INTRODUCTION
The importance of advancing drug delivery systems lies in their potential to address existing challenges related to current drug formulations. The challenges arise from the inherent physical and chemical properties of drug molecules and the natural barriers present within the human body. These limitations manifest as poor drug solubility and permeability. A considerable number of pharmaceutical compounds (up to 70%) encounter difficulties due to limited water solubility, potentially influencing their absorption within the gastrointestinal system, emphasizing the importance of overcoming them for successful drug development [1]. Several approaches have been developed thus far, among which, lipid-based drug delivery systems have emerged as a fundamental formulation method aimed at overcoming challenges related to poor bioavailability.
Self-emulsifying drug delivery systems (SEDDSs) are lipid-based drug delivery systems made up of oil, surfactant, and co-surfactant. SEDDS possess the ability to self-emulsify spontaneously within the gastrointestinal tract (GIT), resulting in the formation of an oil-in-water emulsion that improves the absorption of drugs [2]. Although SEDDS is not considered novel, there has been an increasing interest in developing it for therapeutic applications in recent years. The potential of SEDDS to enhance the dissolution rate of Biopharmaceutics classification system (BCS) II and IV drugs has prompted the growing interest in their development. The lipid component of SEDDS stimulates chylomicron/lipoprotein, resulting in micellar solubilization in the duodenum, and the drug becomes entrapped in colloidal micelles, as a result, the drug becomes more soluble, and its absorption is also improved [3]. The small globule size of the emulsion formed upon dilution of SEDDS offers a large surface area for interaction with the GIT, Improving absorption and reducing drug absorption variability [4].
SEDDS are generally classified into two types based on the emulsion formed upon dilution: self-nanoemulsifying drug delivery systems (SNEDDSs) and self-microemulsifying drug delivery systems (SMEDDSs). The characteristic of SMEDDS is the formation of an optically clear to slightly translucent microemulsion when exposed to the aqueous medium. Microemulsion offers small droplet sizes that range from 100 to 400 nm. SMEDDS requires low interfacial tension for the formation of emulsion which offers a wide surface area for absorption. SNEDDS forms an oil-in-water nanoemulsion with droplet sizes less than 100 nm. SNEDDS are kinetically stabilized, whereas SMEDDS is thermodynamically stable [5,6].
Commercially available formulations of SEDDS are liquids, encapsulated in hard or soft gelatin capsules. Table 1 shows various marketed formulations of SEDDS.
The limited number of marketed products is primarily a result of their high cost of manufacturing, stability, and portability challenges, the risk of drug precipitation upon dilution, the absence of accurate in-vitro prediction tools, and the complexity of manufacturing equipment. The prevalent approach for overcoming these challenges is the solidification of liquid SEDDS. The primary objective is to improve both its physicochemical stability and reduce the overall manufacturing costs [15]. In addition, solidification has several advantages such as extended gastric residence time [16], controlled release of drug [17], improved solubility and bioavailability [18], ease of handling, dose accuracy, targeting drugs to a specific absorption region in the GIT, protecting the drugs from the harsh gut environment, spontaneous emulsification, [19] and so on. Solidification of liquid SEDDS can reduce the surfactant amount and enhance the oxidative stability of lipids by protecting them from degradation [20]. Achieving effective oral delivery of liquid SEDDS of macromolecules such as proteins and peptides is challenging due to the precipitation and conformational changes. Solid SEDDS (S-SEDDS) demonstrate the ability to stabilize peptides and proteins [21]. This review aims to build on earlier review articles in the field by highlighting current developments and challenges associated with the solidification of SEDDS. The focus of the article is to review various applications of S-SEDDS found in existing works of literature.
BUILDING BLOCKS OF S-SEDDS
The building blocks of S-SEDDS are lipid, surfactant, cosurfactant, and solid carrier. These components synergistically contribute to the formulation and performance of S-SEDDS. Types of oil and surfactant, along with their concentrations, are important for designing a highly effective self-emulsifying system. Adequate solubility drugs in lipids, surfactants, and co-surfactants and the self-emulsification efficiency of surfactants and cosurfactants are to be considered before formulation [22]. The performance, stability, and release profile of S-SEDDS are impacted by the types of solid carriers. It is crucial to choose a solid carrier that guarantees the best drug solubility, dispersion, and bioavailability after administration in addition to offering a stable matrix for the formulation. The compatibility of solid carriers with other components is a critical factor in achieving the intended therapeutic effect [23].
Lipids
Lipids play a vital role in the functionality of SEDDS. The solubility of the drug in the oil phase is the key consideration in the selection of oils. The drug solubility in the oil phase has a significant impact on the efficiency of nanoemulsion in maintaining the drug in a solubilized state; this is particularly significant when it comes to oral formulations [15]. The oil phase improves drug bioavailability by enhancing solubility and facilitating lymphatic transport. However, having high solubility does not ensure optimal in vivo efficiency [24]. A variety of long- and medium-chain triglyceride oils that are distinguished by their saturation levels, are used for the development of SEDDS. Natural oils and fats are composed of triglyceride combinations that comprise fatty acids with varying chain lengths and levels of unsaturation. The melting point of a particular oil increases as the chain lengths of its fatty acids lengthen and drop as the degree of unsaturation increases. Furthermore, increased unsaturation makes the oil more susceptible to oxidation [25]. Unmodified vegetable oils, especially those with medium-chain triglycerides (Examples: Palm kernel oil and coconut oil), are considered favorable due to their natural origin. However, limited drug loading and inefficient self-emulsification, reduce their usage in SEDDS formulation [15]. Long-chain lipids (Examples: Oleic acid and castor oil) were found to be able to maintain drug concentrations at significant levels, avoiding precipitation, in contrast to medium-chain triglycerides [20]. The use of modified vegetable oils (Campul MCM, Acconon CC-6) has become prevalent recently due to their high emulsification capabilities when combined with suitable surfactants [26]. Table 2 represents commonly used natural, semi synthetic, and synthetic oils for the formulation of SEDDS.
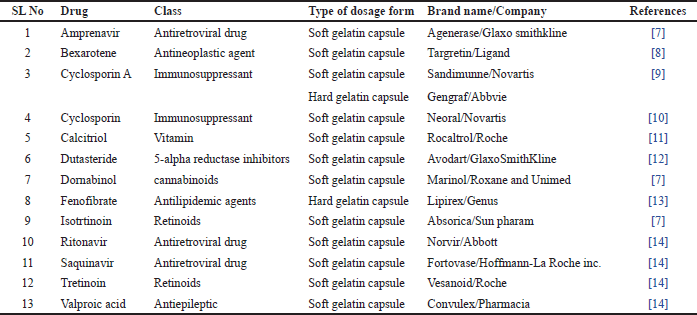 | Table 1. Commercially available SEDDS formulations. [Click here to view] |
Surfactants
SEDDS requires a high concentration of surfactant to create fine oil-in-water emulsions in the GIT, reducing the chance of drug precipitation after dilution. The concentration of surfactants, which normally varies from 30% to 60% in SEDDS formulations. For optimal SEDDS formulation, the surfactant must lower interfacial tension, ensure smooth droplet dispersion, and have flexible, lipophilic properties [28]. A surfactant with strong drug solubility may not have good oil affinity. The self-emulsification efficiency rather than the drug solubilizing ability will determine the criterion for selecting the surfactant and co-surfactant [4]. Nonionic surfactants, particularly those with Hydrophilic lipophilic balance value (HLB) >12, are highly favorable for SEDDS formulations, given their lower toxicity compared to ionic surfactants. The high HLB surfactants facilitate fast and easy dispersion within the aqueous gastrointestinal fluid, forming an extremely fine oil-in-water emulsion. Consequently, this leads to good self-emulsification performance [1]. Polyoxyethylene (10) oleyl ether, polyoxyethylene (20) oleyl ether, Cremophor® EL, Cremophor® RH40, and polysorbates are examples of nonionic surfactants commonly used for SEDDS formulations [29]. Higher concentrations of surfactants in SEDDS may cause gastric irritation, underscoring the importance of addressing safety concerns related to cytotoxicity. Solidifying SEDDS by substituting a portion of surfactants with solid carriers, such as hydroxypropyl methylcellulose (HPMC), can reduce toxicity [30].
Cosurfactants
Cosurfactants aid in the dissolution of drugs and higher concentrations of hydrophilic surfactants. Cosurfactants also improve the fluidity of the interface between the two phases of the emulsion, which inhibits the generation of liquid crystals and speeds up the emulsification process [20]. The choice of the co-surfactant depends on its ability to optimize the nanoemulsion area along with the surfactant. Both the surfactant and co-surfactant create a mechanical barrier against coalescence and reduce interfacial energy, resulting in good thermodynamic stability. Co-surfactants are often alcohols with medium-to-short chain lengths (C3–C8) such as propylene glycol, transcutol HP, ethanol, and isopropyl alcohol [15]. However, alcohols and other volatile cosolvents in conventional SEDDS have a disadvantage, as they can evaporate into the shells of soft gelatin or hard gelatin capsules, resulting in drug precipitation [1].
 | Table 2. Various oils used for the formulation of SEDDS. [Click here to view] |
Solid carriers
Solid carriers have a critical role in the conversion of liquid SEDDS into solid form. Solidification enhances the physical and chemical stability of SEDDS, preventing them from deterioration. Furthermore, controlling the rate of drug diffusion from the matrix provides sustained drug release. Various solid carriers used for the transformation of liquid SEDDS into solid are mainly divided into two types: silica-based carriers and nonsilica-based solid carriers (Fig. 1).
Silica-based solid carriers
Solid carriers made of silicon dioxide (SiO2), also known as silica, are porous substances with a large surface area and a highly porous structure. Silica-based solid carriers provide various advantages in the formulation of S-SEDDS, including enhanced drug solubility, stability, and drug release. The porous nature of silica-based solid carriers can help in drug dissolution and release from the SEDDS, resulting in increased drug bioavailability. Fumed silica nanoparticles (Aerosil), porous amorphous silica gels (Syloid and Sylysia), mesoporous silica materials, and magnesium aluminum silicate (Neusilin) are the commonly used silica-based solid carriers [31] The utilization of Aerosil 200 as the solid carrier in the solid SNEDDS of olmesartan exhibited good flow properties and a significant drug content [4]. Snela et al. [32] and colleagues developed two novel SEDDSs for atorvastatin calcium. The SEDDSs were adsorbed onto solid inorganic carriers such as Aerosil® 200, Neusilin® UFL2, and Neusilin® US2 to improve stability and effectiveness. Scanning electron microscopic images of SEDDS formulations adsorbed on three different solid carriers revealed the absence of crystals, which was also confirmed by differential scanning calorimetry. Inverse gas chromatography on solid SEDDSs (S-SEDDSs) demonstrated that the strongest interactions were observed for Neusilin® UFL2 samples, while the weakest were observed for Aerosil®-based materials [32]. A study focused on the development of solid supersaturable SEDDS of celecoxib via the adsorption method, utilizing various grades of silica-based solid carriers (Sylysia 350 fcp, Aerosil 200, Aerosil 300, Aerosil R 972), were chosen based on their features such as porosity, hydrophobicity-hydrophilicity and surface area. The drug release characteristics of solid supersaturable SEDDS made from these solid carriers showed significant variances, despite the fact that they were various grades of the same solid carrier. The variations in drug release were due to their unique physicochemical properties. The solid supersaturable SEDDS with solid carrier Sylysia 350 fcp demonstrated a higher and more consistent drug release, resulting in improved oral bioavailability among all solid supersaturable SEDDS. This study proves that the characteristics of solid carriers are crucial in influencing the drug release profile of S-SEDDS and can help to make formulation development more rational [33].
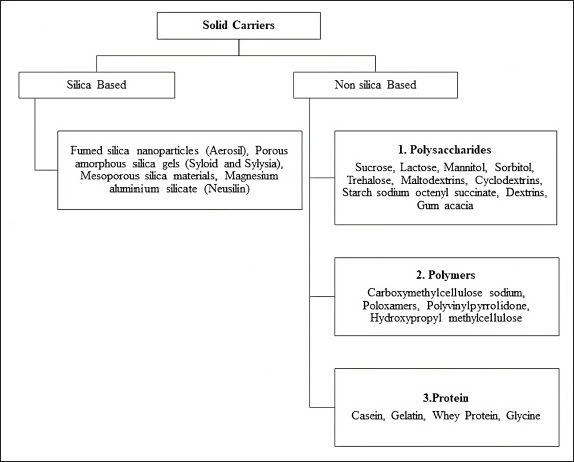 | Figure 1. Solid carriers used for the conversion of liquid SEDDS into solid SEDDS. [Click here to view] |
Nonsilica-based solid carriers
Nonsilica-based solid carriers such as polysaccharides, polymers, and proteins are also used in the formulation of S-SEDDS. Polysaccharides are commonly considered safe for pharmaceutical applications due to their high biocompatibility and low toxicity. Polysaccharide-based solid carriers include low molecular weight sucrose, lactose, mannitol, sorbitol, and trehalose, as well as higher molecular weight maltodextrins, cyclodextrins, starch sodium octenyl succinate, dextrins, and gum acacia. The crystal structure and moisture sensitivity of polysaccharide solid carriers should be considered while designing formulations. When subjected to spray-drying, lyophilization, and rotary evaporation, these carriers are susceptible to undergoing partial or total transformation into an unstable, amorphous state with high energy levels [34]. Polymer solid carriers can be used to generate a matrix in which the liquid SEDDS may be incorporated to develop solid dosage forms such as tablets, capsules, or granules. Polymer solid carriers are effective as emulsifiers, solid carriers, and inhibitors of drug precipitation. Carboxymethylcellulose sodium, poloxamers, polyvinylpyrrolidone, and HPMC are a few examples of polymer-based solid carriers. At high temperatures and humidity levels, polymer-based solid carriers tend to absorb moisture, due to their hygroscopic nature. Amphiphilic properties of protein-based solid carriers allow them to serve as both emulsifiers and solid carriers for lipid formulations. Casein, whey protein, gelatin, glycine, and soy protein are examples of protein-based solid carriers that provide improved biocompatibility and biodegradability. Although they might not be stable enough to function effectively with some drug molecules [35]. Gelatin and glycine are less frequently reported for the solidification of lipid colloids due to their poor compressibility [34].
SOLIDIFICATION STRATEGIES FOR LIQUID SEDDS
Physical adsorption
Physical adsorption is an uncomplicated solidification method wherein liquid SEDDS is mixed with solid carriers, including high surface-area colloidal inorganic adsorbent substances, microporous inorganic substances cross-linked polymers, or nanoparticle adsorbents (Fig. 2) [20]. Cross-linked polymers such as polymethyl methacrylate and carboxymethyl cellulose facilitate sustained drug release and prevent drug re-precipitation. Porous SiO2, fullerenes, charcoal, and carbon monomers are nanoparticle adsorbents [36].
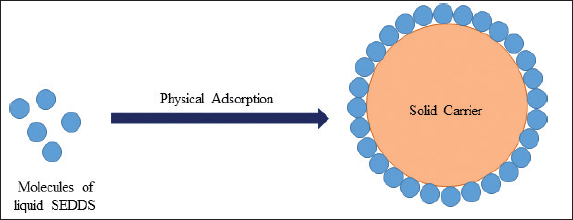 | Figure 2. Schematic representation of solidification of liquid SEDDS by physical adsorption. [Click here to view] |
The selection of solid carriers is important as the nature (hydrophilic/hydrophobic) of solid carriers can influence the properties of the drug. X-ray diffraction studies on a self-nanoemulsifying granule of ezetimibe using hydrophobic colloidal SiO2 as a solid carrier revealed that the presence of the drug is in its amorphous form. Intriguingly, when the same SNEDDS adsorbed onto a different solid carrier, led to the formation of a eutectic mixture [37]. Furthermore, it is essential to thoroughly analyze the porosity of the chosen adsorbent, considering the significant influence of pore length, shape, and specific surface area on the dissolution of S-SEDDS [38]. A novel S-SNEDDS of valsartan utilizing different porous solid carriers Aerosil 200, Sylysia 350, Sylysia 550, Sylysia 730, and Neusilin US2 exhibited a 3–3.5 fold increase in bioavailability of valsartan [39]. The drug dissolution rate of S-SEDDSs can be affected by interactions between solid carriers and the components of SEDDS. Therefore, it is important to consider the characteristics of solid carriers such as their particle size, nature, quantity, and specific surface area. In addition, an important factor to be considered is drug crystallinity. Amorphous drugs are more prone to re-crystallization and chemical instability. The stability and dissolution rate of S-SEDDS formulations must, therefore, be optimised with careful consideration of these aspects [40].
The physical adsorption method uses a minimal number of excipients and avoids the use of organic solvents to produce the final formulation. Moreover, good content uniformity and significantly high drug loading are obtained using only basic equipment.
The leakage of liquid components during the compression of S-SEDDS is a major limitation of this approach. It may result in the development of soft tablets and can give rise to problems such as sticking, chipping, and variable hardness. In certain cases, a substantial quantity of solid carriers may be required, which undesirably increases the volume of the final product [20].
Spray drying
Spray drying is thought to be an efficient and cost-effective way to produce micro-particle systems that can be easily scaled up. This method is suitable for solidifying SEDDS, and the end product can be developed into tablets [41]. The spray drying apparatus is made up of a feeding system, hot air source, atomizer, drying chamber, solid–gas separator, and final product collection chamber. The characteristics of the material being dried and the desired features of the resulting powder are taken into consideration while choosing the specific layout of the drying chamber, as well as the temperature, airflow pattern, and atomizer orifice size [1].
The spray drying process of SEDDS involves the mixing and the solubilization of lipids, surfactants, active constituents, and solid carriers. The solubilized formulation is atomized to produce a spray of droplets that travel into a drying chamber and facilitate water evaporation from SEDDS to form the dried particles with the aid of controlled temperature and airflow (Fig. 3) [42].
The selection of solid carriers has a significant impact on drug release in addition to drug bioavailability since it affects the entrapment efficiency of SEDDS and the droplet size of the reconstituted emulsion. Microcrystalline cellulose, HPMC, hydroxypropyl cellulose, lactose, and maltodextrin are a few examples of hydrophilic solid carriers used in spray drying. These carriers promote rapid emulsion formation and water penetration [43].
Spray drying offers highly accurate control over particle size, crystallinity level impurities, bulk density, and residual solvent. In addition, it maintains a good quality powder throughout the drying process [44].
Spray-dried SEDDS offers enhanced stability, solid particles with a narrow size distribution, good flow properties, and an enhanced dissolution rate. However, a reduced yield may be obtained because of the elimination of the nonencapsulated drug with the exhausted gas. Reduction in the volume of volatile surfactant in liquid SEDDS at high temperatures may also affect the drug loading capacity [19]. High temperatures and rapid drying rates hinder the solidification of temperature-sensitive substances. Clogging of the nozzle with oil components poses a challenge in this approach [45].
Successful SEDDS solidifications using the spray drying approach are shown in Table 3.
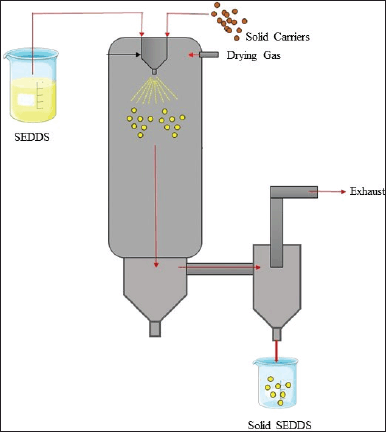 | Figure 3. Process of conversion of liquid SEDDS into solid by Spray Drying method. [Click here to view] |
Spray congealing
The spray congealing method is mainly used for the preparation of self-emulsifying microparticles. The process involves the spraying of molten SEDDS into a cooling chamber, where the liquefied droplets undergo solidification and are followed by re-crystallization to form fine powders. The absence of solvent evaporation makes congealed particles robust and impermeable. Spray congealing holds promise for improving the bioavailability of weakly soluble drugs through different mechanisms. These include improving wettability, forming dispersed systems, reducing the size of drug crystals, and converting the drug into metastable polymorphs or the amorphous form [53].
The spray congealing approach has a continuous flow, is simple to use, and can be adjusted for automatic procedures with rapid response. Despite the longer cooling times required, the quality of the powder does not deteriorate during cooling. The fine powder can then be subjected to further processes that produce a variety of solid oral dosage forms, such as tablets. Spray congealing technology was employed in the development of S-SEDDS of glibenclamide to enhance the solubilization rate. It is suggested that spray congealing could serve as a promising approach for designing S-SEDDS, offering a remedy to challenges associated with liquid SEDDS [54]. Spray congealing is a solvent-free, low-cost, and quick manufacturing method for increasing the bioavailability of poorly water-soluble drugs like praziquantel [55].
Although molten SEDDS usually melt at temperatures higher than their melting point, this is not always a viable option as certain drugs have higher melting points than lipids. However, the use of spray-congealing as a unit process to increase the solubility of active ingredients is possible [56].
Gelucire®, vitamin E tocopheryl polyethyleneglycol succinate, solutol, and poloxamer are commonly used excipients for the production of spray-congealed SEDDS [43]. Among them, Gelucire 50/13, with an average HLB of 11 and a melting temperature of around 50°C, is frequently employed [57].
Lyophilization
The lyophilization method, commonly known as freeze drying, is a novel approach for solidifying SEDDS. SEDDS can be easily solidified through lyophilization, which also increases their solubility, stability, and patient compliance [13]. In this method, the liquid SEDDS is frozen and followed by conversion into a dry powder via sublimation at low temperatures and pressures. On reconstitution, dry powder can be easily turned back into a fine emulsion. The lyophilization process consists of three stages: fast freezing of the product is the first stage, and then it goes through a primary drying phase where most of the water content is sublimated out. The third stage, known as secondary drying, eliminates any leftover water molecules to produce a stable and dehydrated powder [58].
Lyophilization has several benefits, one of which is the use of an aseptic procedure that reduces the chances of contamination. This technique helps stabilize chemicals that are prone to oxidation, and a slow cooling rate of the lyophilization method stabilizes the SEDDS formulation throughout the process. In addition, the preservation duration is prolonged because 95%–99.5% of the water content is removed, and chemicals that are heat-sensitive are particularly well-suited for lyophilization [15]. Cryoprotectants are used to avoid the mild stresses that the lipid bilayers experience during freezing and drying states. Sugars such as maltose, sucrose, mannitol, trehalose, and glucose are commonly used as cryoprotectants [1].
S-SMEDDS of Lornoxicam was formulated utilizing the lyophilization method for solidification and assessed its bioavailability in Wistar rats. In vitro drug release assessment of S-SMEDDS of Lornoxicam showed significant improvement in solubility and dissolution rate. In vivo pharmacokinetic studies also exhibited higher bioavailability of S-SMEDDS compared with marketed tablets [59]. Furthermore, a study on S-SNEDDS of meloxicam investigated the use of water-soluble and water-insoluble solid carriers in lyophilization. The study found that the S-SNEDDS formulations prevented Meloxicam crystallization and increased the drug release by three to four times. In comparison to the insoluble solid carrier, the soluble carrier did not affect the formation of the nano-emulsion [60].
Though it has benefits, lyophilization has some disadvantages as well. This method involves extended handling and processing time and also lyophilization apparatus can be highly complex and costly [61]
 | Table 3. A literature review on successful SEDDS solidification using the spray drying method and its outcome. [Click here to view] |
Melt extrusion-spheronization method
Melt extrusion-spheronization is a method of choice for producing pellets and one of the most investigated approaches for producing self-emulsifying pellets. This method involves mixing SEDDS with a carrier, agglomerating the mixture, and then spheronizing it. During spheronization, the extrudate is broken down to generate pellets or spheroids. Spheroids have a narrow particle size distribution, favorable flow characteristics, and low friability. In addition, the process allows for achieving high drug loading [19]. In addition, extrusion/spheronization is a potential strategy for developing self-emulsifying pellets of phytotherapeutic agents [62].
The interplay between the proportion of SEDDS and water holds an important role in determining the extrusion force, size distribution, disintegration time, and surface roughness of pellets. It is important to highlight that, the maximum amount of SEDDS that can be solidified by extrusion spheronization method is 42% of the dry pellet weight [36]. Achieving favorable physical characteristics and high drug loading in pellets necessitates a reduction in the solid carrier quantity and a concurrent increase in the liquid SEDDS content. In contrast to other excipients, a formulation with a higher proportion of absorbent material permits the inclusion of a greater portion of the SEDDS.
According to Abdalla et al. [63] more than 40 % of the SEDDS load in pellets may result in the sticking of the extrudate to the apparatus. Pellets without suitable adsorbents may have poor hardness, poor flowability, and a tendency to agglomerate. Commonly used adsorbents are lactose, methylcellulose, microcrystalline cellulose, HPMC, croscarmellose sodium, and crospovidone [64]. The tensile strength and irregularity of the spheroids can be affected by the ratio of SEDDS to water as well as the relative proportions of absorbent and other components. The extrusion force and the sphericity of the pellets are positively correlated with the proportion of SEDDS. Rapid drug release is an additional advantage of using a disintegrant [63].
Melt extrusion is a closed process that uses minimal equipment, provides high blending efficiency, and minimizes the risk of cross-contamination. The processing time of this method is less, and the variables are easy to control. [1]. However, melt extrusion has some limitations, such as high-energy input, a limited number of polymers, and the challenges of using thermal-sensitive material. Extensive research should be done on the various properties of components, such as stability, flow characteristics, wettability, and compatibility before the selection [65].
Melt granulation
Melt granulation is a single-step method, that can be used to produce S-SEDDS. This process does not include the liquid addition and drying phases, making it an effective substitute for methods that rely on solvents [66]. Nonswellable and water-insoluble properties of the dried product are considered safe for human use. The melt granulation process produces agglomerates through two mechanisms: immersion and dispersion. The primary particle is submerged onto the binder surface in immersion mode, while the molten binder spreads on the primary particle’s surface in dispersion mode [65]. Neutral solid carriers such as silica and magnesium aluminometasilicate and lipid-based carriers such as gelucire and lecithin are common adsorbents used in meltgranulation [67].
Regardless of whether high or low impeller speeds are used, the viscosity of SEDDS or binders is critical in nucleation and particle size. Binders having lower melting points melt and soften continuously during handling and storage, making them prone to agglomeration, whereas binders with higher melting points require greater temperatures, which might cause instability, especially in heat-sensitive materials [36]. The requirement for high temperatures during the melt granulation process is a main limitation, as it may deteriorate the constituents [66].
The melt granulation method was employed to develop immediate-release self-emulsifying tablets of carbamazepine and it was found that drug stability improved through this process and also melt granulation had no impact on the immediate-release properties of carbamazepine self-emulsifying tablet [68].
Twin screw granulation (TSG)
Common solidification techniques fall under the category of batch manufacturing, where the quantity of material processed is constrained by the capacity of the manufacturing equipment. Furthermore, the optimization of several process parameters is required for these manufacturing processes. This optimization is particularly important when there is a need to either scale up or scale down the batch size. However, these limitations make these methods less acceptable for large-scale production.
TSG has received the greatest attention in recent years as a single-step continuous process. Furthermore, depending on the physical state of the binder, the TSG process is divided into three categories: twin-screw melt granulation (TSMG), twin-screw dry granulation (TSDG), and twin-screw wet granulation (TSWG) [69].
TSMG depends on a low-melting-point binder that acts as a binding liquid after melting, necessitating a low concentration. This process involves a blend of drugs, binders, and excipients processed at a temperature near or above the melting point of the binder, ensuring uniform distribution of binder between the particles and forming granules. TSDG is an economical method of granulating blends as it does not require the use of solvents. It is like the conventional roller compaction process, which was used to achieve dry granulation. In TSDG, a mixture containing the drug, binder, and other components is fed into the extruder using a gravimetric hopper and then conveyed to the mixing zone. The temperature is maintained below the glass transition temperature or melting point of the components to keep all materials in a dry state. The combination of temperature and shear in the mixing zone causes the materials to soften and distribute uniformly in the extruder barrel, resulting in the formation of granules. This method increases the drug loading capacity by using a low binder concentration. Granulating fluid helps produce granules in the TSWG process. Wet granulation involves moving the materials through an extruder’s barrel while a peristaltic pump forces the granulating fluid into the barrel zone. Granule formation results from the complete mixing of the wet mass formed in the extruder [70].
The TSMG approach was confirmed to be suitable for solidifying SEDDS, as demonstrated by the formulation of S-SEDDS of Ibuprofen. Optimized granules of SEDDS were compressed into tablets. The compressed tablets exhibited enhanced dissolution profiles in comparison to the pure drug. The characteristics of liquid SEDDS were maintained within the S-SEDDS [69]. The effectiveness of the TSMG method for increasing fenofibrate solubility was investigated, with Gelucire® 48/16 as the solubilizer and Neusilin® US2 as the adsorbent. The solubility of fenofibrate improved in all formulations when compared to pure drugs. However, the rate of dissolution was primarily influenced by the concentration of adsorbent and the ratio of molten portion to adsorbent in the formulation [71].
Liquisolid method
Liquisolid approach involves mixing liquids with chosen solid carriers and coating materials to develop freely flowing, easily compressible dry powder. A coating material with a high capacity for adsorption and a significant specific surface area gives good flow properties to the formulation. The primary limitation of the liquisolid method lies in the challenge of incorporating high doses of water-insoluble drugs since it demands large quantities of liquid, carrier, and coating material to produce liquisolid powder with desired properties. The proportion of carrier and coating material can be reduced by incorporating additives such as polyethylene glycol 35,000 and polyvinyl pyrrolidone (PVP) into the liquid formulation [72]. Javadzadeh [73] demonstrated that the addition of PVP into the liquid formulation enables the loading of a high quantity of drug into liquisolid tablets. The liquisolid tablets of carbamazepine prepared with the addition of PVP exhibited an increased dissolution rate [73].
Liquisolid approach is preferred for increasing drug solubility and dissolution rate of BCS classes II and IV drugs. The liquisolid method was employed to develop a self-emulsifying tablet of leflunomide, a BCS class II drug, which showed a higher dissolution rate compared to the marketed tablet [74].
Method based on supercritical fluids
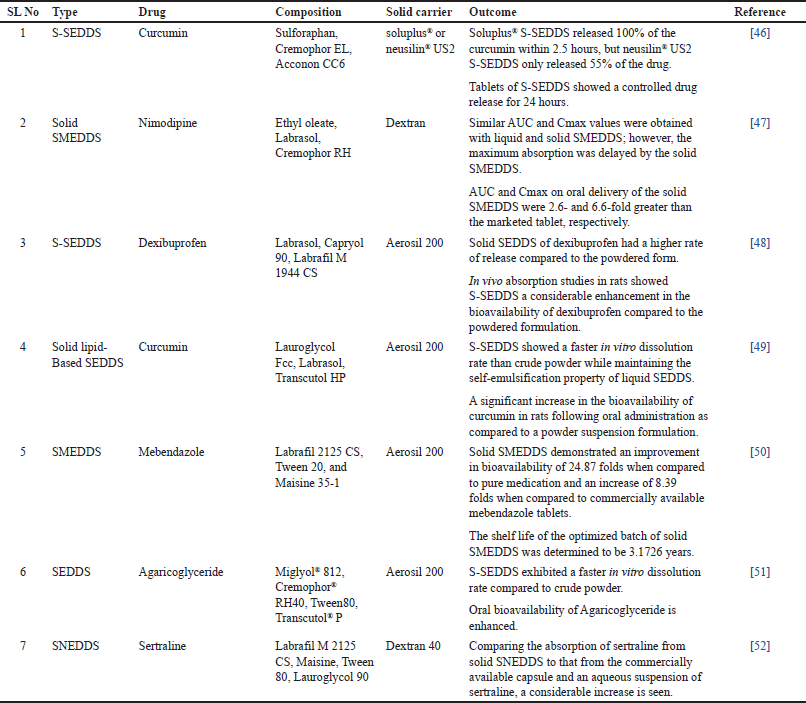
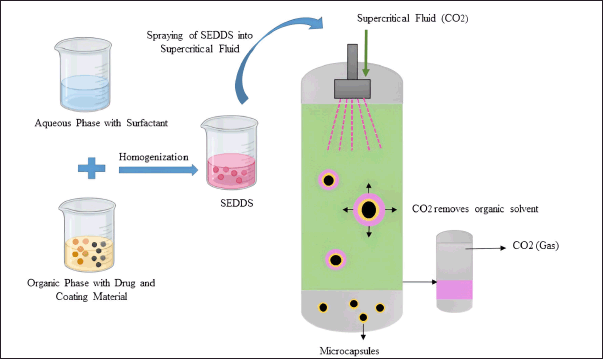
Unique features of supercritical fluids (SCFs) in the solidification of liquid formulations have received a lot of interest nowadays. Various methods have been proposed to exploit the ability to manipulate the properties of SCFs, aiming to produce fine powders characterized by a narrow particle size distribution [75]. Lipids are used in this technique to develop solid dispersions or to give a coating for drugs. In this process, drug particles are dispersed in a SCF such as carbon dioxide which includes one or more coating materials. Carbon dioxide is inexpensive, less toxic, nonflammable, and can be easily recycled. In addition to carbon dioxide commonly used supercritical solvents are nitrous oxide, ethylene, propylene, propane, and n-pentane [76].
SCF acts as an antisolvent and extracts the organic phase of SEDDS. As the solubility of the coating material decreases over time, it is displaced onto the drug particles and precipitates. Precipitation of drug and coating material leads to the formation of microcapsules. Figure 4 shows the solidification of liquid SEDDS based on the SCF method. Solubility of the drug and other components in the SCF is a crucial factor to be considered during this method. Poor drug loading capacity limited this method to low-dose and potent drugs [19].
3D printing (3DP) of SEDDSs
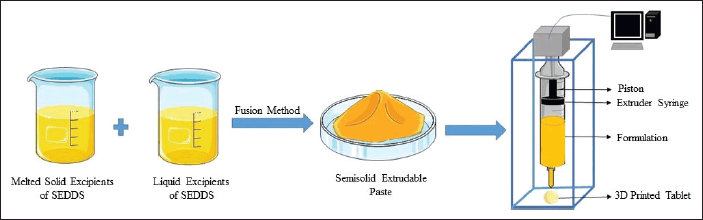
A significant number of solid carriers must frequently be used with several solidification techniques to absorb the liquid formulation. However, the incorporation of these additional carriers may provide issues with dose dilution, tolerance, and potential toxicity. The act of diluting liquid formulations with solid carriers and other excipients might make it difficult to administer high-dose medications accurately, which also impacts the compressibility of the formulation. Optimal reproducibility and increased production time also limit the acceptance of these solidification methods. Therefore, it would be highly desirable to find a way to develop S-SEDDS formulations without the need for high-concentration solid-phase carriers [77]. (Fig. 5) represents the schematic representation of 3D printing of SEDDS.
3DP is an emerging technology that offers digitally controlled personalized doses and geometry. It is an additive method that proceeds through the layer-by-layer development of 3-dimensional structures [78]. The US FDA approved the first 3DP levetiracetam (SPRITAM®) tablet in 2015, which skyrocketed interest in the area of pharmaceutical 3DP [79]. In 3DP, computer-aided design instructions are used to successively deposit layers of materials onto the build plate, resulting in the construction of a three-dimensional model (Fig. 5).
3DP offers numerous advantages in the pharma sector, generation of complex geometries, deposition of a relatively tiny portion of materials, resource conservation, quick production of various compositions for effective assessment activities, and precise control over the drug distribution pattern within the dosage form, as well as the ability to create customized dosage forms. Different technologies, including stereolithography, selective laser sintering, inkjet, fused deposition modeling, binder jetting, and semi-solid extrusion printing, are employed in the 3DP approach. The method of layer generation used to create a 3D object is the key distinction between the technologies [80].
 | Figure 4. Schematic representation of solidification of liquid SEDDS based on the Supercritical fluid method. [Click here to view] |
 | Figure 5. Novel 3D printing technology to convert the liquid SEDDS into solid. [Click here to view] |
Recent research has investigated using pressure-assisted micro syringes (PAM) or extrusion-based 3DP to develop SNEDDS-based lidocaine suppositories for customized drug delivery [81]. A proof of concept by Vithani et al. [80] demonstrates the possibility of 3DP for the creation of customized solid SMEDDS without the use of solid carriers. They used the syringe-based extrusion method and prepared four different geometrical shapes of cinnarizine and fenofibrate-loaded SMEDDS. This proof-of-concept shows that 3DP can provide a new approach to developing optimized solid SMEDDS dosage forms that are complex and geometrically flexible enough to be used for the production of customized lipid-based dosage forms [80]. Algahtani et al. [77] explored 3DP for the development of self-nanoemulsifying tablets of dapagliflozin to overcome poor water solubility. They used a 3DP technique based on semisolid PAM extrusion. 3D-printed self-nanoemulsifying tablet of dapagliflozin showed rapid drug release, and more than 75% the of drug release was within 20 minutes. The study confirmed that 3DP is an alternative method to design solid dosage forms of SEDDS [77].
3DP may be able to manufacture S-SEDDS in a single step by eliminating the solidification processes and their associated limitations. However, the application of 3DP for SEDDS is still in its early stages.
The solidification of SEDDSs is an important turning point in pharmaceutical science, paving the way for the development of different solid dosage forms. This transition not only preserves the favorable properties of SEDDS, but also opens a range of possibilities for other solid dosage forms such as tablets [17], pellets [82], dry emulsion [83], self-emulsifying nanoparticles [84], beads [85], suppositories [86], and implants [87].
FUTURE PERSPECTIVE
In the realm of pharmaceutical advancement, the future of S-SEDDS is quite promising. Solidification provides versatility in the design of tablets, pellets, and even granules. This advancement in solidification broadens the application of SEDDS, leading to the development of patient-friendly formulations with controlled release profiles, increased stability, and enhanced therapeutic efficacy, ultimately redefining the drug delivery landscape. The goal of ongoing research is to improve S-SEDDS formulation to improve drug solubility, stability, and overall performance, with a focus on customizing solutions to drug properties. Careful selection of excipients and the exploring of novel excipients can further optimize S-SEDDS formulations. Unlocking the full potential of S-SEDDS in therapeutic applications requires overcoming the present obstacles. The effective solidification of SEDDS containing temperature-sensitive substances is a significant challenge, which restricts their application in formulations that necessitate precise temperature controls. Overcoming this issue would make it possible to successfully develop S-SEDDS of biologics. Advances in solidification methods are expected to streamline production processes, making S-SEDDS more scalable and accessible in pharmaceutical industries. Furthermore, the possibility of personalized medicine is approaching, wherein formulations of S-SEDDS could be customized to the unique characteristics of each patient to enhance compliance and effectiveness. S-SEDDS has the potential to transform drug delivery techniques and enhance personalized and targeted therapy as regulatory guidance clarifies, opening the door for clearance, and commercialization.
CONCLUSION
In summary, we have explored the components of S-SEDDS and solidification methods, and discussed their advantages and limitations. In addition, we have highlighted several significant earlier studies in this sector. An exciting development in SEDDS formulation is solidification. The transition from liquid to solid formulations addresses key issues with stability, convenience, and dose accuracy. Different approaches to solidification of SEDDS have been investigated to overcome the practical limitations of liquid formulations. Each of these solidification processes has distinct advantages for SEDDS in pharmaceutical formulations. The potential of S-SEDDS lies not only in improving the stability of formulation but also in facilitating controlled release, enhancing solubility and bioavailability, and developing personalized medicine. The selection of the solidification method relies on the unique properties of the drug, the desired release profile, and the intended dosage form. Researchers are constantly developing new approaches to overcome the limits of existing technologies to obtain superior S-SEDDS formulations.
ACKNOWLEDGMENTS
The authors express gratitude to the Manipal Academy of Higher Education for their unwavering support throughout the duration of this study.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
We thank the Manipal Academy of Higher Education for the funding support via Intra-Mural Fund.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest related to the publication.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All information and data discussed in this review have been sourced from the existing literature. The references cited in this article provide a comprehensive overview of the studies and findings included in our analysis.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Gupta S, Kesarla R, Omri A. Formulation strategies to improve the bioavailability of poorly absorbed drugs with special emphasis on self-emulsifying systems. ISRN Pharm. 2013 Dec 26;2013:e848043.
2. ?erpnjak K, Zvonar A, Gašperlin M, Vre?er F. Lipid-based systems as promising approach for enhancing the bioavailability of poorly water-soluble drugs. Acta Pharm. 2013;63(4):427–45.
3. Jo K, Kim H, Khadka P, Jang T, Kim SJ, Hwang SH, et al. Enhanced intestinal lymphatic absorption of saquinavir through supersaturated self-microemulsifying drug delivery systems. Asian J Pharm Sci. 2020;15(3):336–46.
4. Nasr A, Gardouh A, Ghorab M. Novel solid self-nanoemulsifying drug delivery system (S-SNEDDS) for oral delivery of olmesartan medoxomil: design, formulation, pharmacokinetic and bioavailability evaluation. Pharmaceutics. 2016;8(3):20.
5. Souto EB, Cano A, Martins-Gomes C, Coutinho TE, Zieli?ska A, Silva AM. Microemulsions and nanoemulsions in skin drug delivery—PMC [Internet]. Bioengineering (Basel). 2022 Apr 5;9(4):158. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9028917/
6. Dokania S, Joshi AK. Self-microemulsifying drug delivery system (SMEDDS)–challenges and road ahead. Drug Deliv. 2015;22(6):675–90.
7. Savla R, Browne J, Plassat V, Wasan KM, Wasan EK. Review and analysis of FDA approved drugs using lipid-based formulations. Drug Dev Ind Pharm. 2017 Nov 2;43(11):1743–58.
8. Park H, Ha ES, Kim M. Current status of supersaturable self-emulsifying drug delivery systems. Pharmaceutics. 2020 Apr 16;12:365.
9. Grevel J, Nüesch E, Abisch E, Kutz K. Pharmacokinetics of oral cyclosporin a (Sandimmun) in healthy subjects. Eur J Clin Pharmacol. 1986 Mar 1;31(2):211–6.
10. Jaiswal P, Aggarwal G, Sl H, Kaur A. Bioavailability enhancement of poorly soluble drugs by SMEDDS: a review. J Drug Deliv Ther. 2013 Jan 15;3:98–109.
11. Kohli K, Chopra S, Dhar D, Arora S, Khar RK. Self-emulsifying drug delivery systems: an approach to enhance oral bioavailability. Drug Discov Today. 2010 Nov 1;15(21):958–65.
12. Min MH, Park JH, Choi MR, Hur JH, Ahn BN, Kim DD. Formulation of a film-coated dutasteride tablet bioequivalent to a soft gelatin capsule (Avodart®): effect of γ-cyclodextrin and solubilizers. Asian J Pharm Sci. 2019 May 1;14(3):313–20.
13. KAUR M. Self emulsified drug delivery system for the enhancement of oral bioavailability of poorly water soluble drugs. Int J Adv Pharm Biol Chem. 2013 Sep 1;2(3):427–36.
14. Pehlivanov I. Self-emulsifying drug delivery systems as an approach to improve therapeutic effectiveness of orally administrated drugs. J IMAB—Annu Proceeding Sci Pap. 2019 Jun 21;25(2):2575–82.
15. Almeida SRD, Tippavajhala VK. A rundown through various methods used in the formulation of solid self-emulsifying drug delivery systems (S-SEDDS). AAPS PharmSciTech. 2019 Oct 25;20(8):323.
16. Hussain A, Shakeel F, Singh SK, Alsarra IA, Faruk A, Alanazi FK, et al. Solidified SNEDDS for the oral delivery of rifampicin: evaluation, proof of concept, in vivo kinetics, and in silico gastroplusTM simulation. Int J Pharm. 2019 Jul;566:203–17.
17. Nazzal S, Khan MA. Controlled release of a self-emulsifying formulation from a tablet dosage form: stability assessment and optimization of some processing parameters. Int J Pharm. 2006;315(1–2):110–21.
18. Potharaju S, Mutyam SK, Liu M, Green C, Frueh L, Nilsen A, et al. Improving solubility and oral bioavailability of a novel antimalarial prodrug: comparing spray-dried dispersions with self-emulsifying drug delivery systems. Pharm Dev Technol. 2020 Jun;25(5):625–39.
19. Seo EB, du Plessis LH, Viljoen JM. Solidification of self-emulsifying drug delivery systems as a novel approach to the management of uncomplicated malaria. Pharmaceuticals. 2022;15(2):120.
20. Maji I, Mahajan S, Sriram A, Medtiya P, Vasave R, Khatri DK, et al. Solid self emulsifying drug delivery system: superior mode for oral delivery of hydrophobic cargos. J Controlled Release. 2021;337:646–60.
21. Friedl JD, Jörgensen AM, Le-Vinh B, Braun DE, Tribus M, Bernkop-Schnürch A. Solidification of self-emulsifying drug delivery systems (SEDDS): impact on storage stability of a therapeutic protein. J Colloid Interface Sci. 2021;584:684–97.
22. Nardin I, Köllner S. Successful development of oral SEDDS: screening of excipients from the industrial point of view. Adv Drug Deliv Rev. 2019 Mar;142:128–40.
23. Joyce P, Dening TJ, Meola TR, Schultz HB, Holm R, Thomas N, et al. Solidification to improve the biopharmaceutical performance of SEDDS: opportunities and challenges. Adv Drug Deliv Rev. 2019;142:102–17.
24. Izgelov D, Shmoeli E, Domb AJ, Hoffman A. The effect of medium chain and long chain triglycerides incorporated in self-nano emulsifying drug delivery systems on oral absorption of cannabinoids in rats. Int J Pharm. 2020 Apr 30;580:119201.
25. Rahman MdA, Hussain A, Hussain MdS, Mirza MohdA, Iqbal Z. Role of excipients in successful development of self-emulsifying/microemulsifying drug delivery system (SEDDS/SMEDDS). Drug Dev Ind Pharm. 2013 Jan 1;39(1):1–19.
26. Singh B, Bandopadhyay S, Kapil R, Singh R, Katare OP. Self-emulsifying drug delivery systems (SEDDS): formulation development, characterization, and applications. Crit Rev Ther Drug Carr Syst [Internet]. 2009 [cited 2023 Nov 18];26(5):427–521. Available from: https://www.dl.begellhouse.com/journals/3667c4ae6e8fd136,7bc13fbe52b73e32,3ecc45285b4b6014.html
27. Gurram AK, Deshpande P, Kar SS, Nayak U, Udupa N, Reddy M. Role of components in the formation of self-microemulsifying drug delivery systems. Indian J Pharm Sci. 2015 May 1;77:249–57.
28. Pandey V, Kohli S. Lipids and surfactants: the inside story of lipid-based drug delivery systems. Crit Rev Ther Drug Carr Syst. 2018;35(2):99–155.
29. Devraj R, Williams HD, Warren DB, Porter CJ, Pouton CW. Choice of nonionic surfactant used to formulate type IIIA self-emulsifying drug delivery systems and the physicochemical properties of the drug have a pronounced influence on the degree of drug supersaturation that develops during in vitro digestion. J Pharm Sci. 2014;103(4):1050–63 [Internet]. [cited 2023 Nov 20]. Available from: https://sci-hub.se/https://doi.org/10.1002/jps.23856
30. Gao P, Rush BD, Pfund WP, Huang T, Bauer JM, Morozowich W, et al. Development of a supersaturable SEDDS (S-SEDDS) formulation of paclitaxel with improved oral bioavailability. J Pharm Sci. 2003;92(12):2386–98.
31. Dening TJ, Rao S, Thomas N, Prestidge CA. Novel nanostructured solid materials for modulating oral drug delivery from solid-state lipid-based drug delivery systems. AAPS J. 2016 Jan;18:23–40. Available from: https://link.springer.com/article/10.1208/s12248-015-9824-7
32. Snela A, Jadach B, Froelich A, Skotnicki M, Milczewska K, Rojewska M, et al. Self-emulsifying drug delivery systems with atorvastatin adsorbed on solid carriers: formulation and in vitro drug release studies. Colloids Surf Physicochem Eng Asp. 2019 Sep;577:281–90.
33. Chavan RB, Modi SR, Bansal AK. Role of solid carriers in pharmaceutical performance of solid supersaturable SEDDS of celecoxib. Int J Pharm. 2015 Nov 10;495(1):374–84.
34. Tan A, Rao S, Prestidge CA. Transforming lipid-based oral drug delivery systems into solid dosage forms: an overview of solid carriers, physicochemical properties, and biopharmaceutical performance. Pharm Res. 2013 Dec 1;30(12):2993–3017.
35. Akbarbaglu Z, Peighambardoust SH, Sarabandi K, Jafari SM. Spray drying encapsulation of bioactive compounds within protein-based carriers; different options and applications. Food Chem. 2021 Oct 15;359:129965.
36. Tang B, Cheng G, Gu JC, Xu CH. Development of solid self-emulsifying drug delivery systems: preparation techniques and dosage forms. Drug Discov Today. 2008;13(13–14):606–12.
37. Dixit RP, Nagarsenker MS. Self-nanoemulsifying granules of ezetimibe: design, optimization and evaluation. Eur J Pharm Sci. 2008;35(3):183–92.
38. Gumaste SG, Dalrymple DM, Serajuddin AT. Development of solid SEDDS, V: compaction and drug release properties of tablets prepared by adsorbing lipid-based formulations onto Neusilin® US2. Pharm Res. 2013;30:3186–99.
39. Beg S, Swain S, Singh HP, Patra CN, Rao MB. Development, optimization, and characterization of solid self-nanoemulsifying drug delivery systems of valsartan using porous carriers. AAPS PharmSciTech. 2012 Dec;13(4):1416–27.
40. Milovi? M, Djuriš J, Djeki? L, Vasiljevi? D, Ibri? S. Characterization and evaluation of solid self-microemulsifying drug delivery systems with porous carriers as systems for improved carbamazepine release. Int J Pharm. 2012;436(1–2):58–65.
41. Kadian R, Nanda A. A comprehensive insight on recent advancements in self-emulsifying drug delivery systems. Curr Drug Deliv. 2023;20(8):1095–114.
42. Kumar M, Chawla PA, Faruk A, Chawla V. Spray drying as an effective method in the development of solid self-emulsifying drug delivery systems. Curr Drug Deliv. 2023;20(5):508–25.
43. Singh B, Beg S, Khurana RK, Sandhu PS, Kaur R, Katare OP. Recent advances in self-emulsifying drug delivery systems (SEDDS). Crit Rev Ther Drug Carrier Syst. 2014;31(2):121–85.
44. Pawar JN, Shete RT, Gangurde AB, Moravkar KK, Javeer SD, Jaiswar DR, et al. Development of amorphous dispersions of artemether with hydrophilic polymers via spray drying: physicochemical and in silico studies. Asian J Pharm Sci. 2016;11(3):385–95.
45. Sosnik A, Seremeta KP. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv Colloid Interface Sci. 2015 Sep;223:40–54.
46. Kamal MM, Salawi A, Lam M, Nokhodchi A, Abu-Fayyad A, El Sayed KA, et al. Development and characterization of curcumin-loaded solid self-emulsifying drug delivery system (SEDDS) by spray drying using Soluplus® as solid carrier. Powder Technol. 2020 Jun 1;369:137–45.
47. Yi T, Wan J, Xu H, Yang X. A new solid self-microemulsifying formulation prepared by spray-drying to improve the oral bioavailability of poorly water soluble drugs. Eur J Pharm Biopharm. 2008 Oct 1;70(2):439–44.
48. Balakrishnan P, Lee BJ, Oh DH, Kim JO, Hong MJ, Jee JP, et al. Enhanced oral bioavailability of dexibuprofen by a novel solid Self-emulsifying drug delivery system (SEDDS). Eur J Pharm Biopharm. 2009 Aug 1;72(3):539–45.
49. Yan YD, Kim JA, Kwak MK, Yoo BK, Yong CS, Choi HG. Enhanced oral bioavailability of curcumin via a solid lipid-based self-emulsifying drug delivery system using a spray-drying technique. Biol Pharm Bull. 2011;34(8):1179–86.
50. Parakh DR, Patil MP, Dashputre NL, Kshirsagar SJ. Development of self-microemulsifying drug delivery system of mebendazole by spray drying technology: characterization, in vitro and in vivo evaluation. Dry Technol. 2016 Jul 3;34(9):1023–42.
51. Han CC, Han L. Improving oral bioavailability of agaricoglycerides by solid lipid-based self-emulsifying drug delivery system. J Appl Pharm Sci. 2011 Dec 30;1(10):195–9.
52. Rahman MdA, Mujahid Md, Hussain A, Iqbal Z. Development and pharmacokinetic evaluation of spray-dried self-nanoemulsifying drug delivery system of sertraline. J Pharm Investig. 2017 Jul;47(4):325–33.
53. Bertoni S, Albertini B, Passerini N. Spray congealing: an emerging technology to prepare solid dispersions with enhanced oral bioavailability of poorly water soluble drugs. Molecules. 2019 Jan;24(19):3471.
54. Albertini B, Sabatino MD, Melegari C, Passerini N. Formulation of spray congealed microparticles with self-emulsifying ability for enhanced glibenclamide dissolution performance. J Microencapsul. 2015;32(2):181–92.
55. Passerini N, Albertini B, Perissutti B, Rodriguez L. Evaluation of melt granulation and ultrasonic spray congealing as techniques to enhance the dissolution of praziquantel. Int J Pharm. 2006;318(1–2):92–102.
56. Tillotson J. Methodologies for developing s-SEDDS. ONdrugDeliv Mag. 2016;(69):29–31.
57. El Hadri M, Achahbar A, El Khamkhami J, Khelifa B, Faivre V, Abbas O, et al. Lyotropic behavior of gelucire 50/13 by XRD, raman and IR spectroscopies according to hydration. Chem Phys Lipids. 2016 Oct 1;200:11–23.
58. Bjeloševi? M, Pobirk AZ, Planinšek O, Grabnar PA. Excipients in freeze-dried biopharmaceuticals: Contributions toward formulation stability and lyophilisation cycle optimisation. Int J Pharm. 2020;576:119029.
59. Li F, Song S, Guo Y, Zhao Q, Zhang X, Pan W, et al. Preparation and pharmacokinetics evaluation of oral self-emulsifying system for poorly water-soluble drug Lornoxicam. Drug Deliv. 2015 May 19;22(4):487–98.
60. Kuncahyo I, Choiri S, Fudholi A. Solidification of meloxicam self-nano emulsifying drug delivery system formulation incorporated into soluble and insoluble carriers using freeze drying method. In: IOP Conference Series: Materials Science and Engineering. UK: IOP Publishing; 2019. p. 012051.
61. Snyder M. Lyophilization can be a messy process. Drug Discov Dev. 2017 [cited 2023 Nov 21]. Available from: https://www.drugdiscoverytrends.com/lyophilization-the-basics/
62. Iosio T, Voinovich D, Perissutti B, Serdoz F, Hasa D, Grabnar I, et al. Oral bioavailability of silymarin phytocomplex formulated as self-emulsifying pellets. Phytomedicine. 2011;18(6):505–12.
63. Abdalla A, Klein S, Mäder K. A new self-emulsifying drug delivery system (SEDDS) for poorly soluble drugs: characterization, dissolution, in vitro digestion and incorporation into solid pellets. Eur J Pharm Sci. 2008;35(5):457–64.
64. Singh B, Garg B, Kaur R, Jain A, Kumar R, Katare OP. Chapter 4—self-nanoemulsifying systems for oral bioavailability enhancement: recent paradigms. In: Grumezescu AM, editor. Fabrication and self-assembly of nanobiomaterials [Internet]. William Andrew Publishing; 2016 [cited 2023 Nov 21]. pp. 91–115. Available from: https://www.sciencedirect.com/science/article/pii/B9780323415330000040
65. Desai U, Chaudhari P, Bhavsar D, Chavan R. Melt granulation: an alternative to traditional granulation techniques. Indian Drugs. 2013;50(3):5–13.
66. Shanmugam S. Granulation techniques and technologies: recent progresses. BioImpacts. 2015;5(1):55.
67. AboulFotouh K, Allam AA, El-Badry M, AboulFotouh K, Allam AA, El-Badry M. Self-emulsifying drug delivery systems: easy to prepare multifunctional vectors for efficient oral delivery. In: Khalil IA, editor. Current and future aspects of nanomedicine [Internet]. London, UK: IntechOpen; 2019 [cited 2024 Mar 6]. Available from: https://www.intechopen.com/chapters/68412
68. Krstic M, Djuris J, Petrovic O, Lazarevic N, Cvijic S, Ibric S. Application of the melt granulation technique in development of lipid matrix tablets with immediate release of carbamazepine. J Drug Deliv Sci Technol. 2017;39:467–74.
69. Nyavanandi D, Mandati P, Narala S, Alzahrani A, Kolimi P, Vemula SK, et al. Twin screw melt granulation: a single step approach for developing self-emulsifying drug delivery system for lipophilic drugs. Pharmaceutics. 2023 Sep;15(9):2267.
70. Bandari S, Nyavanandi D, Kallakunta VR, Janga KY, Sarabu S, Butreddy A, et al. Continuous twin screw granulation—an advanced alternative granulation technology for use in the pharmaceutical industry. Int J Pharm. 2020 Apr;580:119215.
71. Sarabu S, Kallakunta VR, Butreddy A, Janga KY, Ajjarapu S, Bandari S, et al. A one-step twin-screw melt granulation with gelucire 48/16 and surface adsorbent to improve the solubility of poorly soluble drugs: effect of formulation variables on dissolution and stability. AAPS PharmSciTech. 2021 Apr;22(3):79.
72. Lu M, Xing H, Jiang J, Chen X, Yang T, Wang D, et al. Liquisolid technique and its applications in pharmaceutics. Asian J Pharm Sci. 2017;12(2):115–23.
73. Javadzadeh Y, Jafari-Navimipour B, Nokhodchi A. Liquisolid technique for dissolution rate enhancement of a high dose water-insoluble drug (carbamazepine). Int J Pharm. 2007;341(1–2):26–34.
74. El-Sayyad NMEM, Badawi A, Abdullah ME, Abdelmalak NS. Dissolution enhancement of leflunomide incorporating self emulsifying drug delivery systems and liquisolid concepts. Bull Fac Pharm Cairo Univ. 2017;55(1):53–62.
75. Reverchon E, Adami R, Campardelli R, Della Porta G, De Marco I, Scognamiglio M. Supercritical fluids based techniques to process pharmaceutical products difficult to micronize: palmitoylethanolamide. J Supercrit Fluids. 2015;102:24–31.
76. Selvam P, Kulkarni P, Dixit M. Solidification techniques and dosage form development of solid self-emulsifying drug delivery systems: a technical note. Elixir Pharm. 2011 Jan 1;39:4753–8.
77. Algahtani MS, Mohammed AA, Ahmad J, Abdullah MM, Saleh E. 3D printing of dapagliflozin containing self-nanoemulsifying tablets: formulation design and in vitro characterization. Pharmaceutics. 2021;13(7):993.
78. Jamróz W, Szafraniec J, Kurek M, Jachowicz R. 3D printing in pharmaceutical and medical applications–recent achievements and challenges. Pharm Res. 2018;35:1–22.
79. Fitzgerald S. FDA approves first 3D-printed epilepsy drug experts assess the benefits and caveats. Neurol Today. 2015;15(18):26–7.
80. Vithani K, Goyanes A, Jannin V, Basit AW, Gaisford S, Boyd BJ. A proof of concept for 3D printing of solid lipid-based formulations of poorly water-soluble drugs to control formulation dispersion kinetics. Pharm Res. 2019;36(7):1–13.
81. Chatzitaki AT, Tsongas K, Tzimtzimis EK, Tzetzis D, Bouropoulos N, Barmpalexis P, et al. 3D printing of patient-tailored SNEDDS-based suppositories of lidocaine. J Drug Deliv Sci Technol. 2021;61:102292.
82. Sha K, Ma Q, Veroniaina H, Qi X, Qin J, Wu Z. Formulation optimization of solid self-microemulsifying pellets for enhanced oral bioavailability of curcumin. Pharm Dev Technol. 2021 May 28;26(5):549–58.
83. Pohlen M, Lavri? Z, Prestidge C, Dreu R. Preparation, physicochemical characterisation and DoE optimisation of a spray-dried dry emulsion platform for delivery of a poorly soluble drug, Simvastatin. AAPS PharmSciTech. 2020 Apr 21;21(4):119.
84. Patil SC, Tagalpallewar AA, Kokare CR. Natural anti-proliferative agent loaded self-microemulsifying nanoparticles for potential therapy in oral squamous carcinoma. J Pharm Investig. 2019 Sep 1;49(5):527–41.
85. Dangre PV, Dusad PP, Singh AD, Surana SJ, Chaturvedi KK, Chalikwar SS. Fabrication of hesperidin self-micro-emulsifying nutraceutical delivery system embedded in sodium alginate beads to elicit gastric stability. Polym Bull. 2022 Jan 1;79(1):605–26.
86. Gugulothu D, Pathak S, Suryavanshi S, Sharma S, Patravale V. Self-microemulsifiyng suppository formulation of β-artemether. AAPS PharmSciTech. 2010;11:1179–84.
87. Chae GS, Lee JS, Kim SH, Seo KS, Kim MS, Lee HB, et al. Enhancement of the stabilityof BCNU using self-emulsifying drug delivery systems (SEDDS) and in vitro antitumor activity of self-emulsified BCNU-loaded PLGA wafer. Int J Pharm. 2005 Sep 14;301(1):6–14.