INTRODUCTION
Chrysanthemum is a well-known genus comprising almost 300 species distributed around the world. Several of these species are cultivated as ornamental plants in gardens, while some exist as herbs and under shrubs. A total of 40 species of Chrysanthemum are mainly found in East Asia and belong to the Asteraceae family [1,2]. Studies reported that the entire plant carries therapeutic abilities, and the prominent components can be found mainly in its flower. Traditionally, due to its wide range of beneficial uses, Chrysanthemum is commonly consumed in drinks and medicine. For example, Chrysanthemum flowers were dried and used as herbal tea in North-eastern Asia, such as China and Korea. It reportedly treats several illnesses, such as headaches, inflammation, hypertension, cholera, respiratory disease, and other related diseases [3,4]. The therapeutic effect of Chrysanthemum has been clinically proven for decades based on its medicinal history. According to the World Health Organization (WHO), plant-based pharmacological drugs are now preferred as the safest medication because they are practical and exhibit less toxicity than modern drugs.
Chrysanthemum morifolium and C. indicum are perennial herbs with aromatic alternate, lobed, or serrated leaves. Numerous recent studies demonstrated that extracts or monomeric compounds from both plants have a variety of pharmacological activities. Studies have shown that flavonoids and phenolic acids are major components of secondary metabolites essential to exert therapeutic activities in Chrysanthemum species [5,6]. Identification of phytochemical content from C. indicum isolated around 191 natural compounds, including 42 flavonoids, 96 terpenoids, 21 phenylpropanoids, phenolic acids, 12 spiro ketones, and others [5,6]. Likewise, a previous study also discovered that C. morifolium yielded 60 flavonoid compounds when isolated using column chromatography, most of which were glycosidic derivatives of luteolin, apigenin, acacetin, diosmetin, or eriodictyol in the C7 position [7]. Extracts from C. morifolium and C. indicum can be found in different parts of the Chrysanthemum, such as flowers, aerial parts, or whole herbs. Detection of active compounds, especially with a high level of flavonoid in Chrysanthemum, may lead to understanding how these edible flowers exerted their pharmacological effects.
Detailed discussions on the pharmacological properties and pathways of the Chrysanthemum species are limited. This current review discusses and summarizes the health benefits of two main species of Chrysanthemum: morifolium and indicum. The underlying molecular mechanisms that govern the beneficial effects are also highlighted. Hence, the current review provides additional information to researchers about the therapeutic potential for broadening the use of Chrysanthemum extract.
LITERATURE SEARCH
An updated overview of the pharmacological activities of Chrysanthemums, including anti-inflammatory, antioxidant, antimicrobial, anticancer, and antidiabetic/anti-obesity properties, as well as their underlying mechanisms of action, are presented in this review. A literature search was performed on PubMed and Scopus using the keywords “C. morifolium” or “Chrysanthemum indicum” and “anticancer,” “antimicrobial,” “antioxidant,” “anti-inflammatory,” “anti-obesity,” or “antidiabetic” published between 2008 and 2022. Original research articles on the health effects of Chrysanthemum published in English were included.
PHARMACOLOGICAL POTENTIAL OF CHRYSANTHEMUM
Anti-inflammatory
Living cells constantly produce free radicals, and a healthy immune system requires moderate concentrations of these molecules. Excessive production of free radicals subset from endogenous (primarily mitochondrial) and exogenous (environmental pollution) sources would harm cells, leading to oxidative stress and further activating the inflammatory response in cells [8]. Cellular inflammation that persists for an extended period becomes chronic inflammation, which is the cause of numerous diseases. Lipopolysaccharides (LPS)-induced RAW264.7 cells are frequently used as a model to study anti-inflammation as they form a complex ligand with toll-like receptor (TLR) 4 to induce specific signaling pathways in regulating the inflammatory gene expression [9]. In the mice model of LPS-induced acute lung injury, many pro-inflammatory cytokines of tumor necrosis factor (TNF)-α and interleukin (IL)-6 levels were released, indicating macrophage activation. Treatment with C. morifolium at 50, 100, and 200 mg/kg significantly reduced these pro-inflammatory markers while increasing the tumor growth factor (TGF)-β1 and IL-10 as a compensatory anti-inflammatory response [10]. Maintaining normal physiological homeostasis requires a balance between pro-inflammatory and anti-inflammatory components, suppressing oxygen free radicals. Similarly, in mice models of Cisplatin-induced acute kidney injury, supplementation with C. morifolium reduced the anti-cancer drug’s toxicity by blocking the inflammatory pathway via inhibition of the monocyte chemoattractant protein-1 production [11]. Inhibition of reactive oxygen species (ROS) generation, messenger RNA (mRNA) expression of IL-1β, IL-6, IL-8, and TNF-α suggest regulating the inflammatory responses by C. morifolium [12].
In another study, administration of C. indicum at 200 mg/kg intraperitoneal inhibited the activity of IL-1, TNF-α, and the accumulation of leukocytes in both acute and chronic skin inflammation of mouse models [13]. Wu et al. explored the anti-inflammatory effects of Chrysanthemum in an inflammation animal model by xylene-induced mouse ear edema. Supplementation of C. indicum at 40, 80, and 120 mg/kg dose-dependently decreased the inflammatory response of the edema paw significantly at 2 to 6 hours [14]. A study showed that multiple pro-inflammatory cytokines are highly produced during the late stages of edema (2–6 hours), which may exacerbate the inflammatory response [15]. Significant reduction in the production of inducible nitric oxide (NO) synthase and cyclooxygenase-2 (COX-2) inflammatory factors suppressed the production of IL-6, TNF-α, IL-1β, and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-????B). It is well known that COX-2 and iNOS expression levels are highest in the late stages of carrageenan-induced paw edema [16]. Findings also revealed that the anti-inflammatory effect of C. indicum is related to disinhibition prostaglandin (PGE)-2 and NO syntheses, which play a central role in inflammatory responses. Likewise, other findings demonstrated that oral C. indicum at 42.5, 85, and 170 mg/kg significantly inhibit carrageenan-induced paw edema. Treatment also up-regulated the level of IL-1β while down-regulated PGE-2, mediating the inflammatory response acting at an appropriate level [17]. Vasodilatation inhibition effect in the early stage of acute inflammation response, endowing C. indicum treatment a promising potential for anti-inflammation via decreasing the inflammatory mediator release.
Interestingly, the synergistic effect of C. indicum attenuated the anti-tumor drug bleomycin-induced pulmonary fibrosis by reducing pro-inflammatory cytokines of IL-6 and TNF-α production [18]. Chrysanthemum indicum exhibits stronger anti-inflammatory activity via inhibiting pro-inflammatory mediators of IL-1, IL-6, and TNF-α [19]. In addition, C. indicum reduced inflammatory mediator NO and pro-inflammatory cytokines (IL-1β and IL-6) levels in LPS-stimulated RAW264.7 macrophages. Decreased expression levels of antigen-presenting surface markers like major histocompatibility complex-II and CD80 were also observed [20]. Activated macrophages secrete pro-inflammatory cytokines that stimulate numerous cell signaling pathways to promote inflammation. A decrease in the release of pro-inflammatory cytokines evidences the ability of Chrysanthemum extracts to control inflammation. Further investigations revealed that C. indicum possesses anti-inflammatory effects via hyperproliferation suppression and apoptosis induction in IL-6/sIL-6R-stimulated rheumatoid arthritis fibroblast-like synoviocytes [21]. Targeting the specific blockade of IL-6-regulated signaling pathways may lead to a promising approach for treating diseases since high levels of IL-6/sIL-6R have been reported in several chronic inflammatory conditions [22]. The extracts suppressed the production of TNF-α, IL-1, and IL-6 inflammatory mediators and down-regulated nuclear factor kappa B (NF-κB) activation and TLR4/MyD88 expression [17]. In addition, C. indicum extract was discovered to prevent the activation of both latent infection membrane protein 1 carboxy-terminal activating region (CTAR)-1 and CTAR2-induced translocation of NF-κB by suppressing inhibitory kappa-B kinase (IκK)-α activation [23]. A similar finding showed that the flavonoid compound isolated from Chrysanthemum significantly regulated mucin secretion, production, and gene expression by acting on airway epithelial cells via the NF-κB signaling pathway [24]. NF-κB is an inducible transcription factor that promotes the expression of various pro-inflammatory genes, including those encoding cytokines and chemokines.
Inflammation typically acts as a defense mechanism that helps maintain host health by restoring tissue balance through cellular and molecular processes. However, persistent inflammation contributes to the development of several chronic diseases. The transcription factor NF-κB serves as a mediator of inflammatory responses by inducing the expression of pro-inflammatory genes, including those encoding cytokines and chemokines. Chrysanthemum has been shown to have anti-inflammatory effects, including the ability to target genes involved in inflammation development and progression. Activation of the Janus Kinase (JAK)-signal transducer and activator of transcription (STAT)3 signaling pathway plays a crucial role in cancer development and progression as well as stimulates the expression of genes that promote invasion and metastasis. The activation of NF-κB further regulates inflammation. Oxidative stress may also initiate an inflammatory response, which generates additional free radicals that can cause more oxidative stress, creating a self-perpetuating cycle. Chrysanthemum activates the nuclear factor erythroid 2–related factor (Nrf2) signaling pathway, thereby enhancing the expression of antioxidant enzymes. The various mechanisms of Chrysanthemum in anti-inflammatory response have been demonstrated in Figure 1. It is noteworthy to mention that the correlation among the anti-inflammatory, antioxidant, anticancer, and antidiabetic properties across Chrysanthemum species highlights their potential as versatile therapeutic agents capable of holistically addressing a range of health challenges.
Antioxidant
Intracellular antioxidant enzymes, which include superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), are endogenous antioxidants responsible for protecting the cells from oxidative damage. Small-molecule antioxidants have long been considered compounds that can decrease oxidative stress. Free radicals generate the lipid peroxidation process in an organism where malondialdehyde (MDA) is a byproduct of polyunsaturated fatty acid peroxidation in cells [25]. Increased free radicals caused the overproduction of MDA and were used to evaluate oxidative stress and antioxidant status. Therefore, the ability to interact with different redox signaling pathways by altering redox enzymes’ activity is the primary key to their antioxidant action.
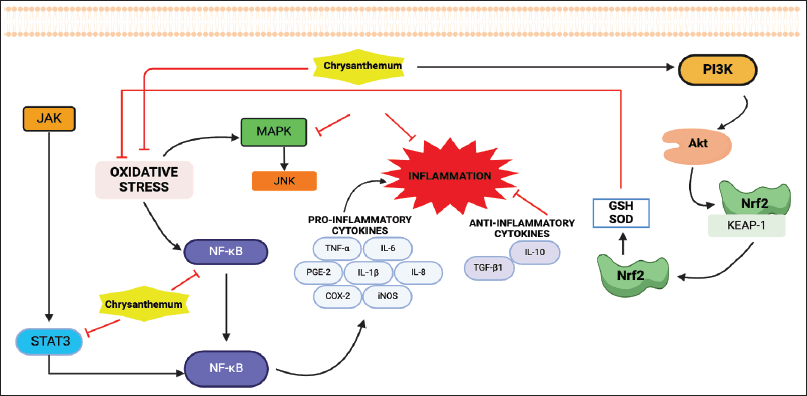 | Figure 1. Molecular mechanism of anti-inflammatory of Chrysanthemum. Abbreviation: Akt: protein kinase B, COX-2: cyclooxygenase-2, IL-6: interleukin-6, IL-8: interleukin-8, IL-10: interleukin-10, JAK: janus kinase, JNK: Jun N-terminal kinase, MAPK: mitogen-activated protein kinases, Nrf2: nuclear factor erythroid 2–related factor 2, NF-κB: nuclear factor kappa B, PI3K: phosphoinositide 3-kinase PGE-2: prostaglandin-2, STAT3: signal transducer and activator of transcription 3, TGF-β1: tumor growth factor-β1, TNF-α: tumor necrosis factor-α. [Click here to view] |
Oxygen free radicals primarily cause damage by destroying the structure and function of the cell membrane, lipid peroxidation, deoxyribonucleic acid (DNA), mitochondria, endoplasmic reticulum, and cell death [26]. To explore the potential mechanism of C. morifolium as an antioxidant effect, Tian et al. investigated the effects of acetaminophen (APAP)-induced on liver injury in hepatocytes HL-7702 cells [27]. Treatment significantly suppresses hepatic oxidative stress by lowering ROS production and increasing the antioxidant enzymes glutathione (GSH) and SOD activity. Results also showed that C. morifolium intensified the nuclear translocation of Nrf2 and protein expression in the nucleus. Under normal conditions, Nrf2 is retained in the cytoplasm by binding to the negative regulator Keap1 [28]. Oxidative stress caused by a high level of ROS resulted in the dissociation of Keap1 from Nrf2, followed by translocation into the nucleus. This event initiates the expression of antioxidant genes and enzymes by binding to the antioxidant response element of the target gene promoter region [29,30]. Further investigations, in a rat model of APAP-induced liver injury, C. morifolium at 110, 220, and 440 mg/kg significantly reduced serum aspartate transaminase (AST) and alanine transaminase (ALT) as well as in ROS levels while upregulated SOD and GSH levels. Treatment inhibited apoptosis induced by APAP through the phosphoinositide 3-kinase/protein kinase B (PI3K–Akt) pathway and restored the ability of mitochondrial biogenesis, promoting nuclear translocation of Nrf2 [31]. Results suggest that the antioxidant capacity protects the liver from hepatotoxicity. Chrysanthemum morifolium was also observed to increase the total antioxidant capacity (T-AOC) activity and decrease MDA content in a dose-dependent manner of 50, 100, and 200 mg/kg [10].
The ability of antioxidants to reduce damage caused by free radicals by giving the hydrogen atoms to convert them into stable molecules is a crucial indicator of their effectiveness. Total antioxidant activity and the scavenging capacity of hydroxyl radicals and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals from Chrysanthemum were reported to be stronger than those of vitamin C as it significantly increased the SOD and T-AOC activity and decreased MDA content [32]. DPPH is the most stable free radical species that can accept electron or hydrogen radicals and is widely used to assess natural antioxidant activity. Besides, C. indicum has been shown to increase the activity of antioxidant enzymes including SOD, CAT, and GPX while decreasing the NF-κB activation [33]. In parallel, the protective effect of C. indicum extract on D-galactose-induced brain and liver damage alleviated the abnormal alterations by restoring normal antioxidant enzyme activities (SOD, GPx, and CAT) and reducing MDA accumulation [19]. Treatment also attenuated the decline of thymus and spleen indexes and reduced the elevated levels of AST and ALT liver markers. Keeping these liver indicators within the normal range significantly predicts favorable clinical outcomes, particularly for liver diseases. Distinctly decreased levels of oxidant enzymes of myeloperoxidase and MDA were also illustrated in another study, indicating the antioxidant properties of C. indicum [18]. Moreover, C. indicum was found to prevent ultraviolet (UV)-induced skin damage by lowering intracellular ROS levels and inhibiting p38 mitogen-activated protein kinases (MAPK) phosphorylation in human immortalized keratinocytes HaCat cells [34].
Anticancer
A type of programmed cell death called apoptosis is crucial for protecting the host against tumor progression. Two main pathways, which include intrinsic and extrinsic, are involved in the mechanism of cancer cells [35]. In response to intracellular stressors such as cytokine deprivation and DNA damage, the intrinsic pathway mediated by the mitochondrial activates caspase-9. Meanwhile, the death receptor-mediated extrinsic pathway activates caspase-8 when ligand-mediated trimerization of TNF family death receptors on the plasma membrane occurs. Both of these initiators caspases cleavage and activate the effector caspase-3, which deconstructs cells by cleaving several vital cellular proteins [36]. In addition, the Jun N-terminal kinase (JNK) signaling pathway is an essential regulator of cellular phenotypes in tumorigenesis. It is well-known for its ability to increase the expression of pro-apoptotic genes through transactivation of c-Jun/AP1 dependent or p53/73 protein-dependent mechanisms, thereby promoting apoptosis in the death receptor-initiated extrinsic pathway. Activated JNK translocate to the mitochondria in pathways that target mitochondrial apoptotic proteins can phosphorylate the BH3-only family to counteract the anti-apoptotic activity of B-cell leukemia/lymphoma 2 (Bcl-2) or B-cell lymphoma-extra-large [37]. Promising anticancer properties on several cancerous cell lines, including breast, lung, gastric, bone, and prostate, were discussed and summarised in Table 1.
Chrysanthemum morifolium is cytotoxic to human breast cancer lines of MCF-7 with the lowest cell viability at 312 µg ml−1 [38]. Target validation of Chrysanthemum phytochemicals using molecular docking demonstrated that it belongs to estrogen receptors (ESR1 and ESR2) and progesterone receptors, which are effective therapeutic targets in breast cancer. Moreover, flavonoids extracted from C. morifolium also inhibited cell proliferation by promoting apoptosis of human gastric cancer MKN45 in a dose (5, 10, and 20 mg/ml) and time (24 and 48 hours) dependent manner [39]. Besides, findings also demonstrated C. morifolium signal autophagic degradation induces apoptosis and causes pre-G1 and G0/G1 arrest in Ub-signaling HepG2 cells [40].
In addition, treatment with C. indicum extracts significantly sensitized multidrug-resistant human breast carcinoma cell line MCF7/ADR at nontoxic concentrations. An increase in Rh123 accumulation and a decrease in Rh123 efflux were observed, indicating a blockage of the P-glycoprotein activity [41]. P-glycoprotein is an established membrane transporter that can efflux drug molecules out of cancer cells, decreasing cancer treatment efficacy. As an adaptive response, cancer cells increase the expression of P-glycoprotein to avoid chemotherapy-induced cell death. Molecular analysis revealed that C. indicum inhibited the increment in both matrix metalloproteinase (MMP)-3 and MMP-9 while decreased tissue inhibitor of metalloproteinase 1 (TIMP-1) of MRC-5 fibroblast cells and A549 lung carcinoma [42]. MMP-induced cell apoptosis by targeting apoptotic proteins of Bax-2, Bcl-2, and cleaved caspase-3 [43]. Results suggest that the treatment suppresses Akt activation and induction of cyclin-dependent kinase inhibitor p27K in A549 human alveolar basal epithelial cells lung cancer [44]. As an apoptosis regulator, Akt activation will phosphorylate several proteins involved in cancer progression. Findings from molecular docking illustrated that the inhibition of the PI3K-Akt signaling pathway and activation of the mitochondrial-mediated apoptosis pathway supported the anti-tumor activity of the active compound in Chrysanthemum on lung cancer [45]. In addition, C. indicum promoted cell apoptosis by mediating the activities of caspases-3/-8, up-regulated the protein expression of p53 and miR-29b gene activation [18].
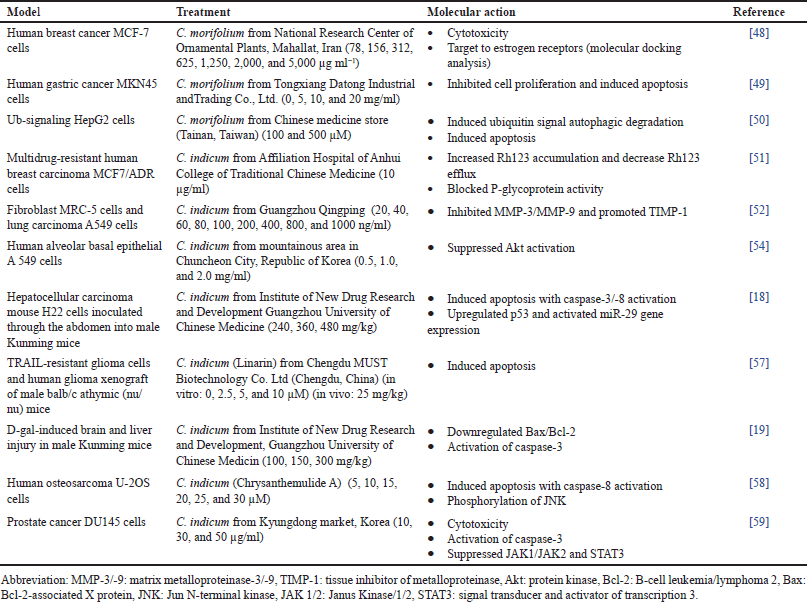 | Table 1. Anticancer properties of Chrysanthemum: morifolium and indicum in various models and their molecular actions. [Click here to view] |
The extrinsic apoptotic pathway involves transmembrane receptor-mediated interactions between death ligands, including the first apoptosis signal ligand, TNF-related apoptosis-inducing ligand (TRAIL), and death receptors [46]. Xu et al. demonstrated the flavonoid compound from C. indicum effectively induced apoptosis against TRAIL-resistant glioma cells [47]. Apoptosis was evidenced by enhanced cleavage of caspase-8/-9/-3 and poly (ADP-ribose) polymerase (PARP) and reduced anti-apoptotic proteins, including Bcl-2. Analysis in vivo further confirmed that treatment significantly suppressed the xenograft growth. Besides, the protective effect of C. indicum in ameliorating D-gal-induced brain and liver impairments down-regulated the rise in Bax/Bcl-2 ratio and activation of cleaved caspase-3 where it was postulated to be associated with the inhibition of apoptosis [19]. Besides, Chrysanthemum also induced apoptotic cells significantly in osteosarcoma U-2OS cells through activated caspase-8-mediated caspase cascade and phosphorylation of JNK [48]. Treatment sensitized the osteosarcoma cells to the death receptor 5 ligands TRAIL-induced apoptotic cell death. Strong cytotoxic activity of C. indicum was exhibited by suppressing the constitutive STAT3 activation against prostate cancer cell DU145 [49]. Treatment caused cell accumulation in the sub-G1 and induced caspase-3-dependent apoptosis. Both the JAK1 and JAK2 signals that function to regulate the inflammatory response were suppressed in a dose-dependent manner. Treatment significantly decreased the ratio of TIMP1 and MMP13, contributing to the caspase-8/-9/-3 cleaved activation. The apoptotic effect was verified by its ability to cleave caspase-3/-9 and suppress the JAK2/STAT3 signaling pathway.
Antidiabetic/anti-obesity
Diabetes is a common chronic metabolic disorder characterized by abnormal glucose and lipid metabolism modulation and has become a significant public health issue. The WHO predicts that diabetes already affects more than 30 million people, and by 2025, that number is anticipated to double [50]. When a person gains weight, the cells in their body predisposed to diabetes become less sensitive to the insulin released by the pancreas. This results in insulin resistance, in which the insulin ratio exceeds the blood sugar level. Obesity is responsible for 80%–85% of the risk of developing type 2 diabetes. The Asian population uses Chrysanthemum as a traditional medicine and herbal tea for obesity-related health benefits. However, the detailed mechanisms underlying the beneficial effects of these edible flowers Chrysanthemum on diabetes, obesity, and dyslipidemia have not thoroughly been discussed.
All lipidomic biomarkers, including cholesteryl esters, lys phosphatidylcholines, phosphatidylcholines, ceramides, and sphingomyelins levels in high-fat diet (HFD) mice, were returned to normal range after supplementation with C. morifolium extract [51]. Findings also demonstrated that C. morifolium significantly reduced body weight gain and epididymal white adipose tissue (eWAT) weight by stimulating mRNA expressions for thermogenesis and energy expenditure via lipid mobilization in HFD male rats. Notably, supplements improved the balance between lipid mobilization and WAT storage by reducing adipocyte inflammation and peripheral tissue lipotoxicity [52]. Flavonoid extract from Chrysanthemum reduced the lipid profile in hyperlipidemia rats relating to improved liver function such as AST, ALT, and alkaline phosphate (ALP). Supplementation also significantly improved the lipid profile levels marked by a reduction in lipid droplets and uniformly the adipocyte morphology in the liver. Lipid-metabolizing enzymes of fatty acid, cholesterol, and triglycerides associated with lipid metabolism were found to be significantly mediated by Chrysanthemum [53]. Consistent findings by Lee et al. indicated that C. morifolium extract significantly decreased serum lipid profile and gene expression involved in adipogenesis, including peroxisome proliferator-activated receptor (PPAR)-γ, sterol regulatory element-binding transcription factor 1 (SREBP-1c), and C/EBP-α. The pro-inflammation factor of adipose tissue macrophage and M1 macrophage phenotype was reduced. The authors suggested that this activity was associated with regulating glycerol-3-phosphate dehydrogenase and NF-κB activities in epididymal adipose tissue of HFD rats.
Moreover, treatment attenuates obesity-associated inflammation by modulating the muscle AMP-activated protein and the histone/protein deacetylase pathway, where the activation limits lipolysis [54]. Free fatty acid (FFA) overflow may lead to lipotoxicity, which causes hepatic steatosis and renal dysfunction in nonadipose tissues like the liver and kidney. Treatment with C. morifolium significantly suppressed the FFA-induced intracellular triacylglycerol accumulation in HepG2 cells by partially inhibiting the gene expression involved in lipid metabolism of SREBP-1c protein-1c and glycerol-3-phosphate acyltransferase [12]. A natural flavonoid compound from C. morifolium showed a potent lipid-lowering capability in the diabetic mice model by reducing serum lipid profile levels and fasting blood glucose, indicating improvement in glucose tolerance. Results suggested flavonoids directly enhance insulin signaling activity with decreased protein tyrosine phosphatase (PTP)-1B protein levels [55]. Moreover, flavonoid treatment induced insulin secretion in a high glucose-treated insulinoma cell line insulinoma (INS)-1 model. The PTP-1B signaling pathway is a potential therapeutic target for type 2 diabetes. The inhibition improved glucose homeostasis and insulin signaling without causing lipid buildup in the liver. In the type 2 diabetic mice model, C. morifolium decreased adipocyte size and improved insulin resistance by significantly reducing blood glucose levels.
Supplementation increased the mRNA levels of adiponectin by upregulating PPAR-γ while decreasing pro-inflammatory adipocytokines [56]. Improvements in insulin resistance and down-regulation of blood glucose levels as well as liver lipid content indicate its potential as antidiabetic in type 2 diabetic mice models. Similarly, an in vitro study showed that C. morifolium increased adipocyte differentiation, adiponectin secretion, and glucose uptake in murine preadipocyte 3T3-L1 cells. Treatment elevated the mRNA levels of PPAR, C/EBP-α, and glucose transporter type (GLUT) 4 expression [57]. The author postulated that the existing extracts contained polyphenols, and corresponding glycosides might be associated with higher PPAR-γ expression. Nevertheless, additional research is required to confirm the mechanism that explains the rising PPAR γ expression. Further study has identified the anti-diabetic components in the isolated compounds from C. morifolium functioned to increase intracellular lipid accumulation and adiponectin secretion while decreasing 3T3-L1 cell diameter during adipocyte differentiation suggested regulating PI3K/Akt Pathway and PPARγ [58].
Nepali et al. examined the effects of C. indicum in high-fat-diet (HFD)-induced obese mice on the basic panels of serum lipid profile. Findings showed that supplementation significantly decreased total cholesterol (TC), triglyceride (TG), and low-density lipoprotein cholesterol (LDLc) while increasing high-density lipoprotein cholesterol (HDLc) levels [59]. LDL particles, which vary in size and density, carry most cholesterol in circulation. On the other hand, HDL particles are high in cholesterol and phospholipids, which aid in reverse cholesterol transport from peripheral tissues to the liver. Significantly inhibited serum leptin and increased adiponectin levels were also observed in HFD mice. Effects on adipogenesis were indicated by upregulated the AMP-activated protein kinase (AMPK) phosphorylation and suppressed transcriptional factors of PPAR-γ and CCAAT/enhancer-binding protein (C/EBP)-α.
Chrysanthemum indicum was highly efficient in converting glycosides to aglycones when combined with enzymes (viscozyme and tannase), potentially suppressing adipogenesis and lipid accumulation. Reduction in body weight, lipid accumulation, serum lipid profile (TC, TG, and LDLc), and leptin levels were observed in HFD male mice by effectively downregulating the adipogenesis-related transcription factors of PPAR-γ and C/EBP-α [60]. Similarly, treatment with Chrysanthemum was found to decrease leptin and increase adiponectin levels by significantly up-regulated the protein expression level of PPAR-α while down-regulated the PPAR-γ and C/EBP-α [61]. Both PPAR and C/EBP factors play a crucial element in regulating adipogenesis. The ligand-activated transcription factor family PPAR is an important target for obesity and metabolic syndrome because it promotes the ligand-dependent transcription of target genes that regulate energy generation and lipid metabolism [62]. In obesity, a high level of leptin will cause leptin resistance, leading to high food intake and decreased energy expenditure. In contrast to leptin, low adiponectin levels lead to gluconeogenesis, hyperlipidemia, and increased glycemia resulting in metabolic syndrome.
Inhibiting the activities of α-glucosidase and pancreatic α-amylase will help manage postprandial hyperglycemia and aggressively delay the breakdown of carbohydrates into absorbable monosaccharides. A study demonstrated that the combination of C. indicum extract produced synergistic inhibition against pancreatic α-amylase [63]. This enzyme hydrolyses alpha bonds of large, α-linked polysaccharides, such as starch and glycogen, yielding shorter chains. The α-amylase inhibition can control the breakdown of dietary starch into smaller oligomers, thereby delaying glucose absorption. A bioactive compound from Chrysanthemum illustrated an inhibitory effect of α-amylase and α-glucosidase in a dose-dependent manner, as the α-glucosidase inhibition is a key to carbohydrate hydrolyzing [64]. Overall, the data suggested that Chrysanthemum administration was beneficial for controlling hyperglycemia and hyperlipidemia as well as possibly preventing diabetic complications by suppressing the transcriptional factors required for adipogenesis that attributed to the anti-adipogenicity properties are summarised in Table 2.
Antimicrobial
Antimicrobial property is not only limited to prevention against bacterial activity, but it comprises all scopes of protection such as antiviral, antifungal, antibiotic, and antibacterial. It prevents the formation of bacterial colonies by stifling or inhibiting the growth of bacteria from spreading within or onto an organism. The main concern on antimicrobial properties globally is the increase of lower effectiveness of antibiotics and microbial resistance to medications [65]. Lack of preventive measures for infection and overuse or misuse of antimicrobial products contribute to the complication. The rising of natural resources awareness in medicines has brought numerous studies on antimicrobial properties. Most anti-bacterial studies were evaluated based on the minimal inhibitory concentration (MIC), minimal bactericidal concentration (MBC) values, and zone of inhibition. The MIC, MBC, and bacteria effects on the inhibition area may vary according to the type of bacteria, the source used, and the dosage applied to the tested microorganisms. Using different bacteria types, the study by Hodaei et al. revealed that C. morifolium has significant antibacterial properties on Salmonella enterica and Bacillus cereus ranging from 5–10 mg/ml−1 and for Staphylococcus aureus and Escherichia coli ranging from 10–20 mg/ml−1 [66]. Higher MIC values mean lower susceptibility to C. morifolium extract [67]. The MIC values represent the ability of an antibacterial agent to inhibit microorganisms’ growth at the lowest concentration of drug or chemical. Similar outcomes were obtained from another study whereby Chrysanthemum buds from morifolium extract exerted inhibitory activity on Cronobacter sakazakii bacteria (a food-borne pathogen that can be hazardous to infants or newborns) at 10 mg/ml MIC values [68]. On the other hand, a study by Yeasmin et al. demonstrated that extract from flower parts of C. morifolium gave out MIC values ranging from 150–250 mg/ml against ten pathogenic bacteria comprising Staphylococcus aureus, Bacillus cereus, Streptococcus-β-haemolytica, Bacillus subtilis, Sarcina lutea, Klebsiella sp, Klebsiella pneumonia, Pseudomonas aeruginosa, Salmonella typhi, and Shigella dysenteriae [69]. They also evaluated the impact of extraction techniques on antibacterial activity, where their data revealed that C. morifolium had a 24.40 mm larger inhibitory zone.
In addition, C. indicum was effective against food-borne pathogens (gram-positive and gram-negative bacteria) of S. typhi, Clostridium perfringens and Listeria monocytogenes [70]. The high content of polyphenolic compounds such as flavonoids can be extracted successfully, indicating its involvement as essential in inducing antibacterial activity. Reactions between the plant-derived compound with enzymes and protein of microbial cell membrane induce antibacterial activity or inhibition [71]. Somehow, variation in antibacterial activity can be due to the various phytochemical compounds in an extract [66]. In the antiviral aspect, a study by Ming et al. demonstrated that phosphorylated C. indicum polysaccharide possesses a better antiviral activity against duck hepatitis A virus [72]. Studies have also revealed that C. indicum extracts have larvicidal and pupicidal effects on the malaria vector of Anopheles stephensi mosquito marked by killing the plasmodium [73].
Other properties
The effect of two Chrysanthemum species on other diseases was limited and poorly documented. In bone metabolism, findings showed that C. morifolium extracts significantly inhibit osteoclast differentiation without cytotoxic effects [74]. Osteoclasts are the cells responsible for the degradation of bone to initiate normal bone remodelling and mediate bone loss. The resorption of bone by osteoclasts and the formation of bone by osteoblasts are essential for bone homeostasis. Imbalances in this tightly coupled process can result in diseases like osteoporosis. Ovariectomized rat was a well-known animal model to induce postmenopausal osteoporosis, in which findings indicated that supplementation could be an alternative to manage this disease. In addition, C. morifolium supplementation was found to attenuate sarcopenia by promoting the phosphorylation of PI3K-Akt and mammalian target of rapamycin pathways, which stimulate the synthesis of muscle proteins [75]. Findings revealed that C. morifolium treatment enhanced grip strength, muscle mass and volume, and cross-sectional area of myofibers in middle-aged C57BL/6J mice, suggesting that it can delay the onset of sarcopenia by enhancing the recovery and functionality of muscle mass.
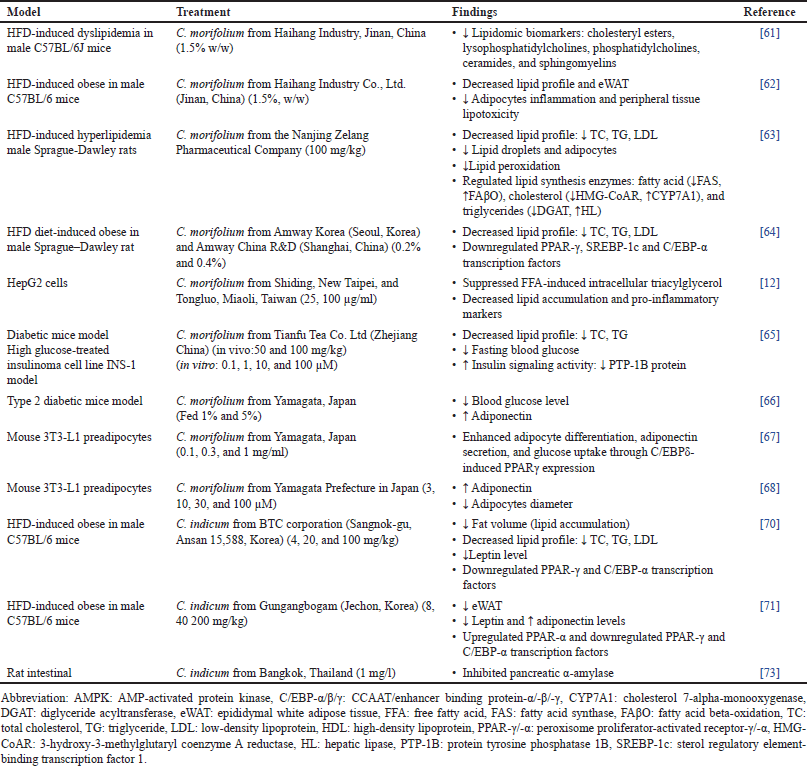 | Table 2. Antidiabetic properties of Chrysanthemum: morifolium and indicum. [Click here to view] |
Li et al. discovered that an active compound from C. indicum increased ALP and extracellular matrix mineralization in MC3T3-E1 cells in a dose-dependent manner, as evidenced by increased osteoblast proliferation and differentiation. The finding also observed the upregulation of osteogenesis-related gene expression, including ALP, runt-related transcription factor-2, osteocalcin, bone sialoprotein and type I collagen in the treatment group, which later preserved the trabecular bone microarchitecture of ovariectomized mice [76]. Furthermore, C. indicum demonstrated antiosteoporosis properties in receptor activator of nuclear factor-B ligand (RANKL)-induced osteoclastic RAW 264.7 cells by inhibiting excessive bone resorption accompanied by a decrease in tartrate-resistant acid phosphatase activity [77].
A major contributing factor to gout, known as hyperuricemia is an abnormally excessive accumulation of uric acid brought on by an imbalance between uric acid production and excretion. Treatment with C. indicum extract significantly increases the expression levels of the organic anion transporter 1 (OAT) and OAT 3 proteins both in vitro and in vivo while suppressing the activity of xanthine oxidase and mRNA expression of xanthine dehydrogenase [78]. A study by Lee et al. also indicated a reduction of serum uric acid levels in normal rats and rats with uricase inhibitor potassium oxonate-induced hyperuricemia while increasing renal uric acid excretion after treatment with C. indicum [79]. In addition, treatment with C. indicum effectively suppressed UV-induced increase in skin thickness and wrinkle grading in a dose-dependent manner, correlated with inhibition of collagen fiber content loss and epidermal thickening on mouse skin [80]. Solar UV radiation is known to cause skin damage primarily through superfluous ROS and chronic low-grade inflammation, which eventually up-regulate the expression of MMPs. Therefore, treatment inhibited the production of inflammatory cytokines, alleviated abnormal changes in anti-oxidative indicators, and down-regulated the levels of MMP-1 and MMP-3, indicating C. indicum role as a novel photoprotective agent. Chrysanthemum indicum also exhibited its function in maintaining healthy skin by increasing the expression of pro-collagen 12, collagen 12, and fibronectin. All these findings may provide valuable data for developing edible flowers as functional products.
TOXICITY AND SAFETY OF CHRYSANTHEMUM
Chrysanthemum toxicity studies have been investigated to characterize and identify potential adverse effects with various doses administrated. In a single-dose administration of C. morifolium at 15 g/kg body weight dose, no death was reported in rats after 14 days of observation. Similarly, no acute toxicity and side effects were observed in rats after orally being treated with Chrysanthemum at 0, 1, and 2 ml/kg body weight after 14 days of observation [81]. Oral administration of C. morifolium for long-term toxicity at doses of 320, 640, and 1280 mg/kg body weight/d for 26 weeks, followed by a 4-week recovery period, was observed to be safe [76]. A study by Hwang et al. reported no mortality and clinical signs of toxicity after oral administration of C. indicum at 2,000 mg/kg body weight/d for 15 days in mice [82]. Another study showed that high C. indicum at 1,024, 1,280, 1,600, and 2,000 mg/kg were reported safe, with no toxic alteration found in mice [83]. To date, no abnormalities were observed in another randomized, double-blind, placebo-controlled trial after 12-week ingestion of Chrysanthemum extract at 100 mg/d doses [84]. Interestingly, the administration of Chrysanthemum extracts at a higher dose of 400 mg/d (200 mg/ml, twice/day) exhibited antioxidant properties by demonstrating its neuroprotective effect on ischemic stroke patients [85]. Several studies have reported no adverse effects related to Chrysanthemum, which indicates its safety. However, there is still limited scientific information to determine an appropriate range of doses for Chrysanthemum, especially in a clinical study. The appropriate dose of Chrysanthemum intake still depends on several factors, including age, health, and other conditions. Nevertheless, further clinical safety studies on Chrysanthemum should still be carried out to validate the findings.
CHRYSANTHEMUM NANOPARTICLES AS GREEN NANOMEDICINE AND OTHER APPLICATION
In the modern era, there has been a growing interest in sustainable and eco-friendly practices including in the field of nanotechnology, leading to the emergence of green nanomaterials. Due to their peculiar physiochemical properties, such as a high ratio of surface area to mass, electric optical, catalytic, and therapeutic properties, nanomaterials are extensively used and studied [86,87]. Green nanomaterials are aimed to be derived from renewable sources, such as plant compounds, and represent a promising strategy for addressing the environmental and health concerns associated with conventional synthetic methods, which usually require high pressure and temperature for operations, expensive equipment and the usage of harsh chemicals and reagents that are noneco-friendly [88]. Among the wide variety of green nanomaterials, those synthesized from plant extracts have garnered the most interest due to their inherent biocompatibility, abundance, and low production costs. Therefore, a burgeoning research field focusing on Chrysanthemum species for nanomaterials synthesis as a candidate has been implied. Utilizing the natural potential of nanoparticles derived from Chrysanthemums for their diverse effects and potential applications will pave the way for more sustainable and eco-friendly nanotechnology.
In previous studies, silver nanoparticles (AgNPs) synthesized from C. indicum extract revealed antimicrobial activity on K. pneumonia, E. coli, and P. aeruginosa, as confirmed by the Kirby–Bauer disc diffusion method. The study emphasized the spherical and hexagonal with the range size of 37.71–71.99 nm AgNP created from the Chrysanthemum aqueous extract that is rich in tannins, saponins, flavonoids, terpenoids, alkaloids, steroids, and glycosides as their active compounds [89]. Moreover, biocompatibility tests conducted on mouse embryo fibroblast cells indicate the safety of nanoparticles. This observation aligns with the most recent observation on C. indicum nanoparticles, highlighting well-dispersed and capping silver nanoparticles with a size range between 71 and 180 nm [86]. The C. indicum extract served as both a reductant and a stabilizing agent for the synthesis of spherical nanoparticles. In addition, further investigation was continued on AgNPs synthesised by Chrysanthemum to optimise various parameters, including extraction concentration, pH, time, and reaction temperature. The investigation yielded 1.98 ± 0.57 nm particle size, demonstrating excellent bacterial resistivity on E. coli and Staphylococcus aureus, thereby aiding in bacterial reduction and azo-contaminated wastewater treatment [90].
The outcome proportionally aligns with another C. morifolium study showing potent bactericidal activity against the same bacteria prioritized in wastewater treatment [91]. The study has yielded 20–50 nm nanoparticle size observed under transmission electron microscopy. This is in agreement with the utilization of Chrysanthemum spp. in the synthesis of ZnFe2O4@ZnO nanocomposite for Congo red dye degradation with a remarkable rate of efficiency, 94.85% discharged in wastewater. The nanocomposite displayed an increased surface area between 7.41–42.66 m2 g−1 and a decreased band gap energy from 1.98 to 1.92 eV, enhancing its efficiency in dye degradation. The dye is often used in printing, textile, color paper, and leather products, which is unfortunately detrimental to the environment and carcinogenic [92]. An additional investigation explored the antioxidant and antibacterial characteristics of AgNPs derived from six distinct varieties of C. morifolium. The study indicated that spherical AgNPs with a size of approximately 40 ± 1.2 nm were successfully generated from the five varieties under examination. Furthermore, the nanoparticles were classified as semiconductors, as observed from scanning electron microscopy images. Notably, all varieties exhibited potent antioxidant and antibacterial activity against E. coli and Staphylococcus aureus bacteria in the study [93]. Furthermore, the most recent discovery concluded that Chrysanthemum spp. extract AgNPs have the potential to be utilized in the semiconductor sector as they allow for size modification and band gap that can be used widely in biomedical instruments and applications [94].
The results of these studies underscore the significance of Chrysanthemum-based nanomaterials as eco-friendly and versatile agents with diverse applications in medicine, environmental protection, and industrial wastewater treatment. Continued research and optimization of these nanoparticles hold the potential for addressing pressing global challenges related to antimicrobial resistance and environmental pollution while promoting sustainable and green nanotechnology practices.
CONCLUSION
Natural products received an overwhelming demand in conjunction with their safety and low toxicity effects compared to modern drugs. This present review provides an up-to-date mechanism study of anti-inflammatory, antioxidant, anticancer, antidiabetic/anti-obesity, and antimicrobial in two common Chrysanthemum species: morifolium and indicum, both in vivo and in vitro studies. The redox-regulated transcription factor Nrf2 plays a crucial role in protecting against oxidative damage by inducing the expression of cytoprotective and antioxidant genes. Chrysanthemum also possesses intriguing anti-inflammatory properties by reducing pro-inflammatory factors and genes. Findings identified that C. indicum study illustrated more as an anticancer property. Chrysanthemum-mediated apoptosis effects are closely related to the activation of caspase cleavage. These molecular properties depend on one another in maintaining the hemostasis process by activating several signaling pathways involved, including JNK, while suppressing the proteins and transcription factors that increase tumor progression. Meanwhile, findings on Chrysanthemum morifolim extracts focus on the antidiabetic/anti-obesity properties. Treatment with Chrysanthemum extracts can regulate lipid metabolism to curb obesity and diabetes, illustrated by a decrement in lipid profiles such as TC, TG, and LDL. Somehow, the clinical trials of these properties must be further validated and properly documented. Two species of Chrysanthemum flowers were similar in origin but differed slightly in their medicinal properties, which can aid in developing a research strategy plan to bridge Chinese medicine with modern precision medicine. The present review summarizes the promising therapeutics properties in the Chrysanthemum genus and will serve as a foundation for future studies on plant-based medicines.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Su J, Jiang J, Zhang F, Liu Y, Ding L, Chen S, et al. Current achievements and future prospects in the genetic breeding of chrysanthemum: a review. Hortic Res. 2019;6:109. CrossRef
2. Zhao H, Liu Z, Hu X, Yin J, Li W, Rao G, et al. Chrysanthemum genetic resources and related genera of Chrysanthemum collected in China. Genet Resour Crop Evol. 2009;56 (7):937–46. CrossRef
3. Dong M, Yu D, Duraipandiyan V, Abdullah Al-Dhabi N. The protective effect of Chrysanthemum indicum extract against ankylosing spondylitis in mouse models. BioMed Res Int. 2017;2017:8206281. CrossRef
4. Hitmi A, Coudret A, Barthomeuf C. The production of pyrethrins by plant cell and tissue cultures of Chrysanthemum cinerariaefolium and Tagetes species. Crit Rev Plant Sci. 2000;19(1):69–89. CrossRef
5. Li Y, Yang P, Luo Y, Gao B, Sun J, Lu W, et al. Chemical compositions of chrysanthemum teas and their anti-inflammatory and antioxidant properties. Food Chem. 2019;286:8–16. CrossRef
6. Yang Y, Sun X, Liu J, Kang L, Chen S, Ma B, et al. Quantitative and qualitative analysis of flavonoids and phenolic acids in snow chrysanthemum (Coreopsis tinctoria Nutt.) by HPLC-DAD and UPLC-ESI-QTOF-MS. Molecules. 2016;21(10):1307. CrossRef
7. Yuan H, Jiang S, Liu Y, Daniyal M, Jian Y, Peng C, et al. The flower head of Chrysanthemum morifolium Ramat. (Juhua): a paradigm of flowers serving as Chinese dietary herbal medicine. J Ethnopharmacol 2020;261:113043. CrossRef
8. Biswas S, Das R, Banerjee ER. Role of free radicals in human inflammatory diseases. Aims Biophys. 2017;4(4):596–614. CrossRef
9. P?óciennikowska A, Hromada-Judycka A, Borz?cka K, Kwiatkowska K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell Mole Life Sci. 2015;72(3):557–81. CrossRef
10. Liu G, Zheng Q, Pan K, Xu X. Protective effect of Chrysanthemum morifolium Ramat. ethanol extract on lipopolysaccharide induced acute lung injury in mice. BMC Complement Med Ther. 2020;20(1):1–11. CrossRef
11. Shi L, Shu Y, Hu X, Akram W, Wang J, Dong S, et al. An Optimized two-herb chinese food as medicine formula reduces cisplatin-induced nephrotoxicity in the treatment of lung cancer in mice. Front Pharmacol. 2022;13:827901. CrossRef
12. Tsai P-J, Chang M-L, Hsin C-M, Chuang C-C, Chuang L-T, Wu W-H. Antilipotoxicity activity of Osmanthus fragrans and Chrysanthemum morifolium flower extracts in hepatocytes and renal glomerular mesangial cells. Mediators Inflamm. 2017;2017:4856095. CrossRef
13. Choi G, Yoon T, Cheon MS, Choo BK, Kim HK. Anti-inflammatory activity of Chrysanthemum indicum extract in acute and chronic cutaneous inflammation. J Ethnopharmacol. 2009;123(1):149–54. CrossRef
14. Wu X-L, Li C-W, Chen H-M, Su Z-Q, Zhao X-N, Chen J-N, et al. Anti-inflammatory effect of supercritical-carbon dioxide fluid extract from flowers and buds of Chrysanthemum indicum Linnén. Evid-Based Complement Altern Med. 2013;2013:413237. CrossRef
15. Morris CJ. Carrageenan-induced paw edema in the rat and mouse. Inflamm Protocols. 2003;225:115–21. CrossRef
16. Loram L, Fuller A, Fick L, Cartmell T, Poole S, Mitchell D. Cytokine profiles during carrageenan-induced inflammatory hyperalgesia in rat muscle and hind paw. J Pain 2007;8(2):127–36. CrossRef
17. Su J-Y, Tan L-R, Lai P, Liang H-C, Qin Z, Ye M-R, et al. Experimental study on anti-inflammatory activity of a TCM recipe consisting of the supercritical fluid CO2 extract of Chrysanthemum indicum, Patchouli Oil and Zedoary Turmeric Oil in vivo. J Ethnopharmacol. 2012;141(2):608–14. CrossRef
18. Yang H-M, Sun C-Y, Liang J-L, Xu L-Q, Zhang Z-B, Luo D-D, et al. Supercritical-carbon dioxide fluid extract from Chrysanthemum indicum enhances anti-tumor effect and reduces toxicity of bleomycin in tumor-bearing mice. Int J Mole Sci. 2017;18(3):465. CrossRef
19. Zhang X, Wu J-Z, Lin Z-X, Yuan Q-J, Li Y-C, Liang J-L, et al. Ameliorative effect of supercritical fluid extract of Chrysanthemum indicum Linnén against D-galactose induced brain and liver injury in senescent mice via suppression of oxidative stress, inflammation and apoptosis. J Ethnopharmacol. 2019;234:44–56. CrossRef
20. Park M-J, Song J-H, Shon M-S, Kim HO, Kwon OJ, Roh S-S, et al. Anti-adipogenic effects of ethanol extracts prepared from selected medicinal herbs in 3T3-L1 cells. Prev Nutr Food Sci. 2016;21(3):227. CrossRef
21. Wu J-Y, Chen Y-J, Fu X-Q, Li J-K, Chou J-Y, Yin C-L, et al. Chrysoeriol suppresses hyperproliferation of rheumatoid arthritis fibroblast-like synoviocytes and inhibits JAK2/STAT3 signaling. BMC Complement Med Ther. 2022;22(1):1–9. CrossRef
22. Rose-John S, Waetzig GH, Scheller J, Grötzinger J, Seegert D. The IL-6/sIL-6R complex as a novel target for therapeutic approaches. Expert Opin Ther Targets 2007;11(5):613–24. CrossRef
23. Kim J-E, Jun S, Song M, Kim J-H, Song Y-J. The extract of Chrysanthemum indicum Linne inhibits EBV LMP1-induced NF-κB activation and the viability of EBV-transformed lymphoblastoid cell lines. Food Chem Toxicol. 2012;50(5):1524–8. CrossRef
24. Lee HJ, Seo H-S, Ryu J, Yoon YP, Park SH, Lee CJ. Luteolin inhibited the gene expression, production and secretion of MUC5AC mucin via regulation of nuclear factor kappa B signaling pathway in human airway epithelial cells. Pulm Pharmacol Ther. 2015;31:117–22. CrossRef
25. Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal Biochem. 2017;524:13–30. CrossRef
26. Wu T, Li J, Li Y, Song H. Antioxidant and hepatoprotective effect of swertiamarin on carbon tetrachloride-induced hepatotoxicity via the Nrf2/HO-1 pathway. Cell Physiol Biochem. 2017;41(6):2242–54. CrossRef
27. Tian Z, Jia H, Jin Y, Wang M, Kou J, Wang C, et al. Chrysanthemum extract attenuates hepatotoxicity via inhibiting oxidative stress in vivo and in vitro. Food Nutr Res. 2019;63. CrossRef
28. Steel RJ, O’Connell MA, Searcey M. Perfluoroarene-based peptide macrocycles that inhibit the Nrf2/Keap1 interaction, Bioorg Med Chem Lett. 2018;28(16):2728–31. CrossRef
29. Zou B, Xiao G, Xu Y, Wu J, Yu Y, Fu M. Persimmon vinegar polyphenols protect against hydrogen peroxide-induced cellular oxidative stress via Nrf2 signalling pathway. Food Chem. 2018;255:23–30. CrossRef
30. Verma AK, Yadav A, Singh SV, Mishra P, Rath SK. Isoniazid induces apoptosis: role of oxidative stress and inhibition of nuclear translocation of nuclear factor (erythroid-derived 2)-like 2 (Nrf2). Life Sci. 2018;199:23–33. CrossRef
31. Zhou Y, Wang C, Kou J, Wang M, Rong X, Pu X, et al. Chrysanthemi Flos extract alleviated acetaminophen-induced rat liver injury via inhibiting oxidative stress and apoptosis based on network pharmacology analysis. Pharm Biol. 2021;59(1):1376–85. CrossRef
32. Jing S, Zhang X, Yan L-J. Antioxidant activity, antitumor effect, and antiaging property of proanthocyanidins extracted from Kunlun Chrysanthemum flowers. Oxid Med Cell Longev. 2015;2015:983484.
33. Wu X-L, Feng X-X, Li C-W, Zhang X-J, Chen Z-W, Chen J-N, et al. The protective effects of the supercritical-carbon dioxide fluid extract of Chrysanthemum indicum against lipopolysaccharide-induced acute lung injury in mice via modulating Toll-like receptor 4 signaling pathway. Mediators Inflamm. 2014;2014:246407.
34. Sun S, Jiang P, Su W, Xiang Y, Li J, Zeng L, et al. Wild chrysanthemum extract prevents UVB radiation-induced acute cell death and photoaging. Cytotechnology. 2016;68(2):229–40. CrossRef
35. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. CrossRef
36. Parrish AB, Freel CD, Kornbluth S. Cellular mechanisms controlling caspase activation and function. Cold Spring Harb Perspect Biol. 2013;5(6):a008672. CrossRef
37. Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27(48):6245–51. CrossRef
38. Hodaei M, Rahimmalek M, Behbahani M. Anticancer drug discovery from Iranian Chrysanthemum cultivars through system pharmacology exploration and experimental validation. Sci Rep. 2021;11(1):1–11. CrossRef
39. Liu YH, Mou X, Zhou DY, Zhou DY, Shou CM. Extraction of flavonoids from Chrysanthemum morifolium and antitumor activity in vitro. Exp Ther Med. 2018;15(2):1203–10. CrossRef
40. Chang T-L, Liou P-S, Cheng P-Y, Chang H-N, Tsai P-J. Borneol and Luteolin from Chrysanthemum morifolium regulate ubiquitin signal degradation. J Agric Food Chem. 2018;66(31):8280–90. CrossRef
41. Yang L, Wei D-D, Chen Z, Wang J-S, Kong L-Y. Reversal of multidrug resistance in human breast cancer cells by Curcuma wenyujin and Chrysanthemum indicum. Phytomedicine. 2011;18(8–9):710–8. CrossRef
42. Nie J, Liu Y, Sun C, Zheng J, Chen B, Zhuo J, et al. Effect of supercritical carbon dioxide fluid extract from Chrysanthemum indicum Linné on bleomycin-induced pulmonary fibrosis. BMC Complement Med Ther. 2021;21(1):1–14. CrossRef
43. Chen Y, Wang W, Liu F, Tang L, Tang R, Li W. Apoptotic effect of mtrix metalloproteinases 9 in the development of diabetic retinopathy. Int J Clin Exp Pathol. 2015;8(9):10452.
44. Seo D-W, Cho Y-R, Kim W, Eom SH. Phytochemical linarin enriched in the flower of Chrysanthemum indicum inhibits proliferation of A549 human alveolar basal epithelial cells through suppression of the Akt-dependent signaling pathway. J Med Food. 2013;16(12):1086–94. CrossRef
45. Wufuer Y, Yang X, Guo L, Aximujiang K, Zhong L, Yunusi K, et al. The antitumor effect and mechanism of total flavonoids from Coreopsis tinctoria nutt (snow Chrysanthemum) on lung cancer using network pharmacology and molecular docking. Front Pharmacol. 2022;13. CrossRef
46. Rubio-Moscardo F, Blesa D, Mestre C, Siebert R, Balasas T, Benito A, et al. Characterization of 8p21. 3 chromosomal deletions in B-cell lymphoma: TRAIL-R1 and TRAIL-R2 as candidate dosage-dependent tumor suppressor genes. Blood. 2005;106(9):3214–22. CrossRef
47. Xu Z-F, Sun X-K, Lan Y, Han C, Zhang Y-D, Chen G. Linarin sensitizes tumor necrosis factor-related apoptosis (TRAIL)-induced ligand-triggered apoptosis in human glioma cells and in xenograft nude mice. BiomedPharmacother. 2017;95:1607–18. CrossRef
48. Zhuo FF, Zhang C, Zhang H, Xia Y, Xue GM, Yang L, et al. Chrysanthemulide A induces apoptosis through DR5 upregulation via JNK-mediated autophagosome accumulation in human osteosarcoma cells. J Cell Physiol. 2019;234(8):13191–208. CrossRef
49. Kim C, Kim MC, Kim SM, Nam D, Choi SH, Kim SH, et al. Chrysanthemum indicum L. extract induces apoptosis through suppression of constitutive STAT3 activation in human prostate cancer DU145 cells. Phytother Res. 2013;27(1):30–8. CrossRef
50. Cockram C. The epidemiology of diabetes mellitus in the Asia-Pacific region. Hong Kong Med J. 2000;6(1):43–52.
51. Shon JC, Kim WC, Ryu R, Wu Z, Seo J-S, Choi M-S, et al. Plasma lipidomics reveals insights into anti-obesity effect of Chrysanthemum morifolium Ramat leaves and its constituent luteolin in high-fat diet-induced dyslipidemic mice. Nutrients. 2020;12(10):2973. CrossRef
52. Ryu R, Kwon E-Y, Choi J-Y, Shon JC, Liu K-H, Choi M-S. Chrysanthemum leaf ethanol extract prevents obesity and metabolic disease in diet-induced obese mice via lipid mobilization in white adipose tissue. Nutrients. 2019;11(6):1347. CrossRef
53. Sun J, Wang Z, Chen L, Sun G. Hypolipidemic effects and preliminary mechanism of Chrysanthemum flavonoids, its main components Luteolin and Luteoloside in hyperlipidemia rats. Antioxidants. 2021;10(8):1309. CrossRef
54. Lee Y, Lee J, Lee M-S, Chang E, Kim Y. Chrysanthemum morifolium flower extract ameliorates obesity-induced inflammation and increases the muscle mitochondria content and AMPK/SIRT1 activities in obese rats. Nutrients. 2021;13(10):3660. CrossRef
55. Chen M, Wang K, Zhang Y, Zhang M, Ma Y, Sun H, et al. New insights into the biological activities of Chrysanthemum morifolium: Natural flavonoids alleviate diabetes by targeting α-glucosidase and the PTP-1B signaling pathway. Eur J Med Chem. 2019;178:108–15. CrossRef
56. Yamamoto J, Tadaishi M, Yamane T, Oishi Y, Shimizu M, Kobayashi-Hattori K. Hot water extracts of edible Chrysanthemum morifolium Ramat. Exert antidiabetic effects in obese diabetic KK-Ay mice. Biosci Biotechnol Biochem. 2015;79(7):1147–54. CrossRef
57. Yamamoto J, Yamane T, Oishi Y, Shimizu M, Tadaishi M, Kobayashi-Hattori K. Chrysanthemum promotes adipocyte differentiation, adiponectin secretion and glucose uptake. Am J Chin Med. 2015;43(02):255–67. CrossRef
58. Nishina A, Sato D, Yamamoto J, Kobayashi-Hattori K, Hirai Y, Kimura H. Antidiabetic-like effects of Naringenin-7-O-glucoside from Edible Chrysanthemum ‘Kotobuki’and Naringenin by Activation of the PI3K/Akt Pathway and PPARγ. Chem Biodivers. 2019;16(1):e1800434. CrossRef
59. Nepali S, Cha J-Y, Ki H-H, Lee H-Y, Kim Y-H, Kim D-K, et al. Chrysanthemum indicum inhibits adipogenesis and activates the AMPK pathway in high-fat-diet-induced obese mice. Am J Chin Med. 2018;46(01):119–36. CrossRef
60. Lee J-H, Moon J-M, Kim Y-H, Lee B, Choi S-Y, Song B-J, et al. Effect of enzymatic treatment of Chrysanthemum indicum linne extracts on lipid accumulation and adipogenesis in high-fat-diet-induced obese male mice. Nutrients. 2019;11(2):269. CrossRef
61. Cha JY, Nepali S, Lee HY, Hwang SW, Choi SY, Yeon JM, et al. Chrysanthemum indicum L. ethanol extract reduces high-fat diet-induced obesity in mice. Exp Ther Med. 2018;15(6):5070–6. CrossRef
62. Jay MA, Ren J. Peroxisome proliferator-activated receptor (PPAR) in metabolic syndrome and type 2 diabetes mellitus. Curr Diabetes Rev. 2007;3(1):33–39. CrossRef
63. Adisakwattana S, Ruengsamran T, Kampa P, Sompong W. In vitro inhibitory effects of plant-based foods and their combinations on intestinal α-glucosidase and pancreatic α-amylase. BMC Complement Med Ther. 2012;12(1):1–8. CrossRef
64. Yu Q, Chen W, Zhong J, Qing D, Yan C. Structural elucidation of three novel oligosaccharides from Kunlun Chrysanthemum flower tea and their bioactivities. Food Chem Toxicol. 2021;149:112032. CrossRef
65. Mohammed S, Gorski L. Antimicrobial resistance and antimicrobial stewardship in home healthcare. Home Healthcare Now. 2021;39(5):238–46. CrossRef
66. Hodaei M, Rahimmalek M, Arzani A. Variation in bioactive compounds, antioxidant and antibacterial activity of Iranian Chrysanthemum morifolium cultivars and determination of major polyphenolic compounds based on HPLC analysis. J Food Sci Technol. 2021;58(4):1538–48. CrossRef
67. Mummed B, Abraha A, Feyera T, Nigusse A, Assefa S. In vitro antibacterial activity of selected medicinal plants in the traditional treatment of skin and wound infections in eastern Ethiopia. BioMed Res Int. 2018;2018. CrossRef
68. Chang Y, Xing M, Hu X, Feng H, Wang Y, Guo B, et al. Antibacterial activity of Chrysanthemum buds crude extract against Cronobacter sakazakii and its application as a natural disinfectant. Front Microbiol. 2021;11:632177. CrossRef
69. Yeasmin D, Swarna R, Nasrin M, Parvez S, Alam M. Evaluation of antibacterial activity of three flower colours Chrysanthemum morifolium Ramat. Against multi-drug resistant human pathogenic bacteria. Int J Biosci. 2016;9:78–87. CrossRef
70. Karnwal A. In vitro antibacterial activity of Hibiscus rosa sinensis, Chrysanthemum indicum, and Calendula officinalis flower extracts against Gram negative and Gram positive food poisoning bacteria. Orien Pharm Exp Med. 2022;22(3):607–19. CrossRef
71. Tresch M, Mevissen M, Ayrle H, Melzig M, Roosje P, Walkenhorst M. Medicinal plants as therapeutic options for topical treatment in canine dermatology? A systematic review. BMC Vet Res 2019;15(1):1–19. CrossRef
72. Ming K, Chen Y, Shi J, Yang J, Yao F, Du H, et al. Effects of Chrysanthemum indicum polysaccharide and its phosphate on anti-duck hepatitis a virus and alleviating hepatic injury. Int J Biol Macromole. 2017;102:813–21. CrossRef
73. Arokiyaraj S, Dinesh Kumar V, Elakya V, Kamala T, Park SK, Ragam M, et al. Biosynthesized silver nanoparticles using floral extract of Chrysanthemum indicum L.—potential for malaria vector control. Environ Sci Poll Res. 2015;22(13):9759–65. CrossRef
74. Jang H-Y, Lee H-S, Noh E-m, Kim J-M, You Y-O, Lee G, et al. Aqueous extract of Chrysanthemum morifolium Ramat. Inhibits RANKL-induced osteoclast differentiation by suppressing the c-fos/NFATc1 pathway. Arch Oral Biol. 2021;122:105029. CrossRef
75. Kwon D, Kim C, Woo YK, Hwang J-K. Inhibitory effects of Chrysanthemum (Chrysanthemum morifolium Ramat.) Extract and its active compound isochlorogenic acid A on Sarcopenia. Prev Nutr Food Sci. 2021;26(4):408. CrossRef
76. Li L, Gu L, Chen Z, Wang R, Ye J, Jiang H. Toxicity study of ethanolic extract of Chrysanthemum morifolium in rats. J Food Sci. 2010;75(6):T105–9. CrossRef
77. Luyen BTT, Tai BH, Thao NP, Lee YM, Lee SH, Jang HD, et al. The anti-osteoporosis and antioxidant activities of chemical constituents from Chrysanthemum indicum flowers. Phytother Res. 2015;29(4):540–8. CrossRef
78. Kim O-K, Yun J-M, Lee M, Kim D, Lee J. Hypouricemic effects of Chrysanthemum indicum L. and Cornus officinalis on hyperuricemia-induced HepG2 cells, renal cells, and mice. Plants. 2021;10(8):1668. CrossRef
79. Lee Y-S, Kim S-H, Yuk HJ, Kim D-S. DKB114, a mixture of Chrysanthemum indicum linne flower and Cinnamomum cassia (L.) J. presl bark extracts, improves hyperuricemia through inhibition of xanthine oxidase activity and increasing urine excretion, Nutrients. 2018;10(10):1381. CrossRef
80. Zhang X, Xie Y-L, Yu X-T, Su Z-Q, Yuan J, Li Y-C, et al. Protective effect of super-critical carbon dioxide fluid extract from flowers and buds of Chrysanthemum indicum linnen against ultraviolet-induced photo-aging in mice. Rejuvenation Res. 2015;18(5):437–48. CrossRef
81. Kwon J-K, An I-J, Lee J-S, Kim H-R, Park H-S, Kim D-C, et al. Acute oral toxicity and skin irritation studies on natural dyes extracted from chrysanthemum. J Food Hygiene Safety. 2012;27(2):188–93. CrossRef
82. Hwang ES, Kim GH. Safety evaluation of Chrysanthemum indicum L. flower oil by assessing acute oral toxicity, micronucleus abnormalities, and mutagenicity. Prev Nutr Food Sci. 2013;18(2):111–6. CrossRef
83. Ma J-Y, Park H-Y, Choi H, Zee O-P, Lee J-H. Acute toxicity study on Mud Chrysanthemum indicum in mice. Korea J Herbol. 2008;23(2):81–5.
84. Takara T, Yamamoto K, Suzuki N, Yamashita S-I, Iio S-I, Kakinuma T, et al. Effects of luteolin-rich chrysanthemum flower extract on purine base absorption and blood uric acid in Japanese subjects. J Funct Foods Health Dis. 2022;12(1):12–25. CrossRef
85. Zhu Z, Qian S, Lu X, Xu C, Wang Y, Zhang X, et al. Protective properties of the extract of Chrysanthemum on patients with ischemic stroke. J Healthcare Eng. 2021;2021.
86. John Alphonso K. Green synthesis of silver nanoparticles from Chrysanthemum indicum and its characterization. J Pharm Negat Res. 2022;13:4095–101. CrossRef
87. Rajeshkumar S, Bharath L. Mechanism of plant-mediated synthesis of silver nanoparticles–a review on biomolecules involved, characterisation and antibacterial activity. Chem-Biol Interact. 2017;273:219–27. CrossRef
88. Chowdhury A, Ara J, Islam MS. Green synthesis of phytochemical nanoparticles and their antimicrobial activity, a review study. Biomed J Sci Tech Res. 2021;34(4):26929–35. CrossRef
89. Arokiyaraj S, Arasu MV, Vincent S, Prakash NU, Choi SH, Oh Y-K, et al. Rapid green synthesis of silver nanoparticles from Chrysanthemum indicum L and its antibacterial and cytotoxic effects: an in vitro study. Int J Nanomed. 2014;9:379–88. CrossRef
90. Wan H, Huang Q, Mia R, Tao X, Mahmud S, Liu H. Bioreduction and stabilization of nanosilver using Chrysanthemum phytochemicals for antibacterial and wastewater treatment. Chem Select. 2022;7(29):e202200649. CrossRef
91. He Y, Du Z, Lv H, Jia Q, Tang Z, Zheng X, et al. Green synthesis of silver nanoparticles by Chrysanthemum morifolium Ramat. Extract and their application in clinical ultrasound gel. Int J Nanomed. 2013;8:1809–15. CrossRef
92. Yakupova EI, Bobyleva LG, Vikhlyantsev IM, Bobylev AG. Congo red and amyloids: history and relationship. Biosci Rep. 2019;39 (1):BSR20181415. CrossRef
93. Kandiah M, De Silva LN. Microwave-assisted ecofriendly silver nanoparticle synthesis by varieties of Chrysanthemum morifolium Ramat: assessing their antioxidant, photocatalytic and antibacterial activities. J Metals Mater Miner. 2021;31(4):51–61.
94. López-López JR, Tejeda-Ochoa A, Cervantes-Gaxiola ME, Herrera-Ramirez JM, Méndez-Herrera PF. Photocatalytic activity of ZnO nanoparticles synthesized from zinc nitrate and botanical extracts of neem, chrysanthemum, Mexican marigold and shiitake mushroom. J Chem Technol Biotechnol. 2023;98(8):1810–8. CrossRef