INTRODUCTION
The view of target-based drug discovery where new drugs are discovered initially began with a simple search of therapeutic agents from plants, animals, microbes and naturally occurring minerals for numerous disorders [1]. In the present research, obsessive-compulsive disorder (OCD) is taken into account. Approximately in worldwide 2.3% of the population has OCD and in India, 4% of the population has OCD, which means that one in every 25 people suffers. One study found that approximately two-thirds of people with OCD had the majority of symptoms before the age of 24. This disorder is considered an intellectual, behavioral, and relatively rare disease as well as a chronic condition until about two decades ago [2]. OCD is not only a genetic disease but can also be caused due to several environmental factors such as bacteria, stress, and trauma. Several genes play an important role progression of environment-induced OCD. OCD is distinguished by intrusive thoughts or obsession to reduce the anxiety and distress associated with this notion and emotional sense from which the individual experiences a compelling need to carry out specific routines, which may employ compulsions or rituals [3]. Obsessions are interruptive, unwelcome thoughts, perception, drives, or temptations that occur repeatedly and endlessly. They frequently result in anxiety [4]. Individual attempts various notions and actions to suppress unwanted thoughts, i.e., by overcoming them by compulsion. The cognitive and behavioral patterns that an individual engages in as compulsions or routines serve as a coping mechanism aimed at mitigating distress by avoiding unwanted thoughts. Excessive hand washing, scrubbing, organizing, avoiding triggers, measuring, and devoting are a few examples of common compulsions [5]. OCD symptoms are not caused by the physiological side effects of any chemical, even though the inheritance patterns of OCD are unknown (e.g., medication) [6].
Plant parts have been utilized extensively for therapeutics and vitality throughout their evolutionary history. Plant sections have been the primary focus for treating illnesses and injuries for decades in many civilizations around the domain, and they are still under the consideration of ritualistic medicine in many nations [7]. In this research species of Musa acuminata is taken into the spotlight for medicinal purposes. Musa acuminata is described as a species of wild variety of banana that is native to Southeast Asia. It has a broad range of pharmacological applications, and investigations suggest that the active ingredients in M. acuminata are important in the management of the disease. All plant parts, including the roots, stem, leaves, and flowers, have long been utilized in therapeutics throughout North and South America, India Asia, and Africa [8]. Primary metabolites (nutraceuticals, vitamins, and trace elements) and secondary metabolites (phytochemicals) are examples of naturally occurring active ingredients present in plant-based meals. Studies have revealed the anti-inflammatory and antiproliferative functions of different active ingredients high in antioxidants, such as flavonoids and anthocyanin, in neurological diseases [9]. Bananas contain some active ingredients that are beneficial to depression and neurological disorders [10].
A new approach called network-target-based network pharmacology is a type of systematic analysis and next-generation mode approach that has emerged to examine the network of interactions between various elements, such as medications, protein targets, illnesses, and genes [11]. In this research for investigating the active ingredient and mechanism of M. acuminata against OCD network pharmacology is utilized [12].
METHODOLOGY
The complete methodology opted in the current research work has been described in Figure 1.
Screening of drug-active ingredients
IMPPAT 2.0 [13], NPASS [14], and HMDB [15] databases were used to find the active ingredient of M. acuminata. These databases are freely available and contain a wide variety of active ingredients found in medicinal plants.
Collection of active compound targets of action
The search tool for chemical interactions was used to import the targets of action of active ingredients. STITCH [16] and the comparative toxicogenomics database (CTD) [17] databases. These databases of protein-chemical interactions are used to find out gene targets of any active ingredient.
.jpeg) | Figure 1. The complete workflow of the present study. [Click here to view] |
Collection and acquisition disease target
The gene targets of OCD are taken from the GeneCard database and DisGeNET [18]. This database offers thorough details on all predicted and annotated human genes. The gene targets of the active ingredient of M. acuminata and the gene targets of OCD were compared to identify the common genes. In addition, a Venn diagram is established using the Venny (version 2.1) software to identify the gene that links M. acuminata with OCD.
Constructing the protein–protein interaction (PPI) network and identification of hub genes
The acquired common anticancer targets were imported into Cytoscape [19] in the short form of symbols of genes abbreviation, the examination carried out between proteins interactions and the PPI network diagram of gene targets were acquired. To investigate the mechanism of M. acuminata’s antidepressant impact, a network map of gene targets was created using the Cytoscape software to reveal the relationship between M. acuminata and multiple OCD gene targets. The hub genes were identified by filtering out the gene network obtained through Cytoscape based on maximal cliques’ centrality (MCC) topology by using the Cytohubba plug-in of Cytoscape.
Molecular docking and visualization
The three molecular structures of active ingredients were retrieved from PubChem [20]. The three-dimensional structures of target proteins of OCD were acquired from the protein data bank (PDB) [21]. The acquired ligand and protein structure receptor were been docked and analyzed with the DockThor server, and the lowest binding scores for every active ingredient were recorded against the gene target.
RESULTS
Assembly of drug active ingredients
Musa acuminata active ingredients were recognized to check their potential therapeutic mechanism against OCD. IMPPAT, NPASS, and HMDB were used to identify the active ingredients of M. acuminata. These databases collectively retrieved 24 active compounds of the M. acuminata plant. The list of active ingredients of M. acuminata along with their PubChem ID is given in Table 1.
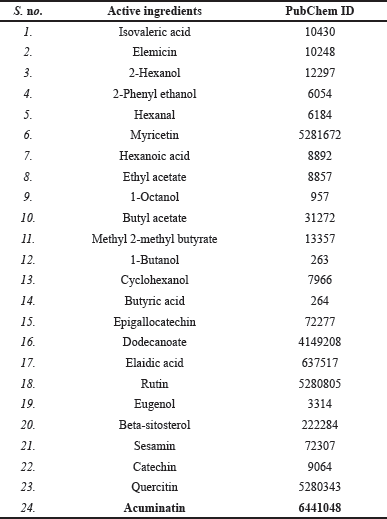 | Table 1. List of active ingredients of M. acuminata along with their PubChem ID. [Click here to view] |
Target collection of M. acuminata
The target genes of the active ingredients of M. acuminata were further identified for therapeutic purposes. Through the STITCH and CTD database, a total of 11,553 gene targets were obtained from the 24 active ingredients. The replicated gene targets in every active ingredient were eliminated, and finally, 8,320 related gene targets were obtained.
Assembly of disease-related targets
With the GeneCards database and DisGeNET, 2,966 OCD genes were found. The 2,966 OCD genes and 8,320 target genes of M. acuminata were compared to identify the common gene targets. This resulted in 1,191 gene targets that play a key role in OCD and are also the target genes of active ingredients of M. acuminata. They were contemplated as the same targets between M. acuminata and OCD. Figure 2 depicts 1,191 common genes between M. acuminata and OCD in the form of a Venn diagram.
PPI network construction and identification of hub genes
The Cytoscape tool was used to generate the active ingredient-target disease network diagram for examination to acquire PPI. The usual targets of M. acuminata 1,196 and OCD were entered. At the confidence score of 0.40, the network encloses a total of 1,155 nodes with 19,906 edges. As the number of edges becomes greater the association will be stronger. The network was further refined at a confidence score of 0.95, the network contains a total of 1,155 nodes with 1,602 edges. Further analysis of targets of action in the gene network is being filtered out of the top nine interacting genes based on MCC topology by using the Cytohubba plug-in of Cytoscape. Out of which tumor necrosis factor (TNF) was found to be the most interactive gene in the network. Musa acuminata and OCD both target on TNF gene. Figure 3 shows the network of interacting genes obtained from CytoHubba.
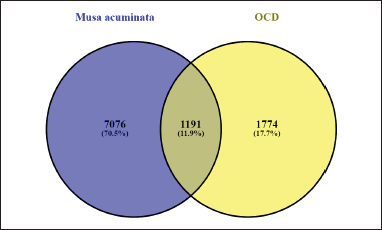 | Figure 2. Venn diagram showed 1,191 common genes between targets of M. acuminata active ingredient and OCD. [Click here to view] |
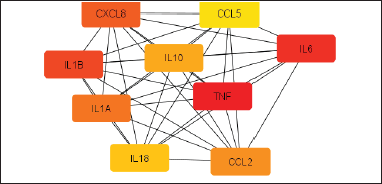 | Figure 3. Construction of interacting gene targets obtained from CytoHubba. The constructed network had 9 nodes and 36 edges. [Click here to view] |
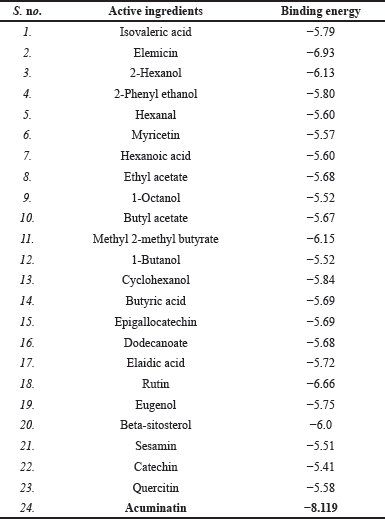 | Table 2. The binding energy of the active ingredients with TNF as obtained from DockThor. [Click here to view] |
Molecular docking and visualization
Molecular docking technologies helped to further validate the involvement of M. acuminata in the treatment of disease. PPI networking was used to filter and analyze the top nine important targets in total. The average betweenness centrality, MCC, and degree of freedom are measured while developing the network. TNF (PDB ID: 1TNF) is the top interacting protein with the highest interactions and is selected for docking with the evaluated 24 active ingredients. Recorded scores of docking suggested that all active ingredients exhibited scores between 5.41 and −8.119 kcal/mol. The molecular docking results indicated that the active ingredient acuminatin (PubChem ID: 6441048) found in M. acuminata shows the best affinity with TNF having the binding energy of −8.119 kcal/mol and interacting with lysine residue at 85 position of TNF receptor through vanderwaal, alkyl, and hydrogen bond interactions. Figure 4 shows the 2D and 3D visualization of molecular interaction between acuminatin active ingredient with TNF protein. The binding energy of the active ingredients with TNF as obtained from DockThor have been tabulated in Table 2.
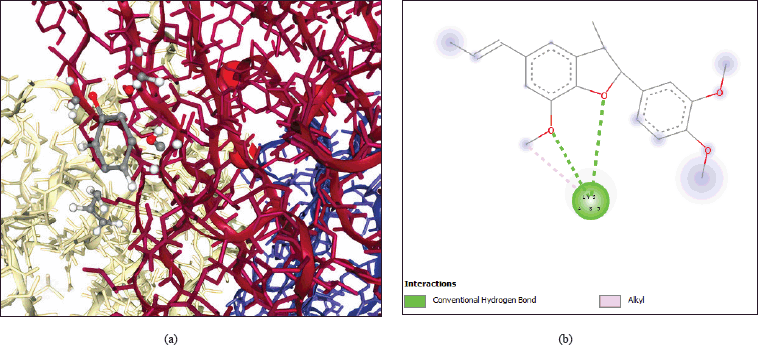 | Figure 4. 2D and 3D visualization of molecular interaction between acuminatin active ingredient with TNF protein. (a) 3D structure. (b) 2D structure. [Click here to view] |
CONCLUSION
Obsessive compulsive disorder is a mental condition that affects a vast population. Since OCD is a lifelong issue, it is important to look for the natural phytochemicals that can help manage the symptoms of the disease. According to a study, banana pulp and peel offer anti-anxiety, antidepressant, and memory-strengthening properties, probably due to their antioxidant mechanisms. Therefore, the present study investigates the significant role of banana supplements in reducing stress (anxiety and depression) and increasing cognitive function, potentially due to phyto-antioxidants [22].
In the present study, both network pharmacology and molecular docking techniques have been applied, and the therapeutic benefits of the M. acuminata on OCD were examined. The present study uses network biology approaches to identify the key targets in OCD. The top nine genes obtained through cytoHubba have fluctuating expression in OCD patients as revealed in several research papers indicating the accuracy of our results. According to a study by Ewa and Oglodek [23], the level of CCL5 in men and women was significantly increased in patients suffering from an obsessive-compulsive personality disorder. A cytokine study of an adult patient with OCD performed by Fontenelle et al. [24], revealed a significant increase in the plasma level of CCL2 and CXCL8. It is also seen that a significant decrease in interleukin-1 beta (IL-1β) levels was observed in individuals with OCD, along with a statistical increase in the plasma levels of IL-6 and IL-10 [25]. The cytoHubba network found TNF as the most interactive gene which is targeted by both M. acuminata and OCD. The TNF gene is located on chromosome number 6, it is the particular position that is associated with OCD [26]. Initially, it was stated that TNF induces programmed cell death, also known as apoptosis [27]. TNF is a cytokine known for its involvement in the development and progression of various autoimmune and inflammatory conditions, playing a significant role in their pathogenesis [28]. The major histocompatibility complex class third area on the chromosome between the HLA-B and HLA-DR genes [29] is where the TNF gene’s coding sequence is situated, the region has been linked to OCD.
Through carrier-mediated transport through the blood–brain barrier, peripheral cytokines can enter the brain and affect complex brain functions. [30]. TNF and other cytokines were observed to be higher in OCD patients as compared to healthy patients. According to Cappi et al. [31], the TNFA polymorphism’s A allele is substantially related to OCD patients. The A allele may cause TNF- to be more often transcribed. OCD and other mental disorders that have been linked to TNF have been shown to exhibit both normal and aberrant physiological processes that depend heavily on the expression of the glutamate and serotonin transporter.
The molecular docking studies revealed the active ingredient of banana, that is, acuminatin having strong binding affinity, that is, −8.119 kcal/mol against the TNF gene. The molecular docking strategy provides evidence for how effectively the active ingredient acts on the target’s expression to obstruct OCD. Acuminatin is a neolignan that is used for the overall wellness. Acuminatin has been shown to have neuroprotective effects on several brain disorders [32]. The neolignan class of phytochemicals has excellent neuroprotective potential and is now being researched for its use in Parkinson’s disease, Alzheimer’s disease, and stroke [33,34]. The neolignans protect brain microvascular endothelial cells (BMECs) and nerve cells thereby preventing several psychiatric disorders. Neolignans protect neuronal cells by regulating neuronal function, reducing neurotoxicity, suppressing neuronal apoptosis, and its anti-inflammatory and antioxidant potential. Neolignans increase blood supply, normalize blood glucose levels, and close the connections between BMECs thereby protecting BMECs. In addition, neolignans also decrease glucocorticoid negative feedback and increase BDNF expression [35].
Therefore, banana especially its active ingredient acuminatin can be effectively used to control and prevent the symptoms of OCD triggered by genetic or environmental factors like stress, trauma, or bacterial pollution. Acuminatin supplements can also be prescribed to OCD patients to manage their obsessive and compulsive episodes.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Burger A. Approaches to drug discovery. N Engl J Med. 1964;270:1098–101. CrossRef
2. Janardhan Reddy YC, Rao NP, Khanna S. An overview of Indian research in obsessive compulsive disorder. Indian J Psych. 2010;52:200–9.
3. Fenske JN, Petersen K. Obsessive-compulsive disorder: diagnosis and management. Am Fam Phys. 2015:896–903.
4. Robbins TW, Vaghi MM, Banca P. Obsessive-compulsive disorder: puzzles and prospects. Neuron. 2019;102(1):27–47. CrossRef
5. Krebs G, Heyman I. Obsessive-compulsive disorder in children and adolescents. Arch Dis Child. 2015;100(5):495–9. CrossRef
6. Fenske JN, Schwenk TL. Obsessive compulsive disorder: diagnosis and management. Am Fam Phys. 2009:239–45.
7. Mathew NS, Negi PS. Traditional uses, phytochemistry and pharmacology of wild banana (Musa acuminata Colla). J Ethnopharmacol. 2017;196:124–40.
8. Pereira A, Maraschin M. Banana (Musa spp) from peel to pulp: ethnopharmacology, source of bioactive compounds and its relevance for human health. J Ethnopharmacol. 2015;160:149–63. CrossRef
9. Oyeyinka BO, Afolayan AJ. Suitability of banana and plantain fruits in modulating neurodegenerative diseases: implicating the in vitro and in vivo evidence from neuroactive narratives of constituent biomolecules. Foods. 2022;11(15):2263. CrossRef
10. Samad N, Muneer A, Ullah N, Zaman A, Ayaz MM, Ahmad I. Banana fruit pulp and peel involved in antianxiety and antidepressant effects while invigorate memory performance in male mice: possible role of potential antioxidants. Pak J Pharm Sci. 2017 May;30(3(Suppl.):989–95.
11. Hopkins AL. Network pharmacology. Nat Biotechnol. 2007;25(10):1110–1. CrossRef
12. Jiang Y, Zhong M, Long F, Yang R, Zhang Y, Liu T. Network pharmacology-based prediction of active ingredients and mechanisms of Lamiophlomis rotata (Benth.) Kudo against rheumatoid arthritis. Ethnopharmacology. 2019;10:1435.
13. Mohanraj K, Karthikeyan BS, Vivek-Ananth RP, Chand RPB, Aparna SR, Mangalapandi P, et al. IMPPAT: a curated database of Indian medicinal plants, phytochemistry and therapeutics. Sci Rep. 2018;8(1):4329. CrossRef
14. Zeng X, Zhang P, He W, Qin C, Chen S, Tao L, et al. NPASS: natural product activity and species source database for natural product research, discovery and tool development. Nucleic Acids Res. 2018;46:1217–22.
15. Wishart DS, Tzur D, Knox C, Eisner R, Guo AC, Young N, et al. HMDB: the human metabolome database. Nucleic Acids Res. 2007;35:521–6.
16. Kuhn M, von Mering C, Campillos M, Jensen LJ, Bork P. STITCH: interaction networks of chemicals and proteins. Nucleic Acids Res. 2008;36:684–8.
17. Davis AP, Grondin CJ, Johnson RJ, Sciaky D, Wiegers J, Wiegers TC, et al. Comparative toxicogenomics database (CTD). Nucleic Acids Res. 2020;49:D1138–43.
18. Piñero J, Bravo À, Queralt-Rosinach N, Gutiérrez-Sacristán A, Deu-Pons J, Centeno E, et al. DisGeNET: a comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 2017;45:833–9.
19. Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–504. CrossRef
20. Kim S. PubChem Substance and Compound databases. Nucleic Acids Res. 2016;44:1202–13.
21. Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, et al. The protein data bank. Nucleic Acids Res. 2000;28(1):235–42. CrossRef
22. Samad N, Muneer A, Ullah N, Zaman A, Ayaz MM, Ahmad I. Banana fruit pulp and peel involved in antianxiety and antidepressant effects while invigorate memory performance in male mice: possible role of potential antioxidants. Pak J Pharm Sci. 2017 May;30(3(Suppl.):989–95.
23. Og?odek EA, Szota AM, Just MJ, Mo? DM, Araszkiewicz A. The MCP-1, CCL-5 and SDF-1 chemokines as pro-inflammatory markers in generalized anxiety disorder and personality disorders. Pharmacol Rep. 2015 Feb;67(1):85–9.
24. Fontenelle LF, Barbosa IG, Luna JV, de Sousa LP, Abreu MN, Teixeira AL. A cytokine study of adult patients with obsessive-compulsive disorder. Compr Psychiatry. 2012;53(6):797–804. CrossRef
25. Rao NP, Venkatasubramanian G, Ravi V, Kalmady S, Cherian A, Yc JR. Plasma cytokine abnormalities in drug-naïve, comorbidity-free obsessive-compulsive disorder. Psychiatry Res. 2015;229:949–52.
26. Hanna GL, Veenstra-VanderWeele J, Cox NJ, Boehnke M, Himle JA, Curtis GC, et al. Genome-wide linkage analysis of families with obsessive-compulsive disorder ascertained through pediatric probands. Am J Med Genet. 2002;114(5):541–52. CrossRef
27. O’Malley WE, Achinstein B, Shear MJ. Action of bacterial polysaccharide on tumors. II. Damage of sarcoma 37 by serum of mice treated with Serratia marcescens polysaccharide, and induced tolerance. Nutr Rev. 1998;46:389–91.
28. Zhang BB, Liu XZ, Sun J, Yin YW, Sun QQ. Association between TNF α gene polymorphisms and the risk of duodenal ulcer: a meta-analysis. PLoS One. 2013;8(2):e57167. CrossRef
29. EI-El-Tahan RR, Ghoneim AM, El-Mashad N. TNF-α gene polymorphisms and expression. Springerplus. 2016;5(1):1508. CrossRef
30. Kronfol Z, Remick DG. Cytokines and the brain: implications for clinical psychiatry. Am J Psychiatry. 2000;157(5):683–94. CrossRef
31. Cappi C, Muniz RK, Sampaio AS, Cordeiro Q, Brentani H, Palácios SA, et al. Association study between functional polymorphisms in the TNF-alpha gene and obsessive-compulsive disorder. Arq Neuro Psiquiatr. 2012;70(2):87–90. CrossRef
32. Zhu S, Liu F, Zhang R, Xiong Z, Zhang Q, Hao L, et al. Neuroprotective potency of neolignans in Magnolia officinalis cortex against brain disorders. Front Pharmacol. 2022;13:857449. CrossRef.
33. Luo H, Wu H, Yu X, Zhang X, Lu Y, Fan J, et al. A review of the phytochemistry and pharmacological activities of Magnoliae officinalis cortex. J Ethnopharmacol. 2019;236:412–42. CrossRef.
34. Rauf A, Olatunde A, Imran M, Alhumaydhi FA, Aljohani ASM, Khan SA, et al. Honokiol: a review of its pharmacological potential and therapeutic insights. Phytomedicine. 2021;90:153647. CrossRef.
35. Zhu S, Liu F, Zhang R, Xiong Z, Zhang Q, Hao L, et al. Neuroprotective potency of neolignans in Magnolia officinalis cortex against brain disorders. Front Pharmacol. 2022 Jun 16;13:857449. CrossRef.