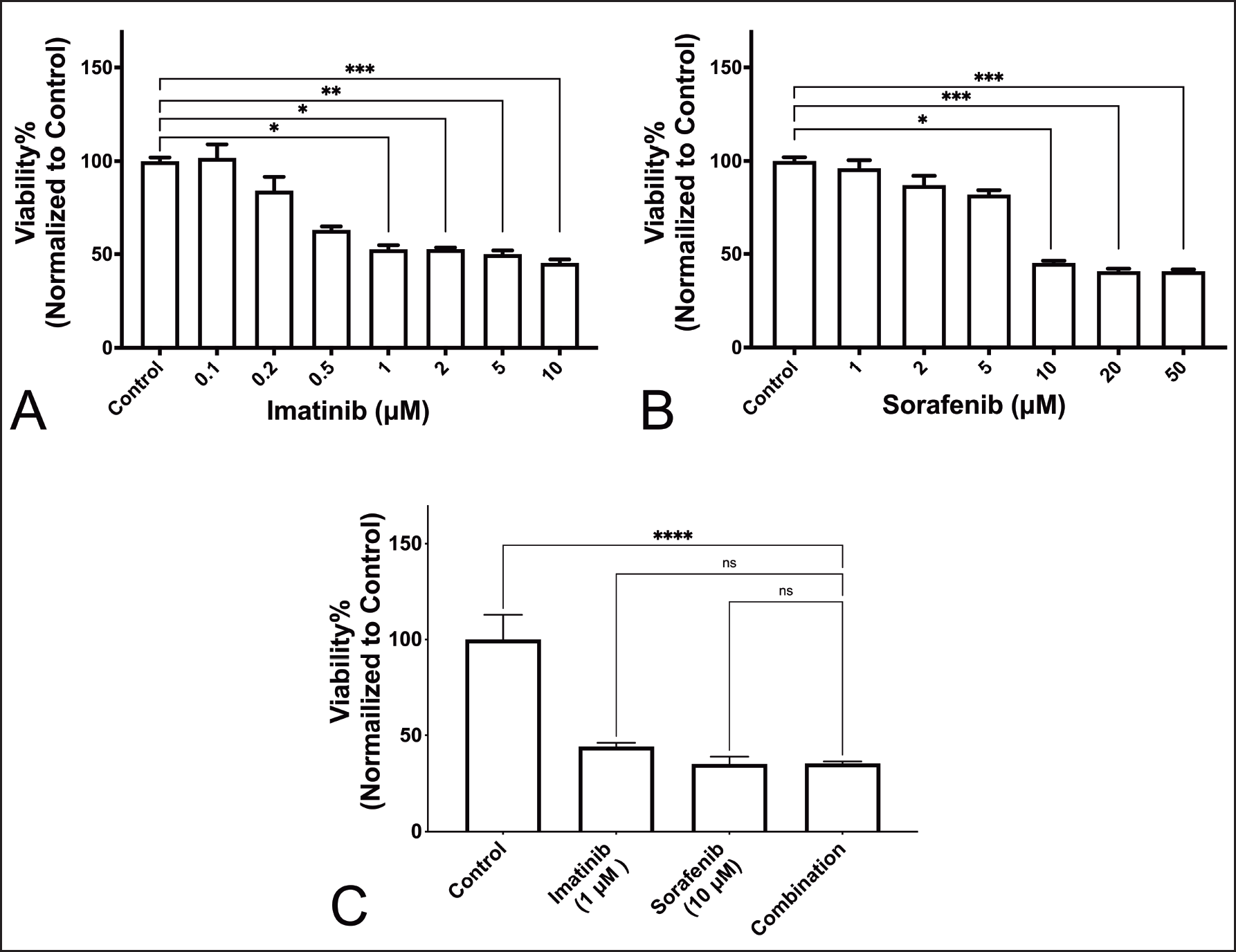 | Figure 1. Viability of K-562 cells after 48 hours incubation. (A) Effect of imatinib versus vehicle. (B) Effect of Sorafenib versus vehicle. (C) Effect of Combination treatment (*:P <0.05; **: P < 0.005; ***: P < 0.0005; ****: P < 0.0001). [Click here to view] |
Reverse transcription quantitative real-time PCR (RT-qPCR)
Messenger RNA extraction
A total of 10 ml of 6.25 × 106 cells/10 ml were seeded into a 90mm dish plate. Imatinib and sorafenib were added with a concentration of 1 and 10 µM, respectively. Combination therapy was performed also by adding both drugs to each other with the same concentration. Cells were incubated with drugs for 24 and 48 hours. After the incubation time was completed, cells were harvested into two ml Eppendorf tubes then RNA was extracted according to Qiagen RNeasy Plus Mini Kit (Cat No. 74134). After extraction, RNA was stored at −80°C. RNA quality control was investigated using Nanodrop.
Complementary DNA (cDNA) preparation
Messenger RNA (mRNA) was converted to complementary DNA using QuantiTect Reverse Transcription Kit (Cat No.: 205311). The cDNA concentration was estimated by nanodrop. Then, cDNA was used to perform RT-qPCR.
Quantitative polymerase chain reaction
PCR conditions to be applied include: 30 seconds at 95°C for initial denaturing, 10 seconds at 95°C for denaturing, and 60 seconds at 60°C for annealing and extension for a total of 40 cycles. Primers that used are: BCR/ABL-sense: 5′-TCCGCTGACCATCAAYAAGGA-3′; antisense: 5′-CACTCAGACCCTGAGGCTCAA-3′; β-actin (as a housekeeping gene)-sense: 5′-GTCATCACCATTGGCAATGAG-3′; antisense: 5′-CGTCACACTTCATGATGGAGTT -3′. Bio-Rad CFX96 real-time detection system was used and the 2−ΔΔCt method was used for interpretation of results.
STATISTICAL ANALYSIS
Experiments were conducted in multiplicate. For the viability assay, number of replicates (n) was 5, while other experiments had an n of 3. Analyses were performed using GraphPad Prism version 9.0 for Windows, GraphPad Software, San Diego, CA, www.graphpad.com. For viability assay, apoptosis essay, and BCR-ABL expression, one-way ANOVA followed by Dunn’s multiple comparisons test was used for analysis. For cell cycle analysis, a 2-way ANOVA followed by Dunnett’s multiple comparisons test was used. To simplify the interpretation of data analysis, comparisons were made in relation to combination therapy.
RESULTS
Treatment combination has no synergistic effect on viability of K-562 cells
Figure 1A and B show the effect of treatment with imatinib and sorafenib on the viability of K-562 cells. Results show that there was a significant reduction of viability that started at 1 µM for imatinib and 10 µM for sorafenib. Figure 1C shows, however, that combination therapy was not associated with a significant reduction in viability.
For the effect on cell cycle, apoptosis, and BCR-ABL expression, we used the lowest most effective concentrations that significantly affected the viability of K-562.
Treatment combination arrests cell cycle of K-562 cells at G1 phase
Figure 2 shows the effect of treatment on cell cycle. When comparing the combination to other treatments, results show that the combination significantly arrested the cell cycle of K-562 cells at the G1 phase. Results show that there was an arrest of the cell cycle in the S and G2/M phases, but the combination was not significantly different than the sorafenib treatment arm.
Treatment combination significantly affects apoptosis of K-562 cells
Figure 3 shows the effect of treatment on apoptosis of K-562 cells. Combination therapy had a significant effect on the induction of apoptosis compared to control. The combination also caused a significant increase in apoptosis compared to individual agents.
Treatment combination has no synergistic effect on BCR-ABL mRNA expression in K-562 cells
Figure 4 shows changes in the expression of BCR-ABL mRNA with different treatment groups. Combination therapy did not show a significant reduction in the BCR-ABL mRNA expression in comparison to sorafenib therapy. Combination treatment, however, showed a significant reduction in the expression of BCR-ABL mRNA in comparison to imatinib and control.
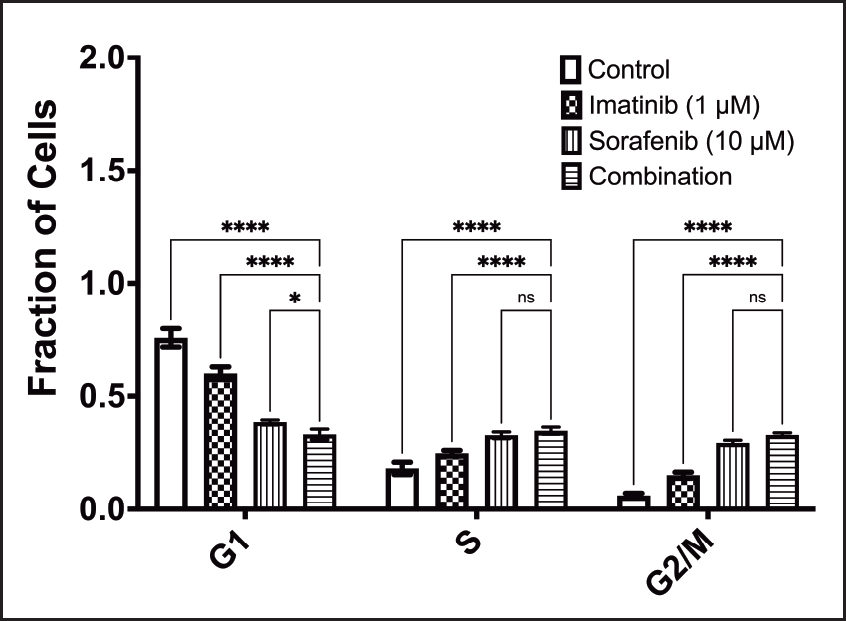 | Figure 2. Effect of combination treatment on cell cycle (ns: nonsignificant; *: P <0.05; ****: P < 0.0001). [Click here to view] |
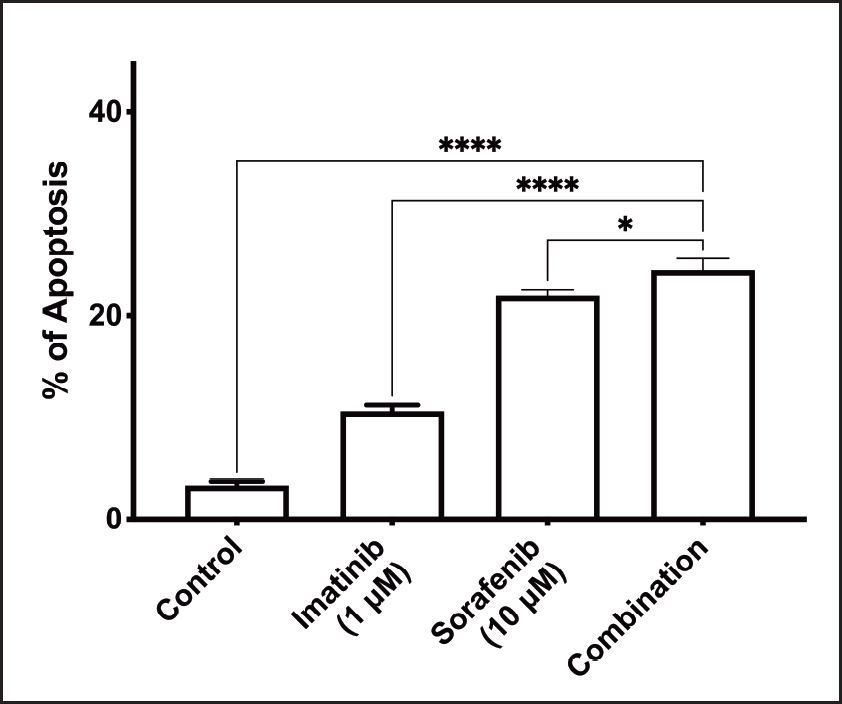 | Figure 3. Effect of combination treatment on apoptosis (*: P <0.05; ****: P < 0.0001). [Click here to view] |
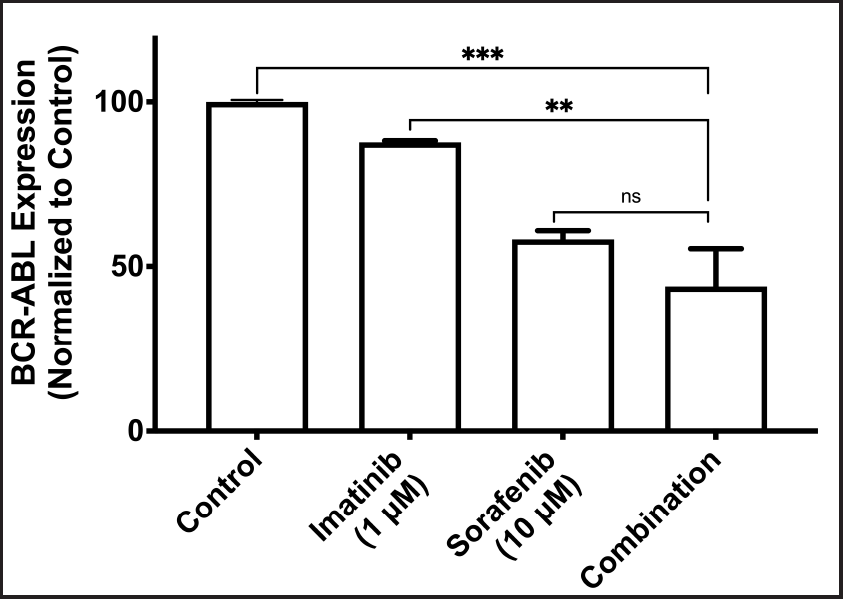 | Figure 4. Effect of combination treatment on BCR-ABL mRNA levels (ns: nonsignificant; **: P < 0.005; ***: P < 0.0005). [Click here to view] |
DISCUSSION
Tyrosine kinase inhibitor (TKI) were developed to target CML’s Philadelphia chromosome product, bcr-abl tyrosine kinase. However, resistance can develop along the course of therapy and renders TKIs ineffective [12]. Mutations leading to resistance occur as part of the course of the disease and accumulate as the disease shifts from the chronic phase to the accelerated phase and then to the blastic phase [13]. Second and third-generation bcr-abl TKIs, such as dasatinib, nilotinib, bosutinib, and ponatinib were developed to overcome resistance. Activating alternate pathways could lead to resistance to those TKIs, and other research explored the combination therapy to restore the efficacy of imatinib [14,15]. In this study, the potential for sorafenib to enhance the effects of imatinib in CML therapy was evaluated using the K-562 cell line. The synergistic effect was evaluated via studying viability, cell cycle, apoptosis, and BCR-ABL mRNA expression level to demonstrate the mechanism for such an effect. To the best of our knowledge, the effect of the combination of imatinib and sorafenib was not previously studied in any type of CML cell lines.
Previous work studied the potential of combination therapy to enhance imatinib’s effect using cisplatin and oxaliplatin (both are platinum alkylating agents), in both imatinib-sensitive and resistant K-562 cells [16]. To our advantage, the combination with sorafenib could offer additional benefits compared to chemotherapy combinations. Sorafenib, as well as other TKIs, are associated with less side effects compared to conventional chemotherapy [17]. Add to that, sorafenib alone had the potential to induce apoptosis in K-562 cells, through the downregulation of MCL-1, MEK1/2/ERK1/2, and STAT5, in a mechanism unrelated to bcr-abl signaling [15]. One other approach has studied the combination of imatinib and dasatinib in a patient with CML and showed some additive/synergistic effects [18]. This approach, however, is questionable since both imatinib and dasatinib generally share similar molecular targets but with different affinities to that target [19]. The addition of sorafenib, on the other hand, could have the potential to synergize imatinib’s effect, as it is a multikinase inhibitor with the ability to target many tyrosine kinase domains in many other receptors, as well as nonreceptor kinases [20].
Results of our viability assay show that cell viability was reduced in a statistically significant manner, starting at 1 µM for imatinib and 10 µM for sorafenib. The combination of imatinib and sorafenib in our study, however, was not associated with a significant reduction of viability compared to individual drugs.
Regarding flow cytometry, treated K-562 cells showed a pattern of accumulation of cells in the G2/M phase, and to a lesser extent, in the S phase that was not significant and a significant reduction in the G1 phase when compared to control-treated cells. The results indicated that there is a pattern of accumulation of cells in the corresponding cell cycle phase with a statistically significant effect on the interaction. Accumulation of cells in the G2/M phase comes in agreement with previous work around that concentration with sorafenib in hepatocytes [21]. With imatinib, however, lower concentrations of 0.128 µM [22] and 0.5 µM [23] showed arrest of cells in the G0 Phase.
The effect of the combination therapy of imatinib and sorafenib on apoptosis in K-562 cells was also evaluated using flowcytometry. The addition of sorafenib to imatinib enhances CML cell apoptosis compared to imatinib or sorafenib separately. These findings confirm the results of another study which indicated that sorafenib induces apoptosis of multiple BCR-ABL-expressing cells, through activation of Bax and caspase-3, leading to activation of mitochondria-dependent apoptotic pathway [24].
It is well known that measuring the clinical response of CML to therapy is done by assessing the reduction of mRNA expression level of BCR-ABL as a marker of molecular response [25]. Compared to control, there was a trend of reduction in the expression of BCR-ABL with individual treatments and then with combination. Compared to the combination, however, BCR-ABL mRNA expression was significantly reduced in the control and imatinib arms. For combination vs. sorafenib, on the other hand, there was no significant reduction in the expression of BCR-ABL mRNA levels. We suggest since the effect of sorafenib is more pronounced compared to imatinib’s, the addition of imatinib has no additional benefit on the overall effect of the combination compared to sorafenib alone on BCR-ABL mRNA levels of expression.
Finally, we believe that there is a synergistic effect of imatinib and sorafenib that could not be detected in in-vitro settings, but we think it would be detectable in an in vivo model. Sorafenib is an antiangiogenic agent, and knowing that angiogenesis is involved in the pathogenesis of CML [26], we believe an in vivo model will be more capable of finding synergism for the combination than an in vitro one.
CONCLUSION
In conclusion, our study outcomes revealed that the addition of sorafenib to imatinib does not produce a significant synergistic effect on viability and levels of BCR-ABL mRNA expression in K-562 cells, but rather produces an effect on cell-cycle arrest and apoptosis. We also found that sorafenib has a more significant effect than the drug of choice, which is imatinib, in K-562 CML cells.
AUTHOR CONTRIBUTIONS
BAA and AA contributed to conceptualizations. BAA got the grant funding. BAA and FSR worked on the experimental design and conducted experiments. All authors performed data analysis. All authors wrote the manuscript. All authors revised and approved the manuscript.
FINANCIAL SUPPORT
The study was funded by grant number 20170083 to B.A.A from the Deanship of Research of the Jordan University of Science and Technology. The research was conducted in laboratories of Jordan University of Science and Technology and Princess Haya Biotechnology Center.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Faderl S, Kantarjian HM, Talpaz M. Chronic myelogenous leukemia: update on biology and treatment. Oncology. 1999;13(2):169–80; discussion 81, 84.
2. Emole J, Talabi T, Pinilla-Ibarz J. Update on the management of philadelphia chromosome positive chronic myelogenous leukemia: role of nilotinib. Biol Targets Ther. 2016;10:23–31.
3. Melo JV, Gordon DE, Cross NC, Goldman JM. The ABL-BCR fusion gene is expressed in chronic myeloid leukemia. Blood. 1993;81(1):158–65.
4. Pinilla-Ibarz J, Sweet KL, Corrales-Yepez GM, Komrokji RS. Role of tyrosine-kinase inhibitors in myeloproliferative neoplasms: comparative lessons learned. Onco Targets Ther. 2016;9:4937.
5. You L, Liu H, Huang J, Xie W, Wei J, Ye X, et al. The novel anticancer agent JNJ-26854165 is active in chronic myeloid leukemic cells with unmutated BCR/ABL and T315I mutant BCR/ABL through promoting proteosomal degradation of BCR/ABL proteins. Oncotarget. 2017;8(5):7777.
6. Cortes J, Lipton JH, Rea D, Digumarti R, Chuah C, Nanda N, et al. Phase 2 study of subcutaneous omacetaxine mepesuccinate after TKI failure in patients with chronic-phase CML with T315I mutation. Blood. 2012;120(13):2573–80.
7. Jabbour E, Kantarjian H, Cortes J. Use of second-and third-generation tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia: an evolving treatment paradigm. Clin Lymphoma Myeloma Leukemia. 2015;15(6):323–34.
8. Qi X, Guo X. Sorafenib for the treatment of hepatocellular carcinoma with portal vein tumour thrombosis: a systematic review of comparative studies. Prz Gastroenterol. 2015;10(3):142–7.
9. Juengel E, Kim D, Makarevi? J, Reiter M, Tsaur I, Bartsch G, et al. Molecular analysis of sunitinib resistant renal cell carcinoma cells after sequential treatment with RAD001 (everolimus) or sorafenib. J Cell Mole Med. 2015;19(2):430–41.
10. Halbach S, Hu Z, Gretzmeier C, Ellermann J, Wöhrle FU, Dengjel J, et al. Axitinib and sorafenib are potent in tyrosine kinase inhibitor resistant chronic myeloid leukemia cells. Cell Commun Signal. 2016;14(1):6.
11. Frazer R, Irvine AE, McMullin MF. Chronic myeloid leukaemia in the 21st century. Ulster Med J. 2007;76(1):8.
12. Wieczorek A, Uharek L. Management of chronic myeloid leukemia patients resistant to tyrosine kinase inhibitors treatment. Biomark Insights. 2015;10(Suppl 3):49– 54.
13. DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. Pharmacotherapy: a pathophysiologic approach, 10e. Pharmacotherapy: A Pathophysiologic Approach 10e New York, NY: McGraw-Hill Education; 2017:255–8.
14. Weisberg E, Catley L, Wright RD, Moreno D, Banerji L, Ray A, et al. Beneficial effects of combining nilotinib and imatinib in preclinical models of BCR-ABL+ leukemias. Blood. 2007;109(5):2112–20.
15. Rahmani M, Nguyen TK, Dent P, Grant S. The multikinase inhibitor sorafenib induces apoptosis in highly imatinib mesylate-resistant bcr/abl+ human leukemia cells in association with signal transducer and activator of transcription 5 inhibition and myeloid cell leukemia-1 down-regulation. Mole Pharmacol. 2007;72(3):788–95.
16. Wei Y, To KK, Au-Yeung SC. Synergistic cytotoxicity from combination of imatinib and platinum-based anticancer drugs specifically in Bcr-Abl positive leukemia cells. J Pharmacol Sci. 2015;129(4):210–5.
17. American Cancer Socienty. Targeted therapy side effects 2020 Available from: https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/targeted-therapy/side-effects.html
18. Zhu Y, Pan L, Hong M, Liu W, Qiao C, Li J, et al. The combination therapy of imatinib and dasatinib achieves long-term molecular response in two imatinib-resistant and dasatinibintolerant patients with advanced chronic myeloid leukemia. J Biomed Res. 2016;30(6):525.
19. Laurini E, Posocco P, Fermeglia M, Gibbons DL, Quintás-Cardama A, Pricl S. Through the open door: preferential binding of dasatinib to the active form of BCR-ABL unveiled by in silico experiments. Mol Oncol. 2013;7(5):968–75.
20. Ravi S, Singal AK. Regorafenib: an evidence-based review of its potential in patients with advanced liver cancer. Core Evid. 2014;9:81–7.
21. Sonntag R, Gassler N, Bangen JM, Trautwein C, Liedtke C. Pro-apoptotic Sorafenib signaling in murine hepatocytes depends on malignancy and is associated with PUMA expression in vitro and in vivo. Cell Death Dis. 2014;5(1):e1030-e.
22. Chen Y, Zhou Q, Zhang L, Wang R, Jin M, Qiu Y, et al. Idelalisib induces G1 arrest and apoptosis in chronic myeloid leukemia K562 cells. Oncol Rep. 2016;36(6):3643–50.
23. Zhang M, Luo Z, Liu H, Croce CM, Burke TR, Bottaro DP. Synergistic anti-leukemic activity of imatinib in combination with a small molecule Grb2 SH2 domain binding antagonist. Leukemia. 2014;28(4):948–51.
24. Kurosu T, Ohki M, Wu N, Kagechika H, Miura O. Sorafenib induces apoptosis specifically in cells expressing BCR/ABL by inhibiting its kinase activity to activate the intrinsic mitochondrial pathway. Cancer Res. 2009;69(9):3927–36.
25. Narl? Özdemir Z, K?l?çaslan NA, Y?lmaz M, E?kazan AE. Guidelines for the treatment of chronic myeloid leukemia from the NCCN and ELN: differences and similarities. Int J Hematol. 2023;117(1):3–15.
26. Krasowska-Kwiecie? A, Kijowski J, ?ukasiewicz E, Sacha T, Foryciarz K, Majka M, et al. Angiogenesis in different clinical phases of chronic myeloid leukemia. Przegl Lek. 2009;66(9):471–8.