INTRODUCTION
Potentilla fulgens Wall. (Rosaceae) which is commonly known in Hindi (India) as “Bajradanti” and locally (Khasi, Meghalaya) as “Lynniang” is gaining scientific attention for its therapeutic potential. This delicate herb has a long history of traditional use in Asian countries such as India, China, Nepal, Japan, Bhutan, Tibet, Malaysia, and Korea. It is found in various regions of India particularly the Western Himalayas and North Eastern regions of India and has been used in folk medicine to address and treat diabetes, digestive issues, ulcers, wounds, cancer, and stomach worms [1]. Studies highlight the presence of polyphenolic compounds in Potentilla species, suggesting their potential as antioxidants [2]. Several studies have shown that the ethyl acetate and methanol root extracts of P. fulgens contain high levels of polyphenols, demonstrating various pharmacological actions such as anti-ulcer, anti-diabetes, anti-diarrhea, anti-cancer, and wound healing [3–6]. The plant’s abundant polyphenol content is the main contributor to its diverse range of biological effects. According to research, polyphenolic ingredients have gained significance in medicines and cosmetics due to their advantageous and multifunctional biological features and their widespread availability in a variety of dietary sources. Although polyphenols appear to provide several benefits, excessive consumption may have negative effects [7]. The effects of polyphenols on skin and hair are mostly determined by their physicochemical characteristics. Therefore, it is crucial to evaluate the efficiency of systemic and/or topical administration of polyphenolic substances in treating skin and hair disorders [8]. Conversely, research also revealed the presence of saponins and steroids in the root of P. fulgens [9]. Saponins are well known for their detergent effect, and their potential toxicity can lead to cellular membrane damage through the formation of pores and vesicles, ultimately resulting in disruption of membrane integrity [10,11].
The growing popularity of herbal treatments is attributed to the concept that they are safe and effective due to their natural origins and long history of usage. However, the inherent natural quality of these remedies does not inherently guarantee their safety, as evidenced by the associated risks linked to their usage. It is important to verify and validate the safety and efficacy of these plant remedies for usage as alternative medicines through scientific data before promoting them as prospective new therapies [12]. Following the Organization for Economic Cooperation and Development (OECD) guidelines, acute toxicity refers to adverse reactions observed shortly after a single dose of the test substance is applied to the skin. As outlined in OECD guideline No. 410, a substance is considered harmful in sub-acute dermal toxicity when repeated dermal exposures within a short timeframe are expected to significantly compromise well-being. In both studies, particularly sub-acute toxicity assessments, it is essential to examine changes in blood parameters conduct histological examinations of the skin, and select vital organs. This is particularly important for substances derived from plant products that are applied to the skin over an extended period, allowing for a comprehensive evaluation of potentially toxic components. Furthermore, closely observing any physical or behavioral changes in the experimental animals holds paramount importance [13]. The acute oral toxicity profile of the ethanol root extract derived from P. fulgens was investigated in Charles-Foster albino rats as per the OECD guidelines, as documented by Laloo et al. [3]. The study indicated that animals receiving a single oral dose of the extract at 2,000 and 5,000 mg/kg exhibited no signs of abnormal behavior, toxicity, or fatalities. Similarly, in 2014, Tangpu and colleagues conducted acute oral toxicity studies in mice, administering a single dose of the extract at 3,200 mg/kg. The results demonstrated the absence of observable signs of toxicity in the majority of the animals, although mortality was reported with one animal at the tested dose. The study reported an LD50 of 5355.97 mg/kg for the extract [4]. Furthermore, in 2016, researchers assessed the acute dermal toxicity of a topical ointment formulated with ethanol and ethyl acetate root extracts rich in polyphenolics from P. fulgens in experimental rats. The findings indicated the absence of toxicity signs in the tested extracts up to 2,000 mg/kg [5]. However, this investigation remains incomplete, as it omitted a thorough examination of the impacts of these extracts on critical parameters such as hematological and serum biochemical values, as well as the structural composition of the skin. These parameters are essential for gauging the potential toxicity of substances, following OECD guidelines. Without complete sets of toxicological data, the suitability of P. fulgens for use in topical applications cannot be substantiated. In addition, apart from acute toxicity, it is imperative to assess the sub-acute toxicity of the tested extracts in in vivo models. Although P. fulgens root extracts demonstrated promising results when applied topically to treat skin issues such as wounds, no prior attempt has been made to explore its possible acute and sub-acute dermal toxicity effects. To evaluate and establish the safety properties of P. fulgens root extracts in an experimental rat model, the current study was conducted.
MATERIAL AND METHODS
Plant material and extraction process
Potentilla fulgens plant was gathered from the East Khasi Hill district of Meghalaya, India, in December 2019. The plant was identified as P. fulgens Wall. Ex Hook. by the Botanical Survey of India, Shillong, Meghalaya (Letter No.: BSI/ERC/2019/Plant identification/281) and a voucher specimen for future references was preserved at the Central Museum Hall of Girijananda Chowdhury Institute of Pharmaceutical Science, Guwahati, India.
To prepare the plant’s root for extraction, it was thoroughly washed with tap water to remove any unwanted inorganic particles. The cleaned root was then dried in a tray drier for three days at a controlled temperature not exceeding 40°C. Once completely dried, the root material was finely ground using a mechanical grinder and subsequently sieved through a mesh with a size of 60. The powdered medicinal substance (500 g) was defatted with petroleum ether followed by successive Soxhlet extraction with ethyl acetate and methanol for 72 hours. Following the extraction, each successive extract was concentrated in a rotary vacuum evaporator and the resulting dried extracts were weighed and stored in sealed containers until use [1].
HPTLC standardization of EAPF and MEPF extracts
EAPF and MEPF extracts were standardized using high-performance thin layer chromatography (HPTLC) against the reference standard epicatechin (Tokyo Chemical Industry Pvt. Ltd., India). Epicatechin (0.5 mg/mL), EAPF (5 mg/mL), and MEPF (5 mg/mL) stock solutions were prepared by dissolving in methanol. The mobile phase for chromatogram development consisted of chloroform: acetone and formic acid mixture in the ratio 130:53:17 (v/v/v). The study utilized HPTLC instrumentation [CAMAG®, Anchrom Enterprises (I) Pvt. Ltd., Mumbai, India] equipped with a Linomat V sample applicator (maintaining a dosage speed of 150 nL/s and a pre-dosage volume of 0.2 μL), a twin trough chamber (20 × 10 cm with a saturation time of 20 min), Camag TLC scanner 4 (maintaining a scanning speed of 20 mm/s, slit dimensions of 6 × 0.45 mm, and a deuterium lamp with a wavelength of 254 nm), Camag TLC visualizer, and VisionCATS software for data interpretation. The Rf values were recorded, and the developed plate was screened and photographed in the ultraviolet range at a wavelength (λmax) of 254 nm [3].
Animal preparation, experimental design, and dosage
The acute and sub-acute dermal toxicity studies were assessed as per the OECD guidelines [14] and the experimentation was executed in male albino Wistar rats (150 to 220 g). The experimental protocol (Approval number: GIPS/IAEC/PhD/ASTU/PRO/01/2021) was approved by the Institutional Animal Ethical Committee of Girijananda Chowdhury Institute of Pharmaceutical Science, Guwahati, India. Before the study, the rats were acclimatized for 2 weeks within a controlled environment featuring consistent temperature (22°C ± 2°C), humidity (55% ± 10%), and lighting conditions (12 hours of light followed by 12 hours of darkness). Throughout the study, the animals received a standard pellet diet and had unrestricted access to water. To assess both acute and sub-acute dermal toxicity, the rats were divided evenly into five distinct groups (n = 5) designated as follows: group I (normal control), group II (EAPF 2,000 mg/kg), group III (EAPF 5,000 mg/kg), group IV (MEPF 2000 mg/kg), and group V (MEPF 5,000 mg/kg). These groups were individually housed in cages for observation. Before proceeding, the rats were anesthetized using intramuscular injections of Ketamine (50 mg/kg) and xylazine (5 mg/kg). This was done before shaving the fur in the dorsal thoracic region to minimize any potential discomfort. The shaved area was uniformly treated with two different doses (2,000 and 5,000 mg/kg) of ethyl acetate and methanol extracts of the plant, both of which were prepared using water as a solvent. A separate control group of animals was included to facilitate the comparison of physical, biological, and behavioral changes such as salivation, tremors, convulsions, diarrhea, lethargy, and breathing patterns. For acute dermal toxicity assessment, the animals received a calculated dosage of extract powder mixed with water and were observed for the following 14 days. On the other hand, sub-acute dermal toxicity was evaluated by applying the calculated dosage daily, mixed with water, over a continuous period of 28 days. Dosage calculations were based on the body weight of each animal, and a paste was formulated by blending the extract with an appropriate amount of distilled water. This paste was then applied topically to the shaved skin. Consistent with OECD guidelines No. 434, dosages of 2,000 and 5,000 mg/kg were selected for conducting both acute and sub-acute dermal toxicity studies [12].
Assessment of general signs and behavioral changes of rats
Each rat underwent daily examinations to monitor mortality, alterations in fur condition, eye health, and mucous membrane state, as well as any shifts in behavior including symptoms such as salivation, convulsions, tremors, diarrhea, lethargy, and changes in breathing rhythms. The continuous behavior activity of the rat was initially monitored for 30 minutes and later continued for 6 hours using a video tracing system (Maze master software, version 5.0.0). The rat body weight was also examined weekly from day 0 to day 14 (for acute dermal toxicity) and up to day 28 (for sub-acute dermal toxicity). The weight fluctuations for each rat were computed and juxtaposed with the weight variations observed in the control group of [13].
Euthanasia and necropsy
After the completion of both the study, the experimental animals were humanely euthanized by using the halogenated ether inhalation method [15]. An incision was made in the caudal region of the abdominal wall, and the abdominal viscera were then moved out of the abdominal cavity to reveal the posterior vena cava, allowing the abdominal cavity to be viewed. Following that, blood was collected from the posterior vena cava of each necropsied rat and stored in ethylene diamine tetraacetic acid blood tubes for hematology testing [12]. For the serum biochemical analysis, blood samples were also collected in blood tubes devoid of anticoagulants. The blood samples were centrifuged for 5 minutes at 5,000 rpm to separate the sera after they had clotted for at least 30 minutes at room temperature. The collected blood samples were stored at −80°C for analysis of biochemical parameters. Moreover, critical gross inspection of vital organs such as the kidney, liver, and spleen was introduced by excising them from the corpses. The organs were dried and their relative weight was recorded and preserved at 10% formalin solution along with the treated region of skin for histopathological analysis [13].
Hematological analysis
The blood samples that were collected underwent analysis using an automatic hematology Analyzer (Erba Mannheim-H560, Canada). Various hematological parameters were assessed, including white blood cell (WBC) count, WBC differential counts encompassing lymphocyte, monocyte, neutrophil, eosinophil, and basophil counts, red blood cell count, mean corpuscular volume, hematocrit, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration (MCHC), hemoglobin (Hb) concentration, and platelet (Pct) for both study types.
Serum biochemistry analysis
The serum profile was assessed using commercially available biochemical kits and the reading was examined using an auto-analyzer (Microlab 300, Merck, Netherlands). Various serum biochemical parameters were scrutinized including creatinine, urea, total bilirubin, total protein, albumin, globulin, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and creatine kinase [12,13,15–17].
Histopathological examination
On the last day of the experiment, various major vital organs including skin, kidney, liver, and spleen were excised and preserved in 10% formalin as per the standard procedure. An automated rotary microtome (Leica Biosystems, USA) was used to process the histopathological section of the tissues, and a section slice of 4 μm thickness was cut. The tissue samples were mounted on glass slides and allowed to stand overnight on a hot plate heated to 42°C. After being deparaffinized with xylene for 5 minutes, the tissue slices were rehydrated twice for 5 minutes each with various ethanol dilutions (100% and 80%). Before staining with hematoxylin and eosin (H&E), the tissue slices were washed in tap water. Lesions in all tissues were scored on a scale of 0 (no lesion found) to 1 (mild), 2 (moderate), and 3 (severe) [12].
RESULTS AND DISCUSSION
Traditional medicine provides primary health care to around 80% of the world’s total population. Even though the usage of these plants has demonstrated significant potential with considerable worldwide demand, there are still questions regarding their use as well as their safety [18]. Herbal products are often regarded as harmless or low toxicity due to their lengthy history of human use. Nonetheless, recent investigations have revealed that many of these traditional medicine items have undesirable side effects [19]. Although the frequency of harm from natural products and extracts is lower when compared to synthetic medications. As consumers’ interest in skin care products grows, in-vivo experimental studies are required to give solid scientific proof, notably on cutaneous toxicity studies. Therefore, this study explores the dermatological safety of P. fulgens root extracts. Although the extracts from the plant are beneficial for skin disorders, their safety when applied topically is currently uncertain. This assessment is vital for the development and future use of topical medications of P. fulgens extracts.
Phytochemical analysis of EAPF and MEPF
Successive Soxhlet extraction of P. fulgens roots (Fig. 1) produces ethyl acetate extract (EAPF, 6.31% w/w) and methanol extracts (MEPF, 8.75% w/w). HPTLC analysis (Fig. 2) of both the extracts were standardized against the major phytoconstituents of P. fulgens taken as epicatechin as standard reference. The Rf value of epicatechin corresponding with both EAPF and MEPF was found to be in the range of 0.079–0.082. The percentage yield of epicatechin in the extracts was determined using the HPTLC regression equation (y = 8.606 × 10-10 x + 3.361 × 10-4, R 2= 0.9925), revealing that EAPF contained 2.27%w/w and MEPF contained negligible amount of epicatechin. The presence of epicatechin in the P. fulgens extract was identified and confirmed, signifying the successful standardization of the plant. This finding aligns with the analytical data documented in previous research studies [3].
 | Figure 1. Plant of Potentilla fulgens Wall. [Click here to view] |
Effect of EAPF and MEPF body weight and organ weight
In both acute and sub-acute dermal toxicity studies, topical administration of EAPF and MEPF at tested doses of 2,000 and 5,000 mg/kg for the first three crucial hours when compared to normal control showed no sign of dermal toxicity or mortality on the rodents (Fig. 3). Furthermore, throughout the study period, it was found that the animals of both the study did not show any noteworthy changes or impairment in their behavior, posture, breathing pattern, feed intake, skin and fur, itching, inflammation, water consumption, lethargy, salivation, urination, and so on. The OECD guidelines (no. 434) for chemical testing prescribe an upper fixed dose level of 5000 mg/kg for acute dermal toxicity studies, aligning with regulatory requirements and the direct relevance to safeguard human health [14]. Despite the traditional consumption of P. fulgens roots and their incorporation into ‘Vicco vajradanti tooth powders’ for treating oral diseases [9], no investigations have explored dermal toxicity at the upper dose level of 5000 mg/kg. Our current findings demonstrate the safety of dermal testing for the P. fulgens extract at the maximum dose of 5,000 mg/kg. This aligns with previous research indicating an oral LD50 of the plant extract at 5355.97 mg/kg [4], providing support for our assertions.
Effect of EAPF and MEPF body weight and organ weight
The body weights of the animals in the acute dermal toxicity study (Fig. 4A) were recorded at intervals of 0th, 7th, and 14th days, while animals that were subjected to sub-acute dermal toxicity (Fig. 4B) were reported at intervals of 0th, 9th, 18th, and 28th days by using a calibrated digital balance. Report from the current study revealed that there were no significant (nsp>0.05) changes in the body weight of the animals when rats were administered topically with EAPF (2,000 and 5,000 mg/kg) and MEPF (2,000 and 5,000 mg/kg) in comparison to normal control. All the results were statistically analyzed and expressed as Mean ± SEM. In acute (day 14) and sub-acute (day 28) dermal toxicity studies, topical application of EAPF and MEPF each at 2000 and 5000 mg/kg to the shaved surface of the rat when compared to normal control rats showed no significant (nsp>0.05) changes in the relative organ weight of liver, kidney, and spleen (Fig. 5A and B). An abnormal alteration in the body weight of the animals following the administration of test substances is a key parameter in toxicity studies for assessing the animals’ health status. Variations in body weight are indicative of adverse effects resulting from drugs and chemicals. If the observed body weight loss exceeds 10% of the initial body weight, it is deemed statistically significant about the animals’ health [20]. Moreover, the weights of essential organs such as the kidney, liver, heart, lungs, and spleen serve as indicators of both physiological and pathological conditions. Relative organ weight is a key metric employed to assess whether these internal organs have incurred damage due to exposure to toxic substances [21]. The current investigation found that rats administered with EAPF and MEPF showed no significant changes in the body weights of the animals recorded every 7th day for two weeks in the case of acute dermal toxicity testing and every 9th day for four weeks in the case of sub-acute dermal toxicity studies. Moreover, no significant changes were observed in the relative weights of the tested vital organs (kidney, liver, and spleen) implying that the tested extracts had no toxic effects on the rat organs.
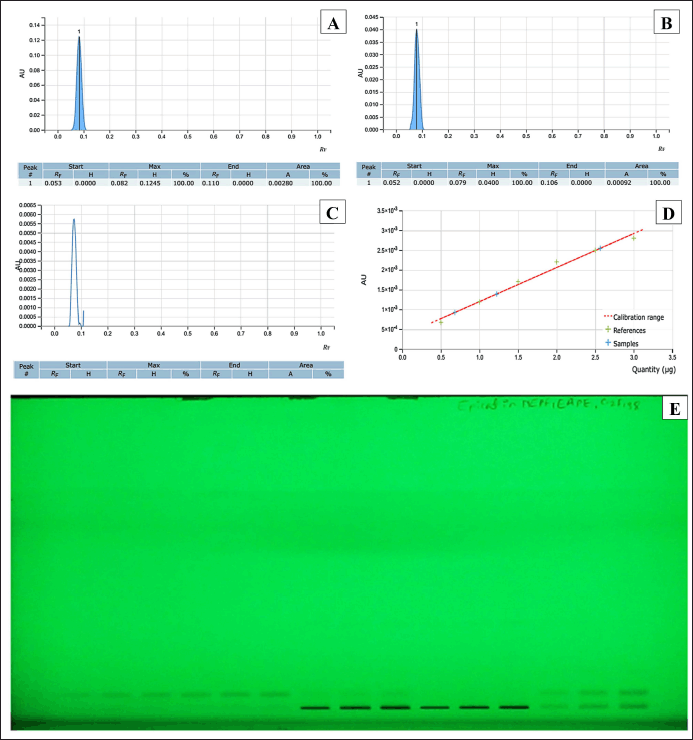 | Figure 2. HPTLC standardization of EAPF and MEPF extracts with epicatechin as reference. (A) Chromatogram of standard epicatechin; (B) Chromatogram of epicatechin in EAPF; (C) Chromatogram of epicatechin in MEPF; (D) Standard calibration curve of epicatechin; (E) HPTLC analysis of standard epicatechin, EAPF and MEPF under UV 254 nm. [Click here to view] |
Effect of EAPF and MEPF on hematological and serum biochemical parameters
The hematological parameters of the animals in different experimental groups, which underwent acute and sub-acute dermal toxicity assessments, are presented in Tables 1 and 2, respectively. The findings indicated that the groups of rats treated topically with EAPF (2,000 and 5,000 mg/kg) and MEPF (2,000 and 5,000 mg/kg) did not exhibit any statistically significant (nsp > 0.05) alterations in their hematological parameters when compared to the control group. However, a noticeable increase in significance (*p > 0.05) in Pct count was observed with both EAPF (2,000 and 5,000 mg/kg). Moreover, the serum biochemical parameters, including liver enzymes (ALT, AST, and ALP), muscle enzyme (creatine kinase), and kidney markers (creatinine and urea), did not demonstrate significant deviations (nsp > 0.05) from the levels observed in the control group (Table 3). Anomalies in the composition of blood and its components can cause a variety of medical problems. The anomalies arise from changes in the blood cell function or structure in erythrocytes, leukocytes, thrombocytes, and clotting factors resulting in leukopenia, neutropenia, thrombocytopenia, anemia, leukocytosis, and other blood cell related abnormalities [22]. When evaluating the potentially toxic effects of medicinal herbs, their extracts, or isolated components, it is crucial to consider hematological tests as a vital indicator. Blood serves as the primary conduit for the distribution of many drugs and foreign substances throughout the body [23,24]. In addition to hematology, the assessment of various biochemical markers such as enzyme activity in tissues and blood (such as AST, ALT, ALP, bilirubin, creatinine, and CK) plays a substantial role in disease diagnosis. In the present study, it was observed that both the EAPF and MEPF extracts did not induce significant alterations in hematological parameters or serum biochemical profiles, indicating the absence of systemic toxicity in rats. Furthermore, our study aligns with research that demonstrated no alterations in the serum biochemical parameters of rodents when orally administered P. fulgens root extract [5]. This further confirms the safety of using the plant topically.
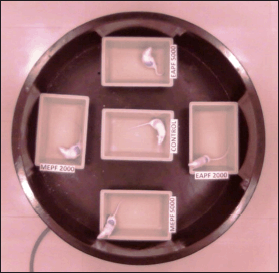 | Figure 3. Image extracted from Maze master (5.0.0.) video tracing system demonstrating the comparative behavioral activity of the control group (normal) and groups treated topically with EAPF (2,000 and 5,000 mg/kg) and MEPF (2,000 and 5,000 mg/kg). [Click here to view] |
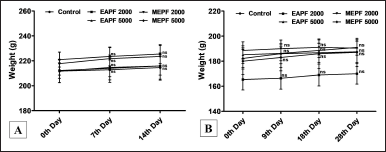 | Figure 4. Effects of EAPF and MEPF on the body weights of the rats on (A) the 0th, 7th, and 14th day acute dermal toxicity study, and (B) the 0th, 9th, 18th, and 28th day sub-acute dermal toxicity study. All results are expressed as Mean ± SEM (n = 5). Statistical comparison was determined by one-way ANOVA followed by Tukey’s Post-test for multiple comparison between groups. Significance level was taken when *p < 0.05 and non-significant value was expressed as nsp >0.05. [Click here to view] |
Effect of EAPF and MEPF on macroscopic and microscopic examination
Fig. 6 illustrates the histological examination of the liver, kidney, spleen, and skin in animals subjected to topical treatment with PFEA (5,000 mg/kg) and MEPF (5,000 mg/kg) over 28 days during the sub-acute dermal toxicity study. The histological sections of the administered substances were compared to microscopic observations of the normal control group. Topical administration with PFEA and MEPF reveals the epidermis appears to be normal with a normal appearance of the skin with multiple visible keratinocytes (black arrows). The dermal layer appears to be in its normal architecture with intact collagen fibers (red arrows). There is the presence of sweat glands and sebaceous glands (blue arrows), and also hair follicle extensions. The histological section of the liver signifies that the lobules are seen to appear in polygonal prisms. The central vein (red dotted arrow) appears to be clear and aligned by hepatic cells in the form of hepatic cords. The hepatic nuclei (black dotted arrows) appear to be normal, in a few shows binucleation. The portal triads (blue dotted arrow) appear to be intact and clear from any hemorrhage and hyperplasia. The portal triad is surrounded by a loose connective tissue. Few inflammatory cells can be seen excluding neutrophils and plasma cells. Microscopic section through the kidney demonstrated that the kidney of rats applied topically with PFEA and MEPF appears to be in its normal architectural detail which is comparable with the normal group kidney. The tubular epithelial cell appears to be normal in the proximal (indigo blue arrows) as well as the distal convoluted tubules (green arrows). The nucleus of the renal tubules is also seen to be intact and adhered to the epithelium. The glomerulus (white-colored arrows) appears to be normal with an intact glomerular tuft. The capillaries seem to be in their normal structure. The spleen histological section showed that the lining capsule is seen to be normal with various trabeculae (green arrows) and follicle (green asterisks). The germinal centers can be seen in the white pulp (blue asterisks) with the presence of an arteriole. The red pulp (red asterisks) also appears to be normal with the presence of sinusoids (black arrow). Histopathology is the study of disease markers through the microscopical examination of a biopsy or surgically removed material that has been processed and mounted to glass slides. Histopathology images, on the other hand, provide a more complete view and in-depth information about a disease and its influence on tissues since the preparation procedure preserves the underlying tissue structure. Furthermore, a diagnosis based on a histopathological picture remains the gold standard for a large variety of disorders including cancers [25,26]. Findings from the current study demonstrated that there were no abnormal changes in the cellular structure of the liver, kidney, spleen, or skin tissues following 28 days of continuous topical delivery of EAPF and MEPF, signifying the complete safety profile of the plant.
Species falling under the genus Potentilla have been worldwide reported to be non-toxic with their long-term uses in traditional medicine for the treatment of diseases [27]. Potentilla is classified within the Rosaceae family and the Rosoideae subfamily, and extensive research has unequivocally demonstrated the absence of toxic compounds in many species belonging to this genus. Remarkably, while a limited number of species, such as Potentilla anserina and Potentilla erecta, have been documented to exhibit cardiac toxicity when their extracts were administered continuously through intravenous routes, the majority of the species showed minimal or negligible toxicity [28]. Notably, despite the high content of polyphenols reported, Potentilla species are devoid of toxic substances such as alkaloids, cyanogenic glycosides, oxalate crystals, and other compounds that have been shown to elicit harmful effects in experimental studies. Traditionally, extracts from Potentilla species have been widely used for a variety of exterior purposes. These include bathing for conditions such as dermatoses, mycoses, and excessive perspiration; irrigation to address issues of white leucorrhea and vulvo vaginitis; compresses for conditions to treat burns, frostbite, and skin injuries; and ointments are prepared to treat purulent and allergic dermatitis [27]. P. fulgens is a significantly noteworthy species, backed by extensive research focusing on both its root and aerial components. This plant is abundant in polyphenols and triterpenoids, while studies have confirmed the absence of alkaloids, cyanogenic glycosides, and other harmful acids, but the presence of calcium oxalate crystals was reported [9]. It is widely recognized that oxalate crystals, whether in acicular or raphide form, can induce motion in the eyes when ingested or when they come into contact with the skin externally. One notable example of this phenomenon is documented in cases involving the dumb cane plant (Dieffenbachia species) [29,30]. While there is no defined correlation between the presence and specific types of calcium oxalate crystals and toxic plant organs, it is worth noting that druse crystals could potentially serve as the primary irritants in these toxic plant parts [31]. Nevertheless, pharmacognostic investigations have revealed the presence of druse-shaped calcium oxalate crystals in the root portion of P. fulgens [9]. Although the plant’s root extract is non-toxic in acute oral toxicity studies, it is imperative to conduct a comprehensive investigation into the plant’s dermal toxicity in experimental animals. Our results showed no indications of mortality, dermal irritation, or systemic toxicity linked to both EAPF and MEPF extracts. This supports the assertion that it can be safely applied externally.
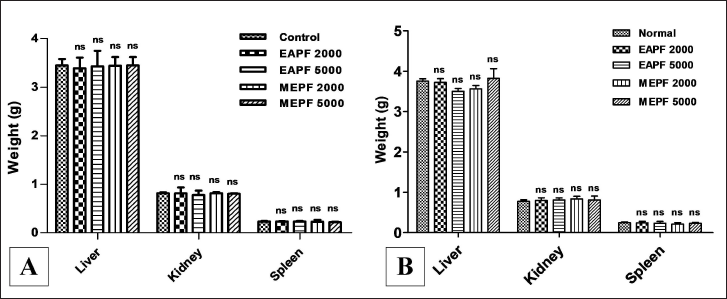 | Figure 5. Effects of EAPF and MEPF on the rat’s vital organs weight on (A) the 14th day acute dermal toxicity study, and (B) the 28th day sub-acute dermal toxicity study. All results are expressed as Mean ± SEM (n = 5). Statistical comparison was determined by one-way ANOVA followed by Tukey’s Post-test for multiple comparison between groups. Significance level was taken when *p < 0.05 and non-significant value was expressed as nsp >0.05. [Click here to view] |
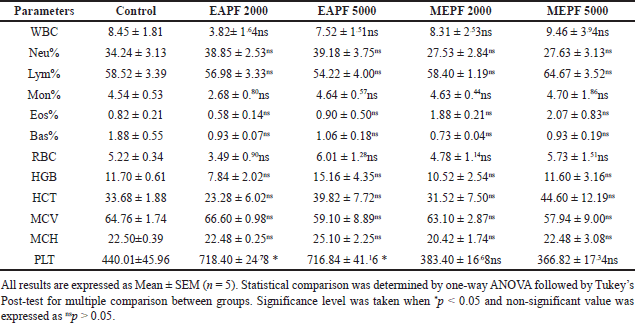 | Table 1. Effects of EAPF and MEPF on the rat’s hematological parameters in 14th day acute dermal toxicity study. [Click here to view] |
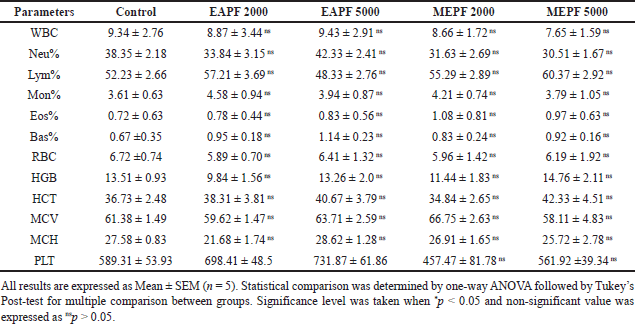 | Table 2. Effects of EAPF and MEPF on the rat’s hematological parameters in 28th day sub-acute dermal toxicity study. [Click here to view] |
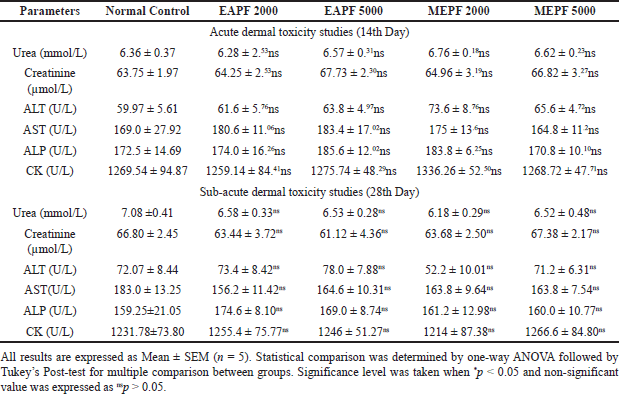 | Table 3. Effects of EAPF and MEPF on the rat’s serum biochemical parameters in 14th day acute dermal toxicity and 28th day sub-acute dermal toxicity studies. [Click here to view] |
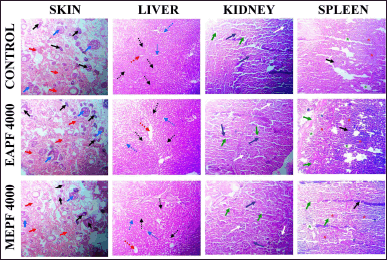 | Figure 6. Effects of EAPF and MEPF on histological architecture of skin, liver, kidney and spleen on 28th day sub-acute dermal toxicity studies in rats. Skin (Black arrow: Keratinocytes, Blue arrow: Sweat/Sebaceous glands, Red arrow: Collagen fibres); Liver (Red dotted arrow: Central vein, Hepatic nuclei: Black dotted arrow, Blue dotted arrow: Portal traids); Kidney (Green arrow: Distal convoluted tubules, Indigo blue arrow: Proximal tubules, White arrows: Glomerulus); Spleen (Black arrow: Sinusoids, Green arrow: Trabeculae, Red asterisks: Red pulp, Green asterisks: Follicle, Blue asterisks: White pulp). [Click here to view] |
CONCLUSION
The current findings show that ethyl acetate and methanol extracts obtained from the root portion of P. fulgens have a consistently safe profile, as evidenced by evaluations conducted in both acute and sub-acute dermal toxicity models employing experimental rats. Our findings suggest that even at large dosages (5,000 mg/kg) applied topically, P. fulgens root extract did not cause any mortality or morbidity, nor did it have any significant adverse effects on biochemical and hematological markers. Furthermore, no changes in the histological structure of important organs such as the skin, liver, kidney, and spleen were seen. Finally, it can be concluded that P. fulgens plant extracts are extremely safe for cutaneous applications and have the potential to serve as a valuable source for drugs intended for topical dermal delivery.
AUTHOR CONTRIBUTIONS
Each author contributed significantly to the conception and design, data acquisition, analysis, and interpretation. They actively participated in drafting or critically revising the article for important intellectual content. Furthermore, all authors consented to the submission to the present journal, provided final approval for the published version, and committed to being accountable for all aspects of the work.
FINANCIAL SUPPORT
Mr. Suman Kumar (DST-INSPIRE SRF: No.-DST/IF190138) received research fellowship support from DST-INSPIRE for pursuing Ph.D. under Dr. Damiki Laloo (Principal Investigator). The fellowship funded by the Department of Science and Technology (DST), Government of India, New Delhi, India, is greatly acknowledged. The All India Council for Technical Education (New Delhi, Government of India) under the grant Research Promotion Scheme (File No. 8-19/FDC/RPS/SC&ST (POLICY-1)/2019-20) is also greatly acknowledged for providing financial assistance.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The experimentation was executed in male albino Wistar rats (150 to 220 g). The study protocol was approved by the Institutional Animal Ethical Committee of Girijananda Chowdhury Institute of Pharmaceutical Science, Guwahati, India (Approval No.: GIPS/IAEC/PhD/ASTU/PRO/01/2021).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Laloo D, Tahbildar R, Smith K, Ahmed AU, Nath J, Shil D, et al. Impact of quality control standardization parameters and antioxidant potential of the aerial parts of Potentilla fulgens wall.: a comprehensive monographic study. JBAPN. 2020;10(4):338–56. CrossRef
2. Choudhary A, Radhika M, Chatterjee A, Banerjee UC, Singh IP. Qualitative and quantitative analysis of Potentilla fulgens roots by NMR, matrix-assisted laser desorption/ionisation with time-of-flight MS, electrospray ionization MS/MS and HPLC/UV. Phytochem Anal. 2015;26(2):161–70. CrossRef
3. Laloo D, Prasad SK, Krishnamurthy S, Hemalatha S. Gastroprotective activity of ethanolic root extract of Potentilla fulgens Wall. ex Hook. J Ethnopharmacol. 2013;146(2):505–14. CrossRef
4. Tangpu V, Deori K, Yadav AK. sEvaluation of safety and protective effects of Potentilla fulgens root extract in experimentally induced diarrhea in mice. J Intercult Ethnopharmacol. 2014;3(3):103. CrossRef
5. Kundu A, Ghosh A, Singh NK, Singh GK, Seth A, Maurya SK, et al. Wound healing activity of the ethanol root extract and polyphenolic rich fraction from Potentilla fulgens. Pharm Biol. 2016;54(11):2383–93. CrossRef
6. Ganguly B, Chaudhary A, Dakhar H, Singh IP, Chatterjee A. Methanolic extract of Potentilla fulgens root and its ethyl-acetate fraction delays the process of carcinogenesis in mice. Sci Rep. 2019;9(1):16985. CrossRef
7. Bertelli A, Biagi M, Corsini M, Baini G, Cappellucci G, Miraldi E. Polyphenols: from theory to practice. Foods. 2021;10(11):2595. CrossRef
8. Sun M, Deng Y, Cao X, Xiao L, Ding Q, Luo F, et al. Effects of natural polyphenols on skin and hair health: a review. Molecules. 2022;27(22):78–32. CrossRef
9. Laloo D, Kumar M, Prasad SK, Hemalatha S. Quality control standardization of the roots of Potentilla fulgens wall: a potent medicinal plant of the Western Himalayas and North-eastern India. Pharmacogn J. 2013;5(3):97–103. CrossRef
10. Jiang X, Cao Y, von Gersdorff Jørgensen L, Strobel BW, Hansen HC, Cedergreen N. Where does the toxicity come from in saponin extract? Chemosphere. 2018;204:243–50. CrossRef
11. Coondoo A, Phiske M, Verma S, Lahiri K. Side-effects of topical steroids: a long overdue revisit. Indian Dermatol Online J. 2014;5(4):416. CrossRef
12. Nurul SA, Hazilawati H, Mohd RS, Mohd FH, Noordin MM, Norhaizan ME. Subacute oral toxicity assesment of ethanol extract of Mariposa christiavespertilionis leaves in male Sprague Dawley rats. Toxicol Res. 2018;34(2):85–95. CrossRef
13. Reduan FH, Shaari RM, Sayuti NS, Mustapha NM, Abu Bakar MZ, Sithambaram S, et al. Acute and subacute dermal toxicity of ethanolic extract of Melastoma malabathricum leaves in Sprague-Dawley rats. Toxicol Res. 2020;36(2):203–10. CrossRef
14. OECD guidelines for testing of chemicals: acute dermal toxicity—Fixed dose procedure (No. 434), OECD, Paris 2004.
15. Marquardt N, Feja M, Hu¨nigen H, Plendl J, Menken L, Fink H, et al. Euthanasia of laboratory mice: Are isoflurane and sevoflurane real alternatives to carbondioxide? PLoSONE. 2018;13(9):e0203793. CrossRef
16. Renne RA, Sumner WA, Harkema JR, Davis CA, AG BS. Draft OECD guidance document on histopathology for inhalation toxicity studies, Supporting TG 412 (Subacute Inhalation Toxicity: 28-Day) and TG 413 (Subchronic Inhalation Toxicity: 90-Day).
17. Banerjee S, Chattopadhyay P, Ghosh A, Pathak MP, Singh S, Veer V. Acute dermal irritation, sensitization, and acute toxicity studies of a transdermal patch for prophylaxis against (±) anatoxin-A poisoning. Int J Toxicol. 2013;32(4):308–13. CrossRef
18. Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014;4(1):177. CrossRef
19. Ugwah-Oguejiofor CJ, Okoli CO, Ugwah MO, Umaru ML, Ogbulie CS, Mshelia HE, et al. Acute and sub-acute toxicity of aqueous extract of aerial parts of Carallumadalzielii NE Brown in mice and rats. Heliyon. 2019;5(1):e01179. CrossRef
20. Raza M, Al-Shabanah OA, El-Hadiyah TM, AlMajed AA. Effect of prolonged vigabatrin treatment on hematological and biochemical parameters in plasma, liver and kidney of Swiss albino mice. Sci Pharm. 2002;70(2):135–45.
21. Dybing E, Doe J, Groten J, Kleiner J, O’brien J, et al. Hazard characterization of chemicals in food and diet: dose response, mechanisms and extrapolation issues. FCT. 2002;40(2):237–82. CrossRef
22. Yanuhar U, Raharjo DK, Caesar NR, Junirahma NS. Hematology response of catfish (Clarias sp.) as an indicator of fish health in tuban regency. In IOP Conference Series: Earth Environ Sci. 2021;718(1):012059. CrossRef
23. Djerrou Z, Djaalab H, Riachi F, Serakta M, Chettou A, Maameri Z, et al. Irritancy potential and sub-acute dermal toxicity study of Pistacia lentiscus fatty oil as a topical traditional remedy. Afr J Tradit Complement Altern Med. 2013;10(3):480–89. CrossRef
24. Han SM, Lee GG, Park KK. Acute dermal toxicity study of bee venom (Apis mellifera L.) in rats. Toxicol Res. 2012;28(2):99–102. CrossRef
25. Padol AR, Jayakumar K, Shridhar NB, Swamy HN, Swamy MN, Mohan K. Safety evaluation of silk protein film (a novel wound healing agent) in terms of acute dermal toxicity, acute dermal irritation and skin sensitization. Toxicol Int. 2011;18(1):17. CrossRef
26. Teshome K, Gebre-Mariam T, Asres K, Perry F, Engidawork E. Toxicity studies on dermal application of plant extract of Plumbago zeylanica used in Ethiopian traditional medicine. J Ethnopharmacol. 2008;117(3):236–48. CrossRef
27. Tomczyk M, Latté KP. Potentilla-A review of its phytochemical and pharmacological profile. J Ethnopharmacol. 2009;122(2):184–204. CrossRef
28. Über die Wirkung von. Extrakten von Potentilla anserine be iInfusionsversuchen am Meerschweinchen. Pharmazie. 1950;5(1):538–542.
29. Prychid CJ, Rudall PJ. Calcium oxalate crystals in monocotyledons: a review of their structure and systematics. Ann Bot. 1999;84(3):725–39. CrossRef
30. Unlu U, Kocaba? A. Dieffenbachia plant poisoning cases and effects on human health. Anatolian J Bot. 2020;4(3):65–68. CrossRef
31. Tütüncü Konyar S, Öztürk N, Dane F. Occurrence, types and distribution of calcium oxalate crystals in leaves and stems of some species of poisonous plants. Bot Stud. 2014;55(1):1–9. CrossRef