INTRODUCTION
Among noncommunicable diseases, ischemic heart disease and stroke have remained in the top three causes of death in 2021 globally [1]. Hypercholesterolemia is the major cause of these cardiovascular diseases. For individuals with moderately high cholesterol, controlling blood cholesterol levels can be achieved through lifestyle changes and dietary modifications [2]. In addition, there has been increasing interest in recent years in supplementing these efforts with natural alternatives. Natural cholesterol-lowering compounds from plant extracts are being explored as functional foods or nutraceuticals due to their safety profile.
Oxyresveratrol is a natural polyphenols found in various plants, including the heartwood of Artocarpus lakoocha Roxb and mulberry trees [3,4]. Oxyresveratrol exhibits significant antihyperlipidemic properties, effectively lowering serum triglyceride, and cholesterol levels in hyperlipidemic rats [5]. These studies also showed that oxyresveratrol-treated rats had decreased fat accumulation in the liver and improved expression of cholesterol metabolism-related genes and proteins. Its antioxidant [6], anti-inflammatory [7], and anti-adipogenesis [8,9] activities further contribute to preventing cardiovascular disease development. Although oxyresveratrol exhibits antihyperlipidemic activity, its underlying mechanism requires further investigation. Observed changes in hepatic histological features and protein expression in animal models [10] may be a consequence of improved lipid levels in the circulation but may not fully explain its mechanism of action. The direct impact of oxyresveratrol on lipid and cholesterol processing in the intestinal tract, which could influence blood cholesterol homeostasis, has yet to be thoroughly explored.
In addition to testing the commercially purified oxyresveratrol, Puag-Haad, an aqueous extract from the heartwood of A. lakoocha, was investigated to discover a novel natural source of such an active compound. Puag-Haad is a dried aqueous extract obtained by boiling the heartwood of A. lakoocha with water. It is a rich source of oxyresveratrol, with concentrations ranging from 70% to 80% [4]. In addition to being enriched in oxyresveratrol, Puag-Haad has demonstrated low toxicity with an LD50 of over 5 g/kg body weight in rats [11], making it an excellent candidate as a natural remedy in the pharmacological field. Therefore, the present study investigated the inhibitory activity of oxyresveratrol and Puag-Haad (an oxyresveratrol-enriched product) on lipid digestion enzyme, cholesterol solubility in lipid micelle, bile acids binding, and cholesterol absorption.
MATERIALS AND METHODS
Chemicals
Oxyresveratrol, gallic acid, porcine pancreatic lipase, lipase substrate (1, 2 di-O-lauryl-rac-glycero-3 glutaric acid 6′-methylresorufin ester), orlistat, cholesterol, l-α-phosphatidylcholine, taurocholic acid sodium salt hydrate, 3-[4,5-dimethylthiazol-2-yl]-2,3-diphenyl tetrazolium bromide, taurocholic acid, taurodeoxycholic acid, glycodeoxycholic acid, hydrazine hydrate solution, 3-α-hydroxysteroid dehydrogenase, and β-nicotinamide adenine dinucleotide (NAD) were purchased from Sigma-Aldrich (Steinheim, Germany). The cholesterol test kit was purchased from HUMAN GmbH Co (Wieabaden, Germany). Dulbecco-modified Eagle medium (DMEM), fetal bovine serum (FBS), trypsin/EDTA, and penicillin/streptomycin were purchased from GIBCO (Grand Island, NY). TopFluor® Cholesterol 23-(dipyrrometheneboron difluoride)-24-norcholesterol (Bodipy Cholesterol; Bdp-Chol) was purchased from Avanti Polar Lipids, Inc. Puag-Haad was obtained from Research and Innovation Center in Cosmetic Sciences and Natural Products (University of Phayao, Thailand). The heartwood of A. lakoocha was dried, ground into a fine powder, and extracted by decoction technique in boiling water (100°C) for 5 hours. After rapid filtration through a Whatman No. 5 membrane, the filtrate was cooled at room temperature, allowing the collection of insoluble components. These precipitates were then dissolved in ethanol, filtered, and the solvent was evaporated under reduced pressure. The resulting dried powder was yellowish–brown and contained 85.34 ± 1.07 g of oxyresveratrol per 100 g of extract, according to HPLC analysis.
Pancreatic lipase inhibitory activity
The pancreatic lipase activity assay was conducted using the method described by Aubry et al. [12]. Lipase substrate, oxyresveratrol, Puag-Haad, and orlistat were dissolved in DMSO, while pancreatic lipase was prepared in a pH 8.0 buffer consisting of 0.8 M Tris-HCl, 150 mM NaCl, and 1.3 mM CaCl2. Oxyresveratrol, Puag-Haad, and orlistat at various concentrations were mixed with 50 U/ml pancreatic lipase and 400 µM lipase substrate at 37°C in the dark for 60 minutes. The level of methylresorufin was determined by fluorescence at Ex 535 nm and Em 595 nm. Orlistat, a known lipase inhibitor, was used as a positive control.
Cholesterol solubility in lipid micelles
The assay of cholesterol solubility in lipid micelle was adapted from the method described by Kirana et al. [13]. Lipid micelles were composed of 1 mM cholesterol, 1 mM sodium taurocholate, and 0.6 mM phosphatidylcholine. The micelle solutions were sonicated, combined with the tested compounds, and incubated at 37°C for 3 hours. Precipitated cholesterol was separated from the intermicellar cholesterol by filtering through a 0.22 µm syringe filter and the cholesterol remaining in the micelles was then quantified using a cholesterol assay kit.
Bile acid binding determination
The assay to determine the bile acid binding was modified from the methods described by Adisakwattana et al. [14] and Yoshie-Stark and Wäsche [15]. In this experiment, the bile acids used included taurocholic acid, taurodeoxycholic acid, and glycodeoxycholic acid. Briefly, Puag-Haad and cholestyramine were mixed with each bile acid (2 mM) in 100 mM PBS, pH 7.0, at 37°C for 2 hours. The mixtures were then centrifuged and filtrated through a 0.22 µm membrane filter to separate the bound bile acids from the free bile acids. The fifth-generation Randox total bile acids method was used to measure the bile acids in the filtrates [16]. The free bile acids were mixed with a reaction solution consisting of 0.133 M tris buffer (pH 9.5), 1 M hydrazine hydrate, and 7.7 mM NAD. Then, 1 unit/ml 3α-hydroxysteroid dehydrogenase was added and the mixture was incubated at 30°C for 90 minutes. The rate of thio-NADH formation was measured by determining the absorbance at 405 nM. Cholestyramine was used as a positive control.
Caco-2 cell culture preparation
Caco-2 cells from the American Type Culture Collection were cultured and maintained at 37°C in a 5% CO2 atmosphere in DMEM/F12 medium supplemented with 10% heated-inactivated FBS and 1% penicillin–streptomycin. Cells were sub-cultured every 3–4 days and those used for testing were in passages 20–40.
Cholesterol uptake determination
The cholesterol uptake was conducted in Caco-2 cells using a previously described method with minor modifications [17]. Caco-2 cells (1 x 104 cells/well) were seeded into a 96-well plate and maintained for 14–21 days to achieve differentiation. Differentiated cells were starved overnight in serum-free DMEM and then treated with oxyresveratrol, Puag-Haad, or ezetimibe at various concentrations. After 1 hour of treatment, cholesterol uptake was initiated by adding 0.4 mM Bdp-Chol in the presence of 0.5 mM taurocholic acid and incubated at 37°C for 3 hours. The cells were subsequently washed twice with cold PBS, and fluorescence was measured at Ex 485 nm and Em 535 nm.
Statistical analysis
All data are represented as mean ± standard deviation from at least three independent experiments. Statistical analysis was performed by analysis of variance followed by LSD tests. A p-value of ≤ 0.05 was considered statistically significant. The half-maximal inhibitory concentration (IC50) of tested compounds for pancreatic lipase and cholesterol micellar solubility was calculated using GraphPad Prism (Version 2.01) software.
RESULTS
Oxyresveratrol and Puag-Haad inhibit pancreatic lipase activity
Pancreatic lipase is essential for the digestion of dietary fats in the small intestine. Orlistat, a standard pancreatic lipase inhibitor, showed high potency with IC50 2.05 nM and complete inhibition at > 100 nM (Fig. 1A). Both Puag-Haad and oxyresveratrol exhibited dose-dependent inhibitory effects on pancreatic lipase and oxyresveratrol is more potent than Puag-Haad, as reflected by its lower IC50 value (Fig. 1B). The IC50 of oxyresveratrol is 2.33 mg/ml, which is equivalent to 9.54 mM and is much higher than the IC50 of orlistat, which is 2.5 nM. At the highest concentration (10 mg/ml) of tested compounds, lipase activity was not completely inhibited (~60%–70%). Although Puag-Haad and oxyresveratrol demonstrated significantly less potency than orlistat, their consumption in large amounts as natural substances might still be sufficient to inhibit lipase activity. This inhibition could interfere with the digestion and absorption of fats, potentially leading to an indirect influence on lipid levels in the body.
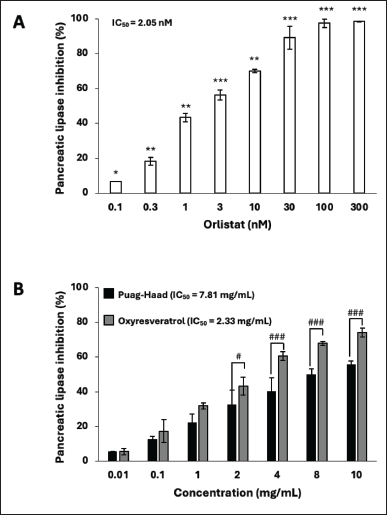 | Figure 1. Oxyresveratrol and Puag-Haad inhibited pancreatic lipase activity. (B) Oxyresveratrol and Puag-Haad at various concentrations were mixed with pancreatic lipase and substrate for 60 minutes. The amount of fluorescent product was determined and calculated as % inhibition. (A) Orlistat was used as a positive control. Values are mean ± SD from 3 to 5 experiments, * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, compared to control and # p ≤ 0.05, ## p ≤ 0.01, ### p ≤ 0.001, indicates a significant difference between groups. IC50 values were calculated using Prism Graph Pad. [Click here to view] |
Oxyresveratrol and Puag-Haad inhibit cholesterol micellar solubility
The solubility of cholesterol in lipid micelles is important for lipid digestion and absorption in the intestinal tract. Once solubilized in the micelle, cholesterol can be transported to the intestinal brush border membrane, where it can be absorbed into intestinal cells. Our data demonstrated that oxyresveratrol and Puag-Haad effectively inhibited the solubility of cholesterol in lipid micelles with IC50 77.23 µg/ml and 56.11 µg/ml, respectively (Fig. 2A). For cholesterol micellar solubility inhibition, there was no standard compound that could be used as a positive control. Gallic acid was tested as it was previously reported for this activity [17], and the result showed that it inhibited the solubility of cholesterol in lipid micelles with IC50 233.1 µg/ml (Fig. 2B). Cholestyramine, a bile acid binder, was also tested with the expectation to interfere micellar forming since bile salts help solubilize cholesterol by incorporating it into the micelle but it here showed low activity (Fig. 2C). These data suggest that consumption of oxyresveratrol and Puag-Haad might directly interfere lipid micelle formation and cholesterol solubility and consequently reduce lipid digestion and absorption.
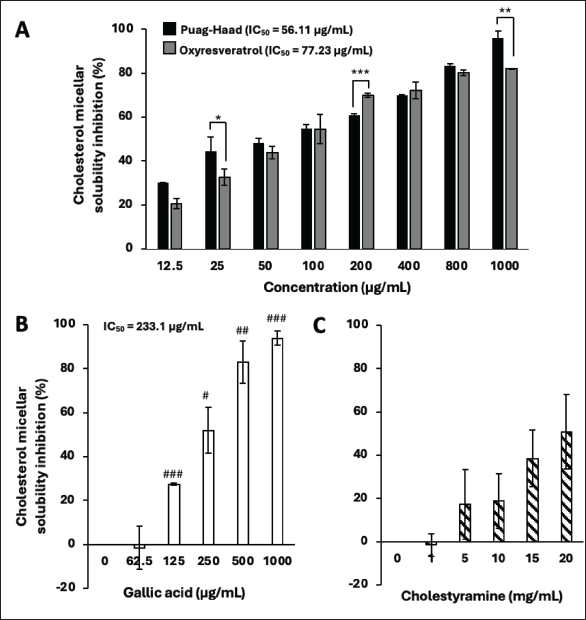 | Figure 2. Oxyresveratrol and Puag-Haad decrease cholesterol micellar solubility. (A) Oxyresveratrol and Puag-Haad at various concentrations were mixed with lipid micelle. The precipitated cholesterol (large lipid droplet) was separated from the intermicellar cholesterol by a syringe filter. (B) Gallic acid and (C) cholestyramine were tested as reference compounds. Values are mean ± SD from 3 to 5 experiments, * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, indicates a significant difference between groups and # p ≤ 0.05, ## p ≤ 0.01, ### p ≤ 0.001, compared to control. IC50 values were calculated using Prism Graph Pad. [Click here to view] |
Bile acid binding capacity of Puag-Haad
Bile acids, in their various forms, play a crucial role in the emulsification and absorption of fats in the small intestine. To determine bile acid binding capacity, tested compounds were tested in comparison with cholestyramine, a standard bile acid binding insoluble resin. The results demonstrated that cholestyramine, at a high concentration of 10 mg/ml, effectively bound to all three types of bile acids (Fig. 3). Remarkably, Puag-Haad, even at a much lower concentration of 0.5 mg/ml, exhibited the same binding intensity to all three types of bile acids as cholestyramine (Fig. 3). In this experiment, oxyresveratrol (in DMSO) interfered with the reaction, making it unsuitable for accurately assessing its activity. Puag-Haad, dispersed in water, exhibited low interference in the bile acid assay. Based on this observation, Puag-Haad demonstrated its potential as a potent bile acid binding agent and could be a promising candidate for further development in managing cholesterol levels.
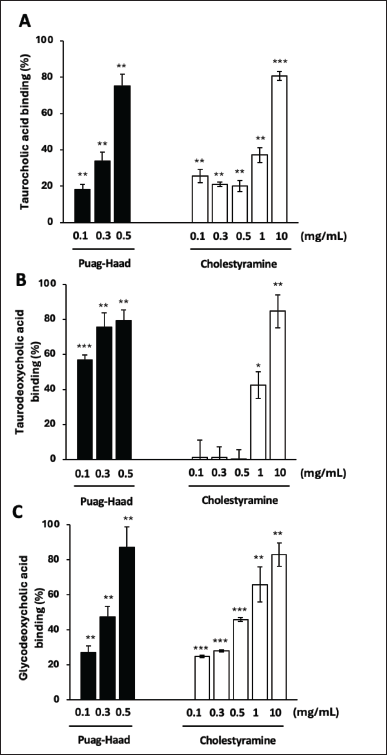 | Figure 3. Bile acids binding capacity of Puag-Haad in comparison with cholestyramine. Puag-Haad and cholestyramine were incubated with (A) taurocholic acid, (B) taurodeoxycholic acid, or (C) glycodeoxycholic acid for 3 hours and free and bound bile acids were separated by syringe filter. Values are mean ± SD from 3 to 5 experiments, * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, compared to control. [Click here to view] |
Oxyresveratrol and Puag-Haad inhibit cholesterol uptake into intestinal cells
In this study, differentiated Caco-2 cells were used as an in vitro model of the human intestinal epithelium to determine cholesterol uptake. The results showed that oxyresveratrol, Puag-Haad, and ezetimibe, a cholesterol transporter inhibitor, demonstrated dose-dependently cholesterol uptake inhibition (Fig. 4A). The concentration at 100 µg/ml of oxyresveratrol and Puag-Haad was the highest that could be used based on cell viability experiments (data not shown). The activity of oxyresveratrol at 100 µg/ml (~400 µM) is comparable to that of ezetimibe at the same concentration. We further investigated the additive effect of both tested compounds on ezetimibe’s activity. The results showed that oxyresveratrol and Puag-Haad at 100 µg/ml increase the inhibitory activity of 100 µM (~40 µg/ml) ezetimibe (Fig. 4B). Since cholesterol uptake into the Caco-2 cell monolayer can predict its absorption in the human intestine, the data suggest that oxyresveratrol and Puag-Haad have the potential to reduce the absorption of dietary cholesterol.
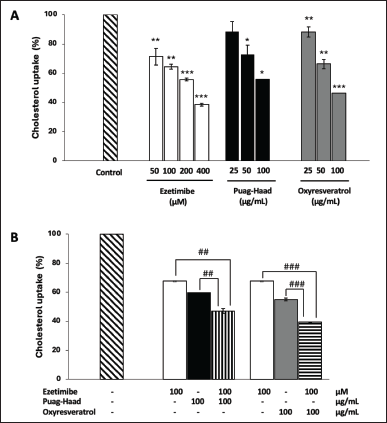 | Figure 4. Oxyresveratrol and Puag-Haad inhibit cholesterol uptake in differentiated Caco-2 cells. The uptake of fluorescent-cholesterol into differentiated Caco-2 epithelial was determined in the presence of oxyresveratrol and Puag-Haad or ezetimibe. Values are mean ± SD from 3 to 5 experiments, * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, compared to control (untreated cell) and # p ≤ 0.05, ## p ≤ 0.01, ### p ≤ 0.001, indicates a significant difference between groups. [Click here to view] |
DISCUSSION
Based on previous studies in high-cholesterol animal models, oxyresveratrol has demonstrated potential as an antihyperlipidemic agent [5]. The mechanism by which oxyresveratrol controls cholesterol levels is not yet fully understood. Therefore, this study focuses on investigating its direct effect on lipid digestion and absorption processes in the intestinal tract, which could subsequently influence blood cholesterol levels. Oxyresveratrol exhibits significant potential as an antihypercholesterolemic agent due to its ability to disrupt several key processes in lipid digestion and absorption. It reduces pancreatic lipase activity, interferes with the solubility of cholesterol in lipid micelles, and decreases the uptake of cholesterol into the intestinal epithelium. The oxyresveratrol-enriched extract (Puag-Haad) not only demonstrated the same activities as oxyresveratrol but also showed an impressive bile acid binding capacity.
Among 20 natural compounds isolated from Morus alba leaves, oxyresveratrol showed low-moderate pancreatic lipase inhibitory activity [18]. The present study supports that oxyresveratrol is not a potent pancreatic lipase inhibitor, suggesting that its antihyperlipidemic activity may be attributed to other pathways.
Cholesterol is transported to the intestinal brush border membrane in the form of lipid micelles, making cholesterol solubility within these micelles crucial for lipid absorption. The solubility of cholesterol in micelles depends on the presence of bile salts and the lipid composition [19]. Cholestyramine, a standard bile acid binding resin, showed much lower effectiveness in reducing cholesterol solubility compared to oxyresveratrol and Puag-Haad. This reduced effectiveness could be due to the large particle size of the resin, which may limit its ability to effectively access and bind to bile salts within the lipid micelles in this experimental condition. Interestingly, certain natural substances have demonstrated this activity. For example, previous studies reported that phytosterol glycosyl derivatives interact with bile salts, leading to reduced cholesterol solubilization in lipid micelles [20]. Gallic acid, a natural polyphenol, was previously demonstrated inhibitory effect against cholesterol solubility [17], it was tested as a comparison in the present study. The interference of oxyresveratrol and Puag-Haad with cholesterol solubility in lipid micelles could reduce the efficient absorption of lipids and cholesterol in the intestinal tract supporting their potential as cholesterol-lowering agent.
Bile acid binding agents, like cholestyramine, are non-absorbable resins that bind bile acids in the intestine. This interaction prevents the reabsorption of bile acids and playing a role in managing blood cholesterol levels [21]. To investigate the bile acid sequestrant from natural sources, insoluble or non-digestible fibers were often the target based on their property’s similarity to pharmaceutical resin, cholestyramine. There have been reporting many types of polysaccharide-based and protein-based biopolymers possessing bile acid binding ability with different mechanisms of interaction [22]. In the present study, Puag-Haad is effectively bound to bile acids with higher potency compared to the positive control drug. Puag-Haad contains several phytochemical components, including polyphenols, flavonoids, catechin [23], and tannins, with oxyresveratrol being the most prominent polyphenolic compound [4,24]. There have been no reports on the presence of biopolymers in Puag-Haad. A study on tea polyphenols revealed a positive correlation between the phenolic content and their bile acid-binding ability [25]. Given that approximately 80% of Puag-Haad consists of oxyresveratrol, this polyphenol is likely the active ingredient responsible for its bile acid-binding activity. Further in-depth studies are needed to identify the active compound or explore the underlying mechanism, which could support the development of Puag-Haad as an anti-hypercholesterolemic product.
Inhibition of cholesterol absorption is one of the key mechanisms for managing blood cholesterol levels. The Caco-2 intestinal epithelial cells were widely used to determine cholesterol uptake inhibitory activity of various natural compounds, such as Prunus domestica L fruit extract [17] and coffee leave extract [26]. The present study demonstrated the activity of oxyresveratrol and Puag-Haad in inhibiting cholesterol uptake in differentiated Caco-2 epithelial cells. The efficacy of both tested compounds appeared to be comparable to the standard drug, ezetimibe. In addition, they enhanced the activity of ezetimibe, suggesting that they may be beneficial for reducing the required dose of this therapeutic drug. Further investigation is needed to determine whether their site of action is the cholesterol transporter NPC1L1, which is also the target of ezetimibe.
Taken together, the data suggest that oxyresveratrol and Puag-Haad interfere with several processes involved in dietary fat digestion and absorption. These target sites of action occur in the intestinal lumen, which is the advantage of oxyresveratrol having low oral bioavailability (~10%) although this bioavailability increases with higher doses [27]. Coupled with their low toxicity [11], oxyresveratrol and Puag-Haad are excellent candidates for further development as dietary supplements or therapeutic agents.
CONCLUSION
In conclusion, oxyresveratrol and Puag-Haad demonstrate significant potential as natural antihyperlipidemic agents by interfering with multiple processes involved in lipid digestion and absorption. The study reveals that both compounds exhibit dose-dependent inhibition of pancreatic lipase, reduce cholesterol solubility in lipid micelles, and inhibit cholesterol uptake in intestinal cells, with Puag-Haad also showing strong bile acid-binding capacity. These findings highlight the promise of oxyresveratrol and Puag-Haad in managing hypercholesterolemia, offering a natural alternative for controlling blood cholesterol levels.
AUTHOR CONTRIBUTIONS
All authors conceptualized the study and took part in drafting the article. Trisat K performed all experiments, collected data, and conducted statistical analysis. Limpeanchob N interpreted results and revised the manuscript. All authors agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
FINANCIAL SUPPORT
This work was supported by Naresuan University (NU), and National Science, Research and Innovation Fund (NSRF). Grant number; R2566B017.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data are available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declare that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. WHO. The top 10 causes of death. Geneva: World Health Organization; 2024 [cited 2024 August 7]. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
2. Mannu GS, Zaman MJ, Gupta A, Rehman HU, Myint PK. Evidence of lifestyle modification in the management of hypercholesterolemia. Curr Cardiol Rev. 2013;9(1):2–14.
3. Likhitwitayawuid K. Oxyresveratrol: sources, productions, biological activities, pharmacokinetics, and delivery systems. Molecules. 2021;26:4212.
4. Maneechai S, Likhitwitayawuid K, Sritularak B, Palanuvej C, Ruangrungsi N, Sirisa-Ard P. Quantitative analysis of oxyresveratrol content in Artocarpus lakoocha and “Puag-Haad.” Med Princ Pract. 2009;18(3):223–7.
5. Jo SP, Kim JK, Lim YH. Antihyperlipidemic effects of stilbenoids isolated from Morus alba in rats fed a high-cholesterol diet. Food Chem Toxicol. 2014;65:213–8.
6. Lorenz P, Roychowdhury S, Engelmann M, Wolf G, Horn TF. Oxyresveratrol and resveratrol are potent antioxidants and free radical scavengers: effect on nitrosative and oxidative stress derived from microglial cells. Nitric Oxide. 2003;9(2):64–76.
7. Chung KO, Kim BY, Lee MH, Kim YR, Chung HY, Park JH, et al. In vitro and in vivo anti-inflammatory effect of oxyresveratrol from Morus alba L. J Pharm Pharmacol. 2003;55(12):1695–700.
8. Choi HJ, Song NJ, Lee RA, Lee HD, Seo M, Kim S, et al. Oxyresveratrol increases energy expenditure through Foxo3a-mediated Ucp1 induction in high-fat-diet-induced obese mice. Int J Mol Sci. 2018;20(1):26.
9. Tan H, Tse IM, Li ET, Wang M. Inhibitory effects of oxyresveratrol and cyanomaclurin on adipogenesis of 3T3-L1 cells. J Funct Foods. 2015;15:207–16.
10. Charoenlarp P, Radomyos P, Bunnag D. The optimum dose of Puag-Haad in the treatment of taeniasis. J Med Assoc Thai. 1989;72(2):71–3.
11. Nilvises N, Panyathanya R, Wamnutchinda W. Toxicity test of Puag Haad (Artocarpus lakoocha). Bull Dept Med Sci. 1985;27(1):49–55.
12. Aubry S, Aubert G, Cresteil T, Crich D. Synthesis and biological investigation of the beta-thiolactone and beta-lactam analogs of tetrahydrolipstatin. Org Biomol Chem. 2012;10(13):2629–32.
13. Kirana C, Rogers PF, Bennett LE, Abeywardena MY, Patten GS. Naturally derived micelles for rapid in vitro screening of potential cholesterol-lowering bioactives. J Agric Food Chem. 2005;53(11):4623–7.
14. Adisakwattana S, Intrawangso J, Hemrid A, Chanathong B, Mäkynen K. Extracts of edible plants inhibit pancreatic Llpase, cholesterol Eeterase and cholesterol micellization, and bind bile acids Food Sci Biotechnol. 2012;50(1):11–6.
15. Yoshiestark Y, Wäsche A. In vitro binding of bile acids by lupin protein isolates and their hydrolysates. Food Chem. 2004;88(2):179–84.
16. Porter JL, Fordtran JS, Santa Ana CA, Emmett M, Hagey LR, MacDonald EA, et al. Accurate enzymatic measurement of fecal bile acids in patients with malabsorption. J Lab Clin Med. 2003;141(6):411–8.
17. Chamnansilpa N, Aksornchu P, Adisakwattana S, Thilavech T, Mäkynen K, Dahlan W, et al. Anthocyanin-rich fraction from Thai berries interferes with the key steps of lipid digestion and cholesterol absorption. Heliyon. 2020;6(11):e05408.
18. Jeong JY, Jo YH, Kim SB, Liu Q, Lee JW, Mo EJ, et al. Pancreatic lipase inhibitory constituents from Morus alba leaves and optimization for extraction conditions. Bioorg Med Chem Lett. 2015;25(11):2269–74.
19. Coreta-Gomes FM, Vaz WLC, Wasielewski E, Geraldes CFG, Moreno MJ. Quantification of cholesterol solubilized in dietary micelles: dependence on human bile salt variability and the presence of dietary food ingredients. Langmuir. 2016;32(18):4564–74.
20. Hu Y, Ma C, Yang R, Guo S, Wang T, Liu J. Impact of molecular interactions between hydrophilic phytosterol glycosyl derivatives and bile salts on the micellar solubility of cholesterol. Food Res Int. 2023;167:112642.
21. Tiwari V, Khokhar M. Mechanism of action of anti-hypercholesterolemia drugs and their resistance. Eur J Pharmacol. 2014;741:156–70.
22. Li M, Wang L, Chen G, Chen Z. Biopolymer-based sequestrants for lowering cholesterol: structures, in vitro bile acid anion binding effects, and interaction mechanisms. J Funct Foods. 2024;113:106002.
23. Islam S, Shajib MS, Rashid RB, Khan MF, Almansur MA, Datta BK, et al. Antinociceptive activities of Artocarpus lacucha Buch-ham (Moraceae) and its isolated phenolic compound, catechin, in mice. BMC Complement Altern Med. 2019;19(1):214.
24. Duangdee N, Chamboonchu N, Kongkiatpaiboon S, Prateeptongkum S. Quantitative (1) HNMR spectroscopy for the determination of oxyresveratrol in Artocarpus lacucha heartwood. Phytochem Anal. 2019;30(6):617–22.
25. Wu Z, Teng J, Huang L, Xia N, Wei B. Stability, antioxidant activity and in vitro bile acid-binding of green, black and dark tea polyphenols during simulated in vitro gastrointestinal digestion. RSC Adv. 2015;5(112):92089–95.
26. Sansri V, Sroyraya M, Phisalprapa P, Yosboonruang A, Ontawong A, Saokaew S, et al. Suppressive effect of coffee leaves on lipid digestion and absorption in vitro. Foods. 2024;13(15):2445.
27. Chen W, Yeo S, Mai G, Lin H. Oxyresveratrol: a bioavailable dietary polyphenol. J Funct Foods. 2016;22:122–31.