INTRODUCTION
A colloidal drug delivery system is an innovative approach in the field of pharmaceuticals and nanotechnology that aims to enhance the therapeutic effectiveness of drugs. It involves the utilization of colloidal particles, typically ranging in size from 1 to 1,000 nanometers, to encapsulate and deliver drugs to specific target sites within the body [1]. This technology has gained considerable attention due to its ability to overcome many challenges associated with traditional drug delivery methods. A colloidal drug delivery system includes liposomes, niosomes, micelles, dendrimers, in-situ gels, and so on [2–5]. It provides significant advancements in the field of ocular drug delivery, offering promising solutions (Sols) for treating various ocular disorders by overcoming numerous barriers such as corneal, precorneal, conjunctival, and formulation challenges [6]. Niosomes have grown in prominence as the most effective drug carriers in ocular therapies. The small vesicle size of niosomes and their inability to permeate connective tissue and epithelium deliver the drug at the administration site [7].
However, niosomes suffer from poor precorneal retention and nasolacrimal drainage [8]. Therefore, to overcome this problem, niosomes are being incorporated into an in-situ gel [9]. A system known as “in-situ gel” is one that, when exposed to physiological circumstances such as variations in temperature, pH, or ion concentration, goes through a phase transition from a Sol or liquid state to a gel or semi-solid state. These systems provide controlled and sustained release of drugs for an extended period. In situ, gel formulations are predominantly based on biocompatible polymers, such as thermosensitive polymers, mucoadhesive polymers, and biodegradable hydrogels, which provide the necessary mechanical strength, stability, and biodegradability [10].
The potential for a paradigm shift in ocular treatments occurs with the combination of colloidal and in-situ delivery systems which overcome physiological and anatomical limitations of ocular delivery [11]. In terms of improving solubility, stability, targeting, prolonged release, and adaptability, colloidal drug delivery methods are a promising new direction for the pharmaceutical industry. This current review provides an overview of combining in situ gel with niosomes for ocular delivery of many therapeutic agents. An in-depth review has been made focusing on various formulation, characterization, safety, and development prospects of in situ gels loaded with niosomes for ocular administration.
Niosomes have small vesicle sizes and allow limited penetration into the epithelium and connective tissue, keeping the medication at the administration site. However, precorneal and nasolacrimal drainage limit the applicability of niosomes in ocular delivery because of their low viscosity. The inclusion of niosomes in in-situ gels ensures prolonged retention on the ocular surface, resulting in improved medicine penetration and extended contact. Furthermore, because of their ability to minimize systemic side effects, lower dose frequency, and delayed drug release, make them potential candidates for long-term therapies [12]. Continued research and development in this field hold great potential for optimizing drug therapies and improving patient outcomes across various medical conditions.
NIOSOMES IN OCULAR DRUG DELIVERY
The eyes are the key sensory organ responsible for vision. In the skull, the eyes are in bony spaces known as orbits. Its shape is roughly spherical, with one of its inner layers—the cornea and sclera make up the outermost layer, also referred to as the fibrous tunic [13]. The choroid, ciliary body, pigmented epithelium, and iris make up the middle layer, also referred to as the vascular tunic or uvea. The retina is the innermost layer. Retinal blood arteries (anteriorly) and choroid blood vessels (posteriorly) supply oxygen to the retina. The vitreous body, a jelly-like material, fills the entire posterior chamber of the eye, whereas the aqueous humor fills the area anteriorly, between the cornea and lens. Conjunctiva, a thin clear layer lies on top of this [14].
Ocular drug delivery is a challenging field due to the presence of various static and dynamic barriers present in the eye. Static barriers include nasolacrimal drainage, blinking, cornea, sclera, and blood-queous barriers, whereas dynamic barriers include conjunctival blood flow, lymphatic clearing, and tear drainage [13,6]. Overcoming the ocular barrier using nanotechnology holds a revolutionizing approach for the treatment of a variety of eye disorders [15]. Nanotechnology plays a significant role in ocular drug delivery through nanoparticles, niosomes, liposomes, micelles, dendrimers, and other polymeric vesicles. Due to their affordability and stability in Sols and during storage niosomes are becoming more and more popular as drug-delivery nanocarriers [16]. These are non-ionic surfactant vesicles that can encapsulate drugs and protect them from degradation while facilitating their transport across ocular barriers [17]. Small unilamellar vesicles, large unilamellar vesicles, and multilamellar vesicles are the three categories into which niosomes are divided according to their size or number of bilayers. These non-ionic vesicles have particles that are submicron in size. Commonly employed methods for formulating niosomes are the thin film hydration method, reverse phase evaporation (REV), ether injection method, handshaking method, and hydration by freezing and thawing [17]. Drug loading in niosomes is a crucial step in formulating niosomes for targeted and controlled delivery. Proper drug loading ensures the efficient delivery of drugs to the target site [18]. Active loading and passive loading are some drug loading techniques [19]. Passive drug loading involves passively encapsulating the drug inside the niosomal vesicles during the hydration process. Active loading involves encapsulating medicinal molecules into niosomes using a transmembrane gradient [20]. The drug diffuses across the niosome membrane and builds up inside the niosome through protonation. The primary benefit of this approach over passive loading techniques is its high drug loading efficiency [21].
IN-SITU GELLING SYSTEM
In situ, gelling formulations are drug delivery systems that are generally liquid at ambient temperature but convert to gel after being applied to the body in response to numerous stimuli such as temperature, pH, and ionic composition. The term “in situ gel-forming systems” refers to low-viscosity Sols that, in reaction to the physiological environment, experience conformational changes in polymers and undergo a phase transition in the conjunctival cul-de-sac to create viscoelastic gels [10]. In situ gels can be made using both natural and synthetic polymers. Gelation imparts new viscoelastic properties, restricting pre-corneal removal and resulting in a longer residence time on the ocular surface [22].
MECHANISMS OF SOL-GEL FORMULATION
In situ, gels undergo a phase transition from a liquid/sol state to a gel state upon specific trigger or environmental conditions. Mechanisms of sol-gel formation for niosomal in-situ gel can vary depending on the specific formulation and the stimuli used. The most common and briefly described method for the formation of gel is physiological stimuli, i.e., through pH, temperature, and ion-induced triggers.
Physiological stimuli
Temperature-triggered in-situ gel
In some in-situ gel formulations, the sol-gel transition is triggered by a change in temperature. This mechanism relies on the thermal responsiveness of certain polymer or gelling agents. Liquid can be injected into the eye with precision and ease, without producing pain or blurriness [23]. After administration, the liquid is transformed into gel at a precorneal temperature (35°C) to withstand lachrymal fluid dilution without causing the drug to be rapidly eliminated from the precorneal [24]. The thermoreversible polymers undergo a transition from sol-gel above their lower critical Sol temperature (LCST). LCST or lower consolute temperature is the critical temperature below which all the combinations are miscible. As the temperature of the Sol is raised above the LCST of the thermoreversible polymer, a critical temperature is reached [25]. At this point, the polymer undergoes a phase transition and becomes hydrophobic. This transition is driven by the dehydration of the polymer chains, causing them to collapse and aggregate. The collapsed polymer chains start to associate with each other forming a 3D network throughout the Sol [26]. This network entraps water and other solutes within its structure, resulting in the formation of the gel. The gelation process is highly reversible, it can be converted into a sol state when the temperature is lowered below LCST [27]. Poly-N-isopropylacrylamide (PNIPAAm) has benefits including high aqueous solubility and a LCST that is near to body temperature. PNIPAAm is not frequently employed, though, which may be due to potential drawbacks including low biodegradability and low drug loading capacity [28]. Elmotasem Heba et al. [29] formulated niosomal in-situ gel loaded with fluconazole to treat fungal keratitis using thermosensitive polymers such as poloxamer 407. The formulated gel showed enhanced corneal permeation with good entrapment efficiency (EE) and good gelling capacity with a gelation temperature of about 35.7°C [29]. Another interesting study performed by Gugleva et al. [30] formulated a niosome-loaded in-situ gel of doxycycline for ocular delivery using poloxamer 407 as a polymer alone and in combination with hydroxy propyl methyl cellulose (HPMC). The gelation temperature of the prepared gel was determined to be 34 0 C, suitable for ocular delivery. In-vitro investigation showed sustained release of drugs from the system [30].
pH-triggered in-situ gel
pH-sensitive in-situ gels are a type of advanced drug delivery system that undergoes gelation in response to changes in pH. These gels are particularly useful for controlled drug release in different parts of the body where pH levels vary. These gels contain biocompatible polymers that undergo pH-dependent conformational changes [31]. These polymers may contain acidic or basic groups that can ionize or deionize in response to variations in pH. In an acidic environment, polymers with the acidic group become protonated leading to increased repulsion between polymer chains, which promotes gelation. Conversely, in a basic environment, these polymers deprotonate, disrupting the cross-linking and causing the gel to revert to the sol state [32,33]. Significant potential is required for the in-situ pH-triggered gelling technology to maintain drug release and stability of pharmacological products. In this regard, Zafar et al. [34] formulated a pH-triggered niosomal loaded in-situ gel of moxifloxacin using chitosan to improve ocular residence time in the treatment of bacterial infection of the eye. Optimized formulation was evaluated for antimicrobial properties, gelling capacity, Ex vivo penetration, and in vitro drug release. Results showed good antimicrobial activity with a sustained release pattern and increase in permeability. It also showed good gelling capacity within seconds [34]. Allam and coworkers, [12] focused on developing a niosome-loaded in-situ gel of Betaxolol hydrochloride for the treatment of glaucoma to increase precorneal residence of drug using Carbopol 934P and Hydroxy ethyl cellulose as a pH-sensitive polymer. The formed gel showed an in-vitro sustained release pattern. Whereas, the in-vivo study performed in rabbit eyes showed a remarkable decrease in intraocular pressure and significant advancement in bioavailability when compared to the marketed formulation [12].
Ion-triggered in-situ gel
An ion-triggered in-situ gel is a system that triggers gelation in response to changes in the ionic environment. Ion-induced gelation relies on the use of ion-responsive polymers, also known as ion-sensitive or ionotropic polymers [35]. These polymers possess functional groups or side chains that can interact with ions in the surrounding environment. These polymers often carry negatively charged groups, such as carboxylates (–COO-) or sulfate (–SO3-) groups which can interact with positively charged ions (cations) in the Sol. This interaction involves electrostatic attraction between negatively charged polymer and positively charged ions [36]. The ion-polymer complex results in the formation of a 3D network, transforming a liquid Sol into a gel. The extent of gelation and gel properties can be controlled by adjusting the concentrations and type of ions added [37].
Balasubramaniam et al. [38] devised an ion-activated in-situ gelling system, for bacterial conjunctivitis to deliver ciprofloxacin hydrochloride for a longer duration of action. Gelrite, gellan gum was employed both alone and in combination with sodium alginate as the gelling agent. The formulations showed an 8-hours sustained drug release in vitro and were clinically effective [38]. Furthermore, Phenylephrine hydrochloride niosome-loaded in-situ gel was formulated using gellan gum as an ion-sensitive polymer. The formulation exhibited sustained drug release and was found to be effective in the treatment of mydriasis when performed in vivo study in the rabbit eye [39].
Physical stimuli
Swelling triggered
Swelling triggered in situ gel undergoes swelling forming a gel matrix that can sustainably release encapsulated drug over time due to changes in temperature or pH. In this approach, the material begins to gel as it takes water from the environment and then expands to fill the desired space. Polar lipid Myverol 18–99 (glycerol mono-oleate) expands in water to create lyotropic liquid crystalline phase formations. It has some bio-adhesive qualities and is susceptible to enzymatic degradation in vivo [40]. Myverol 18–99/water gel bio adhesion appeared to be caused by secondary chemical bonding, such as van der Waals forces but was restricted by their cohesive strength [41].
Diffusion/Solvent exchange triggered
This approach involves dispersing solvent from a polymer arrangement into adjacent tissue, which results in polymer grid precipitation. N-methyl pyrrolidone is used as a diffusion-triggered in-situ gel [42].
Chemical Stimuli
Ionic cross-linking
Polymers may undergo a phase transition in the presence of different ions. For example, gellan gum (an anionic polysaccharide) undergoes gelation due to the presence of various cations such as Mg+2, Ca+2, Na+, and K+, whereas pectins undergo gelation due to the presence of divalent cations. Likewise, Kappa-carrageenan forms a hard gel in response to a monovalent cation and forms an elastic gel in the presence of a divalent cation [43].
Enzymatic cross-linking
To formulate a reversible gelling system, enzymatic cross-linking is the most effective technique. In this approach formation of gel occurs by cross-linking of enzymes present in body fluid. An enzymatic cycle operates effectively in physiologic conditions without the requirement for potentially hazardous chemicals such as monomers and initiators [44].
Photoinitiated polymerization
To formulate in-situ gel, electromagnetic radiation can be used. With the use of electromagnetic radiation, the gel can be prepared by injecting a Sol of reactive macromere or monomers and invaders into a tissue location. Ideal polymers for polymerization are those that undergo dissociation of functional groups in the presence of light initiators like acrylate or similar monomers and macromers [45].
POLYMERS USED IN THE FORMULATING REVERSIBLE GELLING SYSTEM
Ideal polymer is selected based on the type of in-situ gelling system that is to be formulated. Various types of polymers can be used as per their characteristic as shown in Table 1.
Nano formulations based on in situ gels
In situ, gel systems include a delivery vehicle made of polymers (natural, semi-synthetic, or synthetic) that have the unique feature of converting the sol into a gel when impacted by a biological stimulus [46]. Gels help the nanocarriers to be administered locally to the target tissue, and to maintain their release. When applied to the eyes, the formulation initially remains liquid, allowing for easy administration. However, upon contact with the ocular surface, it undergoes gelation, resulting in sustained drug release, improved drug absorption, and increased therapeutic effectiveness [47,48]. This approach is particularly useful in treating ocular conditions and diseases where prolonged drug releases and increased drug retention on the eye surface are essential for successful treatment [49]. Nano formulations such as liposomes, niosomes, and nanostructured lipid carriers are some novel methods of ocular delivery. Liposome as a carrier, is incorporated into an in-situ gelling system which undergoes phase transition upon contact with the ocular surface transforming into a gel-like consistency, increasing the retention time of the drug into the ocular surface which in turn increases drug bioavailability [50,51]. However, liposomes possess certain prevalent limitations such as a lack of targeting strategies, production challenges, stability, and poor drug loading capacity. Unlike liposomes, niosomes face issues of instability and aggregation, leading to changes in vesicle size and reduced drug encapsulation efficiency. By combining niosome with in-situ gel formulations, these challenges can be effectively addressed, leading to improved stability, drug release, and overall efficacy in ocular drug delivery. The synergistic effect of both systems can enhance the therapeutic benefits of the delivered drug [52,53]. Nanostructure lipid carriers (NLCs) are colloidal drug delivery systems that consist of a blend of solid and liquid lipids. Formulating NLC-loaded in-situ gel for ocular delivery can address specific challenges associated with NLCs in the context of ocular drug delivery. Incorporating NLCs into an in-situ gel can provide a stabilizing matrix, reducing the likelihood of aggregation and enhancing the stability of the NLCs during storage and administration [54,55]. Table 2 gives details of some nano formulation-based in-situ gels for ocular application.
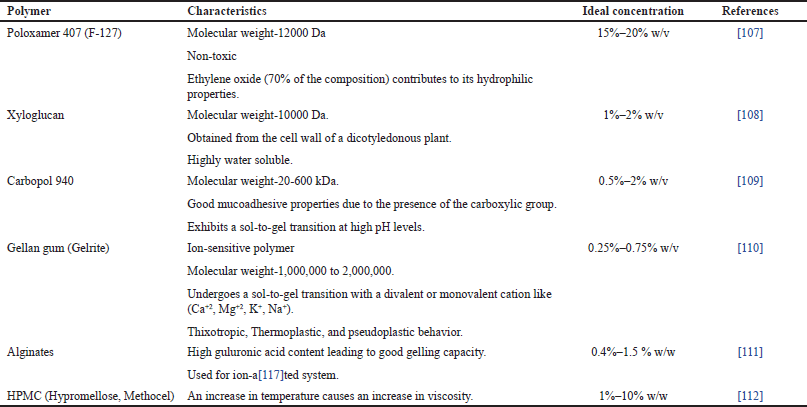 | Table 1. Polymers of In-situ gel formulation. [Click here to view] |
NIOSOME LOADED IN-SITU GEL
Niosomes are the bilayer vesicles that can entrap amphiphilic drugs intended for ocular delivery within their aqueous core [56]. These nanosized vesicles are made up of a non-ionic surfactant [57]. These serve as carriers to protect the drug from degradation, improve solubility, and enhance its bioavailability. Gugleva and collaborators [58] formulated doxycycline hyclate niosome using different surfactants and cholesterol in different ratios using the thin film hydration method followed by a REV method. Their findings suggest that niosomes may be a viable drug delivery platform for doxycycline ophthalmic use [58]. Another interesting study was performed by Kattar et al. [59] on epalrestat niosomes to treat diabetic retinopathy by inhibiting the polyol pathway. Formulated niosomes were characterized for various parameters which proved niosomes to be a promising carrier to carry and encapsulate epalrestat through the eye in a controlled manner [59]. However, niosomes suffer from their physical fragility and the risk of drugs that may cause their poor precorneal retention [60]. This problem can be overcome by incorporating niosomes into the gelling system. Several investigations have revealed that this combination approach has considerably improved ocular bioavailability for niosomal-loaded in-situ gel [61]. Doxycycline-loaded niosomal in situ gel formulated by a modified thin film hydration method for treating corneal infections, keratoconjunctivitis sicca showed enhanced antibacterial activity and sustained in vitro drug release drugs, ensuring sufficient therapeutic concentration [30]. In-vivo studies of Vancomycin-loaded niosomal in situ gel performed in MRSA-infected rabbits showed an increase in antibacterial activity after treatment as compared with those rabbits that were treated with free drug Sol [62]. Niosomes-loaded in-situ gel offers a promising approach by addressing many of the challenges associated with conventional dosage forms and providing a more effective and patient-friendly option for treating various eye conditions and diseases [63]. Various research has been conducted based on in situ niosomes in ocular delivery as shown in Table 3.
Factors affecting formulation of niosomes
Effect of type and structure of surfactant
The critical packing characteristics of surfactant can be used to predict the geometry of the vesicle to be generated. The type of micellar structure that forms can be determined from the critical packing parameter, if critical packaging parameter (CPP) < ½ then spherical micelles are formed, if CPP > 1/2, then bilayer micelles are formed, and if CPP >1, then inverted micelles are formed. An increase in the alkyl chain length and transition temperature of the surfactant leads to an increase in the EE of the drug. An increase in concentration of surfactant beyond normal value leads to the formation of micelles [64]. The effect of surfactant in formulating niosomes is shown in Figure 1.
Effect of cholesterol
Cholesterol serves several important roles in the structure and properties of niosomes. Cholesterol helps in stabilizing the bilayer structure of niosomes. It is well-known for its capacity to intercalate between the alkyl chains of surfactant molecules, hence increasing the lipid bilayer’s rigidity and stability. This can enhance the overall structural integrity of niosomes and prevent substances that are encapsulated from leaking [65]. The amount of cholesterol to be added is determined by the surfactant’s hydrophilic-lipophilic balance (HLB) value. To account for the larger head groups, the minimum quantity of cholesterol that must be added must be increased as the HLB number rises over 10. The rate of encapsulated material release decreases when the stiffness of the bilayers increases in response to an increase in cholesterol content within the bilayers [66]. The kinetics of drug release may be affected by the cholesterol in niosomes. It may have a barrier effect, decreasing the permeability of the niosome membrane and therefore delaying the release of the medication that has been encapsulated. Cholesterol has two effects: it causes gel-state bilayers’ chain order to decrease and liquid-state bilayers’ chain order to increase [67].
Hydration medium
Additional crucial factors are the niosomes volume and the duration of hydration. The use of aqueous Sols with different pH levels and ionic strengths as hydration medium can affect vesicle properties. Improper hydration can result in the production of fragile niosomes or drug leakage from niosomes [68].
Temperature of hydration medium
The temperature of hydration affects the niosomes shape and dimensions. For best results, it should be higher than the gel-to-liquid phase transition temperature of the system. The temperature of the hydration medium is essential to optimize to obtain the proper niosome properties. Temperature variations have an impact on both the assembly of surfactants into vesicles and the modification of vesicle shape [69].
Nature of encapsulated drug
Molecular weight, lipophilicity, hydrophilicity, and chemical structure of drugs influence the size of niosomes. The hydrophilic drug decreases stability and increases leakage from vesicles, while the hydrophobic drug increases stability and decreases leakage from vesicles. Amphiphilic drugs decrease leakage from vesicles and increase encapsulation [70]. The entrapment of drugs in niosomes causes an increase in vesicle size, most likely as a result of the solute interacting with the head groups of the surfactant, which raises the charge and mutual repulsion of the surfactant bilayers [68].
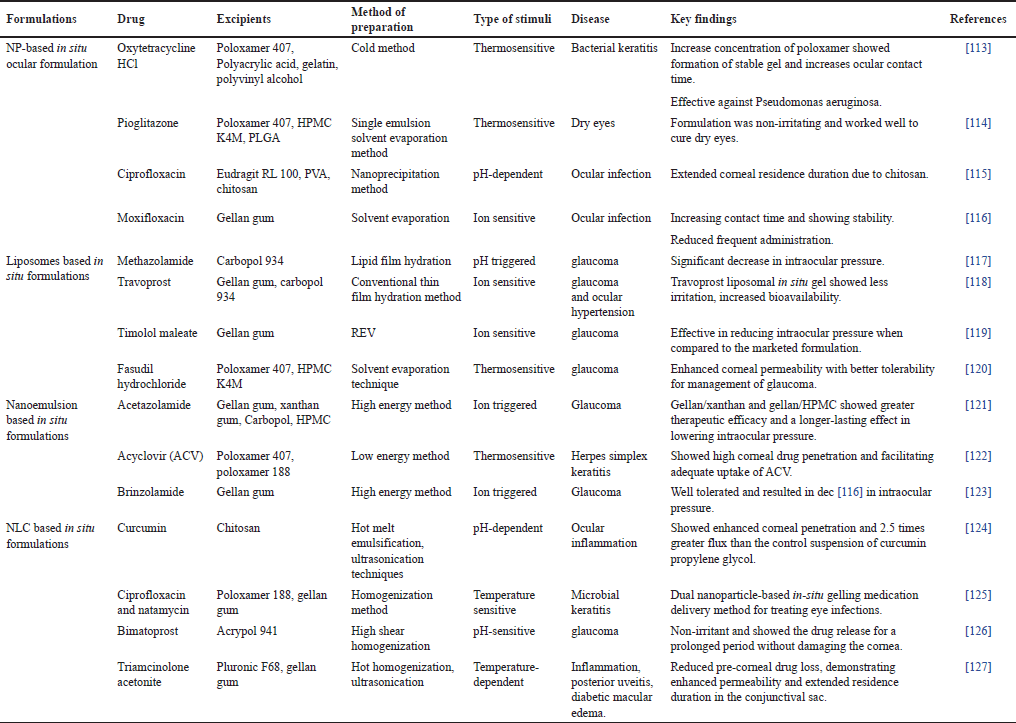 | Table 2. Nano formulations based in situ gels for ocular delivery. [Click here to view] |
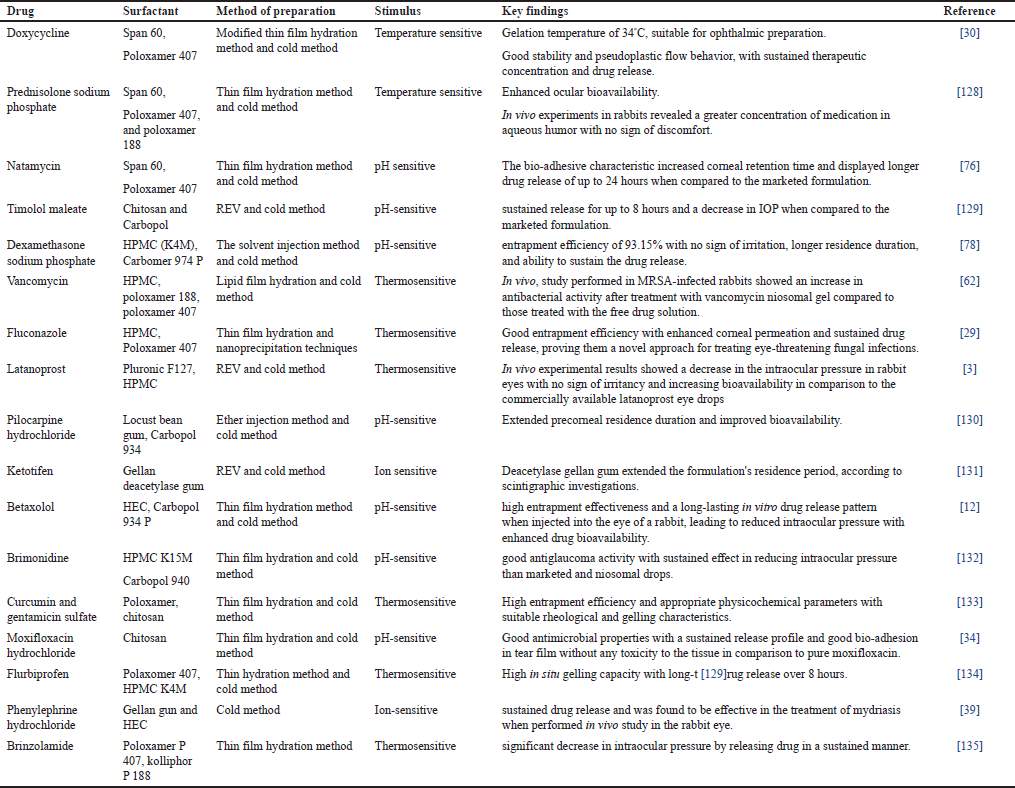 | Table 3. Niosomal in situ gels for ocular administration. [Click here to view] |
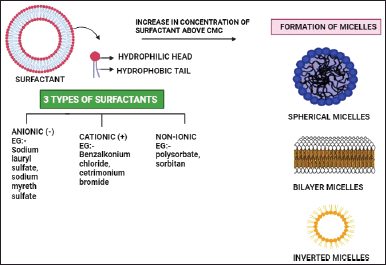 | Figure 1. Effect of surfactant in formulating niosomes. [Click here to view] |
Drug loading method
The efficiency of drug loading can be affected by the process utilized to incorporate medications or active ingredients into niosomes [71]. Based on the properties of the encapsulated substance, techniques such as remote loading or post-insertion can be used [71]. A study [72] has been conducted on niosome preparation techniques, including sonication, ether injection, and handshaking. Vesicles formed by the handshaking approach had a larger diameter (0.35–23 nm) than those formed by the ether injection method (50–1000 nm). The REV method can produce small-sized niosomes. The microfluidization process results in more homogeneity and smaller vesicles [72].
METHOD OF PREPARATION OF NIOSOMAL LOADED IN SITU GELS
Niosome-loaded in situ gel involves a combination of two distinct drug delivery approaches together. Incorporation of drug-loaded niosome in the in-situ gel offers advantages of both dosage forms. In the first step of niosome-loaded in situ gel formation, niosomes are prepared by any method such as the handshaking method, REV, or ether injection method as shown in Figure 2. The second step involves formulation of in situ gel by accurately weighing and mixing in situ polymer with a suitable amount of water or buffer Sol to form a gel and adjust pH if needed. In the final step, the drug-loaded niosomes are incorporated into the in-situ gel formulation with continuous stirring as shown in Figure 2. This uniform dispersion was kept overnight at 4°C to get a clear Sol. Formulated niosomes loaded in situ gel are then characterized for various evaluation parameters [73–76].
CHARACTERIZATION
Characterization of niosome-loaded in situ gel is a comprehensive process that involves accessing physicochemical properties, drug release kinetics, safety, and efficacy. A thorough evaluation is crucial for optimizing the formulation and ensuring its suitability for delivering drugs with enhanced therapeutic outcomes. In addition, quality control standards and regulatory guidelines should be followed to guarantee the safety and efficacy of this drug delivery system.
In-vitro evaluation
pH
Measuring the pH of niosome-loaded in-situ gel for ocular delivery is an important parameter for ensuring the stability and compatibility of the formulation with the ocular environment. Immerse the pH meter in a sample and allow it to stabilize to display a stable pH reading. Be sure to take multiple measurements and calculate the average for accuracy. Ideally, ophthalmic formulations must possess a pH equivalent to lacrimal fluid value, i.e., 7.4 [77].
Gelling Time
The time taken by the formulation to convert into gel is noted. Gelling time should be in the range of 5–7 seconds. The gelling time of the sample will be tested according to the method described by Patel et al. [78] using the test tube inversion method. This method involves transferring of sample into the test tube and placing it in a water bath at 35°C ± 0.5°C. The test tube was inverted at 90°C and the time when no fluidity of the sample was observed was evaluated as the gelling time.
Gelling capacity
A drop of prepared niosome-loaded in-situ gel is placed in a vial containing freshly developed simulated tear fluid (STF) (sodium chloride, 0.67 g, sodium bicarbonate, 0.20 g, and calcium chloride dihydrate, 0.008 g in 100 ml of distilled water) to determine the prepared formulation’s gelling capability. The gelling capability of the Sol was assessed based on the stiffness of the created gel and the time duration during which the formed gel remains thus [78].
Isotonicity adjuster
For ocular delivery, isotonicity is a crucial factor to ensure that the formulation does not damage or irritate ocular tissues. The formulation was mixed with a few drops of blood to determine isotonicity, and the morphology of the blood cell was studied under a microscope. Blood cells retain their integrity in isotonic fluids, whereas cells shrink in hypertonic and bulge in hypotonic Sols [79].
Viscosity
Measurement of viscosity is critical for assuring the consistency and quality of in-situ gels in manufacturing operations. Consistent viscosity aids in the production of gels with the desired qualities and performance characteristics. Viscosity can be measured by using a Brookfield viscometer. After allowing the prepared system to gel in the STF, the viscosity is measured at various angular velocities [80].
Polydispersity index (PDI), and zeta potential (ZP)
Dynamic light scattering and Malvern zeta sizer are used to analyze the PDI and ZP of niosome-loaded in-situ gel. The homogeneity of the size distribution was measured using PDI. A lower PDI value indicates a narrower size range, implying more uniform particle sizes (PSs). The PDI scale goes from 0 (completely monodisperse) to 1 (extremely polydisperse). A higher ZP value indicates better stability because it suggests strong repulsive forces between particles, preventing aggregation. A ZP value of ±30 mV or higher is often considered desirable for good stability [80].
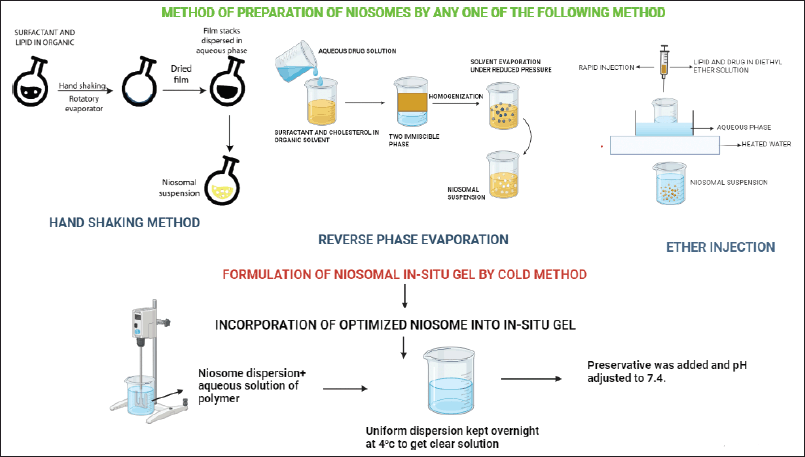 | Figure 2. Different method of preparation of niosomes loaded in situ gels. [Click here to view] |
EE
It is defined as the total amount of drug entrapped inside the vesicle. It can be determined using the centrifugation method. Centrifuge the niosomal in-situ gel at a specific speed and duration that separates the niosomes from the gel. After centrifugation two fractions are obtained: one is the precipitate containing niosomes and the other is the supernatant containing an unencapsulated drug. Carefully collect the supernatant and measure the amount of drug in the collected supernatant by diluting the supernatant and accessing it in HPLC or UV-VIS spectroscopy. Calculate EE using the following formula:
Higher EE indicates that a larger portion of the drug is encapsulated within the niosomal in-situ gel, which is typically desirable [81].
In-vitro drug release study
In vitro drug release study is carried out using a Franz diffusion cell or by using dialysis method.
Dialysis method This method typically involves encapsulating the drug formulation within a semipermeable membrane, followed by immersion in a release medium or physiological buffer Sol. The semi-permeable membrane acts as a barrier that permits the passage of the drug molecule while retaining the drug carrier or any other large molecule present in the formulation. This membrane is then placed in the dialysis bag and the release medium is constantly stirred to ensure uniform condition throughout the experiment. Release of the drug from the formulation is monitored at specific time intervals by analyzing the drug concentration in the release medium using UV-visible spectroscopy, HPLC, and so on [82,83].
Franz diffusion cell It is the most widely accepted apparatus for conducting in-vitro drug release studies. This method allows for the evaluation of the rate and extent of drug release from formulation through synthetic membranes, often mimicking the biological barrier. The apparatus consists of two compartments, i.e., the donor compartment that contains drug formulation, while the receptor compartment contains a suitable medium. The procedure involves placing drug formulation on the surface of the membrane in the donor compartment and maintaining the receptor compartment under constant stirring to ensure uniform drug concentration. Samples are collected at a predetermined interval from the receptor compartment for analysis using techniques such as UV-visible spectroscopy, HPLC, or other suitable methods depending on the nature of the drug [84,85].
Morphology study
Microscopical methods, including transmission electron microscopy (TEM) and scanning electron microscopy (SEM), can be used to analyze the morphology of niosomal vesicles. For SEM characterization, the niosome-loaded in-situ gel should first be transformed into a dry powder, which is put on a sample holder and then coated with a conductive metal (sputter coating either with gold, or gold-palladium). The coating prevents charging effects during the SEM analysis. Set the appropriate voltage and beam current suitable for the gel under investigation. For TEM analysis samples were diluted using distilled water. A droplet of the specimen was positioned on a copper grid coated with carbon, dyed with 2% phosphotungestic acid, and allowed to dehydrate. The photomicrographs demonstrate that the niosome-loaded in situ gel was distinct, spherical, and free of aggregations with a smooth surface [29].
Ex-vivo evaluation
Ex-vivo corneal permeability
Freshly removed rabbit corneas were used in a Franz diffusion cell for ex-vivo corneal permeability research. The dissected rabbit cornea was placed facing the donor compartment [29]. It was then placed on a magnetic stirrer set to 100 rpm and maintained at a constant temperature of 35.1°C. The chosen formulations were applied to the ocular surface of the donor compartment, and the receptor medium was isotonic phosphate buffer (pH 7.4). At prearranged intervals, aliquots of the receptor medium were taken out of the sampling port and replaced with an equivalent volume of fresh medium to maintain a consistent volume. Spectrophotometric analysis was performed on the samples [29]. The permeability coefficient (P) was calculated using the following formula:
P = (dQ/dt)/AC = J/C
dQ/dt = Rate of permeation
A = Surface area of diffusion membrane
J = Slope
C = concentration of drug in the donor compartment.
In-vivo evaluation
Eye irritancy test (Draize test)
The Draize test is a typical method for determining the potential irritancy and toxicity of chemicals, especially those that may encounter the eyes. While performing the Draize test in rabbits it is important to note ethical and animal welfare considerations [86]. Healthy adult rabbits free from ocular injuries are selected for study. A small amount of the test chemical is directly injected into the rabbit’s conjunctival sac. Over many days, the rabbits are observed at predetermined intervals, noting any indications of discomfort, inflammation, or unpleasant reactions. The rabbits should receive the proper post-test care, including any necessary medication for any negative test results [87].
Histopathology
A histopathology study is performed to verify the safety profile of the formulation. The sample of tissue or cells that have been exposed to the formulation is collected and placed in the formalin Sol for 24 hours to maintain structural integrity. Tissue is then bisected and mounted in a paraffin block; this process is known as paraffin embedding. The section is transferred to a microscope slide and stained with hematoxylin and eosin to visualize tissue morphology and cellular structure. The histopathological alterations brought on by the in-situ gel filled with niosomes are analyzed [88].
Stability studies
Niosomes loaded in-situ gel must undergo a stability study to assess the formulation’s long-term physical, chemical, and biological stability. Storage of the niosome-loaded in-situ gel is observed over three months at two distinct temperatures (4°C in a refrigerator and 25°C). Samples are collected every, two, and three months, and any physical alterations in the PS, PDI, ZP, color, smell, or percentage of EE were assessed. Three measurements of each parameter were made, and the mean value will be calculated [63,89]. A stability study performed by A.A. Omnia et al. [90] on levofloxacin-loaded niosomal in-situ gel when kept in various storage situations, revealed no changes in appearance. There was a negligible change (0.05) in the EE%, PS, PDI, or ZP at 4°C indicating good stability compared to freshly prepared formulation [90].
TOXICITY OF NIOSOMES
Surfactants are widely used in the formulation of niosomes. These amphiphilic molecules are used to construct the bilayer membrane of the niosomes. Based on charge, surfactants can be classified into ionic and non-ionic surfactants. Ionic surfactants are of three types- anionic, cationic, and amphoteric. Ionic surfactants have limited ocular applications because of toxicity issues. Ocular tissues are extremely sensitive, and their intricate structure necessitates great caution when choosing components for ocular administration. Surfactants potentiate to cause corneal and conjunctival cellular changes, especially at high concentrations [91]. Cationic surfactants like benzalkonium chloride are responsible for corneal epithelial cell death. A study assessing the cytotoxicity of different surfactants on rabbit corneal cells found nonionic surfactants to be the least irritant, followed by amphoteric, anionic, and finally cationic surfactants to be the most irritant [92]. Surfactants with polar head groups that are not electrically charged are known as non-ionic surfactants.
Brij, polyglycerol alkyl ethers, ester-linked surfactants, glucosyl dialkyl ethers, Spans (sorbitan esters), crown ethers, Tweens (polysorbates), and polyoxyethylene alkyl ethers are examples of non-ionic surfactants used to manufacture niosomes. These surfactants are classified as generally recognized as safe by United State Food and Drug Administration. As previously stated, nonionic surfactant contains no charge hence, are considered to be less irritating and less toxic compared to other surfactants but, can still potentially cause mild irritation when coming into contact with sensitive areas such as the eyes. The specific toxicity of non-ionic surfactants to the eyes depends on various factors, including the concentration of surfactants, the duration of exposure, chemical structure, and individual sensitivity [93]. Non-ionic surfactants are toxic for LC50 between 1 and 10 mg/l [94]. In a study, performed by Hamad Alyami and co-workers formulated non-ionic surfactant vesicles of pilocarpine hydrochloride for ocular drug delivery and the purpose of the study was to characterize the toxicological profile of non-ionic surfactant (span 60). The toxicity of optimized niosomes was tested on the human corneal epithelium-2 cell line as a model of corneal epithelium. Results showed no corneal cellular toxicity with a span 60 [52]. Another interesting study conducted in 2012 by Abdelkader assessed the ocular toxicity of niosomes by examining the potential for conjunctival and corneal irritation caused by Span 60 niosomes and Span 60 niosomes containing ranging proportions of bilayer membrane components such as Solulan C24, dicetyl phosphate, and sodium cholate. They did this by using an acceptable in vitro conjunctival model (hen’s egg chorioallantoic membranes) and excised bovine corneal opacity and permeability models. The results of this investigation indicate that niosomes can be tolerated by the eyes with minimum ocular irritations [95].
CORNEAL PENETRATION AND ABSORPTION MODELS
In the prior stages of formulation development, robust in vitro and Ex vivo models are needed to generate more effective drug carriers. Compared to in vivo experiments, these techniques are more expedient, less expensive, and more humane. The standard protocol for conducting corneal permeation investigations involves preparing the tissue either in vitro or Ex vivo and then putting it in an assembly, like a Ussing chamber or a Franz diffusion cell [96,97]. Transcorneal penetration investigations are increasingly being conducted using in vitro cell culture models. Studies on corneal penetration can be greatly aided by artificially created human corneal equivalents, even if research and development on these corneas are still underway [98]. In vitro and Ex vivo models frequently aid in reducing the number of laboratory animals utilized and have proven to be a more pertinent, affordable, efficient, and ethical option for investigating ocular formulation absorption and penetration [53]. Various corneal permeation and absorption models utilized are as follows:
1) In-vitro models
2) Ex-vivo models.
IN-VITRO MODELS
The In-vitro model serves to reduce the associated cost and number of laboratory animals employed for permeation studies. Rabbit, rat, bovine, human, and porcine corneal cultures are generally utilized for this purpose. Rabbit cells are readily used due to their easy availability whereas, human cells are difficult to obtain. To date, a variety of in vitro models have been developed, each model has its pros and cons. In vitro, models as specified in Table 4 can be broadly categorized into three groups: immortalized cell line, reconstructed tissue culture, and primary cell culture [99]. Primary cultures do not fully represent ocular tissue since they are often multilayered (5–6 layers) cell cultures of a single kind of cell. The cells in immortalized cell lines have undergone genetic modification [66]. Usually, tumors are the source of these cell lines, or they are created through viral oncogene transformation. Reconstructed tissue culture entails creating an analogous human cornea composed of endothelial, stromal, and epithelial cells [100]. Isolated ocular tissues and corneal epithelial cell cultures from rabbits and pigs have been used by Scholz et al. [101] to examine the penetration of the hydrophilic drug pilocarpine hydrochloride (p-HCl). A strong correlation was observed in P-HCl transport across the isolated tissues and cell cultures [101]. Human corneal epithelial cells (HCE-T), Statens Serum Institut rabbit corneal (SIRC) cells, SkinEthic human corneal epithelium (S-HCE), and Clonetics human corneal epithelium (C-HCE) were compared with excised rabbit and human corneas in terms of their permeability and barrier characteristics by Becker et al. [102]. According to their findings, SIRC and S-HCE’s barrier characteristics were not sufficiently developed to distinguish between the permeability of distinct drug compounds because of leaky gap junctions [102].
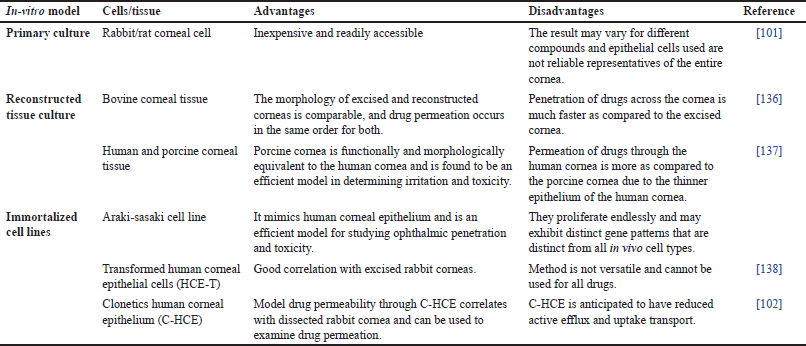 | Table 4. In-vitro corneal permeation and absorption model. [Click here to view] |
 | Table 5. Ex-vivo corneal permeation and absorption model. [Click here to view] |
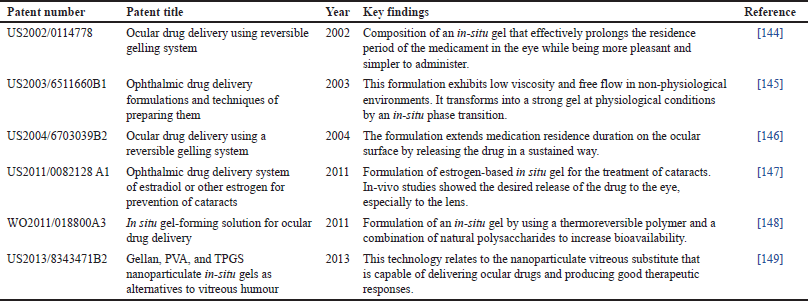 | Table 6. Patent on in-situ gel for ocular delivery. [Click here to view] |
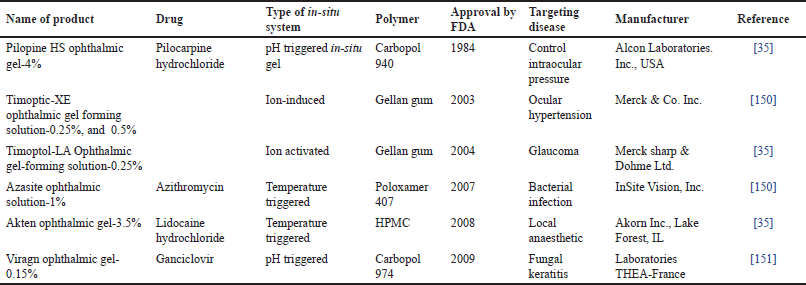 | Table 7. Marketed formulation of reversible gelling system for ocular delivery. [Click here to view] |
EX-VIVO MODELS
Ex vivo ocular models are a significant tool for studying corneal penetration and developing ocular medication delivery techniques. Since human corneas are usually exclusively used for transplantation, ex-vivo models usually use removed animal corneas in the proper diffusion cell. In ex-vivo models, rabbit, porcine, and cow corneas are the most often employed tissues [103]. Table 5 discusses the most often utilized tissues in ex-vivo models. Despite being smaller than human eyes, rabbit eyes are the most popular choice for Ex vivo models. However, rabbits have substantially greater penetration because their eyes lack Bowman’s layer. Pig’s eyes and human eyes are most comparable structurally. Contrarily, the size of a cow’s eye and its corneal epithelium are nearly twice as huge as those of a human being [103]. Ghada A. Abdelbary et al. [104] performed an ex-vivo corneal permeation study of proniosomal gel for ocular delivery on excised rabbit’s cornea using a Franz diffusion cell. A significantly higher permeability coefficient (p < 0.05) and a steady state flow of 2.44 mcg/cm2h and 0.000244 cm2/h, have been observed respectively [104]. Ex vivo experiments on fresh and thawed corneal tissues of pigs by Aller Rodriguez et al. [105] discovered that transcorneal penetration of cyclosporine A prodrug is a simple and reliable model.
Future prospective
A combination of niosome and in-situ gels has emerged as a compelling area of research in the realm of pharmaceutical sciences. With their unique properties, this amalgamation presents a plethora of opportunities for advancing drug delivery mechanisms [61,35]. The future of niosome-loaded in-situ gel holds significant promise in revolutionizing therapeutic approaches, ensuring targeted and controlled drug delivery, and enhancing patient compliance [11]. With the continuous progress in nanotechnology and pharmaceutical sciences, the future of niosome-loaded in-situ gel envisages the development of personalized medicine. Beyond their pharmaceutical applications, niosome-loaded in-situ gels hold promise in the fields of biomedical research and cosmetics [106,26]. Some of the patented and marketed formulations of reversible gel are shown in Tables 6 and 7.
CONCLUSION
The incorporation of niosome into an in-situ gel system provides a novel and synergistic method for boosting the distribution of therapeutic agents, overcoming the drawbacks of traditional drug delivery methods, and enhancing patient outcomes. Niosomes’ capacity to encapsulate a variety of pharmaceuticals and the in-situ gels’ ability to gel at different temperatures or pH levels enable fine control over drug release kinetics and the localization of drug activity. Niosome-loaded in situ gel has proven tremendous potential in several biological applications due to its combined benefits, which include improved stability, targeted drug delivery, sustained release, and increased bioavailability. Furthermore, niosome-loaded in-situ gel systems have shown promise in overcoming biological barriers, facilitating localized and sustained drug release at the target region, and encouraging prolonged residence time, notably in mucosal and ocular drug delivery applications. At present there is no marketed formulation based on niosome in situ gels for ocular delivery. Therefore, to make optimal use of this novel strategy and to satisfy the changing healthcare needs of various patient populations, multidisciplinary research and collaboration efforts are necessary. There is an urgent need to explore the potential of this delivery method using clinical trials.
List of Abbreviations
CPP: Critical packaging parameter; EE: Entrapment efficiency; HLB: Hydrophilic-lipophilic balance; HPLC: High-performance liquid chromatography; HPMC: Hydroxy propyl methyl cellulose; LCST: Lower critical solution temperature; NLC: Nanostructure lipid carrier; PDI: Polydispersity index; PNIPAAm: Poly-N-Isopropylacrylamide; PS: Particle size; REV: Reverse phase evaporation; SEM: Scanning electron microscopy; Sol: Solution; STF: Simulated tear fluid; TEM: Transmission electron microscopy; ZP: Zeta potential.
Acknowledgment
All authors conveyed special thanks to Mr. Jitender Joshi (Chancellor) and Prof. (Dr.) Dharam Buddhi (Vice Chancellor) of Uttaranchal University for their research-associated encouragement.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Al-Kinani AA, Zidan G, Elsaid N, Seyfoddin A, Alani AWG, Alany RG. Ophthalmic gels: past, present and future. Adv Drug Deliv Rev. 2018;126:113–26.
2. Qiao H, Xu Z, Sun M, Fu S, Zhao F, Wang D, et al. Rebamipide liposome as an effective ocular delivery system for the management of dry eye disease. J Drug Deliv Sci Technol. 2022;75:103654.
3. Fathalla D, Fouad EA, Soliman GM. Latanoprost niosomes as a sustained release ocular delivery system for the management of glaucoma. Drug Dev Ind Pharm. 2020;46(5):806–13.
4. Ghezzi M, Ferraboschi I, Delledonne A, Pescina S, Padula C, Santi P, et al. Cyclosporine-loaded micelles for ocular delivery: investigating the penetration mechanisms. J Control Release 2022;349:744–55.
5. Dhaval M, Devani J, Parmar R, Soniwala MM, Chavda J. Formulation and optimization of microemulsion based sparfloxacin in-situ gel for ocular delivery: in vitro and ex vivo characterization. J Drug Deliv Sci Technol. 2020;55:101373.
6. Kandpal N, Ale Y, Semwal YC, Padiyar N, Jakhmola V, Farswan AS, et al. Proniosomes: a provesicular system in ocular drug delivery. J Adv Biotechnol Exp Ther. 2023;6(3):622–37.
7. Kwon S, Kim SH, Khang D, Lee JY. Potential therapeutic usage of nanomedicine for glaucoma treatment. Int J Nanomed. 2020;15:5745–65.
8. Izhar MP, Hafeez A, Kushwaha P, Simrah. Drug delivery through niosomes: a comprehensive review with therapeutic applications. J Clust Sci. 2023;34(5):2257–73.
9. Sharma A. Recent innovation in niosomes-A comprehensive review of advancements and applications. Int J Pharm Prof Res. 2023;14(3):20–34.
10. Majeed A, Khan NA. Ocular in situ gel: an overview. J Drug Deliv Ther. 2019;9(1):337–47.
11. Chaudhari P, Shetty D, Lewis SA. Recent progress in colloidal nanocarriers loaded in situ gel in ocular therapeutics. J Drug Deliv Sci Technol. 2022;71:103327.
12. Allam A, Elsabahy M, El Badry M, Eleraky NE. Betaxolol-loaded niosomes integrated within pH-sensitive in situ forming gel for management of glaucoma. Int J Pharm. 2021;598:120380. CrossRef
13. Willoughby CE, Ponzin D, Ferrari S, Lobo A, Landau K, Omidi Y. Anatomy and physiology of the human eye: effects of mucopolysaccharidoses disease on structure and function—a review. Clin Exp Ophthalmol. 2010;38(SUPPL. 1):2–11.
14. Wilson CG. Back of the eye anatomy and physiology: impact on product development. AAPS Adv Pharm Sci Ser. 2021;37:67–92.
15. Diwan P, Jangde R, Khunte S, Bhardwaj H, Suresh PK, Diwan P, et al. Ocular Drug Delivery System: Barrier for Drug Permeation, Method to Overcome Barrier. Drug Development Life Cycle. 2022. doi:10.5772/INTECHOPEN.105401.
16. Verma A, Tiwari A, Saraf S, Panda PK, Jain A, Jain SK. Emerging potential of niosomes in ocular delivery. Expert Opin Drug Deliv. 2021;18(1):55–71.
17. Durak S, Rad ME, Yetisgin AA, Sutova HE, Kutlu O, Cetinel S, et al. Niosomal drug delivery systems for ocular disease-recent advances and future prospects. Nanomaterials (Basel). 2020;10(6):1–29.
18. Marianecci C, Di Marzio L, Rinaldi F, Celia C, Paolino D, Alhaique F, et al. Niosomes from 80s to present: the state of the art. Adv Colloid Interface Sci. 2014;205:187–206.
19. Dehaghi MH, Haeri A, Keshvari H, Abbasian Z, Dadashzadeh S. Dorzolamide loaded niosomal vesicles: comparison of passive and remote loading methods. Iran J Pharm Res. 2017;16(2):413.
20. Fritze A, Hens F, Kimpfler A, Schubert R, Peschka-Süss R. Remote loading of doxorubicin into liposomes driven by a transmembrane phosphate gradient. Biochim Biophys Acta. 2006;1758(10):1633–40.
21. Rajera R, Nagpal K, Singh SK, Mishra DN. Niosomes: a controlled and novel drug delivery system. Biol Pharm Bull. 2011;34(7):945–53.
22. Destruel PL, Zeng N, Seguin J, Douat S, Rosa F, Brignole-Baudouin F, et al. Novel in situ gelling ophthalmic drug delivery system based on gellan gum and hydroxyethylcellulose: innovative rheological characterization, in vitro and in vivo evidence of a sustained precorneal retention time. Int J Pharm. 2020;574:118734.
23. Anbarasan B, Thanka J. Optimization and evaluation of temperature triggered in situ hydrogels for an effective treatment of ophthalmic preparations—a perlustration. Int J Pharm Sci Rev Res. 2018;50(2):34–9.
24. Saraf S, Ajazuddin AA, Khan J, Giri TK, Tripathi DK, et al. Advancement in stimuli triggered in situ gelling delivery for local and systemic route. Expert Opin Drug Deliv. 2012;9(12):1573–92.
25. Ward MA, Georgiou TK. Thermoresponsive gels based on ABA triblock copolymers: Does the asymmetry matter? J Polym Sci A Polym Chem. 2013;51(13):2850–9.
26. Almeida H, Amaral MH, Lobão P, Lobo JMS. In situ gelling systems: a strategy to improve the bioavailability of ophthalmic pharmaceutical formulations. Drug Discov Today. 2014;19(4):400–12.
27. Wei G, Xu H, Ding PT, Li SM, Zheng JM. Thermosetting gels with modulated gelation temperature for ophthalmic use: the rheological and gamma scintigraphic studies. J Control Release. 2002;83(1):65–74.
28. Pandey M, Choudhury H, Aziz ABA, Bhattamisra SK, Gorain B, Su JST, et al. Potential of stimuli-responsive in situ gel system for sustained ocular drug delivery: recent progress and contemporary research. Polymers. 2021;13(8):1340.
29. Elmotasem H, Awad GEA. A stepwise optimization strategy to formulate in situ gelling formulations comprising fluconazole-hydroxypropyl-beta-cyclodextrin complex loaded niosomal vesicles and Eudragit nanoparticles for enhanced antifungal activity and prolonged ocular delivery. Asian J Pharm Sci. 2020;15(5):617–36.
30. Gugleva V, Titeva S, Ermenlieva N, Tsibranska S, Tcholakova S, Rangelov S, et al. Development and evaluation of doxycycline niosomal thermoresponsive in situ gel for ophthalmic delivery. Int J Pharm. 2020;591. doi:10.1016/j.ijpharm.2020.120010.
31. Hemant K, Mukesh G, Rakesh G. pH Sensitive in situ ocular gel: a review. 2014.
32. Garge LV, Saudagar R. Ophthalmic pH sensitive in-situ gel: a review. J Drug Deliv Ther. 2019;9(2-s):682–9.
33. Suresh C, Abhishek S, Chand S. pH sensitive in situ ocular gel: a review. J Pharm Sci Biosci Res. 2016;6(5):684–94.
34. Zafar A, Alsaidan OA, Imam SS, Yasir M, Alharbi KS, Khalid M. Formulation and evaluation of moxifloxacin loaded bilosomes in-situ gel: optimization to antibacterial evaluation. Gels. 2022;8(7):418. CrossRef
35. Wu Y, Liu Y, Li X, Kebebe D, Zhang B, Ren J, et al. Research progress of in-situ gelling ophthalmic drug delivery system. Asian J Pharm Sci. 2019;14(1):1–15.
36. Gupta H, Velpandian T, Jain S. Ion- and pH-activated novel in-situ gel system for sustained ocular drug delivery. J Drug Target. 2010;18(7):499–505.
37. Salunke SR, Patil SB. Ion activated in situ gel of gellan gum containing salbutamol sulphate for nasal administration. Int J Biol Macromol. 2016;87:41–7.
38. Balasubramaniam J, Pandit JK. Ion-activated in situ gelling systems for sustained ophthalmic delivery of ciprofloxacin hydrochloride. Drug Deliv. 2003;10(3):185–91.
39. Destruel PL, Zeng N, Brignole-Baudouin F, Douat S, Seguin J, Olivier E, et al. In situ gelling ophthalmic drug delivery system for the optimization of diagnostic and preoperative mydriasis: in vitro drug release, cytotoxicity and mydriasis pharmacodynamics. Pharmaceutics. 2020;12(4):360.
40. Padmasri B, Nagaraju R, Prasanth D. A comprehensive review on in situ gels. Int J Appl Pharm. 2020;12(6):24–33.
41. Clogston J, Rathman J, Tomasko D, Walker H, Caffrey M. Phase behavior of a monoacylglycerol: (Myverol 18-99K)/water system. Chem Phys Lipids. 2000;107(2):191–220.
42. Phaechamud T, Setthajindalert O. Antimicrobial in-situ forming gels based on bleached shellac and different solvents. J Drug Deliv Sci Technol. 2018;46:285–93.
43. Hassan RM, Khairou KS, Awad AM. New aspects to physicochemical properties of polymer gels in particularly the coordination biopolymeric metal–alginate ionotropic hydrogels. Polym Gels Synth Charact. 2018;275–354.
44. Patel LD, Shastri DH, Patel LD. A novel alternative to ocular drug delivery system: hydrogel. Int J Pharm Res. 2010;2(1):1–13. Available from: https://www.researchgate.net/publication/286322402
45. Hasanji FM, Patel AK, Patel VM. In-situ gel: popular novel sustained release technique. Int J Pharm Res Appl.7:601.
46. Ahmad W, Singh A, Jaiswal KK, Gupta P. Green synthesis of photocatalytic TiO2 nanoparticles for potential application in photochemical degradation of ornidazole. J Inorg Organomet Polym Mater. 2021;31(2):614–23.
47. Meshram S, Thorat S. Review article ocular in situ gels: development, evaluation and advancements. Sch Acad J Pharm. 2015;4(7):340–46.
48. Shaikh MAJ, Alharbi KS, Almalki WH, Imam SS, Albratty M, Meraya AM, et al. Sodium alginate based drug delivery in management of breast cancer. Carbohydr Polym. 2022;292:119689.
49. Bashir R. An in sight into novel drug delivery system: in situ gels. Cellmed Orthocell Med Pharm Assoc. 2021;1–7.
50. Pande S. Liposomes for drug delivery: review of vesicular composition, factors affecting drug release and drug loading in liposomes. Artif Cells Nanomed Biotechnol. 2023;51(1):428–40.
51. Sana SS, Kumbhakar DV, Pasha A, Pawar SC, Grace AN, Singh RP, et al. Crotalaria verrucosa leaf extract mediated synthesis of zinc oxide nanoparticles: assessment of antimicrobial and anticancer activity. Molecules. 2020;25(21):4896.
52. Alyami H, Abdelaziz K, Dahmash EZ, Iyire A. Nonionic surfactant vesicles (niosomes) for ocular drug delivery: development, evaluation and toxicological profiling. J Drug Deliv Sci Technol. 2020;60:102069.
53. Dave V, Paliwal S. A novel approach to formulation factor of aceclofenac eye drops efficiency evaluation based on physicochemical characteristics of in vitro and in vivo permeation. Saudi Pharm J. 2014;22(3):240–5.
54. Han H, Li S, Xu M, Zhong Y, Fan W, Xu J, et al. Polymer- and lipid-based nanocarriers for ocular drug delivery: current status and future perspectives. Adv Drug Deliv Rev. 2023;196:114770.
55. Ana R da, Fonseca J, Karczewski J, Silva AM, Zieli?ska A, Souto EB. Lipid-based nanoparticulate systems for the ocular delivery of bioactives with anti-inflammatory properties. Int J Mol Sci. 2022;23(20):12102. CrossRef
56. Patel D. Niosome drug delivery system: basics, advantage, disadvantage, applications. Gandhinagar, India: SIPS; 2020.
57. Sharma D, Ali AAE, Aate JR. Niosomes as novel drug delivery system: review article. Pharmatutor. 2018;6(3):58.
58. Gugleva V, Titeva S, Rangelov S, Momekova D. Design and in vitro evaluation of doxycycline hyclate niosomes as a potential ocular delivery system. Int J Pharm. 2019;567:118431.
59. Kattar A, Quelle-Regaldie A, Sánchez L, Concheiro A, Alvarez-Lorenzo C. Formulation and characterization of epalrestat-loaded polysorbate 60 cationic niosomes for ocular delivery. Pharmaceutics. 2023;15(4):1247.
60. Bhardwaj P, Tripathi P, Gupta R, Pandey S. Niosomes: a review on niosomal research in the last decade. J Drug Deliv Sci Technol. 2020;56:101581.
61. Durak S, Rad ME, Yetisgin AA, Sutova HE, Kutlu O, Cetinel S, et al. Niosomal drug delivery systems for ocular disease—recent advances and future prospects. Nanomaterials. 2020;10(6):1191. CrossRef
62. Allam A, El-Mokhtar MA, Elsabahy M. Vancomycin-loaded niosomes integrated within pH-sensitive in-situ forming gel for treatment of ocular infections while minimizing drug irritation. J Pharm Pharmacol. 2019;71(8):1209–21.
63. Pitorre M, Gondé H, Haury C, Messous M, Poilane J, Boudaud D, et al. Recent advances in nanocarrier-loaded gels: Which drug delivery technologies against which diseases? J Control Release. 2017;266:140–55.
64. Nowroozi F, Almasi A, Javidi J, Haeri A, Dadashzadeh S. effect of surfactant type, cholesterol content and various downsizing methods on the particle size of niosomes. Iran J Pharm Res. 2018;17(Suppl2):1.
65. El-Laithy HM, Shoukry O, Mahran LG. Novel sugar esters proniosomes for transdermal delivery of vinpocetine: preclinical and clinical studies. Eur J Pharm Biopharm. 2011;77(1):43–55.
66. Žiniauskait? A, C?pla V, Jelinskas T, Eimont R, Ul?inas A, Aldonyt? R, et al. Introducing an efficient in vitro cornea mimetic model for testing drug permeability. Sci. 2021;3(3):30.
67. Biswal S, Murthy PN, Sahu J, Sahoo P, Amir F. Vesicles of non-ionic surfactants (niosomes) and drug delivery potential. Int J Pharm Sci Nanotechnol. 2008;1(1):1–8.
68. Kaur D, Kumar S. Niosomes: present scenario and future aspects. J Drug Deliv Ther. 2018;8(5):35–43.
69. Chandu VP, Arunachalam A, Jeganath S, Yamini K, Tharangini K, Chaitanya G. Niosomes: a novel drug delivery system. Int J Novel Trends Pharm Sci. 2012;2(1):25–31.
70. Ramana Reddy KV, Reddy V, Ramana Reddy KV. Factors affecting in formation of niosomes. Indo Am J Pharm Res. 2014;2014(04):4.
71. Mokhtar M, Sammour OA, Hammad MA, Megrab NA. Effect of some formulation parameters on flurbiprofen encapsulation and release rates of niosomes prepared from proniosomes. Int J Pharm. 2008;361(1–2):104–11.
72. Yadav JD, Kulkarni PR, Vaidya KA, Shelke GT. Niosomes: a review. J Pharm Res. 2011;4(3):632–6.
73. Shreedevi HM, Adlin J, Nesalin J, Mani TT. Development and evaluation of stavudine niosome by ether injection method. Int J Pharma Sci Res. 2016;7(1):38–46.
74. Rao AA, Arvapalli S, Rao GSNK, Malothu N, Bandaru NR. Design and evaluation of acyclovir niosomes. Res J Pharm Technol. 2021;14(8):4185–8.
75. Sharma R, Dua JS, Prasad DN, Kaushal S, Puri A. Formulation and evaluation of clindamycin phosphate niosomes by using reverse phase evaporation method. J Drug Deliv Ther. 2019;9(3-s):515–23.
76. Paradkar MU, Parmar M. Formulation development and evaluation of Natamycin niosomal in-situ gel for ophthalmic drug delivery. J Drug Deliv Sci Technol. 2017;39:113–22.
77. Wada AM, Chanana A, Singh RP. A review on niosomes in ocular drug delivery system (ODDS). Int J Pharm Res Appl. 2022;7(6):2023–44.
78. Patel VP, Pande V V, P. V. K. Design, Development and Evaluation of Dexamethasone Sodium Phosphate Niosomal in-situ Gel for Visual Medication Conveyance. International Journal of Pharmaceutical Sciences and Nanotechnology. 2018;11(5):4274–4279.
79. Bharath S, Karuppaiah A, Siram K, Hariharan S, Santhanam R, Veintramuthu S. Development and evaluation of a pH triggered in situ ocular gel of brimonidine tartrate. J. Res. Pharm. 2020;24:416–24. CrossRef
80. Kumar BS, Krishna R, Lakshmi PS, Vasudev DT, Nair SC. Formulation and evaluation of niosomal suspension of cefixime. Asian J Pharm Clin Res. 2017;10(5):194–201.
81. Article O, Acharya A, Kumar K, Gulzar Ahmed M, Paudel S. A novel approach to increase the bioavailability of candesartan cilexetil by proniosomal gel formulation: in-vitro and in-vivo evaluation. Int J Pharm Pharm Sci. 2016;8(1):241–46.
82. Souza SD’, Keck CM. A review of in vitro drug release test methods for nano-sized dosage forms. Adv Pharm. 2014;2014:1–2. CrossRef
83. Kim Y, Park EJ, Kim TW, Na DH. Recent progress in drug release testing methods of biopolymeric particulate system. Pharmaceutics. 2021;13(8):1313. CrossRef
84. More A, Ambekar AW. Development and characterization of nanoemulsion gel for topical drug delivery of nabumetone. Int J Pharm Pharm Res. 2016;7(3):126–57.
85. Kar M, Chourasiya Y, Maheshwari R, Tekade RK. Current developments in excipient science: Implication of quantitative selection of each excipient in product development. Basic fundamentals of drug delivery. Cambridge, MA: Academic Press; 2018. pp 29–83.
86. Schafer KA, Bolon B. Special senses-ear. Fundamentals of toxicologic pathology, 3rd ed. Cambridge, MA: Academic Press; 2017. pp 729–47.
87. Lee M, Hwang JH, Lim KM. Alternatives to in vivo draize rabbit eye and skin irritation tests with a focus on 3D reconstructed human cornea-like epithelium and epidermis models. Toxicol Res. 2017;33(3):191.
88. Aboali FA, Habib DA, Elbedaiwy HM, Farid RM. Curcumin-loaded proniosomal gel as a biofreindly alternative for treatment of ocular inflammation: in-vitro and in-vivo assessment. Int J Pharm. 2020;589. CrossRef
89. Dantas MGB, Reis SAGB, Damasceno CMD, Rolim LA, Rolim-Neto PJ, Carvalho FO, et al. Development and evaluation of stability of a gel formulation containing the monoterpene borneol. Sci World J. 2016;2016:7394685 CrossRef
90. Agha OA, Girgis GNS, El-Sokkary MMA, Soliman OAEA. Spanlastic-laden in situ gel as a promising approach for ocular delivery of Levofloxacin: in-vitro characterization, microbiological assessment, corneal permeability and in-vivo study. Int J Pharm X. 2023;6:100201. CrossRef
91. Ibrahim SS. The role of surface active agents in ophthalmic drug delivery: a comprehensive review. J Pharm Sci. 2019;108(6):1923–33.
92. Roguet R, Dossou KG, Rougier A. Prediction of eye irritation potential of surfactants using the SIRC-NRU cytotoxicity test. Alternat Lab Animals. 1992;20(3):451–56; CrossRef
93. Sonia TA, Sharma CP. Lipids and inorganic nanoparticles in oral insulin delivery. Oral Delivery of Insulin. Amsterdam, The Netherlands: Elsevier; 2014. pp 219–56.
94. Jahan K, Balzer S, Mosto P. Toxicity of nonionic surfactants. WIT Trans Ecol Environ. 2008;110:281.
95. Abdelkader H, Ismail S, Hussein A, Wu Z, Al-Kassas R, Alany RG. Conjunctival and corneal tolerability assessment of ocular naltrexone niosomes and their ingredients on the hen’s egg chorioallantoic membrane and excised bovine cornea models. Int J Pharm. 2012;432(1–2):1–10.
96. Agarwal P, Rupenthal ID. In vitro and ex vivo corneal penetration and absorption models. Drug Deliv Transl Res. 2016;6(6):634–47.
97. Ahuja M, Sharma SK, Majumdar DK. In vitro corneal permeation of diclofenac from oil drops. Yakugaku Zasshi. 2007;127(10):1739–45.
98. Bíró T, Bocsik A, Dukovski BJ, Gróf I, Lovri? J, Csóka I, et al. New approach in ocular drug delivery: in vitro and ex vivo investigation of cyclodextrin-containing, mucoadhesive eye drop formulations. Drug Design Dev Ther 2021; 15: 351–60. CrossRef
99. Tho I, Škalko-Basnet N. Cell-based in vitro models for vaginal permeability studies. Concepts and Models for Drug Permeability Studies: Cell and Tissue based in Vitro Culture Models. Cambridge, MA: Woodhead Publishing; 2015. pp 115–28.
100. Veit JGS, Birru B, Singh R, Arrigali EM, Serban MA. An in vitro model for characterization of drug permeability across the tympanic membrane. Pharmaceuticals. 2022;15(9):1114.
101. Scholz M, Lin JEC, Lee VHL, Keipert S. Pilocarpine permeability across ocular tissues and cell cultures: influence of formulation parameters. J Ocul Pharmacol Ther. 2002;18(5):455–68.
102. Becker U, Ehrhardt C, Schneider M, Muys L, Gross D, Eschmann K, et al. A comparative evaluation of corneal epithelial cell cultures for assessing ocular permeability. Altern Lab Anim. 2008;36(1):33–44.
103. De Hoon I, Boukherroub R, De Smedt SC, Szunerits S, Sauvage F. In vitro and ex vivo models for assessing drug permeation across the Cornea. Mol Pharm. 2023;20(7):3298–319.
104. Abdelbary GA, Amin MM, Zakaria MY. Ocular ketoconazole-loaded proniosomal gels: formulation, ex vivo corneal permeation and in vivo studies. Drug Deliv. 2017;24(1):309–19. CrossRef
105. Rodriguez-Aller M, Guillarme D, El Sanharawi M, Behar-Cohen F, Veuthey JL, Gurny R. In vivo distribution and ex vivo permeation of cyclosporine A prodrug aqueous formulations for ocular application. J Control Release. 2013;170(1):153–9.
106. Weng Y, Liu J, Jin S, Guo W, Liang X, Hu Z. Nanotechnology-based strategies for treatment of ocular disease. Acta Pharm Sin B. 2017;7(3):281–91.
107. Bruschi ML, Borghi-Pangoni FB, Junqueira MV, de Souza Ferreira SB. Nanostructured therapeutic systems with bioadhesive and thermoresponsive properties. Nanostruct Novel Ther Syn Charact Appl. 2017;313–42.
108. Nishinari K, Takemasa M, Zhang H, Takahashi R. Storage plant polysaccharides: xyloglucans, galactomannans, glucomannans. Comprehen Glycosci Chem Syst Biol. 2007;2–4:613–52.
109. Kumar VV, Chetty CM, Reddy YD, Ugandar RE, Gladiola BD. Formulation and in vitro characterization of ocular in situ gels of valcyclovir. J Pharm Sci Res. 2019;11(8):2974–9.
110. Khare P, Chogale MM, Kakade P, Patravale VB. Gellan gum–based in situ gelling ophthalmic nanosuspension of Posaconazole. Drug Deliv Transl Res. 2022;12(12):2920–35.
111. Liu Y, Liu J, Zhang X, Zhang R, Huang Y, Wu C. In situ gelling gelrite/alginate formulations as vehicles for ophthalmic drug delivery. AAPS PharmSciTech. 2010;11(2):610.
112. Kurniawansyah IS, Rusdiana T, Sopyan I, Ramoko H, Wahab HA, Subarnas A. In situ ophthalmic gel forming systems of poloxamer 407 and hydroxypropyl methyl cellulose mixtures for sustained ocular delivery of chloramphenicole: optimization study by factorial design. Heliyon. 2020;6(11):e05365.
113. Abbas MN, Khan SA, Sadozai SK, Khalil IA, Anter A, El Fouly M, et al. Nanoparticles loaded thermoresponsive in situ gel for ocular antibiotic delivery against bacterial keratitis. Polymers (Basel). 2022;14(6). CrossRef
114. Laddha UD, Kshirsagar SJ. Formulation of nanoparticles loaded in situ gel for treatment of dry eye disease: in vitro, ex vivo and in vivo evidences. J Drug Deliv Sci Technol. 2021;61:102112. CrossRef
115. Datta S, Bhowmik R, Nath R, Chakraborty R, Chakraborty A. Formulation and evaluation of a nanoparticle laden in situ gel system for enhancing the ocular delivery of ciprofloxacin. Int J Pharm Sci Rev Res. 2021;70(2):156–63. CrossRef
116. Kesarla R, Tank T, Vora PA, Shah T, Parmar S, Omri A. Preparation and evaluation of nanoparticles loaded ophthalmic in situ gel. Drug Deliv. 2016;23(7):2363–70.
117. Londhe VY, Sharma S. Formulation, characterization, optimization and in-vivo evaluation of methazolamide liposomal in-situ gel for treating glaucoma. J Drug Deliv Sci Technol. 2022;67:102951.
118. Shukr MH, Ismail S, El-Hossary GG, El-Shazly AH. Design and evaluation of mucoadhesive in situ liposomal gel for sustained ocular delivery of travoprost using two steps factorial design. J Drug Deliv Sci Technol. 2021;61:102333.
119. Yu S, Wang QM, Wang X, Liu D, Zhang W, Ye T, et al. Liposome incorporated ion sensitive in situ gels for opthalmic delivery of timolol maleate. Int J Pharm. 2015;480(1–2):128–36.
120. Khallaf AM, El-Moslemany RM, Ahmed MF, Morsi MH, Khalafallah NM. Exploring a novel fasudil-phospholipid complex formulated as liposomal thermosensitive in situ gel for glaucoma. Int J Nanomed. 2022;17:163–81.
121. Morsi N, Ibrahim M, Refai H, El Sorogy H. Nanoemulsion-based electrolyte triggered in situ gel for ocular delivery of acetazolamide. Eur J Pharm Sci. 2017;104:302–14.
122. Mahboobian MM, Mohammadi M, Mansouri Z. Development of thermosensitive in situ gel nanoemulsions for ocular delivery of acyclovir. J Drug Deliv Sci Technol. 2020;55:101400.
123. Bhalerao H, Koteshwara K, Chandran S. Design, optimisation and evaluation of in situ gelling nanoemulsion formulations of brinzolamide. Drug Deliv Transl Res. 2020;10(2):529–47.
124. Lakhani P, Patil A, Taskar P, Ashour E, Majumdar S. Curcumin-loaded Nanostructured Lipid Carriers for ocular drug delivery: design optimization and characterization. J Drug Deliv Sci Technol. 2018;47:159–66.
125. Youssef AAA, Dudhipala N, Majumdar S. Dual drug loaded lipid nanocarrier formulations for topical ocular applications. Int J Nanomedicine. 2022;17:2283–99.
126. Wadetwar RN, Agrawal AR, Kanojiya PS. In situ gel containing Bimatoprost solid lipid nanoparticles for ocular delivery: in-vitro and ex-vivo evaluation. J Drug Deliv Sci Technol. 2020;56:101575.
127. Tatke A, Dudhipala N, Janga KY, Balguri SP, Avula B, Jablonski MM, et al. In situ gel of triamcinolone acetonide-loaded solid lipid nanoparticles for improved topical ocular delivery: tear kinetics and ocular disposition studies. Nanomaterials. 2018;9(1):33.
128. Chaudhari PD, Desai US. Formulation and evaluation of niosomal in situ gel of prednisolone sodium phosphate for ocular drug delivery. Int J Appl Pharm. 2019;11(2):97–116.
129. Aggarwal D, Kaur IP. Improved pharmacodynamics of timolol maleate from a mucoadhesive niosomal ophthalmic drug delivery system. Int J Pharm. 2005;290(1–2):155–9.
130. Jain N, Verma A, Jain N. Formulation and investigation of pilocarpine hydrochloride niosomal gels for the treatment of glaucoma: intraocular pressure measurement in white albino rabbits. Drug Deliv. 2020;27(1):888–99.
131. Zhu L, Ao J, Li P. A novel in situ gel base of deacetylase gellan gum for sustained ophthalmic drug delivery of ketotifen: in vitro and in vivo evaluation. Drug Des Devel Ther. 2015;9:3943–9.
132. Sathyavathi V, Hasansathali AA, Ilavarasan R, Sangeetha A. Formulation and evaluation of niosomal in situ gel ocular delivery system of brimonidine tartrate. Int J Life Sci Pharma Res. 2012;2(1):82–95.
133. Gugleva V, Michailova V, Mihaylova R, Momekov G, Zaharieva MM, Najdenski H, et al. Formulation and evaluation of hybrid niosomal in situ gel for intravesical co-delivery of curcumin and gentamicin sulfate. Pharmaceutics. 2022;14(4):747. CrossRef
134. Bisht M, Kanyal R, Bhardwaj M, Kumar A. Development and characterization of flurbiprofen loaded niosomal in-situ gel for ophthalmic drug delivery system. Annal Romanian Soc Cell Biol. 2021;25(6):20635–55. Available from: http://annalsofrscb.ro
135. Gupta P, Yadav KS. Formulation and evaluation of brinzolamide encapsulated niosomal in-situ gel for sustained reduction of IOP in rabbits. J Drug Deliv Sci Technol. 2022;67:103004.
136. Tegtmeyer S, Papantoniou I, Müller-Goymann CC. Reconstruction of an in vitro cornea and its use for drug permeation studies from different formulations containing pilocarpine hydrochloride. Eur J Pharm Biopharm. 2001;51(2):119–25.
137. Reichl S, Bednarz J, Müller-Goymann CC. Human corneal equivalent as cell culture model for in vitro drug permeation studies. Br J Ophthalmol. 2004;88(4):560–5.
138. Saarinen-Savolainen P, Järvinen T, Araki-Sasaki K, Watanabe H, Urtti A. Evaluation of cytotoxicity of various ophthalmic drugs, eye drop excipients and cyclodextrins in an immortalized human corneal epithelial cell line. Pharm Res. 1998;15(8):1275–80.
139. Van Der Bijl P, Engelbrecht AH, Van Eyk AD, Meyer D. Comparative permeability of human and rabbit corneas to cyclosporin and tritiated water. J Ocul Pharmacol Ther. 2002;18(5):419–27.
140. Van Der Bijl P, Van Eyk AD, Seifart HI, Meyer D. In vitro transcorneal penetration of metronidazole and its potential use as adjunct therapy in Acanthamoeba keratitis. Cornea. 2004;23(4):386–9.
141. Loch C, Zakelj S, Kristl A, Nagel S, Guthoff R, Weitschies W, et al. Determination of permeability coefficients of ophthalmic drugs through different layers of porcine, rabbit and bovine eyes. Eur J Pharm Sci. 2012;47(1):131–8.
142. Remington LA. Cornea and Sclera. Clinical anatomy and physiology of the visual system. New York, NY: Elsevier Health Sciences; 2012. pp 10–39.
143. Pawar PK, Majumdar DK. Effect of formulation factors on in vitro permeation of moxifloxacin from aqueous drops through excised goat, sheep, and buffalo corneas. AAPS Pharm Sci Tech. 2006;7(1):E89–E94. CrossRef
144. Xia E, Furr RB. United States (12) Patent Application Publication (10) Pub. 2002;(43).
145. Lin. Date of Patent. 2003;511(45):9–10.
146. Xia E et al. Reversible gelling system for ocular drug delivery. 2004;1–6.
147. Adeyeye M et al. Ophthalmic drug delivery system of estradiol or other estrogen for prevention of cataracts. United States Patent Application Publication. 2011.
148. Mohan N. In-situ gel forming solution for ocular drug delivery—oogle Patents. WIPO (PCT). 2011. Available from: https://patents.google.com/patent/WO2011018800A3/en. Accessed 23 September 2023.
149. Banerjee et al. Nanoparticulate in-situ gels of TPGS, gellan and PVA as vitreous humor substitutes. United states patent. 2013.
150. Verma S, Nainwal N, Kikon NY, Ali A, Jakhmola V. Hopes and hurdles of nanogels in the treatment of ocular diseases. J Appl Pharm Sci. 2024;14,(2):001–012.
151. Ahmed TA, Aljaeid BM. A potential in situ gel formulation loaded with novel fabricated poly(lactide-co-glycolide) nanoparticles for enhancing and sustaining the ophthalmic delivery of ketoconazole. Int J Nanomed. 2017;12:1863–75.