INTRODUCTION
Obesity has become a prevalent health problem due to its close relationship with a number of disorders, including type 2 diabetes, hypertension, and atherosclerosis [1]. Prolonged periods of positive energy balance resulting from excessive consumption of high-calorie foods and lack of physical activity increase adiposity, which finally leads to the development of obesity [2,3]. The amount of triacylglycerol (TAG) stored in the adipocytes depends on the balance between lipogenesis (the biosynthesis, incorporation, and storage of TAG in the lipid droplet in the cytoplasm) and lipolysis (the sequential hydrolysis of TAG) [4,5]. Thus, substances suppressing lipogenesis and/or promoting lipolysis of adipocytes have the potential to demonstrate anti-obesity.
Oxyresveratrol is one of the natural bioactive agents being explored for the potential of anti-adipogenesis activity [6,7]. In addition, oxyresveratrol has several biological activities including anti-inflammatory [8], anti-oxidative [9], neuroprotective [10,11], and anti-postprandial hyperglycemic activities [12,13]. According to previous evidence, oxyresveratrol down-regulated the expression of adipogenesis transcription factors peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT-enhancer-binding protein α (C/EBPα), and induced cell cycle arrest of 3T3-L1 adipocyte cells [7]. The energy expenditure was increased through Foxo3a-mediated Ucp-1 induction in obese mice supplemented with oxyresveratrol [6]. Thus, the present study aimed to further investigate the effect of oxyresveratrol-enriched extract so-called Puag-Haad along with standard oxyresveratrol.
Puag-Haad is a dried aqueous extract prepared from the heartwood of Artocarpus lacucha containing oxyresveratrol in the range of 70%–80% [14]. Artocarpus lacucha is a synonym for A. lakoocha, primarily found in South and Southeast Asia, and possesses various pharmacological activities [15]. Puag-Haad has been traditionally used as an anti-helminthic [16], and our research group has demonstrated its neuroprotective [17] and anti-diabetic [12] effects. With a wide margin of safety (LD50 > 5 g/kg bodyweight in rat) [18] and enrichment of oxyresveratrol, Puag-Haad was, therefore, a good candidate as a natural source of a pharmacological agent, particularly anti-adipogenic compounds.
Accordingly, the present study determines the adipogenesis inhibitory effects of Puag-Haad and oxyresveratrol as well as their promoting effect on lipolysis in cultured 3T3-L1 adipocytes. Adipocytes were treated with tested substances at different periods of adipogenesis to further clarify and understand their mechanisms of action.
MATERIALS AND METHODS
Chemicals
Oxyresveratrol, 3-Isobutyl-1-methylxanthine (IBMX), dexamethasone, insulin, Oli Red O, isopropanol (Isop), formalin, 3-[4,5-dimethylthiazol-2-yl]-2,3-diphenyl tetrazolium bromide (MTT), propranolol, and adipolysis assay kit were purchased from Sigma-Aldrich, St. Louis, MO. Dulbecco modified Eagle medium (DMEM), calf bovine serum (CBS), fetal bovine serum (FBS), trypsin/EDTA, Hank’s Balanced Salt Solution (HBSS) and penicillin/streptomycin were purchased from GIBCO, Grand Island, NY. Puag-Haad was purchased from Origin Plant Co., Bangkok, Thailand, containing ~65% oxyresveratrol according to HPLC analysis [13].
3T3-L1 pre-adipocyte cell culture and differentiation
Mouse embryo 3T3-L1 cell line was obtained from the American Type Culture Collection (ATCC, CL-173). 3T3-L1 pre-adipocytes were grown in high glucose-DMEM, supplemented with 10% CBS. Cells were maintained at 37°C in a humidified 5% CO2 atmosphere throughout the experiments. For cell differentiation, 3T3-L1 pre-adipocytes were cultured for 3 days with differentiation media (high glucose-DMEM supplemented with 10% FBS, 1 µM dexamethasone, 0.5 mM IBMX, and 10 µg/ml insulin). At the end of day 3, the media was replenished with maintenance media (high glucose-DMEM with 10% FBS and 10 µg/ml insulin). Media was then changed every 2 days until day 10.
Adipocytes viability determination
Cell viability was determined using the MTT assay. Cells were grown in 96-well plates at an initial density of 2 × 103 cells/cm2. After cell confluence, oxyresveratrol (25–100 µM) or Puag-Haad (12.5–50 µg/ml) were added to the medium. Cells were exposed to tested compounds for the entire 10 days of the adipogenesis process. Two hours before the end of the treatments, MTT (5 µg/ml) was added to the culture medium. After treatments, removed medium, added 200 µl isopropanol, and measured the absorbance at 595 nm using a microplate reader. Cell viability was expressed as a percentage of control.
Lipid accumulation by Oil Red O staining
3T3-L1 cells were treated with various concentrations of oxyresveratrol or Puag-Haad for a certain time period depending on the experimental conditions. Cells were washed twice with ice-cold PBS, fixed with 10% v/v formalin for 1 hour, rinsed with 60% isopropanol, and stained with 3.5 mg/ml Oil Red O solution for 10 minutes. The stained cells were rinsed three times with PBS and then photographed. After that, stained oil droplets in 3T3-L1 cells were lyzed with 100% isopropanol, and the absorbance was measured at 510 nm using a microplate reader. Lipid accumulation was expressed as a percentage of control.
Measurement of lipolysis by glycerol assay
Fully differentiated adipocytes were overnight cultured in serum-free medium before incubating with various concentrations of oxyresveratrol or Puag-Haad in HBSS for 4 hours. HBSS was then collected for glycerol measurement by adipolysis assay kit. According to the manufacturer’s instructions, glycerol concentration is determined by a coupled enzyme assay of glycerol kinase and glycerol phosphate oxidase, resulting in a colorimetric product (570 nm absorbance) proportional to the glycerol level. Isoproterenol was used as a positive control.
Statistical analysis
All data were represented as mean ± standard error of the mean of at least three separate experiments. The data were statistically analyzed by analysis of variance (ANOVA) followed by LSD tests. p values ≤ 0.05 were considered statistically significant.
RESULTS
Oxyresveratrol and Puag-Haad reduced lipid accumulation.
After treating 3T3-L1 cells with oxyresveratrol or Puag-Haad for 10 days, lipid accumulation and cell viability were determined by Oil Red O staining and MTT assay, respectively (Fig. 1). It should be noted that Puag-Haad was tested at concentration equivalent to the level of oxyresveratrol found in the extract (Puag-Haad at 50 μg/ml contained approximately 100 µM oxyresveratrol). The result showed that oxyresveratrol and Puag-Haad dose-dependently inhibited lipid accumulation in a similar intensity at equivalent concentrations (Fig. 1C). At the highest concentration used in this experiment, Puag-Haad and oxyresveratrol reduced lipid accumulations by 47% and 36%, respectively. In this experiment, adipocytes were exposed to Puag-Haad or oxyresveratrol for 10 days with no cytotoxicity observed (Fig. 1D), suggesting the ability of both compounds to suppress the accumulation of intracellular lipid during adipogenesis of adipocytes.
Adipocyte differentiation period was crucial for lipid accumulation inhibitory activities of oxyresveratrol and Puag-Haad
According to the above-mentioned finding, oxyresveratrol and Puag-Haad demonstrated an inhibitory effect on lipid accumulation when cells were incubated with tested compounds for an entire 10-day adipogenesis period. Thus, to further explore the site of action of oxyresveratrol and Puag-Haad on adipogenesis, adipocytes were treated with each compound covering either differentiation (D0-D3) or lipogenesis (D3-D10) periods (Fig. 2A). The results showed that the degree of lipid accumulation inhibitory activities of oxyresveratrol and Puag-Haad when adipocytes were treated for 3 days at differentiation (D0-D3) period were similar to that when cells were exposed for the entire adipogenesis (D0-D10) period (Fig. 2B, C). When adipocytes were exposed to tested compounds for 7 days at lipogenesis (D3-D10) period, lipid accumulation was reduced not so much as when adipocytes were treated covering the differentiation period. Inhibition on lipid accumulation of oxyresveratrol and Puag-Haad was dominant when cells were exposed at the differentiation period, suggesting that this early stage of adipogenesis is the important site of action.
Oxyresveratrol and Puag-Haad stimulated intracellular lipolysis
In addition to the effect of oxyresveratrol and Puag-Haad on adipogenesis, their effect on lipolysis was investigated. Mature adipocytes (D10) were treated with oxyresveratrol or Puag-Haad for 4 hours and the glycerol released to culture buffer were determined (Fig. 3A). The result showed that the releases of glycerol from adipocytes were increased depending on the concentration of oxyresveratrol and Puag-Haad (Fig. 3B). The remaining intracellular lipid content at 4 hours was not changed (data not shown) but slightly decreased after 24 hours treatments (Fig. 3C). The glycerol release was considerably increased (~2 folds) whereas lipid content reduced ~20% at highest concentration of tested compounds (Fig. 3B, C). Isoproterenol (10 µM), a β-adrenergic agonist, a positive control as lipolysis inducer, effectively induced glycerol release by ~3 folds and decreased lipid content by ~25%. These data suggested that oxyresveratrol and Puag-Haad promoted intracellular lipolysis of mature adipocytes which subsequently led to the reduction of intracellular lipid content.
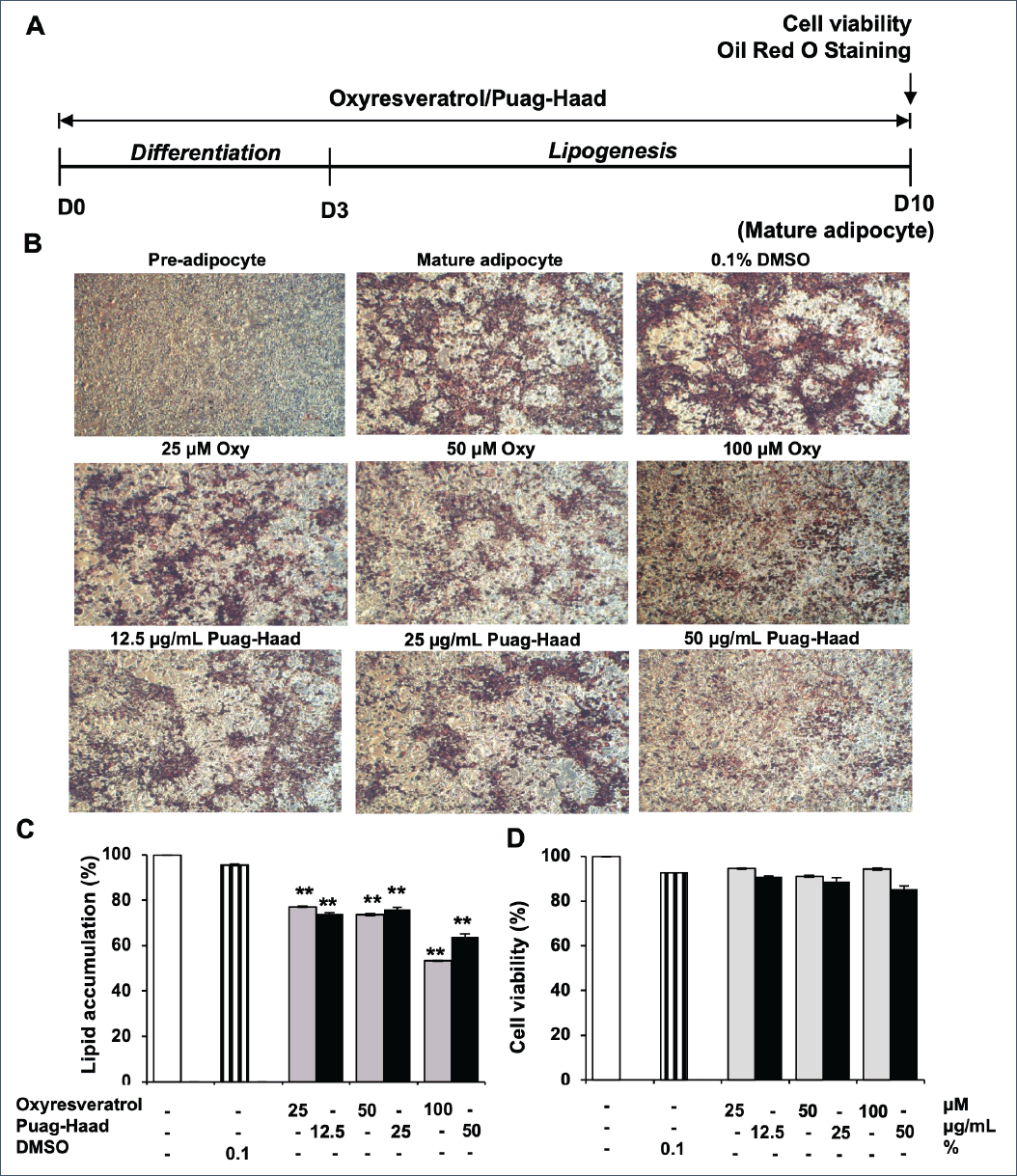 | Figure 1. Effects of oxyresveratrol and Puag-Haad on lipid accumulation and viability of 3T3-L1 adipocyte. (A) Schematic of the 3T3-L1 cells treatments. (B) Photography taken at D10 after Oil Red O staining (4x). (C) Lipid accumulation quantified by Oil Red O staining with absorbance measured at 570 nm. (D) Viability of adipocytes quantified by an MTT assay. Data are mean ± SEM (n = 4–10), ∗ p ≤0.05, ∗∗ p ≤ 0.01, compared to control (untreated cells). [Click here to view] |
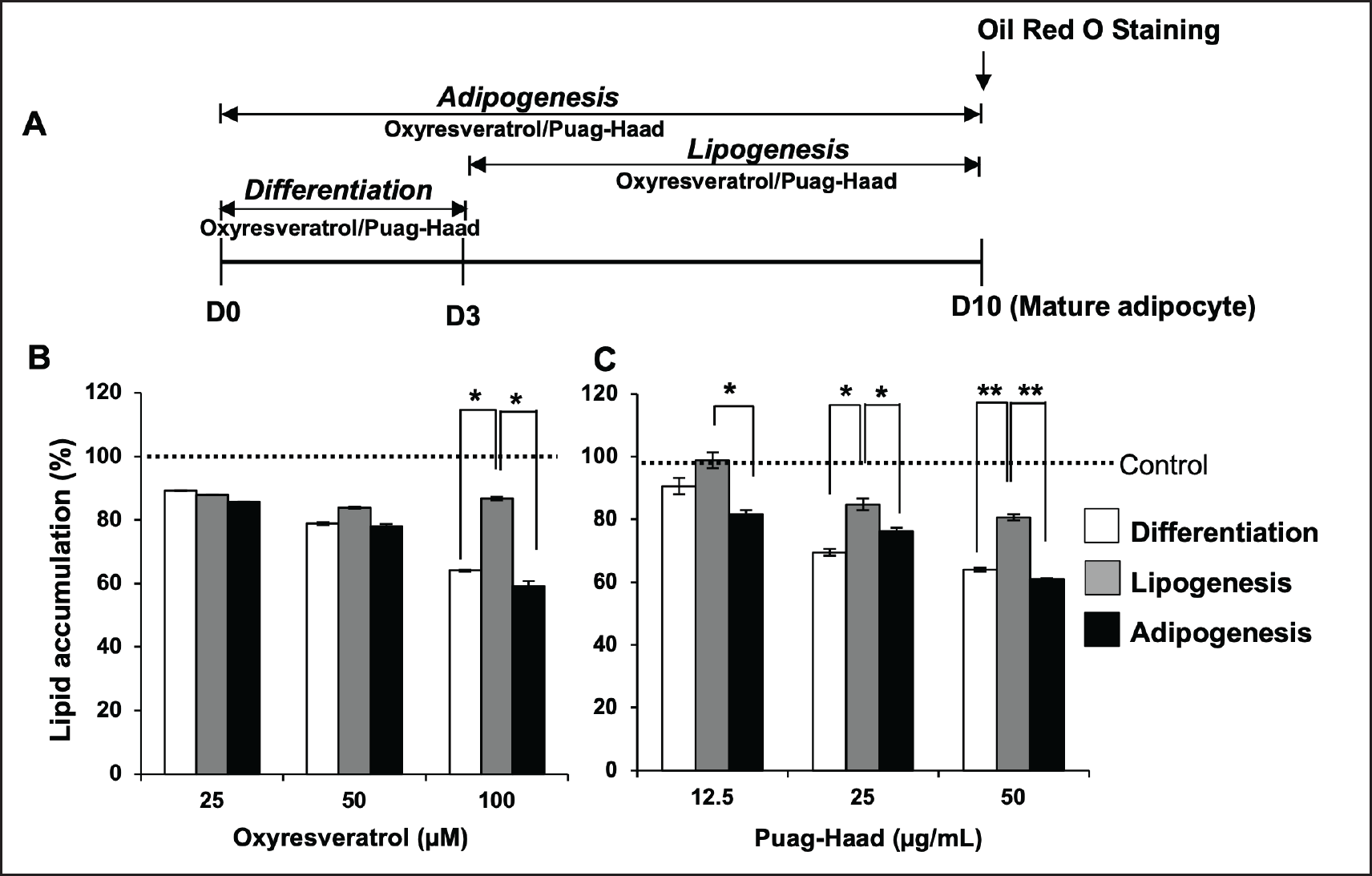 | Figure 2. Lipid accumulation of oxyresveratrol- and Puag-Haad-treated 3T3-L1 adipocyte at different time periods. (A) Schematic of the 3T3-L1 cells treatments. (B, C) Lipid accumulation in 3T3-L1 adipocyte after treatment with oxyresveratrol and Puag-Haad at differentiation (D0-3), lipogenesis (D3-10), and adipogenesis (D0-10) periods. Data are mean ± SEM (n = 3), ∗ p ≤ 0.05, ∗∗ p ≤0.01, compared to control (untreated cell). [Click here to view] |
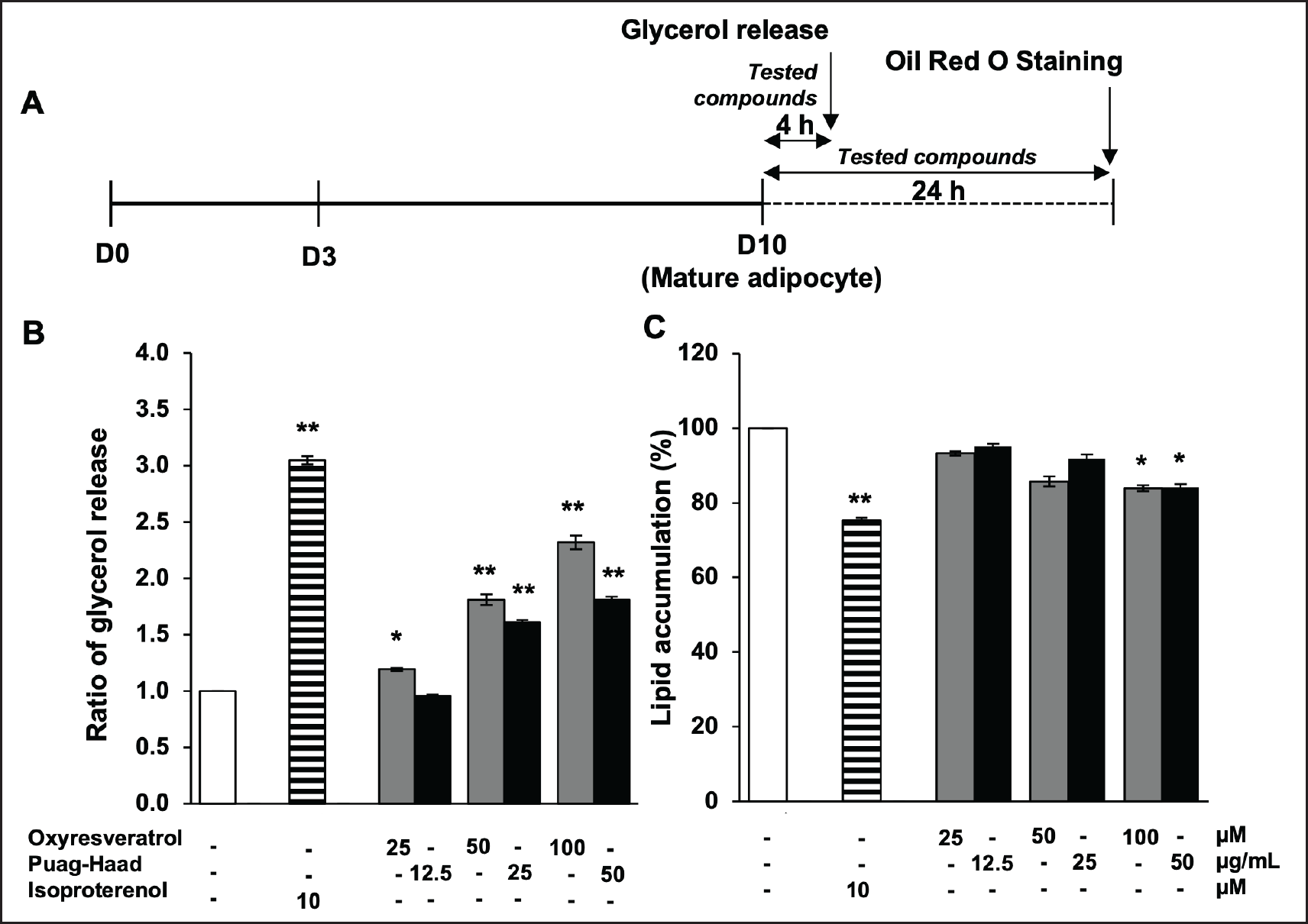 | Figure 3. Lipolysis stimulating activity of oxyresveratrol and Puag-Haad. (A) Schematic of the 3T3-L1 cells treatments. (B) The glycerol release at 4 h and (C) intracellular lipid accumulation at 24 h after treatmenting cells with isoproterenol (Isop), oxyresveratrol, and Puag-Haad. Data are mean ± SEM (n = 3–6), ∗ p ≤ 0.05, ∗∗ p ≤ 0.01, compared to control (untreated cell). [Click here to view] |
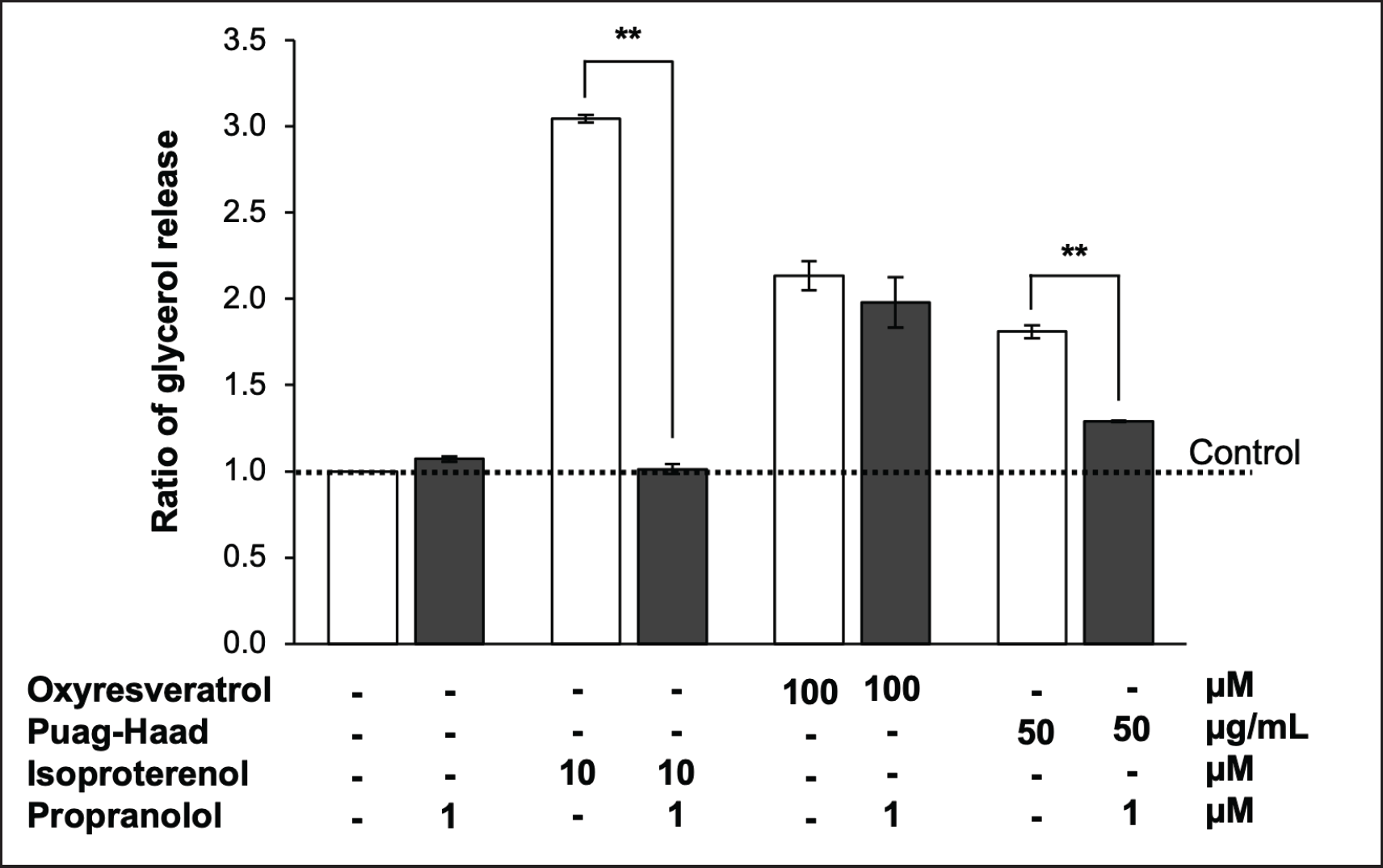 | Figure 4. Role of β-adrenergic receptor on oxyresveratrol and Puag-Haad-induced lipolysis. The release of glycerol from 3T3-L1 mature adipocytes was determined after 4 hours of treatment with 100 μM oxyresveratrol, 50 μg/ml Puag-Haad, or 10 μM isoproterenol in the presence and absence of 1 μM propranolol (β-adrenergic receptor agonist). Data are mean ± SEM (n = 3-6), ∗ p ≤ 0.05, ∗∗ p ≤ 0.01, compared to control (untreated cell). [Click here to view] |
Puag-Haad-induced adipocyte lipolysis potentially through β-adrenergic receptor
To investigate whether lipolysis induced by oxyresveratrol and Puag-Haad mediated through β-adrenergic receptors or not, propranolol (β-adrenergic antagonist) was used to block the function of these receptors. The result showed that propranolol completely blocked isoproterenol-induced lipolysis as expected. Propranolol also inhibited the glycerol release induced by Puag-Haad but surprisingly failed to inhibit oxyresveratrol-induced lipolysis (Fig. 4). These data indicated that some other components but not oxyresveratrol in Puag-Haad might play a role to induce intracellular lipolysis via β-adrenergic receptor.
DISCUSSION
In this study, an oxyresveratrol-enriched aqueous extract from A. lacucha called Puag-Haad exhibited anti-adipogenesis activity corresponding to standard oxyresveratrol at equivalent concentrations. Disruption of adipocyte differentiation possibly was the site of actions as their anti-adipogenesis effects demonstrated by the reduction of lipid accumulation were most prominent when treatment covered the differentiation stage. Oxyresveratrol and Puag-Haad also promoted glycerol release from mature adipocytes indicating lipolysis-inducing activity. Oxyresveratrol-induced lipolysis was independent of β-adrenergic receptors whereas such effect of Puag-Haad depends on these receptors.
Anti-adipogenesis on oxyresveratrol demonstrated by the reduction of intracellular lipid accumulation in this study was consistent with previous reports where 3T3-L1 cells were differentiated into adipocytes and exposed to oxyresveratrol for 6 days [6], and 9 days [7] covering entire period of adipogenesis. We here additionally explored and discovered that adipocyte differentiation was the main target of action of oxyresveratrol. Generally, adipocyte differentiation takes a long time and usually overlaps with lipogenesis. The differentiation phase could be separated into early and late differentiation stages. The early stage of differentiation starts when cells are induced with hormones such as insulin and steroids while in late differentiation, cells begin to express adipocyte-specific protein that leads to fat droplet formation [19]. The time-dependent suppression effect on protein expression of oxyresveratrol was previously reported. During 9 days of 3T3-L1 cells planting (3 days differentiation and 6 days maintenance), oxyresveratrol (3 days treatment) strongly suppressed the expression of PPARγ at the early stage (Day1) whereas down-regulation of C/EBPα was observed at the later stage (Day4) [7]. Taken together with our finding, PPARγ expression during the differentiation period seemed to be the critical signaling for lipid accumulation at a later lipogenesis stage.
Lipolysis is one mechanism of anti-adipogenesis resulting from the reduction of intracellular fat. Generally, during times of energy deprivation, white adipose tissue undergoes a shift toward greater net rates of lipolysis, which can be defined as the hydrolysis of TAG to generate fatty acids and glycerol that are released into the vasculature for use by other organs as energy substrates [20]. Our results clearly showed that oxyresveratrol and Puag-Haad significantly stimulated the lipolysis of fat stored in lipid droplets of mature adipocytes. Lipolysis is essential for thermogenesis, and a previous study suggested that oxyresveratrol increased energy expenditure in obese mice and induced thermogenic gene expression including Ucp-1 a thermogenic regulator in mitochondria in adipocytes [6]. Further investigation also reported the roles of Foxo3a as a regulator of oxyresveratrol-modulated expression [6].
Resveratrol, being similar in structure to oxyresveratrol, has also been shown to induce lipolysis of cultured adipocytes and animal adipose tissue [21,22]. Due to the similarity in the structure, these two bioactive compounds were thought to share similar molecular mechanisms. However, resveratrol induced only free fatty acid (FFA) release but, not glycerol release from adipocytes under basal conditions [21,22], which were different from our study showing that oxyresveratrol directly induced glycerol release. Resveratrol upregulated the expression of adipose triglyceride lipase (ATGL), the key enzyme that cleaves the first acyl chain of a TG, but, not hormone-sensitive lipase (HSL) [22], subsequently preferably effected on FFA release. Glycerol release could be observed by resveratrol at high concentration (1 mM in culture medium) or when assembled with β-adrenergic stimulation [21]. In contrast to ATGL, HSL could hydrolyze various types of substrates such as triacylglycerols, diacylglycerols, monoacylglycerols, and cholesteryl ester [23]. HSL-induced lipolysis is regulated by multiple mechanisms including β-adrenergic receptor activation [23]. Because our study demonstrated that β-adrenergic antagonists failed to inhibit oxyresveratrol-induced lipolysis, thus HSL might not be the target molecule of oxyresveratrol. Thus, further study is needed to identify the molecular target of oxyresveratrol-mediated lipolysis.
Because oxyresveratrol is the major constituent of Puag-Haad, underlining mechanisms on the lipid accumulation inhibitory effect and lipolysis promoting effect were expected to be similar. Surprisingly, only lipolysis mediated by Puag-Haad but not by oxyresveratrol was blocked by the β-adrenergic antagonist. This observation indicated the role of other chemical components in Puag-Haad exhibiting β-adrenergic-mediated lipolysis. Due to limited information on identified phytochemical constituents in Puag-Haad, bioactive compounds such as rutin, resorcinol, pyrogallol, gallic acid, catechins, or caffeic acid that were found in ethanol extract of A. lacucha heartwood [24] might be possible candidates.
In addition to β-adrenergic receptor regulation, there are other receptors and/or signaling proteins associated with lipolysis of adipocytes. Our previous finding demonstrated the role of GABA-A and -B receptors on intracellular lipolysis of adipocytes [21]. Alternatively, inhibition of α1-adenosine receptors and phosphodiesterase activity are also potential mechanisms of lipolysis. Caffeine and methylxanthines derived from tea and coffee were demonstrated to stimulate lipolysis by antagonism of α1-adenosine receptors resulting in a depression of adenylyl cyclase activity and an increase in lipolysis [22]. Methylxanthines also inhibited phosphodiesterase activity, prevented the breakdown of cAMP, and stimulated lipolysis in fat cells [25]. As such, high intakes of methylxanthines may also contribute to improving weight loss through enhancing fat oxidation and thermogenesis [26].
CONCLUSION
Oxyresveratrol and Puag-Haad inhibited adipogenesis and induced lipolysis in culture adipocytes. Suppression at the adipocyte differentiation seems to be the site of their anti-adipogenesis activities. Although oxyresveratrol was primarily thought to be an active ingredient of Puag-Haad, different action on lipolysis induction pathways was observed. Β-adrenergic receptors possibly play a key role in Puag-Haad-mediated but not in oxyresveratrol-mediated lipolysis. According to their potential to prevent lipid accumulation in adipocytes, both oxyresveratrol and Puag-Haad should be further investigated and developed as anti-obesity agents.
AUTHOR CONTRIBUTIONS
All authors conceptualized the study, and took part in drafting the article. Trisat K performed all experiments, collected data, and conducted statistical analysis. Limpeanchob N interpreted results, and revised the manuscript. All authors agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
FINANCIAL SUPPORT
This work was supported by Naresuan University (NU), and National Science, Research and Innovation Fund (NSRF). Grant number; R2565B020.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data are available with the authors and shall be provided upon request.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Walley AJ, Blakemore AI, Froguel P. Genetics of obesity and the prediction of risk for health. Hum Mol Genet. 2006;15 Spec No 2:R124–30.
2. Bergman RN, Ader M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol Metab. 2000;11(9):351–6.
3. Guyenet SJ, Schwartz MW. Clinical review: regulation of food intake, energy balance, and body fat mass: implications for the pathogenesis and treatment of obesity. J Clin Endocrinol Metab. 2012;97(3):745–55.
4. Lafontan M. Advances in adipose tissue metabolism. Int J Obes (Lond). 2008;32 Suppl 7:S39–51.
5. Large V, Peroni O, Letexier D, Ray H, Beylot M. Metabolism of lipids in human white adipocyte. Diabetes Metab. 2004;30(4):294–309.
6. Choi JH, Song NJ, Lee AR, Lee DH, Seo MJ, Kim S, et al. Oxyresveratrol increases energy expenditure through Foxo3a-Mediated Ucp1 induction in high-fat-diet-induced obese mice. Int J Mol Sci. 2018;20(1):26.
7. Tan H-Y, Tse IMY, Li ETS, Wang M. Inhibitory effects of oxyresveratrol and cyanomaclurin on adipogenesis of 3T3-L1 cells. J Funct Foods. 2015;15:207–16.
8. Chung KO, Kim BY, Lee MH, Kim YR, Chung HY, Park JH, et al. In-vitro and in-vivo anti-inflammatory effect of oxyresveratrol from Morus alba L. J Pharm Pharmacol. 2003;55(12):1695–700.
9. Lorenz P, Roychowdhury S, Engelmann M, Wolf G, Horn TF. Oxyresveratrol and resveratrol are potent antioxidants and free radical scavengers: effect on nitrosative and oxidative stress derived from microglial cells. Nitric Oxide. 2003;9(2):64–76.
10. Andrabi SA, Spina MG, Lorenz P, Ebmeyer U, Wolf G, Horn TF. Oxyresveratrol (trans-2,3’,4,5’-tetrahydroxystilbene) is neuroprotective and inhibits the apoptotic cell death in transient cerebral ischemia. Brain Res. 2004;1017(1-2):98–107.
11. Chao J, Yu MS, Ho YS, Wang M, Chang RC. Dietary oxyresveratrol prevents parkinsonian mimetic 6-hydroxydopamine neurotoxicity. Free Radic Biol Med. 2008;45(7):1019–26.
12. Wongon M, Limpeanchob N. Artocarpus lacucha extract and oxyresveratrol inhibit glucose transporters in human intestinal Caco-2 cells. Planta Med. 2021;87(9):709–15.
13. Wongon M, Limpeanchob N. Inhibitory effect of Artocarpus lakoocha Roxb and oxyresveratrol on alpha-glucosidase and sugar digestion in Caco-2 cells. Heliyon. 2020;6(3):e03458.
14. Maneechai S, Likhitwitayawuid K, Sritularak B, Palanuvej C, Ruangrungsi N, Sirisa-Ard P. Quantitative analysis of oxyresveratrol content in Artocarpus lakoocha and ‘Puag-Haad’. Med Princ Pract. 2009;18(3):223–7.
15. Sitorus P, Keliat JM, Asfianti V, Muhammad M, Satria D. A literature review of Artocarpus lacucha focusing on the phytochemical constituents and pharmacological properties of the plant. mol. 2022;27(20):6940.
16. Charoenlarp P, Radomyos P, Bunnag D. The optimum dose of Puag-Haad in the treatment of taeniasis. J Med Assoc Thai. 1989;72(2):71–3.
17. Hasriadi, Wong-on M, Lapphanichayakool P, Limpeanchob N. Neuroprotective effect of Artocarpus lakoocha extract and oxyresveratrol against hydrogen peroxide-induced toxicity in Sh-Sy5y cells. Int J Pharm Pharm. 2017;9(10):229–33.
18. Nilvises N, Panyathanya R, Wamnutchinda W. Toxicity test of Puag Haad (Artocarpus lakoocha). Bull Dept Med Sci. 1985;27(1):49–55.
19. Ntambi JM, Young-Cheul K. Adipocyte differentiation and gene expression. J Nutr. 2000;130(12):3122S–6S.
20. Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS. Regulation of lipolysis in adipocytes. Annu Rev Nutr. 2007;27:79–101.
21. Trisat K, Yomlar S, Limpeanchob N. Role of GABA and its receptors in anti-adipogenesis in cultured adipocytes. Songklanakarin J Sci Technol. 2020;42(5):1053-8.
22. Londos C, Cooper DM, Schlegel W, Rodbell M. Adenosine analogs inhibit adipocyte adenylate cyclase by a GTP-dependent process: basis for actions of adenosine and methylxanthines on cyclic AMP production and lipolysis. Proc Natl Acad Sci USA. 1978;75(11):5362–6.
23. Holm C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem Soc Trans. 2003;31(Pt 6):1120–4.
24. Singhatong S, Leelarungrayub D, Chaiyasut C. Antioxidant and toxicity activities of Artocarpus lakoocha Roxb. heartwood extract. J Med Plants Res. 2010;4(10):947–53.
25. Acheson KJ, Gremaud G, Meirim I, Montigon F, Krebs Y, Fay LB, et al. Metabolic effects of caffeine in humans: lipid oxidation or futile cycling? Am J Clin Nutr. 2004;79(1):40–6.
26. Westerterp-Plantenga MS, Lejeune MP, Kovacs EM. Body weight loss and weight maintenance in relation to habitual caffeine intake and green tea supplementation. Obes Res. 2005;13(7):1195–204.