INTRODUCTION
Pathogenic bacteria, once regarded as contained and manageable in clinical care, have become the most challenging and critical problem in health care following the widespread evolution of antibiotic resistance in the most important species. Now, conceded as a global health crisis and a silent pandemic, the lack of effective drugs renders bacterial infections re-emerging diseases and a rampant cause of mortality [1,2]. After the so-called golden age of antibiotics of the 20th century, the development of new and improved drugs had dwindled with little advancement in the past few decades [3], so the 21st century is posed with an immense global threat of new bacterial infections as the old pathogens revived with renewed virulence that make them more difficult to tackle [4,5]. It is estimated that around 700,000 people die every year due to antibiotic resistance, and the number will increase tenfold by the next five decades [6]. An innovative approach to the development and synthesis of new compounds with better antibacterial activity is of utmost necessity and priority.
Carbohydrates are one of the most important and ubiquitous biological substances, accounting for 75% of all biomolecules on Earth [7]. As integral cellular components, they are crucial in structural and functional properties of all cells [8,9]. They are also the primary source of metabolic fuels and energy storage that are critical in maintaining normal physiological processes, and thereby development of metabolic disorders [10,11]. Organocatalytic experiments in recent years have shown that carbohydrate-based molecules are indicating useful and diverse therapeutic activities that could be exploited in drug development. They have several advantages over other potential sources of drugs for their structural diversity, optical purity and high degree of functional properties [12–14]. Consequently, more than 170 carbohydrate-containing compounds have received approval from various agencies as pharmaceutical medications [15].
Recently, the biological activities of thiourea compounds with a saccharide scaffold have been an area of keen interest, and the pharmacological qualities, including herbicidal, antifungal, antimalarial, antitumor, and insecticidal effects are well documented [16,17]. It has been encountered that the unsubstituted diphenyl thiourea compounds generally lack antibacterial action, but structural changes, notably the introduction of halogen components, have been demonstrated to promote effectiveness against several bacteria [18–20]. In the light of these important precedents, we synthesised a new class of carbohydrate-derived thioureas via coupling of D-fructose-derived primary amines with commercially available isothiocyanates (Figure 1) and were tested for their potential antibacterial activities.
MATERIALS AND METHODS
Synthesis of carbohydrate-derived thioureas 2a–2h
A solution of 1.5 mmol of isothiocyanate in 2 ml of dry dichloromethane was mixed with 1 mmol (0.26 mg) of sugar amines 1 at 0°C, based on the method previously described [21]. The reaction mixture was permitted to reach room temperature and stirred for 48 h. The mixture was then extracted using ethyl acetate and washed with water twice. The organic layer was dried over Na2SO4, filtered and evaporated. The pure product was obtained by column chromatography using 40% ethyl acetate in hexane as an eluent and silica gel (60−120 mesh) as a stationary phase.
Synthesis of thioureas 3a–3h
5 ml of an acetonitrile-water mixture (CH3CN:H2O in 9:1 ratio) was added to a 100 ml round bottom flask charged with 5 mol% of phosphotungstic acid (144 mg) and 1 mmol of the corresponding compounds 2a–2h, following a previously reported procedure [22]. The reaction mixture was allowed to settle at room temperature and be stirred for 6 h. After the reaction was completed, the solvents were evaporated under reduced pressure, diluted with ethyl acetate and washed with water three times. The organic layer was concentrated to give the crude product which was further purified by column chromatography using 40% ethyl acetate in hexane and silica gel.
Spectral analysis
Bruker Advance II, 400 MHz, and JEOL Resonance Inc. (model EC-500R) spectrophotometers were used for recording 1H nuclear magnetic resonance (NMR) and 13C NMR spectra. CDCl3 and DMSO-d6 were used as solvents and chemical shifts were calculated in δ with Me4Si as reference. Fourier-transform infrared (FT-IR) spectra were recorded on an Agilent Cary 630 FTIR spectrometer. Electrospray ionization mass spectrometry (ESI-MS) of the compounds was obtained from an Agilent 6520 Q-TOF mass spectrometer with an Agilent 1200 HPLC system. Optical rotations of the synthesized compounds were measured with a Rudolph AUTOPOL IV Automatic Polarimeter in chloroform and described as [α]D25 (c in mg per 10 ml, solvent).
Antibacterial susceptibility test
Antibacterial activity of the novel carbohydrate-derived thiourea compounds was tested against bacterial strains from both Gram-negative bacteria groups such as Pseudomonas aeruginosa (ATCC 10145), Klebsiella pneumoniae (ATCC 10031), Escherichia coli (ATCC 10536), Salmonella typhi (ATCC 51812), and Gram-positive bacteria such as Bacillus subtilis (ATCC 11774) and Micrococcus luteus (ATCC 10240). A bacterial susceptibility test was performed using disk diffusion assay in a nutrient agar medium [23,24]. All the bacterial strains used were obtained from American Type Culture Collection, grown in an agar broth at 37°C in a microbiological incubator for 24 h prior to the experiment. The turbidity of the test bacterial strains in a nutrient broth was adjusted at 0.5 McFarland standard (~1.4 × 108 bacteria/ml) using normal saline. Ceftriaxone (at 10 μg) was used as the standard antibiotic and a solvent (ethyl acetate) was used as a negative control in every experiment. Ethyl acetate without the tested compounds displayed no antibacterial activity, corroborating that it has no action on the growth of the bacterial strains. For each experiment, a sterilised paper disk of 6 mm was impregnated with the synthesised compounds in three different concentrations, namely 10, 50, and 100 μg. After fixing the papers on the agar, they were incubated at 37°C for 24 h. The resulting inhibition zone were measured with a caliper in mm. Each test was done in triplicate and the results obtained are expressed as the means (± standard deviations) of three observations.
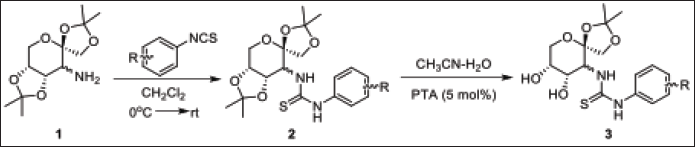 | Figure 1. Scheme of preparation of carbohydrate-based thiourea compounds. [Click here to view] |
RESULTS AND DISCUSSION
Chemistry
16 carbohydrate-based thiourea compounds were synthesised as shown in Figures 1 and 2. The structures of the desired thiourea compounds 2a–2h and 3a–3h were elucidated by the spectral data recorded including NMR, FT-IR, MS, and polarimeter as follows:
1,2;4,5-Di-O-isopropylidene-3-(3,5-bis(trifluoromethyl)phenylthioureido)-3-deoxy-β-D-fructopyranose (2a): Yield %: 96% as white solid; mp: 157°C–159°C. [α]D25 -143.50° (c 0.001, CHCl3). 1H NMR (400 MHz, CDCl3): δ 8.78 (s, 1H), 8.046 (s, 1H), 7.95 (s, 1H), 7.67 (s, 1H), 6.36 (s, 1H), 4.32–3.97 (m, 7H), 1.63–1.36 (m, 12H) ppm. 13C NMR (100 MHz, CDCl3): δ 182.33, 139.02, 132.73, 132.40, 124.54, 124.22, 123.34, 121.49, 119.34, 112.28, 111.33, 110.19, 105.01, 77.99, 75.22, 60.14, 58.13, 29.67, 29.59, 28.19, 27.90 ppm. IR (KBr): 3321.49, 2989.56, 2936.91, 1529.73, 1468.40, 1381.53, 1278.74, 1222.07, 1177.62, 1136.09, 1079.50, 969.78, 885.92, 762.27 cm−1. ESI-MS (m/z): 531.5 (M+). Elemental analysis for C21H24F6N2O5S: C 47.55, H 4.56, F 21.49, N 5.28, O 15.08, S 6.02: Found C 47.35, H 4.86, F 21.22, N 5.68, O 14.68, S 6.20.
1,2;4,5-Di-O-isopropylidene-3-(3,5-bis(trifluoromethyl)phenylthioureido)-3-deoxy-α-D-fructopyranose (2b): Yield: 80% as white solid; mp: 150°C–152°C. [α]D25 -114.63° (c 0.001, CHCl3). 1H NMR (400 MHz, CDCl3): δ 8.63 (s, 1H), 7.95 (s, 2H), 7.68 (s, 1H), 6.70 (d, J = 8.8 Hz, 1H), 4.64–3.93 (m, 7H), 1.72 (s, 3H), 1.51 (s, 3H), 1.42 (s, 3H), 1.33 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 180.60, 138.72, 132.39, 124.54, 124.50, 123.34, 121.79, 119.10, 111.53, 104.95, 72.94, 72.36, 62.27, 55.76, 26.69, 26.11, 25.92, 24.98 ppm. IR (KBr): 3333.10, 2933.83, 1535.59, 1466.80, 1382.22, 1278.33, 1135.27, 1065.27, 878.77, 757.44 cm−1. ESI-MS (m/z): 531.5 (M+). Elemental analysis for C21H24F6N2O5S: C 47.33, H 4.48, F 22.09, N 5.23, O 15.02, S 5.84: Found C 47.45, H 4.20, F 21.98, N 5.02, O 15.34, S 5.99.
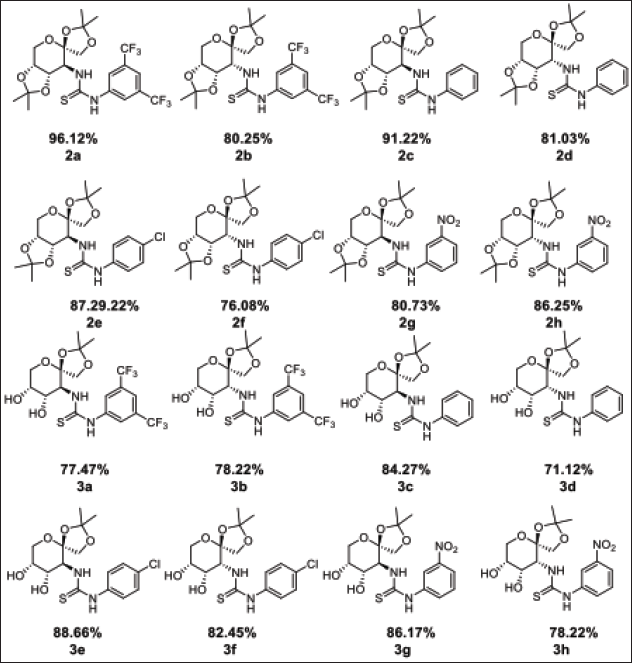 | Figure 2. Structure of the prepared novel D-fructose derived thioureas. [Click here to view] |
 | Figure 3. Inhibitory activity of compound 2a against various pathogenic bacteria. E. coli (EC), K. pneumoniae (KP), M. luteus (ML), and S. typhi (ST). [Click here to view |
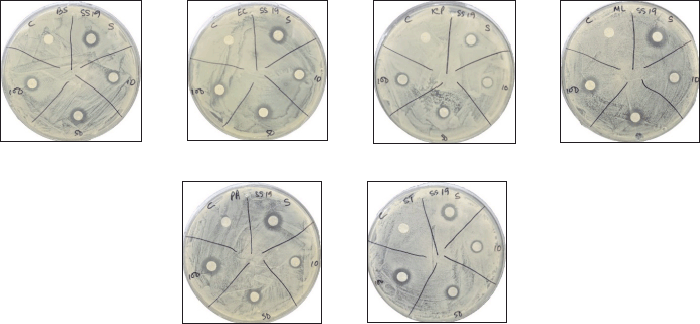 | Figure 4. Inhibitory activity of compound 2b against various pathogenic bacteria. B. subtilis (BS), E. coli (EC), K. pneumoniae (KP), M. luteus (ML), P. aeruginosa (PA), and S. typhi (ST). [Click here to view] |
1,2;4,5-Di-O-isopropylidene-3-(phenylthioureido)-3-deoxy-β-D-fructopyranose (2c): Yield: 91% as white solid; mp: 162°C–165°C. [α]D25 -176.83° (c 0.001, CHCl3). 1H NMR (500 MHz, CDCl3): δ 8.50 (s, 1H), 7.40–7.26 (m, 5H), 6.00 (s, 1H), 4.15–3.93 (m, 7H), 1.66 (s, 3H), 1.38 (s, 3H), 1.32 (s, 3H), 1.06 (s, 3H) ppm. 13C NMR (125 MHz, CDCl3): δ 181.65, 135.67, 130.13, 127.55, 125.80, 111.09, 109.77, 105.05, 75.42, 72.59, 72.11, 60.30, 56.61, 27.88, 26.44, 25.98, 25.46 ppm. IR (KBr): 3378, 3291, 3204, 2932, 2291, 1510, 1358, 1208, 1062, 873, 747 cm−1. ESI-MS (m/z): 395.1 (M+). Elemental analysis for C19H26N2O5S: Calculated C57.85, H 6.64, N 7.10, O 20.28, S 8.13; Found C 57.66, H 6.46, N 7.35, O 20.42, S 8.11.
1,2;4,5-Di-O-isopropylidene-3-(phenylthioureido)-3-deoxy-α-D-fructopyranose (2d): Yield: 81% as white solid; mp: 92°C–95°C. [α]D25 -142.00° (c 0.002, CHCl3). 1H NMR (400 MHz, CDCl3): δ 8.46 (s, 1H), 7.45–7.28 (m, 5H), 6.57 (d, 1H, J = 9.2 Hz), 4.61–3.86 (m, 7H), 1.46 (s, 3H), 1.40 (s, 3H), 1.31 (s, 3H), 1.26 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 180.65, 135.97, 130.05, 129.11, 126.99, 124.37, 111.01, 109.32, 104.59, 72.82, 72.14, 71.79, 61.91, 55.57, 26.50, 25.81, 25.60, 24.78 ppm. IR (KBr): 3370.75, 2987.64, 2934.76, 1534.88, 1379.70, 1310.62, 1212.07, 1105.99, 1064.75, 872.31, 755.08 cm−1. ESI-MS (m/z): 395.5 (M+). Elemental analysis for C19H26N2O5S: Calculated C 57.72, H 6.32, N 7.21, O 19.89, S 8.85; Found C 57.52, H 6.52, N 7.13, O 20.10, S 8.72.
1,2:4,5-Di-O-isopropylidene-3-(4-chlorophenylthioureido)-3-deoxy-β-D-fructopyranose (2e): Yield: 87% as white solid; mp: 165°C–167°C. [α]D25 -146.83° (c 0.002, CHCl3). 1H NMR (400 MHz, CDCl3): δ 7.77 (s, 1H), 7.40–7.24 (m, 4H), 5.87 (s, 1H), 4.19–3.97 (m, 7H), 1.69 (s, 3H), 1.58 (s, 3H), 1.43 (s, 3H), 1.37 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 182.32, 136.00, 132.76, 131.82, 130.62, 128.45, 127.98, 125.88, 110.20, 109.48, 105.64, 72.88, 72.53, 60.65, 57.34, 29.93, 28.19, 26.67, 25.62 ppm. IR (KBr): 3367.43, 2933.39, 1530.50, 1378.17, 1224.43, 1081.58, 882.02, 758.40, 521.71 cm−1. ESI-MS (m/z): 429.5 (M+). Elemental analysis for C19H25ClN2O5S: Calculated C 53.20, H 5.88, Cl 8.26, N 6.53, O 18.65, S 7.47; Found C 53.32, H 5.64, Cl 8.33, N 6.32, O 18.53, S 7.84.
1,2:4,5-Di-O-isopropylidene-3-(4-chlorophenylthioureido)-3-deoxy-α-D-fructopyranose (2f): Yield: 76% as white solid; mp: 169°C–171°C. [α]D25 -106.50° (c 0.001, CHCl3). 1H NMR (400 MHz, CDCl3): δ 8.89 (s, 1H), 7.38–7.28 (m, 4H), 6.58 (d, 1H, J = 9.2 Hz), 4.60–3.88 (m, 7H), 1.46–1.24 (m, 12H) ppm. 13C NMR (100 MHz, CDCl3): δ180.62, 135.09, 132.03, 129.89, 128.98, 128.17, 125.50, 111.01, 109.35, 104.59, 76.80, 72.77, 72.11, 61.87, 60.39, 55.35, 26.47, 25.83, 25.54, 24.76 ppm. IR (KBr): 3362.19, 2932.37, 1530.82, 1379.35, 1225.11, 1081.54, 882.21, 756.84 cm−1. ESI-MS (m/z): 429.4 (M+). Elemental analysis for C19H25ClN2O5S: Calculated C 53.32, H 5.61, Cl 8.11, N 6.83, O 18.28, S 7.84; Found C 53.46, H 5.45, Cl 8.15, N 6.51, O 18.33, S 8.1
1,2:4,5-Di-O-isopropylidene-3-(3-nitrophenylthioureido)-3-deoxy-β-D-fructopyranose (2g): Yield: 81% as yellow solid; mp: 93°C–95°C. [α]D25 -209.50° (c 0.001, CHCl3). 1H NMR (400 MHz, DMSO-d6): δ 10.12 (s,1H), 8.79 (s, 1H), 7.98–7.60 (m, 4H), 4.31–3.81 (m, 7H), 1.51 (s, 3H), 1.48 (s, 3H), 1.44 (s, 3H), 1.41 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 182.56, 148.16, 132.08, 130.37, 129.04, 117.10, 111.53, 109.76, 75.95, 74.79, 60.52, 60.48, 60.33, 27.10, 26.87, 26.66, 26.42 ppm. IR (KBr): 3367.63, 2928.16, 1529.95, 1381.3, 1221.20, 1079.57, 770.4 cm−1. ESI-MS (m/z): 440.5 (M+). Elemental analysis for C19H25N3O7S: Calculated C 51.93, H 5.73, N 9.56, O 25.48, S 7.29; Found C 51.88, H 5.56, N 9.88, O 25.76, S 6.92.
1,2:4,5-Di-O-isopropylidene-3-(3-nitrophenylthioureido)-3-deoxy-α-D-fructopyranose (2h): Yield: 86% as yellow solid; mp: 102°C–104°C. [α]D25 -116.50° (c 0.002, CHCl3). 1H NMR (400 MHz, CDCl3): δ 8.27 (s, 1H), 8.05 (d, 1H, J = 2 Hz), 7.85 (s, 1H), 7.55 (dd, 1H, J = 8, 8.4 Hz), 6.69 (d, 2H, J = 8.8 Hz), 4.65–3.92 (m, 7H), 1.50 (s, 3H), 1.43 (s, 3H), 1.32 (s, 3H), 1.26 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 180.07, 148.95, 139.02, 130.28, 129.64, 120.70, 118.55, 111.46, 110.12, 104.94, 72.98, 72.40, 62.26, 60.68, 55.72, 26.74, 26.14, 25.92, 25.01 ppm. IR (KBr): 3306.03, 2987.98, 2933.68, 1530.63, 1347.44, 1212.06, 1105.57, 1064.44, 1024.77, 867.8, 751.03 cm−1. ESI-MS (m/z): 440.5 (M+). Elemental analysis for C19H25N3O7S: Calculated C 51.73, H 5.46, N 9.86, O 25.24, S 7.70; Found C 51.46, H 5.38, N 9.99, O 25.68, S 7.49.
1,2-O-isopropylidene-3-(3,5-bis(trifluoromethyl)phenylthioureido)-3-deoxy-β-D-fructopyranose (3a): Yield: 77% as white solid; mp: 162°C–164°C. [α]D25 -38.33° (c 0.001, CHCl3). 1H NMR (400 MHz, DMSO-d6): δ 10.33 (s, 1H), 8.37 (s, 2H), 7.75 (s, 1H), 7.68 (d, 1H), 4.92–3.31 (m, 9H), 1.39 (s, 3H), 1.38 (s, 3H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 181.62, 141.75, 130.64, 130.32, 130.13, 129.99, 129.66, 127.31, 124.56, 121.85, 121.66, 119.09, 116.24, 110.33, 106.02, 71.49, 69.23, 67.85, 64.70, 54.35, 26.32, 26.23 ppm. IR (KBr): 3310, 3092, 2349, 1548, 1465, 1374, 1267, 1130, 970, 878, 795, 688 cm−1. ESI-MS (m/z): 491.4 (M+). Elemental analysis for C18H20F6N2O5S: Calculated C 44.18, H 4.07, F 22.84, N 5.39, O 16.51, S 6.99; Found C 44.36, H 4.32, F 22.66, N 5.66, O 16.26, S 6.73.
1,2-O-isopropylidene-3-(3,5-bis(trifluoromethyl)phenylthioureido)-3-deoxy-α-D-fructopyranose (3b): Yield: 78% as white solid; mp: 167°C–169°C. [α]D25 -89.67° (c 0.001, CHCl3). 1H NMR (400 MHz, CDCl3): δ 8.26 (s, 1H), 7.85 (s, 2H), 7.70 (s, 1H), 7.48 (d, 1H, J = 8.4 Hz), 5.12 (s, 1H), 4.30–3.81 (m, 7H), 2.04 (s, 1H), 1.48 (s, 3H), 1.44 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 182.07, 138.87, 136.26, 132.98, 131.58, 125.04, 124.42, 124.14, 119.77, 113.42, 105.19, 73.82, 68.24, 67.07, 64.17, 59.59, 27.05, 26.44 ppm. IR (KBr): 3316, 3097, 3000, 2907, 2374, 1543, 1461, 1374, 1261, 1120, 1057, 951, 868, 791 cm−1. ESI-MS (m/z): 491.1 (M+). Elemental analysis for C18H20F6N2O5S: Calculated C 44.08, H 4.11, F 23.24, N 5.71, O 16.31, S 6.54; Found C 44.13, H 4.04, F 23.38, N 5.89, O 16.28, S 6.27.
1,2-O-isopropylidene-3-(phenylthioureido)-3-deoxy-β-D-fructopyranose (3c): Yield: 84 as white solid; mp: 91°C–93°C. [α]D25 -21.66° (c 0.001, CHCl3). 1H NMR (400 MHz, CDCl3): δ 8.53 (s, 1H), 7.45–7.28 (m, 5H), 6.24 (d, 1H, J = 9.6 Hz), 5.10 (d, 1H, J = 8 Hz), 4.00–3.77 (m, 8H), 1.37 (s, 3H), 0.94 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 181.48, 138.84, 135.37, 130.34, 129.01, 127.87, 125.96, 111.28, 105.12, 76.75, 72. 61, 72.35, 68.61, 64.14, 60.45, 56.05, 25.91, 25.40 ppm. IR (KBr): 3264.31, 2923.94, 1642.98, 1536.88, 1380.98, 1245.29, 1058, 1112.34, 1011.77, 832.28, 678.07 cm−1. ESI-MS (m/z): 355.4 (M+). Elemental analysis for C16H22N2O5S: Calculated C 54.22, H 6.26, N 7.90, O 22.57, S 9.05; Found C 54.48, H 6.06, N 7.45, O 22.97, S 9.03.
1,2-O-isopropylidene-3-(phenylthioureido)-3-deoxy-α-D-fructopyranose (3d): Yield: 74 as white solid; mp: 112°C–115°C. [α]D25 -87.50° (c 0.001, CHCl3). 1H NMR (400 MHz, CDCl3): δ 8.17 (s, 1H), 7.41–7.22 (m, 5H), 5.07 (s 1H), 4.25–3.64 (m, 7H), 2.93 (s,1H), 2.03 (s,1H), 1.46 (s, 3H), 1.43 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 181.70, 135.64, 130.15, 128.58, 127.95, 125.25, 112.80, 106.23, 73.67, 67.46, 67.02, 63.68, 59.27, 26.93, 26.27 ppm. IR (KBr): 3264.31, 2923.94, 1672, 1599.89, 1382.76, 1239.09, 1088, 883.72, 758.07 cm−1. ESI-MS (m/z): 355.4 (M+). Elemental analysis for C16H22N2O5S: Calculated C 54.44, H 5.96, N 7.43, O 22.87, S 9.29; Found C 54.62, H 6.25, N 7.25, O 22.54, S 9.34.
1,2-O-isopropylidene-3-(4-chlorophenylthioureido)-3-deoxy-β-D-fructopyranose (3e): Yield: 89% as white solid; mp: 101°C–103°C. [α]D25 -45.66° (c 0.002, CHCl3). 1H NMR (400 MHz, CDCl3): δ 7.97 (s, 1H), 7.44–7.22 (m, 4H), 6.09 (d, 1H, J = 9.2 Hz), 5.05 (t, 1H, J = 9.6, 9.6 Hz), 4.01–3.78 (m, 7H), 2.39 (s, 1H), 1.40 (s, 3H), 0.99 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 182.42, 134.21, 133.95, 130.84, 127.72, 111.67, 105.17, 73.13, 73.08, 68.72, 64.24, 56.60, 26.24, 25.37 ppm. IR (KBr): 3357.07, 2927.87, 1535.61, 1428.64, 1380.61, 1114.76, 1086.63, 885.91, 801.32, 764.11cm−1. ESI-MS (m/z): 389.4 (M+). Elemental analysis for C16H21ClN2O5S: Calculated C 49.42, H 5.44, Cl 9.12, N 7.20, O 20.57, S 8.24; Found C 49.48, H 5.63, Cl 9.43, N 7.31, O 20.86, S 7.28.
1,2-O-isopropylidene-3-(4-chlorophenylthioureido)-3-deoxy-α-D-fructopyranose (3f): Yield: 83% as white solid; mp: 163°C–165°C. [α]D25 -73.50° (c 0.002, CHCl3). 1H NMR (400 MHz, CDCl3): δ 8.50 (s, 1H), 7.42–7.19 (m, 5H), 5.06 (dd, 1H, J = 3.6, 3.6 Hz), 4.25–3.73 (m, 7H), 1.46 (s, 3H), 1.42 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 181.71, 134.56, 133.25, 130.31, 126.54, 125.73, 115.28, 113.28, 105.09, 73.84, 67.86, 67.15, 64.03, 59.58, 27.07, 26.48 ppm. IR (KBr): 3310.76, 2933.88, 1532.48, 1378.68, 1238.62, 1133.17, 1056.20, 940.01, 862.53, 760.17, 666.90 cm−1. ESI-MS (m/z): 389.4 (M+). Elemental analysis for C16H21ClN2O5S: Calculated C 49.38, H 5.51, Cl 9.09, N 7.14, O 20.87, S 8.00; Found C 49.15, H 5.82, Cl 9.18, N 7.21, O 20.96, S 7.67
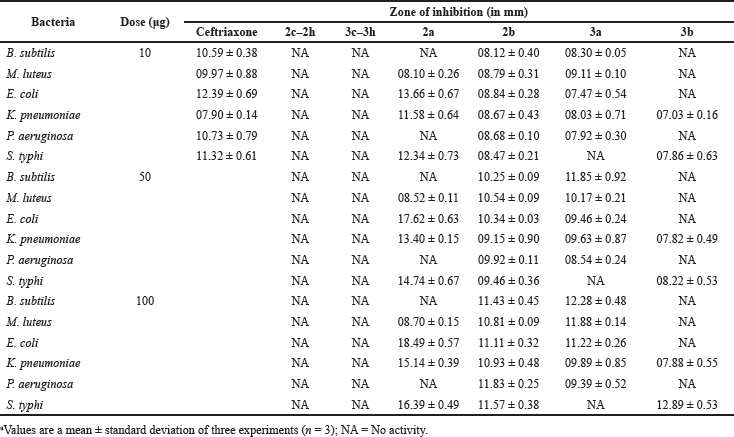 | Table 1. Inhibitory action of standard antibiotic (ceftriaxone) and different carbohydrate-derived thiourea compounds against different bacteria. [Click here to view] |
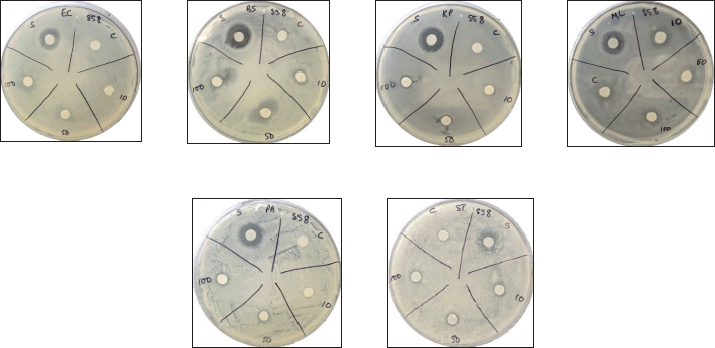 | Figure 5. Inhibitory activity of compound 3a against various pathogenic bacteria: B. subtilis (BS), E. coli (EC), K. pneumoniae (KP), M. luteus (ML), P. aeruginosa (PA), and S. typhi (ST). [Click here to view] |
1,2-O-isopropylidene-3-(3-nitrophenylthioureido)-3-deoxy-β-D-fructopyranose (3g): Yield: 87% as yellow solid; mp: 100°C–102°C. [α]D25 -188.40° (c 0.001, CHCl3). 1H NMR (400 MHz, CDCl3): δ 8.62 (s, 1H), 8.21 (t, 1H, J = 2, 2 Hz), 8.05 (d, 1H, J = 7.6 Hz), 7.74 (s, 1H), 7.54 (t, 1H, J = 8.4, 8 Hz), 6.47 (d, 1H, J = 9.6 Hz), 5.09 (s,1H), 4.07–3.82 (m, 7H), 2.39 (s, 1H), 1.42 (s, 3H), 1.25 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 182.69, 149.06, 131.19, 130.76, 121.61, 120.18, 119.58, 111.73, 105.41, 72.89, 72.29, 68.96, 64.50, 56.33, 26.45, 25.59 ppm. IR (KBr): 3349.80, 2927.97, 1529.70, 1348.26, 1082.21, 882.54 cm−1. ESI-MS (m/z): 398.4 (M+). Elemental analysis for C16H21N3O7S: Calculated C 48.11, H 5.30, N 10.52, O 28.04, S 8.03; Found C 48.51, H 5.09, N 10.23, O 28.00, S 8.17.
1,2-O-isopropylidene-3-(3-nitrophenylthioureido)-3-deoxy-α-D-fructopyranose (3h): Yield: 79% as yellow solid; mp: 142°C–144°C. [α]D25 -151.33° (c 0.002, CHCl3). 1H NMR (400 MHz, CDCl3): δ 8.27–7. 43 (m, 5H), 5.13 (s, 1H), 4.40–3.77 (m, 8H), 2.04 (s, 1H), 1.48 (s, 3H), 1.44 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 181.91, 148.06, 138.29, 130.75, 129.96, 126.92, 121.15, 118.98, 113.28, 105.29, 73.82, 68.16, 66.97, 64.96, 59.89, 26.51, 26.29 ppm. IR (KBr): 3334.98, 2928.06, 1528.68, 1349.50, 1242.72, 1058.03, 863.24, 758.81 cm−1. ESI-MS (m/z): 398.4 (M+). Elemental analysis for C16H21N3O7S: Calculated C 48.23, H 5.41, N 10.22, O 28.12, S 8.02; Found C 48.32, H 5.29, N 10.43, O 27.92, S 8.03.
Antibacterial test
The antibacterial activities of the novel thiourea derivatives were evaluated against Gram-negative bacteria including P. aeruginosa (ATCC 10145), K. pneumoniae (ATCC 10031), E. coli (ATCC 10536), S. typhi (ATCC 51812), and Gram-positive bacteria such as B. subtilis (ATCC 11774) and M. luteus (ATCC 10240). The results obtained from the disk diffusion test as zones of inhibition are shown in Table 1. Of the 16 compounds synthesised, 12 of them, 2c–2h and 3c–3h, did not show any antibacterial activity.
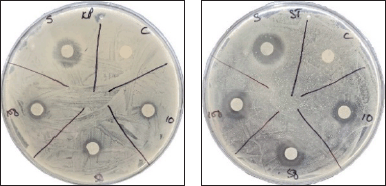 | Figure 6. Inhibitory activity of compound 3a against pathogenic bacteria: K. pneumoniae (KP) and S. typhi (ST). [Click here to view] |
Carbohydrate-based compounds having antibacterial activity are reported but are known to have limited efficacy [12,25,26]. As our data also indicate that the majority of the carbohydrate-derived thiourea moieties possessing an unsubstituted aromatic ring as well as the substituted meta-nitro and para-chloro thiourea series showed no inhibition of the bacterial growth. Surprisingly, the introduction of two trifluoromethyl groups at the meta-position on the phenyl ring displayed a broad spectrum of activity against various species, showing good to excellent inhibitory activity against most of the tested bacteria (Table 1). As independently described earlier, the presence of a trifluoromethyl moiety (–CF3) on the phenyl ring was observed to be crucial for enhancing the antibacterial activity of synthesised compounds [20,25,27].
Among the novel compounds, thiourea 2a showed unique antibacterial properties. Particularly at a concentration of 10 μg, it showed no inhibitory action on Gram-negative bacteria but was the most potent of all the new compounds against K. pneumoniae, E. coli, and S. typhi (Fig. 3). On the other hand, thiourea 2b was found to have less inhibitory effect but with uniform effectiveness against all the bacteria tested (Fig. 4). The use of compounds 3a and 3b, showed a range of results, from complete inactivity to moderate activity in inhibiting the growth of various bacteria tested. This limited activity is expected as most antibiotics are effective only against a particular group, or sometimes against a few species of bacteria [28–30]. Thioureas are typically known to have narrow range of efficacy in different species of bacteria [31,32]. Specifically, thiourea 3b was highly species-specific as it inhibited only K. pneumoniae and S. typhi, while compound 3a showed good activity against both Gram-positive and Gram-negative species, except S. typhi (Figures 5 and 6). The antibacterial inhibitory pattern of the active compounds tested at 10, 50, and 100 μg concentrations showed dose-dependent activities.
To further optimize the antibacterial efficacy of the novel compounds from the above examination, thiourea 2a, having the overall highest activity was selected and further screened at concentrations varying from 5 to 0.31 μg as shown in Figures 3 and 7. Interestingly, the compound showed the best activity against K. pneumoniae, followed by S. typhi and E. coli (Figure 8). The results suggest that thiourea 2a could be a potential antibacterial medication for common pathogenic Gram-negative bacteria.
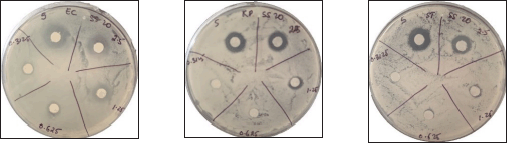 | Figure 7. Inhibitory activity of compound 2a against pathogenic bacteria at lower concentrations, 5 to 0.3125 μg. K. pneumoniae (KP) and S. typhi (ST). [Click here to view] |
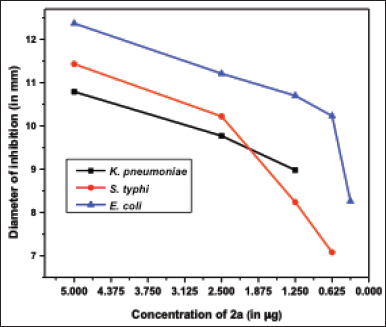 | Figure 8. Comparative inhibitory effect of thiourea 2a against Gram-negative bacteria, E. coli (EC), K. pneumoniae (KP), and S. typhi (ST). [Click here to view] |
CONCLUSION
Our study presents the successful development of a simple technique for the synthesis of a new class of D-fructose-based thiourea compounds. Antibacterial susceptibility tests showed that some of the new compounds exhibit antibacterial activity. Compounds 2b and 3a showed an unusual broad-spectrum activity against Gram-negative and Gram-positive bacteria. However, 2a was found to be the most effective, but only against Gram-negative species. Antibacterial activity was largely due to the addition of trifluoromethyl groups at the meta-position on the phenyl ring. The findings show the promising use of carbohydrate-based thiourea compounds as a new avenue in the development of new or improved antibiotics.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
The study was supported by DBT-BUILDER (BT/INF/22/SP41398/2021) of the Department of Biotechnology, and Science and Engineering Research Board (SERB), Department of Science and Technology (CRG/2022/000821 and EEQ/2017/000505), Government of India.
CONFLICTS OF INTEREST
All authors declares that there is no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
Supplementary materials are available on demand.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Mendelson M, Sharland M, Mpundu M. Antibiotic resistance: calling time on the “silent pandemic”. JAC Antimicrob Res. 2022;4(2):dlac016. CrossRef
2. Lessa FC, Sievert DM. Antibiotic resistance: a global problem and the need to do more. Clin Infect Dis. 2023;77:S1–3. CrossRef
3. Iskandar K, Murugaiyan J, Hammoudi Halat D, Hage SE, Chibabhai V, Adukkadukkam S, et al. Antibiotic discovery and resistance: the chase and the race. Antibiotics. 2022;11(2):182. CrossRef
4. Bhattarai K, Bastola R, Baral B. Antibiotic drug discovery: challenges and perspectives in the light of emerging antibiotic resistance. Adv Genet. 2020;105:229–92. CrossRef
5. Cook MA, Wright GD. The past, present, and future of antibiotics. Science Trans Med. 2022;14:7793. CrossRef
6. Miethke M, Pieroni M, Weber T, Brönstrup M, Hammann P, Halby L, et al. Towards the sustainable discovery and development of new antibiotics. Nat Rev Chem. 2021;5(10):726–749. CrossRef
7. Alén R. Carbohydrate chemistry: fundamentals and applications. New Jersey, USA: World Scientific; 2018, pp 1–5. CrossRef
8. Boysen MMK. Carbohydrates: tools for stereoselective synthesis. Weinhelm, Germany: Wiley-VCH Verlag & Co.; 2012, pp 4–7, 19. CrossRef
9. Su L, Feng Y, Wei K, Xu X, Liu R, Chen G. Carbohydrate-based macromolecular biomaterials. Chem Rev. 2021;121(18):10950–1029. CrossRef
10. Sievenpiper JL. Low-carbohydrate diets and cardiometabolic health: the importance of carbohydrate quality over quantity. Nutr Rev. 2020; 78:S69–77. CrossRef
11. Chandel NS. Carbohydrate metabolism. Cold Spring Harb Perspect Biol. 2021; 13(1):a040568. CrossRef
12. Mishra N, Tiwari VK, Schmidt RR. Recent trends and challenges on carbohydrate-based molecular scaffolding: general consideration toward impact of carbohydrates in drug discovery and development. Carbohydrates in Drug Discovery and Development. Amsterdam, The Netherlands: Elsevier B.V.; 2020, pp 1–69. CrossRef
13. Wang J, Wang D, Zhang Y, Dong J. Synthesis and biopharmaceutical applications of sugar-based polymers: new advances and future prospects. ACS Biomater Sci Eng. 2021;7(3):963–82. CrossRef
14. Wang N, Kong Y, Li J, Hu Y, Li X, Jiang S, et al. Synthesis and application of phosphorylated saccharides in researching carbohydrate-based drugs. Bioorg Med Chem. 2022; 68:116806. CrossRef
15. Pan L, Cai C, Liu C, Liu D, Li G, Linhardt RJ, et al. Recent progress and advanced technology in carbohydrate-based drug development. Curr Opin Biotechnol. 2021;69:191–98. CrossRef
16. Wang PA, Feng JT, Wang XZ, Li MQ. A new class of glucosyl thioureas: synthesis and larvicidal activities. Molecules. 2016;21:925. CrossRef
17. Zahra U, Saeed A, Fattah TA, Flörke U, Erben MF. Recent trends in chemistry, structure, and various applications of 1-acyl-3-substituted thioureas: a detailed review. RSC Adv. 2022; 12(20):12710–45. CrossRef
18. Cunha S, MacEdo FC, Costa GAN, Rodrigues MT, Verde RBV, De Souza Neta LC, et al. Antimicrobial activity and structural study of disubstituted thiourea derivatives. Monatsh Chem. 2007;138:511–16. CrossRef
19. Suresha GP, Suhas R, Kapfo W, Gowda DC. Urea/thiourea derivatives of quinazolinone-lysine conjugates: synthesis and structure-activity relationships of a new series of antimicrobials. Euro J Med Chem. 2011;46:2530–40. CrossRef
20. Qiao L, Huang J, Hu W, Zhang Y, Guo J, Cao W, et al. Synthesis, characterization, and in vitro evaluation and in silico molecular docking of thiourea derivatives incorporating 4-(trifluoromethyl) phenyl moiety. J Mol Struct. 2017;1139:149–59. CrossRef
21. Vanlaldinpuia K, Bora P, Bez G. Monofunctional primary amine: a new class of organocatalyst for asymmetric aldol reaction. J Chem Sci. 2017;129:301–12. CrossRef
22. Vanlaldinpuia K, Bez G. Useful methods for the synthesis of isopropylidenes and their chemoselective cleavage. Tetrahedron Lett. 2011;52:3759–64. CrossRef
23. Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–6. CrossRef
24. CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests. 13th edition. Pasadena, USA: Clinical and Laboratory Standards Institute; 2018, pp 15–22.
25. Bielenica A, Stefanska J, Stepien K, Napiorkowska A, Augustynowicz-Kopec E, Sanna G, et al. Synthesis, cytotoxicity and antimicrobial activity of thiourea derivatives incorporating 3-(trifluoromethyl)phenyl moiety. Euro J Med Chem. 2015;101:111–25. CrossRef
26. de Matos AM. Recent advances in the development and synthesis of carbohydrate-based molecules with promising antibacterial activity. Eur J Org Chem. 2023;26(4):e202200919. CrossRef
27. Khan E, Khan S, Gul Z, Muhammad M. Medicinal importance, coordination chemistry with selected metals (Cu, Ag, Au) and chemosensing of thiourea derivatives. A review. Crit Rev Anal Chem. 2021;51(8):812–834.
28. Vázquez-Laslop N, Mankin AS. How macrolide antibiotics work. Trends Biochem Sci. 2018; 43:668–84. CrossRef
29. Hutchings M, Truman A, Wilkinson B. Antibiotics: past, present and future. Curr Opin Microbiol. 2019;51:72–80. CrossRef
30. Roemhild R, Bollenbach T, Andersson DI. The physiology and genetics of bacterial responses to antibiotic combinations. Nat Rev Microbiol. 2022;20(8):478–90. CrossRef
31. Bonomo MG, Giura T, Salzano G, Longo P, Mariconda A, Catalano A, et al. Bis-thiourea quaternary ammonium salts as potential agents against bacterial strains from food and environmental matrices. Antibiotics. 2021;10(12):1466. CrossRef
32. Tabor W, Katsogiannou A, Karta D, Andrianopoulou E, Berlicki ?, Vassiliou S, et al. Exploration of thiourea-based scaffolds for the construction of bacterial ureases inhibitors. ACS Omega. 2023;8(31):28783–96. CrossRef
SUPPLEMENTARY MATERIAL
The supplementary material can be accessed at the journal's website: Link Here [https://japsonline.com/admin/php/uploadss/4306_pdf.pdf].