INTRODUCTION
Plants have played a significant role in human life, and their utilization for treating various diseases has a long history [1]. The earliest documented records of using medicinal plants date back to 2,600 BC, authored by the Sumerians and Akkadians. In ancient India, the knowledge of medicinal herbs and their application was described in the Rigveda and Atharvaveda (3,500–1,500 B.C.), paving the way for the alternative medical system of Ayurveda [2]. In the present era, over 70% of people worldwide rely on plant-derived medicines to treat various illnesses and health conditions [3]. India stands at the forefront of medicinal plant cultivation and is renowned as the “Botanical Garden of the World.” The medicinal plant market in India is currently valued at 4.2 billion (56.6 million USD) and is projected to reach 14 billion (188.6 million USD) by 2026 [4]. According to a World Health Organization (WHO) estimate, approximately 75% of people all over the world incorporate herbal remedies as alternative or complementary medicine. Plant-derived drugs are enormously consumed, particularly in combating various types of malignancies, due to their antioxidant, immune-modulatory, and cancer-healing properties, which offer minimal side effects.
Each year, more people throughout the world succumb to cancer. According to the “Globocon 2022” survey conducted by the WHO, nearly 19,292,789 new cancer cases were reported worldwide in 2020, resulting in 9,958,133 deaths across genders [5,6]. Lung cancer remains the leading cause of death with 18.4% of cancer cases, followed by breast cancer in women at 11.6%, and stomach and liver cancer at 8.2% each. Nearly 7% of cancer-related deaths are attributable to prostate and colorectal cancers, respectively [7,8].
Three basic categories of etiological factors, namely chemical, physical, and environmental, are responsible for turning normal cells into cancerous cells. Chemical carcinogens such as benzpyrene, asbestos, and over 800 other chemical moieties; physical carcinogens, such as various forms of ionizing radiation; and biological carcinogens, including microorganisms, are to name a few [9,10]. In addition, environmental factors like pollution and lifestyle choices such as smoking, alcohol consumption, sedentary living, obesity, and the adoption of high-fat, high-calorie diets significantly contribute to the incidence of various malignancies [11]. In practical terms, identifying the precise etiology and genetic factors responsible for a specific form of cancer is a complex process with limited treatment options. Modern cancer treatment methods primarily involve chemotherapy, radiation therapy, and immunotherapy, accompanied by surgical tumor removal through different procedures. However, chemotherapeutic agents often produce multiple side effects and adverse drug events, affecting normal cells such as the bone marrow, gastrointestinal tract, and hair follicles. In addition, synthetic drugs face significant challenges, including drug resistance [4].
The utilization of plants by ancient individuals, driven by astute observation and inherited sagacity, holds historical significance. Phyto-origin drugs offer substantial advantages over synthetic medications, displaying minimal side effects, adverse events, cost-effectiveness, efficacy, and low toxicity. The anti-cancer mechanisms of phytoconstituents encompass modulation of cell growth, differentiation, induction of cell death, hindrance of angiogenesis, and obstruction of metastasis. Recently, research on phytodrugs has been escalating exponentially [12]. In addition, more than 50% of the approved new chemical entities registered with the USFDA in the last 15 years originate from phytoconstituents and their derivatives [13]. Phytopharmaceuticals and their derivatives, with their diversified structures and distinctive pharmacological and molecular characteristics, harness potential as chemotherapeutic agents. Alkaloids isolated from Vinca rosea, such as Vincristine, Vinblastine, Taxane diterpenoids (Paclitaxel and Docetaxel), Epipodophyllotoxin (Etoposide and Teniposide), and Camptothecin, have significantly contributed to cancer chemotherapy [14,15]. Drugs such as Paclitaxel and Camptothecin command over 30% of the world’s anti-cancer drug market [16], accounting for nearly $9 billion, underscoring the significance of plant-derived drugs [17,18]. Numerous plant-origin drugs, such as Betulinic acid, Combretstatin, Curcumin, Homoharringtonine, Indirubin, Flavipiridol, Roscovitine, Brucantine, Lycophene, Resveratrol, and Silvestrol, are currently undergoing clinical or preclinical trials, showcasing their potential for future utilization [14,19]. Therefore, access to ethnobotanical information on medicinal plants from both traditional and folklore sources is indispensable for the scientific community to develop innovative drugs and drug delivery strategies. Kerala, renowned as “God’s own country,” is celebrated for its biodiversity and traditional Ayurvedic wisdom. The tribes of Kerala, comprising Malayans, Kurumbas, Karimpalans, Kattunaikans, Mullukkurumans, Malapanickars, Kadars, Koragas, and Cholanaikkans, possess extensive knowledge of medicinal plant usage for diverse ailments, spanning millennia [19]. The Wayanad and Kozhikode districts were chosen and surveyed for this review. This study approach encompassed 50% survey data, 30% meta-analysis reports, 11% observational studies, and 9% interventional studies. The PRISMA web tool (https://prisma-statement.org) was used for the systematic review guidelines [20]. Subsequently, a flow chart was plotted for the review screening process using the Prisma flow chart 2020 protocol. Furthermore, the screening of 552 articles obtained from Medline, Web of Science, and PubMed was done using the Ovid web tool (https://ovidsp.ovid.com/) [21] for duplication removal. The filtered articles underwent manual screening with two reviewers, and the final check was enabled by the third reviewer. The shortlisted articles underwent meta-analysis using the Meta-Mar web tool (https://meta-mar.shinyapps.io/) [22], covering subgroup analysis, effect size analysis, publication bias, and publication correlation.
The Wayanad and Kozhikode regions of Kerala
Kerala is situated on the south-western Malabar coast, with latitudes ranging from 8°18′ N to 12° 48′ N. The longitudes cover the span of 74° 52′ E–77°24′ E. The state is divided into three main regions, namely the eastern highlands, the central midlands, and the western lowlands. The selected territory falls between the eastern highlands and the central midlands, with latitudes ranging from 11°5′ N to 11° N and longitudes spanning from 75°2′ E to 76°5′ E. This region is situated between the Sayadri mountains and the coastal lowlands [23]. The climate in this area experiences a significant variation in temperatures throughout the year, ranging from 17°C to 48°C. During the summer months, temperatures can soar up to 50°C. In contrast, during the winter, the average temperature ranges from 17°C to 21°C in the Wayanad region and slightly higher (around ±5°C) in Kozhikode. The ethnobotanical information for this region was meticulously collected during the period from February 2021 to August 2022. Ethnobotany involves the study of the intricate relationship between plants and people, including the traditional knowledge and uses of plants in local communities. The data gathered during this time frame is expected to encompass the plant usage and traditional knowledge of the local people during that specific period [24].
The selected districts of “Wayanad and Kozhikode” in Kerala are known as the “Malabar Hill regions.” These areas are inhabited by various tribes, each with its own unique culture and practices. In the “Wayanad region,” the tribes include Adiyans, Paniyars, Mullukkurumans, Kattunaikans, Kurichyars of Wayanad, Kanaladi, and Wayanad Kadars. On the other hand, the tribes found in the “Kozhikode district” are Malapanickars, Malayans, Paniyans, Kattunaikans, Karimpalans, and Kurichyars [25,26]. The “Kadars” are inhabitants of various districts, including Malappuram, Wayanad, Thrissur, Kannur, and Kozhikode. The selected Malabar regions are represented in Figure 1.
The “Kattunaikans” tribes reside in the Palakkad, Kannur, and Kozhikode districts of the Malabar hills, where they are primarily involved in the cultivation of food grains and fruits. The “Mullukkurumans” community in Wayanad district is known for their traditional hunting and food gathering practices. However, in modern times, they have also extensively engaged in agriculture, particularly in growing various spices, herbs, medicinal plants, and millets. The “Ayurveda” system of medicine is widely practiced and utilized by both experts and common people in the selected study regions. Moreover, the region is renowned for its enormous spice collection, trading, and exports, making it a significant hub for the spice industry [20,27].
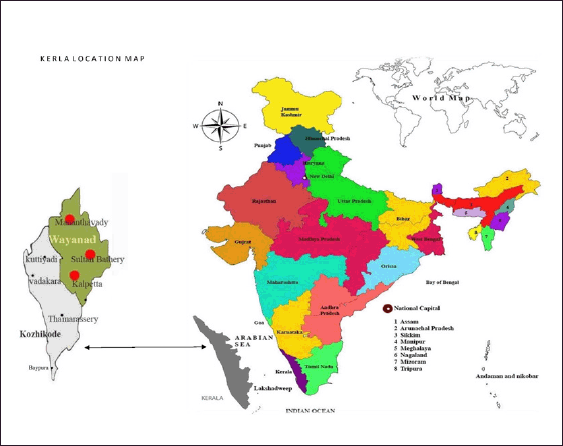 | Figure 1. The selected Malabar regions (Wayanad and Kozhikode) of Kerala. [Click here to view] |
MATERIALS AND METHODS
Data collection methods for the survey
The data collection of this survey enabled the “participatory rural appraisal method,” which proved to be a cost-effective and time-efficient approach. All the data for this study were gathered through structured interviews, semi-structured questionnaires, field surveys, sample collection, and note procurement. The study took place in the selected area of the Malabar Hills, which is inhabited by various tribal communities, including Mullukurumban, Kattunaikans, Karimpalans, Malapanickars, Malayans, Paniyans, Kurichyars, Kadars, and Koragas. Within these two districts, a total of 38 villages were included in the study, each hosting more than five types of indigenous tribes unique to that particular territory. Extensive interviews were conducted with the tribal people using different methods, predominantly semi-structured questionnaires and field surveys. In addition, traditional healers among the tribes were identified, and their knowledge of folklore-related ailments was documented. To ensure the reliability of the data, two separate visits were made to the tribal settlements, and the medicinal plant samples were locally identified. The samples were labeled with ethnobotanical information, and all the acquired information was diligently recorded in field notebooks.
Meta analysis
Literature search methods
The PRISMA systematic review guidelines were followed for this study, and the flow chart was plotted using the PRISMA web tool 2020 [20]. The article search was achieved using PubMed, Ovid/MEDLINE, Scopus, and Web of Science databases. The option for English abstracts from database inception to April 3, 2023, was enabled. The search strategy consisted of keywords related to phytochemicals, the English language, and cancer. Furthermore, screening of the 552 selected articles was carried out using the Ovid web tool (https://ovidsp.ovid.com) [21]. The Prisma flow chart for the screening process is illustrated in Figure 2.
Inclusion and exclusion criteria
The inclusion criteria encompassed full-text articles with phytoconstituents, anticancer activity, and pharmacological uses. The search was enabled using Boolean search terms such as “Phytoconstituents AND anticancer activity OR pharmacological uses” and “Phytochemicals OR plant constituents AND (Antitumor activity OR cytotoxicity) OR medicinal properties.” The articles that are phytoconstituent derivatives, incomplete data, duplicate articles, retracted articles, non-English articles, and articles not of interest were excluded. The inclusion and exclusion criteria were achieved with a manual review with two reviewers, followed by a final check by a third reviewer. The 347 shortlisted articles were screened for inclusion and exclusion criteria. Around 15 articles were excluded. The 331 original articles were subjected to the final screening process. Fifteen articles that were not the outcome of interest and five articles with phytochemical derivatives were excluded after screening, and the 311 articles were included for further analysis using the Meta-Mar web tool [22].
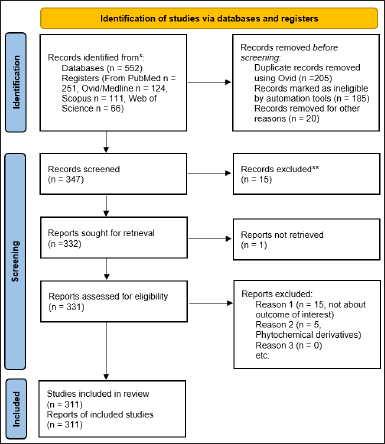 | Figure 2. Prisma flow chart for the systematic review screening process. [Click here to view] |
Meta analysis
The meta-analysis of the included articles covering risk ratio (RR), publication bias, publication correlation, subgroup analysis, effect size prediction, sensitivity, and heterogeneity was performed using the Meta-Mar web tool. The 311 articles were thoroughly analyzed statistically using the Meta-Mar web tool (https://meta-mar.com) [22]. Effect sizes were determined after the articles were chosen based on their quality and relevancy. To evaluate the diversity of the study outcomes and pinpoint heterogeneity, statistical techniques like Cochran’s Q and I² were utilized. To investigate the probable cause of the discrepancy in results among selected articles, subgroup analyses were performed. To examine the potential impact of study-specific factors on the noted effects, a meta-regression analysis was conducted. Analyses using funnel plots and statistical tests were used to determine the likelihood of publication bias and publication correlation. A sensitivity analysis was carried out to guarantee the accuracy of the findings when particular articles were excluded.
RESULTS
There are 4,600 native plants in Kerala, and 900 of them have potent therapeutic properties. More than 180 medicinal plants can be found in Kozhikode and Wayanad, the two districts that were chosen. Among these, 95 plant species from 46 plant families have demonstrated the possibility of possessing anticancer characteristics. The information was gathered and organized, and screening and meta-analysis were performed for phytoconstituents and pharmacological applications, including cancer (Table 1). The common names, vernacular names, folklore applications, and traditional usage were obtained from survey reports, observational, and interventional study data. The tribal communities have used medicinal plants for centuries. They prepare decoctions and pastes from plant parts, such as leaves, flowers, stems, barks, and roots. These preparations are ingested or applied externally for a variety of health conditions. The native tribal people have a deep knowledge of the medicinal properties of plants. They are able to identify the plants that are effective for specific conditions, and they know how to prepare the plants for optimal effectiveness.
The alternative medical science of “Ayurveda” is extensively practiced and embraced by the common people in the selected districts of Wayanad and Kozhikode. Despite this, it is essential to note that many of the selected medicinal plants remain unexplored or only partially explored in terms of their potential benefits and uses. Ayurvedic practitioners in the region have a long history, with records dating back 3,000 years detailing the usage of various medicinal plants found in the region. Some Ayurvedic preparations for the aforementioned medicinal herbs can be procured from Ayurvedic pharmacies. In Ayurveda, medicinal plants and their parts are formulated in different forms depending on the disease condition, need, and efficacy. These formulations include Choornams, Vati, Kashayams, Arishtams, Avaleha, and Tailams [27].
A comprehensive analysis was conducted utilizing 326 articles. In the context of a common effect model, the pooled RR exhibited a value of 0.6503 [95% confidence interval (CI): (0.6007; 0.7040)], indicating a statistically significant association (p < 0.0001). The random effects model demonstrated an RR of 0.4894 [95% CI: (0.3301; 0.7257)], which was also statistically significant (p = 0.0019). Heterogeneity within the study was substantial, with an I² value of 92.1% [95% CI: (88.3%; 94.7%)] and a corresponding Tau2 value of 0.3132 [95% CI: (0.1197; 1.1115)]. The Cochran’s Q test supported the presence of significant heterogeneity [Q: 152.23, degrees of freedom (d.f.): 12, p < 0.0001]. Subgroup analysis under the common effect model revealed varying RR values across different subgroups: subgroup 1 [RR = 0.2644, 95% CI: (0.1449; 0.4825)], subgroup 2 [RR = 0.4118, 95% CI: (0.3602; 0.4708)], subgroup 3 [RR = 0.9836, 95% CI: (0.8704; 1.1115)], and subgroup 4 [RR = 0.6563, 95% CI: (0.5519; 0.7805)]. Significant differences were identified among the subgroups (Q between groups: 97.32, d.f.: 3, p < 0.0001; Q within groups: 54.91, d.f.: 9, p < 0.0001). Similarly, the random effects model’s subgroup analysis demonstrated varying RR values: subgroup 1 [RR = 0.2644, 95% CI: (0.1073; 0.6514)], subgroup 2 [RR = 0.4355, 95% CI: (0.0970; 1.9554)], subgroup 3 [RR = 0.4784, 95% CI: (0.0000; 1482.5025)], and subgroup 4 [RR = 0.6376, 95% CI: (0.3002; 1.3543)]. While the differences in subgroups were not statistically significant in the random effects model (Q: 6.87, d.f.:3, p = 0.0763), a potential publication bias was assessed through Egger’s regression, yielding a nonsignificant result (t = −1.40, d.f.: 11, p = 0.1887). Subgroup meta-regression was carried out, indicating that the intercept was −1.2958 [standard error (SE): 0.4565, t-value: −2.8384, d.f.: 9, p = 0.0195], subgroup2 had a coefficient of 0.4650 (SE:0.5725, t-value: 0.8123, d.f.: 9, p = 0.4376), subgroup3 had a coefficient of 0.6880 (SE: 0.6462, t-value: 1.0646, d.f.: 9, p = 0.3148), and subgroup 4 had a coefficient of 0.8558 (SE: 0.5428, t-value: 1.5766, d.f.: 9, p = 0.1493). This comprehensive analysis underscores the complex interplay of factors influencing the observed outcomes. The relevant data are listed in Supplementary Tables S1–S6. The graphical visualization of the stated data as box plots, drapery plots, funnel plots, and forest plots is depicted in Figure 3a and b. A need for increased sensitivity is indicated by subgroup 1, which displays apparent imbalances between true positives (ranging from 3 to 6) and false negatives (ranging from 119 to 300). From ranges 11 to 29, false positives exist with a control strategy. True negatives exhibit rather an evenly distributed dispersion. The greater true positives (ranging from 33 to 180) and significant false negatives (ranging from 1,361 to 13,536) in subgroup 2 suggest that recall has to be improved. In addition, noteworthy are false positives, which range from 47 to 372. Subgroup 3 has a balanced performance with low false positives and high true negatives, along with respectable true positives and false negatives (between 1,699 and 50,448) in subgroup 4, which highlights the need to improve sensitivity. True negatives and false positives can be controlled. Improved sensitivity in subgroups 1, 2, and 4 while preserving precision is necessary to maximize model performance. Already, subgroup 3 performs in a more even manner (Fig. 3c). The numerical values are categorized and illustrated in Supplementary Table S7. The average “n” value and average “r” value for subgroup 1 are 38 and 0.44, respectively. Similarly, the “n” and “r” values of subgroup 2 are 144 and 0.18. The average “n” for subgroup 3 is 115, while the average “r” is 0.70. The data distribution is depicted as a Pareto chart (Fig. 3c), and the details are listed in Supplementary Table S8.
 | Table 1. Significant anticancer medicinal plants from Wayanad and Kozhikode districts of Kerala. [Click here to view] |
DISCUSSION
This review dives into 4,600 native plants in Kerala’s botanical treasure trove, 900 of which have therapeutic promise. The districts of Wayanad and Kozhikode stand out since they are home to more than 180 medicinal plants, 95 of which may have anticancer qualities. India is a significant exporter of almost 960 medicinal herbs to different regions of the globe, with 178 plant species being traded for more than 100 metric tons. The global market has a significant demand for medicinal herbs such as barberry, senna, isabgol, chandan, long pepper, brahmi, kalmegh, satavari, madhunashini, ashwagandha, sankhpushpi, kokum, and guggal, which are highly sought after. The Malabar Hill region, in particular, plays a crucial role, contributing more than 60% to the trade of medicinal plants [19]. Inspired by folklore origins, several drugs have been adapted for pilot studies to address chronic conditions such as psoriasis, leprosy, HIV infections, cancer, arthritis, tuberculosis, and asthma [23]. Some of these drugs are currently in clinical use, while others are undergoing various stages of clinical trials. In a collaborative effort with India, the WHO established the “Global Centre for Traditional Medicine” in 2022, with a significant contribution of 250 million USD from the Indian government ([email protected]). This initiative aims to promote traditional medicine and explore its potential benefits further. In this study, 552 articles were carefully examined, and the results revealed a statistically significant relationship between specific plants and their anticancer potential. However, the studies’ notable variation highlights the complexity of this topic. Finally, Kerala’s plant diversity offers hope for both conventional and cutting-edge medicine.
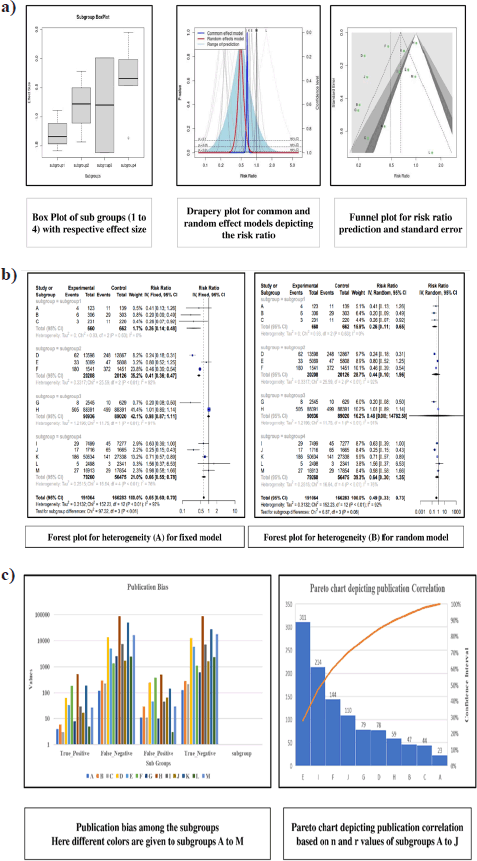 | Figure 3. (a). Box plot (effect size), Drapery (risk ratio), Funnel plot (standard error and risk ratio), (b). Forest plots for heterogeneity (A) fixed model, (B) random model, (c). Publication bias depicted as bar plot and publication correlation depicted as pareto chart. [Click here to view] |
CONCLUSION
The study’s findings emphasized Kerala’s enormous potential for using its rich botanical resources, with an emphasis on the Kozhikode and Wayanad districts. There are 4,600 native species in the area, and 900 of them have plausible therapeutic benefits. The ongoing practice of Ayurveda, a science rooted in history, adds to this treasure. The examination of 552 articles along with the survey emphasized the complexity of this topic and the necessity for careful analysis. Hence, in the existing conditions, the exploration of more medicinal plants with extraordinary phytoconstituents for cancer therapy is crucial. In addition, it is imperative to focus on reinstating medicinal herbs that are on the verge of extinction, utilizing modern, sophisticated methods and tissue culture techniques. The selected region, along with the entire Malabar Mountains, houses an abundance of medicinal plants that remain unexplored or only partially studied. Therefore, scrutinizing these untapped medicinal plants is essential to addressing rare, chronic diseases, including cancer.
ACKNOWLEDGMENT
The authors want to thank the tribal settlement villages of the Wayanad and Kozhikode regions of Kerala for their contribution to the collection of data.
AUTHOR CONTRIBUTION
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest in this research.
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
DECLARATION OF GENERATIVE AI AND AI-ASSISTED TECHNOLOGIES IN THE WRITING PROCESS
During the preparation of this work, the author(s) used Chat-GPT for writing assistance. After using this tool or service, the author(s) reviewed and edited the content as needed and took full responsibility for the content of the publication.
REFERENCES
1. Kumar S, Jawaid T, Dubey SD. Therapeutic plants of Ayurveda; a review on anticancer. Pharmacogn J. 2011;3(23):1–11. CrossRef
2. Akbar S. Introduction. In: Handbook of 200 medicinal plants. Cham, Switzerland: Springer; 2020. CrossRef
3. Majola F, Becker Delwing LK, Marmitt DJ, Bustamante-Filho IC, Goettert MI. Medicinal plants and bioactive natural compounds for cancer treatment: important advances for drug discovery. Phytochem Lett. 2019;31:196–207. CrossRef
4. Pandey G, Madhuri S. Some medicinal plants as natural anticancer agents. Pharmacogn Rev. 2009;3(6):259–63.
5. Chhikara SB, Parang K. Global cancer statistics 2022: the trends projection analysis. Chem Biol Lett. 2023;10(1):451. Available from: https://pubs.thesciencein.org/journal/index.php/cbl/article/view/451
6. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. CrossRef
7. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. CrossRef
8. Mehrotra R, Yadav K. Breast cancer in India: present scenario and the challenges ahead. World J Clin Oncol. 2022;13(3):209–18. CrossRef
9. Bukhtoyarov OV, Samarin DM. Pathogenesis of cancer: cancer reparative trap. J Cancer Ther. 2015;06(05):399–412. CrossRef
10. Pérez-Herrero E, Fernández-Medarde A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm. 2015;93:52–79. CrossRef
11. Kumari I, Kaurav H, Choudhary G. Rubia cordifolia (Manjishtha): a review based upon its Ayurvedic and medicinal uses. Himal J Heal Sci. 2021;6(2):17–28. CrossRef
12. Shaikh R, Pund M, Dawane A, Iliyas S. Evaluation of anticancer, antioxidant, and possible anti-inflammatory properties of selected medicinal plants used in Indian traditional medication. J Tradit Complement Med. 2014;4(4):253–7. CrossRef
13. Pan L, Chai HB, Kinghorn AD. Discovery of new anticancer agents from higher plants. Front Biosci. 2012;4S(1):142–56. CrossRef
14. Demain AL, Zhang L. Natural products and drug discovery. In: Zhang L, Demain AL, editors. Natural products. Totowa, NJ: Humana Press; 2005. pp. 3–29.
15. Butler MS. Natural products to drugs: natural product-derived compounds in clinical trials. Nat Prod Rep. 2008;25(3):475–516. CrossRef
16. Shoeb M. Anticancer agents from medicinal plants. Bangladesh J Pharmacol. 2008;1(2):35–41. CrossRef
17. Mouid MG. Effect of ethanolic extract of aerial parts of Andrographis paniculata on the pharmacokinetics of gliclazide in rats. Asian J Biomed Pharm Sci. 2015;05(51):21–4. CrossRef
18. Lichota A, Gwozdzinski K. Anticancer activity of natural compounds from plant and marine environment. Int J Mol Sci. 2018;19(11):3533. CrossRef
19. Tojo Jose VA, Sebastian A. Ethnobotanical study of traditional medicinal plants used by indigenous people in north Kerala. Indian J Appl Res. 2015;5(10):184–6.
20. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. CrossRef
21. McKeown S, Mir ZM. Considerations for conducting systematic reviews: evaluating the performance of different methods for de-duplicating references. Syst Rev. 2021;10(1):4–11. CrossRef
22. Beheshti A, Chavanon ML, Christiansen H. Emotion dysregulation in adults with attention deficit hyperactivity disorder: a meta-analysis. BMC Psychiatry. 2020;20(1):120. CrossRef
23. Jain DL, Baheti AM, Jain SR, Khandelwal KR. Use of medicinal plants among tribes in Satpuda region of Dhule and Jalgaon districts of Maharashtra-an ethnobotanical survey. Indian J Tradit Knowl. 2010;9(1):152–7.
24. Morvin Yabesh JE, Prabhu S, Vijayakumar S. An ethnobotanical study of medicinal plants used by traditional healers in silent valley of Kerala, India. J Ethnopharmacol. 2014;154(3):774–89. CrossRef
25. Drishya NS, Joseph S, Anusree N, Theertha PC, Atheena K. Ethnobotanical survey of medicinal plants in Urdhook hills, Kuttiady, Kozhikode District, Kerala. Int J Creat Res Thoughts. 2021;9(3):4029–40.
26. Soja S, Saradha M. Documentation of medicinal plants used by the traditional healers, Mayannur forest, Thrissur district, Kerala, India. Kongunadu Res J. 2021;8(2):8–26. CrossRef
27. Raja R, Aswani BS. Identification and conservation status of medicinal plants in Chengottumala hills, Kerala. J Univ Shanghai Sci Technol. 2022;24(8):228–47.
28. Smitha PRB, Madhusoodanan PV. Anticancer activity of Acanthus illicifolius Linn. From chettuva mangroves, Kerala. Int J Bioassays. 2014;3(11):3452–5.
29. Singh D, Aeri V. Phytochemical and pharmacological potential of Acanthus ilicifolius. J Pharm Bioallied Sci. 2013;5(1):17–20. CrossRef
30. Rahamooz Haghighi S, Asadi MH, Akrami H, Baghizadeh A. Anti-carcinogenic and anti-angiogenic properties of the extracts of Acorus calamus on gastric cancer cells. Avicenna J phytomedicine. 2016;7(2):145–6. CrossRef
31. Liu XC, Zhou LG, Liu ZL, Du SS. Identification of insecticidal constituents of the essential oil of Acorus calamus rhizomes against Liposcelis bostrychophila badonnel. Molecules. 2013;18(5):5684–96. CrossRef
32. Sharma V, Singh R. Achyranthes aspera: phytochemical estimation. Am J Pharmtech Res. 2013;3(2):243–51.
33. Tiwari P, Gond P, Koshale S, Tiwari CP. Phytochemical analysis of different parts of Achyranthes aspera. J Pharmacogn Phytochem. 2018;2(1):60–2.
34. Ahmed SM, Tasleem F, Mazhar F, Rizvi SRZ, Azhar I. Pharmacological evaluation on methanol extract of Adenanthera pavonina leaves. Pak J Pharmacol. 2018;35(1&2):57–63.
35. Bhadran S, George SA, Malla S, Harini BP. Screening of bioprotective properties of various plant extracts and gas chromatography-mass spectrometry profiling of Adenanthera pavonina stem extract. Asian J Pharm Clin Res. 2017;10(7):188–94. CrossRef
36. Lindamulage IKS, Soysa P. Evaluation of anticancer properties of a decoction containing Adenanthera pavonina L. and Thespesia populnea L. BMC Complement Altern Med. 2016;16:70. CrossRef
37. Ali A, Khan N, Qadir A, Warsi MH, Ali A, Tahir A. Identification of the phytoconstituents in methanolic extract of Adhatoda vasica L. leaves by GC-MS analysis and its antioxidant activity. J AOAC Int. 2022;105(1):267–71. CrossRef
38. Shoaib A. A systematic ethnobotanical review of Adhatoda vasica (L.). Cell Mol Biol. 2021;67(4):248–63. CrossRef
39. Petricevich VL, Abarca-vargas R. Allamanda cathartica: a review of phytochemistry, pharmacology, toxicology, and biotechnology. Molecules. 2019;24(7):1238. CrossRef
40. Pandey K, Shekar C, Bairwa K, Kate AS. Pharmaceutical perspective on bioactives from Alstonia scholaris: ethnomedicinal knowledge, phytochemistry, clinical status, patent space, and future directions. Phytochem Rev. 2020;19:191–233. CrossRef
41. Wang CM, Yeh KL, Tsai SJ, Jhan YL, Chou CH. Anti-proliferative activity of triterpenoids and sterols isolated from Alstonia scholaris against non-small-cell lung carcinoma cells. Molecules. 2017;22(12):2119. CrossRef
42. Itam A, Wulandari A, Rahman MM, Ferdinal N. Preliminary phytochemical screening, total phenolic content, antioxidant and cytotoxic activities of Alstonia scholaris R. Br leaves and stem bark extracts. J Pharm Sci Res. 2018;10(3):518–22.
43. Riyama Shirin VK, Arthi I, Neethu Krishnan S, Fathima Suman P. Review on Alternanthera brasiliana (L.) Kuntze for its pharmacognostic, phytochemical, pharmacological perspectives. World J Pharm Res. 2021;10(11):382–92. CrossRef
44. Kannan M, Chandran RP, Manju S. Preliminary phytochemical and antibacterial studies on leaf extracts of Alternanthera brasiliana (L.) Kuntze. Int J Pharm Res. 2017;6(7):626–8.
45. Taiwo BJ, Popoola TD, Van Heerden FR, Fatokun AA. Penta galloyl glucose, isolated from the leaf extract of Anacardium occidentale L., could elicit rapid and selective cytotoxicity in cancer cells. BMC Complement Med Ther. 2020;20(1):1–9. CrossRef
46. Salehi B, Gültekin-Özgüven M, Kirkin C, Özçelik B, Morais-Braga MFB, Carneiro JNP, et al. Antioxidant, antimicrobial, and anticancer effects of Anacardium plants: an ethnopharmacological perspective. Front Endocrinol (Lausanne). 2020;11:295. CrossRef
47. Shankar A, Gopinath SM, Shareef MI. Phyto sensitization and cytotoxic studies of Anacardium occidentale L. on cancer cell lines—a herbaceutical approach. Int J Curr Microbiol Appl Sci. 2020;9(2):1589–603. CrossRef
48. Sundaram V, Thiyagarajan D, Lawrence AV, Mohammed SSS, Selvaraj A. In-vitro antimicrobial and anticancer properties of green synthesized gold nanoparticles using Anacardium occidentale leaves extract. Saudi J Biol Sci. 2019;26(3):455–9. CrossRef
49. Souza NC, de Oliveira JM, Morrone MDS, Albanus RD, Amarante MDSM, Camillo CDS, et al. Antioxidant and anti-inflammatory properties of Anacardium occidentale leaf extract. Evid Based Complement Alternat Med. 2017;2017:2787308. CrossRef
50. Khan I, Khan F, Farooqui A, Ansari IA. Andrographolide exhibits anticancer potential against human colon cancer cells by inducing cell cycle arrest and programmed cell death via augmentation of intracellular reactive oxygen species level. Nutr Cancer. 2018;70(5):787–803. CrossRef
51. Wanandi SI, Limanto A, Yunita E, Syahrani RA, Louisa M, Wibowo AE, et al. In silico and in vitro studies on the anti-cancer activity of andrographolide targeting survivin in human breast cancer stem cells. PLoS One. 2020;15(11):e0240020. CrossRef
52. Alaqeel NK, Almalki WH, Binothman N, Aljadani M, Al-Dhuayan IS, Alnamshan MM, et al. The inhibitory and anticancer properties of Annona squamosa L. seed extracts. Braz J Biol. 2023;82:e268250. CrossRef
53. Abd-Elghany AA, Ahmed SM, Masoud MA, Atia T, Waggiallah HA, El-Sakhawy MA, et al. Annona squamosa L. extract-loaded niosome and its anti-Ehrlich Ascites’ carcinoma activity. ACS Omega. 2022;7(43):38436–47. CrossRef
54. Fadholly A, Purnama R, Iskandar D. In vitro anticancer activity Annona squamosa extract nanoparticle on WiDr cells. J Adv Pharm Technol Res. 2019;10(1):149–54. CrossRef
55. Shehata MG, Abu-Serie MM, Abd El-Aziz NM, El-Sohaimy SA. Nutritional, phytochemical, and in vitro anticancer potential of sugar apple (Annona squamosa) fruits. Sci Rep. 2021;11(1):6224. CrossRef
56. Vinodhini R, Saravanan D, Baskaran D, Ramalingam S. In vitro free radical scavenging and anticancer potential of Aristolochia indica against MCF-7 cell line. Int J Pharm Sci. 2015;7(6):392–6.
57. Subramanian PV, AlSalhi MS, Devanesan S, Thomas PA. Evaluation of antioxidant, anticancer and DNA binding potentials of noble metal nanoparticles synthesized using Aristolochia indica and Indigofera tinctoria. J Clust Sci. 2021;32(4):917–27. CrossRef
58. Siwan D, Nandave D, Nandave M. Artemisia vulgaris Linn: an updated review on its multiple biological activities. Future J Pharm Sci. 2022;8(47):1–14. CrossRef
59. Ekiert H, Pajor J, Klin P, Rzepiela A, ?lesak H, Szopa A. Significance of Artemisia vulgaris L. (Common Mugwort) in the history of medicine and its possible contemporary applications substantiated by phytochemical and pharmacological studies. Molecules. 2020;25(19):4415. CrossRef
60. Singh NB, Devi ML, Biona T, Sharma N, Das S, Chakravorty J, et al. Phytochemical composition and antimicrobial activity of essential oil from the leaves of Artemisia vulgaris L. Molecules. 2023;28(5):2279. CrossRef
61. Tandyekkal A, Pandurangan NN, Mohanan J, Nehru J, Botanic T. Asystasia gangetica var. krishnae (Acanthaceae): a new variety from Kerala, India. Rheedea. 2019;29(2):174–7. CrossRef
62. Barbaza MYU, De Castro-Cruz KA, Hsieh CL, Tsai PW. Determination of the chemical constituent contents and antioxidation properties of Asystasia gangetica. Indian J Pharm Educ Res. 2021;55(3):863–1. CrossRef
63. Ahmad Eid NE, Jaradat N. A review of chemical constituents and traditional usage of neem plant (Azardirachta indica). Palest Med Pharm J. 2017;2(2):75–81. CrossRef
64. Gupta A, Ansari S, Gupta S, Narwani M. Therapeutics role of neem and its bioactive constituents in disease prevention and treatment. J Pharmacogn Phytochem. 2019;8(3):680–91.
65. Singh A, Singh N, Pabla D. A review on medicinal uses of Bauhinia variegata Linn. Pharma Tutor. 2019;7(6):12–6. CrossRef
66. Pandey S. In vivo antitumor potential of extracts from different parts of Bauhinia variegata linn. against b16f10 melanoma tumour model in c57bl/6 mice. Appl Cancer Res. 2017;37(1):33–41. CrossRef
67. Shahi NP, Sharma O, Shahi SR. Kanchnara (Bauhinia variegata Linn.)-a review. World J Pharm Res. 2022;11(8):386–94. CrossRef
68. El Khalki L, Maire V, Dubois T. Berberine impairs the survival of triple negative breast cancer cells: cellular and molecular analyses. Molecules. 2020;25(3):506. CrossRef
69. Imenshahidi M, Hosseinzadeh H. Berberine and barberry (Berberis vulgaris): a clinical review. Phytother Res. 2019;33(3):504–23. CrossRef
70. Li J, Yang L, Shen R, Gong L, Tian Z, Qiu H, et al. Self-nanoemulsifying system improves oral absorption and enhances anti-acute myeloid leukaemia activity of berberine. J Nanobiotechnol. 2018;16(1):76. CrossRef
71. Kuo TF, Yang G, Chen TY, Wu YC, Minh HTN, Chen LS, et al. Bidens pilosa: nutritional value and benefits for metabolic syndrome. Food Front. 2021;2(1):32–45. CrossRef
72. Mtenga DV, Ripanda AS. A review on the potential of underutilized Blackjack (Biden Pilosa) naturally occurring in sub-Saharan Africa. Heliyon. 2022;8(6):e09586. CrossRef
73. Mtambo SE, Krishna SB, Govender P. Physico-chemical, antimicrobial and anticancer properties of silver nanoparticles synthesised from organ-specific extracts of Bidens pilosa L. S Afr J Bot. 2019;126:196–206. CrossRef
74. Giridharan B, Sachidanandam M, Meenakumari K. In vitro anticancer activity of Biophytum sensitivum whole plant. Int J Pharm Sci Res. 2016;7(12):2320–5148. CrossRef
75. Saravanan K, Jayabal P, Elavarasi S, Santhi MP, Palanivel K. Anticancer activity of Biophytum sensitivum in breast cancer mcf-7 cell line. J Cell Tissue Res. 2016;16(1):5387–91.
76. Dirar AI, Wada M, Watanabe T, Devkota HP. Phenolic compounds from the aerial parts of Blepharis linariifolia Pers. and their free radical scavenging and enzyme inhibitory activities. Medicines (Basel). 2019;6(113):113. CrossRef
77. Baskar A, Al Numair K, Alsaif M, Ignacimuthu S. In vitro antioxidant and antiproliferative potential of medicinal plants used in traditional Indian medicine to treat cancer. Redox Rep. 2012;17(4):145–56. CrossRef
78. Vidhya RU, Bhuminathan S, Rekha M, Nandhini MS, Ravishankar. Therapeutic and pharmacological efficacy of plant Boerhaavia diffusa -a review. Int J Appl Pharm. 2022;14(TI):58–62. CrossRef
79. Kaur H. Boerhaavia diffusa: bioactive compounds and pharmacological activities. Biomed Pharmacol J. 2019;12(4):1675–82. CrossRef
80. Thuy TT, Thu Trang NT, Hoa PN, Trang PT, Khoi NM, Hoang VD, et al. A new coumaronochromone from Boerhaavia diffusa. Nat Prod Commun. 2019;14(6):2019. CrossRef
81. Friedman JR, Richard SD, Merritt JC, Brown KC, Denning KL, Tirona MT, et al. Capsaicinoids: multiple effects on angiogenesis, invasion and metastasis in human cancers. Biomed Pharmacother. 2019;118:109317. CrossRef
82. Chilczuk B, Marciniak B, Stochmal A, Pecio ?, Kontek R, Jackowska I, et al. Anticancer potential and capsianosides identification in lipophilic fraction of sweet pepper (Capsicum annuum L.). Molecules. 2020;25(13):3097. CrossRef
83. Al-Samydai A, Alshaer W, Al-Dujaili EAS, Azzam H, Aburjai T. Preparation, characterization, and anticancer effects of capsaicin-loaded nanoliposomes. Nutrients. 2021;13(11):3995. CrossRef
84. Abdel-Halim SA, Ibrahim MT, Mohsen MMA, Abou-Setta LM, Sleen AA, Morsey FA, et al. Phytochemical and biological investigation of Carica papaya Linn. leaves cultivated in Egypt (Family Caricaceae). J Pharmacogn Phytochem. 2020;9(5):47–54. CrossRef
85. Gadge S, Game M, Salode V. Marvelous plant Carica papaya Linn: a herbal therapeutic option. Phytopathology. 2020;9(4):629–33. CrossRef
86. Rafiqi UN, Gul I, Saifi M, Nasrullah N, Ahmad J, Dash P, et al. Cloning, identification, and in silico analysis of terpene synthases involved in the competing pathway of artemisinin biosynthesis pathway in Artemisia annua L. Pharmacogn Mag. 2019;15(62):38–46. CrossRef
87. Nalinratana N, Suriya U, Laprasert C, Wisidsri N, Poldorn P, Rungrotmongkol T, et al. In vitro and in silico studies anti—inflammatory lignans from Carallia brachiata as p38 MAP kinase inhibitors. Sci Rep. 2023;13(1):3558. CrossRef
88. Kuthi NA, Chandren S, Basar N. Biosynthesis of gold nanoisotrops using Carallia brachiata leaf extract and their catalytic application in the reduction of 4-nitrophenol. Front Chem. 2022;9:800145. CrossRef
89. Junejo JA, Zaman K, Rudrapal M, Mondal P, Singh KD, Verma VK. Preliminary phytochemical and physicochemical evaluation of Carallia brachiata (Lour.) Merr. leaves. J Appl Pharm Sci. 2014;4:123–7. CrossRef
90. Kanwal A, Azeem F, Nadeem H, Ashfaq UA, Aadil RM, Kober AKMH, et al. Molecular mechanisms of Cassia fistula against epithelial ovarian cancer using network pharmacology and molecular docking approaches. Pharmaceutics. 2022;14(9):1970. CrossRef
91. Kaur S, Kumar A, Thakur S, Kumar K, Sharma R, Sharma A, et al. Antioxidant, antiproliferative and apoptosis-inducing efficacy of fractions from Cassia fistula L. leaves. Antioxidants (Basel). 2020;9(2):173. CrossRef
92. Bahorun T, Neergheen VS, Aruoma OI. Phytochemical constituents of Cassia fistula. Afr J Biotechnol. 2005;4(13):1530–40.
93. Bevelle CA, Handy GA, Segal RA, Cordell GA, Farnsworth NR. Isocentratherin, a cytotoxic germacranolide from Centratherum punctatum (compositae). Phytochemistry. 1981;20(7):1605–7.
94. Krithika KS, Shabir AG, Arun KP, Brindha P, Vijayalakshmi M. In silico and in vitro evaluation of the anti-inflammatory potential of Centratherum punctatum cass-A. J Biomol Struct Dyn. 2017;35(4):765–80. CrossRef
95. Chukwujekwu JC, Ndhlala AR, De Kock CA, Smith PJ, Van Staden J. Antiplasmodial, HIV-1 reverse transcriptase inhibitory and cytotoxicity properties of Centratherum punctatum Cass. and its fractions. S Afr J Bot. 2014;90:17–9. CrossRef
96. Aggarwal S, Bhadana K, Singh B, Rawat M. Cinnamomum zeylanicum extract and its bioactive component cinnamaldehyde show anti-tumor effects via inhibition of multiple cellular pathways. Front Pharmacol. 2022;13:918479. CrossRef
97. Dutta A, Chakraborty A. Cinnamon in anticancer armamentarium: a molecular approach. J Toxicol. 2018;2018:8978731. CrossRef
98. Kubatka P, Kello M, Kajo K, Samec M, Jasek K, Vybohova D, et al. Chemopreventive and therapeutic efficacy of Cinnamomum zeylanicum L. bark in experimental breast carcinoma: mechanistic in vivo and in vitro analyses. Molecules. 2020;25(6):1399. CrossRef
99. Sen Z, Zhan XK, Jing JIN, Yi Z, Wanqi Z. Chemosensitizing activities of cyclotides from Clitoria ternatea in paclitaxel-resistant lung cancer cells. Oncol Lett. 2013;5(1):641–4. CrossRef
100. Alshaya DS, Awad NS. Antiproliferative effect of Clitoria ternatea ethanolic extract against colorectal, breast, and medullary thyroid cancer cell lines. Seperations. 2022;9(11):331. CrossRef
101. Oluwole OB, Obode OC, Elemo GN, Ibekwe D, Adesioye T, Raji FA, et al. Anti-inflammatory and anti-cancer properties of selected green leafy vegetables—a review. J Nutr Food Process. 2021;1(11):1–5. CrossRef
102. Tosoc JPS, Nuñeza OM, Sudha T, Darwish NHE, Mousa SA. Anticancer effects of the Corchorus olitorius aqueous extract and its bioactive compounds on human cancer cell lines. Molecules. 2021;26(19):6033. CrossRef
103. Soykut G, Becer E, Calis I, Yucecan S, Vatansever S. Apoptotic effects of Corchorus olitorius L. leaf extracts in colon adenocarcinoma cell lines. Prog Nutr. 2018;20(4):689–98. CrossRef
104. Ashalatha, Gopinath SM. Phytochemical profiling of Coscinium fenestratum (Gaertn.) Colebr Cultivar, by liquid chromatography-mass spectrometry. Int J Curr Microbiol App Sci. 2019;8(1):3194–201.
105. Tungpradit R, Sinchaikul S, Phutrakul S, Wongkham W, Chen ST. Anti-cancer compound screening and isolation: Coscinium fenestratum, Tinospora crispa and Tinospora cordifolia. Chiang Mai J Sci. 2010;37(3):476–88.
106. Potikanond S, Chiranthanut N, Khonsung P, Teekachunhatean S. Cytotoxic effect of Coscinium fenestratum on human head and neck cancer cell line (HN31). Evid Based Complement Alternat Med. 2015;2015:701939. CrossRef
107. Kumar D, Sharma S, Kumar S. Botanical description, phytochemistry, traditional uses, and pharmacology of Crataeva nurvala Buch. Ham.: an updated review. Futur J Pharm Sci. 2020;6:113. CrossRef
108. Gharge S, Hiremath SI, Kagawad P, Jivaje K, Palled MS, Suryawanshi SS. Curcuma zedoaria Rosc (Zingiberaceae): a review on its chemical, pharmacological and biological activities. Futur J Pharm Sci. 2021;7:166. CrossRef
109. Puspita SD, Yulianti R, Mozartha M. The effectiveness of white turmeric (Curcuma zedoaria) extracts as root canal irrigation alternative material on Streptococcus viridans. J Phys Conf Ser. 2019;1246:012040. CrossRef
110. Mishra J, Bhardwaj A, Misra K. Curcuma sp.: the nature’s souvenir for high-altitude illness. In: Misra K, Sharma P, Bhardwaj A, editors. Management of high-altitude pathophysiology. Cambridge, MA: Academic Press; 2018. pp. 153–69.
111. Venkatadri B, Shanparvish E, Rameshkumar MR, Arasu MV, Al-Dhabi NA, Ponnusamy VK, et al. green synthesis of silver nanoparticles using aqueous rhizome extract of Zingiber officinale and Curcuma longa: in-vitro anti-cancer potential on human colon carcinoma HT-29 cells. Saudi J Biol Sci. 2020;27(11):2980–6. CrossRef
112. Poompavai S, Gowri Sree V. Anti-proliferative efficiency of pulsed electric field treated Curcuma longa (Turmeric) extracts on breast cancer cell lines. IETE J Res. 2020;68(6):4555–69. CrossRef
113. Tong R, Wu X, Liu Y, Liu Y, Zhou J, Jiang X, et al. Curcumin-induced DNA demethylation in human gastric cancer cells is mediated by the DNA-damage response pathway. Oxid Med Cell Longev. 2020;2020:2543504. CrossRef
114. Liu P, Ying Q, Liu H, Yu SQ, Bu LP, Shao L, et al. Curcumin enhances anti-cancer efficacy of either gemcitabine or docetaxel on pancreatic cancer cells. Oncol Rep. 2020;44(4):1393–402. CrossRef
115. Suja S, Varkey IC. Medicinal and pharmacological values of Cyanthillium cinereum (Poovamkurunilla) extracts: investigating the antibacterial and anti-cancer activity in Mcf-7 breast. Int J Res Anal Rev. 2019;6(1):412–5.
116. Ariya SS, Baby J. Anticancer effect of phytochemicals from Cyanthillium cinereum against cancer target matrix metallopeptidase. Int J Adv Res Eng Technol. 2020;11(4):204–17.
117. Bhagya N, Chandrashekar KR, Prabhu A, Rekha PD. Tetrandrine isolated from Cyclea peltata induces cytotoxicity and apoptosis through ROS and caspase pathways in breast and pancreatic cancer cells. In Vitro Cell Dev Biol Anim. 2019;55(5):331–40. CrossRef
118. Jayaraman S, Variyar EJ. Immunomodulatory, anticancer and antioxidant activities of Cyclea peltata (Lam.) Hook. F. and Thomson. Int J Pharm Sci. 2019;11(10):40–6.
119. Yamuna CV, Arthi I, Rajagopal PL, Sajith Kumar PN, Lithashabin PK, Anjana AK. Cyclea peltata (Lam.) Hook.F. & Thomson: a pharmacological review. World J Pharm Res. 2020;9(4):265–73. CrossRef
120. Trang DT, Hoang TKV, Nguyen TTM, Van Cuong P, Dang NH, Dang HD, et al. Essential oils of Lemongrass (Cymbopogon citratus Stapf) induces apoptosis and cell cycle arrest in A549 lung cancer cells. Biomed Res Int. 2020;2020:5924856. CrossRef
121. Alwaili MA. Protective effects of lemongrass (Cymbopogon citratus STAPF) extract mediated mitochondrial fission and glucose uptake inhibition in SW1417. Food Sci Technol. 2023;43:e94522. CrossRef
122. Pan D, Machado L, Bica CG, Machado AK, Steffani JA, Cadoná FC. In vitro evaluation of antioxidant and anticancer activity of Lemongrass (Cymbopogon citratus (D.C.) Stapf). Nutr Cancer. 2022;74(4):1474–88. CrossRef
123. Rojas-Armas JP, Arroyo-Acevedo JL, Palomino-Pacheco M, Herrera-Calderón O, Ortiz-Sánchez JM, Rojas-Armas A, et al. The essential oil of Cymbopogon citratus stapt and carvacrol: an approach of the antitumor effect on 7,12-dimethylbenz-[α]-anthracene (DMBA)-induced breast cancer in female rats. Molecules. 2020;25(14):3284. CrossRef
124. Mukarram M, Choudhary S, Khan MA, Poltronieri P, Khan MMA, Ali J, et al. Lemongrass essential oil components with antimicrobial and anticancer activities. Antioxidants (Basel). 2021;11(1):20. CrossRef
125. Bezzera JJL, Pinheiro AAV. Traditional uses, phytochemistry, and anticancer potential of Cyperus rotundus L. (Cyperaceae): a systematic review. S Afr J Bot. 2022;144:175–86. CrossRef
126. Nafisah W, Pinanti HN, Christina YI, Rifa’i M, Djati MS. Computational biological activity and pharmacological properties analysis for anticancer Cyperus rotundus bioactive compounds. AIP Conf Proc. 2021;2353:030118. CrossRef
127. Simorangkir D, Masfria M, Harahap U, Satria D. Activity of anticancer n-hexane fraction of Cyperus rotundus L. rhizome against breast cancer MCF-7 cell line. Open Access Maced J Med Sci. 2019;7(22):3904–6. CrossRef
128. Lin CH, Peng SF, Chueh FS, Cheng ZY, Kuo CL, Chung JG. The ethanol crude extraction of Cyperus rotundus regulates apoptosis-associated gene expression in HeLa human cervical carcinoma cells in vitro. Anticancer Res. 2019;39(7):3697–709. CrossRef
129. Mannarreddy P, Denis M, Munireddy D, Pandurangan R, Thangavelu KP, Venkatesan K. Cytotoxic effect of Cyperus rotundus rhizome extract on human cancer cell lines. Biomed Pharmacother. 2017;95:1375–87. CrossRef
130. Karim R, Begum MM, Jui Y, Islam T, Billah M, Arafat Y, et al. In-vitro cytotoxic and anti-Vibrio cholerae activities of alcoholic extracts of Desmodium triflorum (L.) whole plant and Terminalia citrina (Roxb.) fruits. Clin. Phytosci. 2021;7:36. CrossRef
131. Dhanabal SP, Dhamodaran P, Chaitnya NL, Duraiswamy B. Ethnopharmacological and phytochemical profile of three potent Desmodium species: Desmodium gangeticum (L.) DC, Desmodium triflorum Linn and Desmodium triquetrum Linn. J Chem Pharm Res. 2016;8(7):91–7.
132. Lai SC, Ho YL, Huang SC, Huang TH, Lai ZR, Wu CR, et al. Antioxidant and antiproliferative activities of Desmodium triflorum (L.) DC. Am J Chin Med. 2010;38(2):329–42. CrossRef
133. Alzandi AA, Taher EA, Al-Sagheer NA, Al-Khulaidi AW, Azizi M, Naguib DM. Phytochemical components, antioxidant and anticancer activity of 18 major medicinal plants in Albaha region, Saudi Arabia. Biocatal Agric Biotechnol. 2021;34:102020. CrossRef
134. Malik FH, Haq I, Fatima H, Ahmad M, Naz I, Mirza B, et al. Bioprospecting Dodonaea viscosa Jacq.; a traditional medicinal plant for antioxidant, cytotoxic, antidiabetic and antimicrobial potential. Arab J Chem. 2022;15(3):103688. CrossRef
135. Kaigongi MM, Lukhoba CW, Ochieng’ PJ, Taylor M, Yenesew A, Makunga NP. LC-MS-based metabolomics for the chemosystematics of Kenyan Dodonaea viscosa Jacq (Sapindaceae) populations. Molecules. 2020;25(18):4130. CrossRef
136. Alghamdi MD, Nazreen S, Ali NM, Amna T. ZnO nanocomposites of Juniperus procera and Dodonaea viscosa extracts as antiproliferative and antimicrobial agents. Nanomaterials (Basel). 2022;12(4):664. CrossRef
137. Christina YI, Rifa’i M, Widodo N, Djati MS. The combination of Elephantopus scaber and Phaleria macrocarpa leaves extract promotes anticancer activity via downregulation of ER-α, Nrf2 and PI3K/AKT/mTOR pathway. J Ayurveda Integr Med. 2022;13(4):100674. CrossRef
138. Silalahi M. Utilization of Elephantopus scaber as traditional medicine and its bioactivity. GSC Biol Pharm Sci. 2021;15(1):112–8. CrossRef
139. Jasmine R, Abarna N, Verghese S. Anticancer potential of Elephantopus scaber L. leaves against MCF-7 cell lines. Asian J Adv Med Sci. 2021;3(4):94–8.
140. Ho WY, Liew SS, Yeap SK, Alitheen NB. Synergistic cytotoxicity between Elephantopus scaber and tamoxifen on MCF-7-derived multicellular tumor spheroid. Evid-Based Complement Altern Med. 2021;2021:6355236. CrossRef
141. Kabeer FA, Rajalekshmi DS, Nair MS, Prathapan R. In vitro and in vivo antitumor activity of deoxyelephantopin from a potential medicinal plant Elephantopus scaber against Ehrlich ascites carcinoma. Biocatal Agric Biotechnol. 2019;19:101106. CrossRef
142. Beeran AA, Maliyakkal N, Rao CM, Udupa N. The enriched fraction of Elephantopus scaber triggers apoptosis and inhibits multi-drug resistance transporters in human epithelial cancer cells. Pharmacogn Mag. 2015;11(42):257–68. CrossRef
143. Almeer RS, Alnasser M, Aljarba N, AlBasher GI. Effects of Green cardamom (Elettaria cardamomum Maton) and its combination with cyclophosphamide on Ehrlich solid tumors. BMC Complement Med. Ther. 2021;21(1):133. CrossRef
144. Qiblawi S, Kausar MA, Shahid SMA, Saeed M, Alazzeh AY. Therapeutic interventions of Cardamom in cancer and other human diseases. J Pharm Res Int. 2020;32(22):60087.
145. Vutakuri N, Somara S. Natural and herbal medicine for breast cancer using Elettaria cardamomum (L.) Maton. Int J Herb Med. 2018;6(2):91–6.
146. Elguindy NM, Yacout GA, El Azab EF, Maghraby HK. Chemoprotective effect of Elettaria cardamomum against chemically induced hepatocellular carcinoma in rats by inhibiting NF-κB, oxidative stress, and activity of ornithine decarboxylase. South African J Bot. 2016;105:251–8. CrossRef
147. Arbade GK, Kumar V, Tripathi V, Menon A, Bose S, Patro TU. Emblica officinalis-loaded poly(?-caprolactone) electrospun nanofiber scaffold as potential antibacterial and anticancer deployable patch. New J Chem. 2019;43(19):7427–40. CrossRef
148. Thoidingjam S, Tiku AB. Therapeutic efficacy of Phyllanthus emblica-coated iron oxide nanoparticles in A549 lung cancer cell line. Nanomedicine. 2019;14(17):2355–71. CrossRef
149. Baby B, Antony P, Vijayan R. Antioxidant and anticancer properties of berries. Crit Rev Food Sci Nutr. 2018;58(15):2491–507. CrossRef
150. Chaitanya MV, Suresh P. The neglected anticancer phytoceutical treasures from the Nilgiris biosphere: a short review. J Pharm Res Int. 2018;22(1):1–13. CrossRef
151. Rajalakshmi P, Sumathi, Pugalenthi MR. Antioxidant activity of Erigeron karvinskianus DC. and Ageratina adenophora (Spreng.) King (leaves). Int J Food Sci Nutr. 2016;1:64–8.
152. Sulaiman CT, Deepak M, Praveen TK, Lijini KR, Salman M, Satheesh N, et al. Metabolite profiling and anti-cancer activity of two medicinally important Euphorbia species. Med Omi. 2023;7:100018. CrossRef
153. Linga Raju K, Naika HR, Nagabhushana H, Nagaraju G. Euphorbia heterophylla (L.) mediated fabrication of ZnO NPs: characterization and evaluation of antibacterial and anticancer properties. Biocatal Agric Biotechnol. 2019;18:100894. CrossRef
154. Aleksandrov M, Maksimova V, Koleva Gudeva L. Review of the anticancer and cytotoxic activity of some species from genus euphorbia. Agric Conspec Sci. 2019;84(1):1–5.
155. Manikandarajan PA, Sathish M, Suresh R, Suresh AJ. Isolation, characterization, docking and anti-cancer activity of quercetin from leaves of Euphorbia heterophylla Linn. Int J Pharm Sci Res. 2018;9(1):197–202. CrossRef
156. Yusuf H, Satria D, Suryawati S, Fahriani M. Combination therapy of eurycomanone and doxorubicin as anticancer on T47D and MCF-7 cell lines. Syst Rev Pharm. 2020;11(10):335–41. CrossRef
157. Moses LB, Abu Bakar MF, Mamat H, Aziz ZA. Unfermented freeze-dried leaf extract of Tongkat Ali (Eurycoma longifolia Jack.) induced cytotoxicity and apoptosis in MDA-MB-231 and MCF-7 breast cancer cell lines. Evid Based Complement Altern Med. 2021;2021:8811236. CrossRef
158. Rahman EY, Kusworini K, Ali M, Purnomo BB, Kania N. The cytotoxic effect of Eurycoma longifolia jack root extract on the prostate adenocarcinoma pc-3 cells through apoptosis enhancement. Open Access Maced J Med Sci. 2020;8(A):317–22. CrossRef
159. Rehman SU, Choe K, Yoo HH. Review on a traditional herbal medicine, Eurycoma longifolia Jack (Tongkat Ali): its traditional uses, chemistry, evidence-based pharmacology and toxicology. Molecules. 2016;21(3):331. CrossRef
160. Andueza N, Giner RM, Portillo MP. Nutraceutical, functional, and therapeutic properties of Garcinia cambogia: a review. Int J Food Prop. 2023;26(1):729–38. CrossRef
161. Baky MH, Fahmy H, Farag MA. Recent advances in Garcinia cambogia nutraceuticals in relation to its hydroxy citric acid level. A comprehensive review of its bioactive production, formulation, and analysis with future perspectives. ACS Omega. 2022;7(30):25948–57. CrossRef
162. Cock IE, Gailot C, Shalom J. An examination of the antimicrobial and anticancer properties of Mangosteen pericarp extracts. Acta Hortic. 2015;1106:231–7. CrossRef
163. Aggarwal V, Tuli HS, Kaur J, Aggarwal D. Garcinol exhibits anti-neoplastic effects by targeting diverse oncogenic factors in tumor cells. Biomedicines. 2020;8(5):103. CrossRef
164. Wang J, Wang L, Ho CT, Zhang K, Liu Q, Zhao H. Garcinol from Garcinia indica downregulates cancer stem-like cell biomarker ALDH1A1 in nonsmall cell lung cancer A549 cells through DDIT3 activation. J Agric Food Chem. 2017;65(18):3675–83. CrossRef
165. Ullah MF, Ahmad A. Critical dietary factors in cancer chemoprevention. Cham, Switzerland: Springer; 2015.
166. Zhang M, Lu Q, Hou H, Sun D, Chen M, Ning F, et al. Garcinol inhibits the proliferation of endometrial cancer cells by inducing cell cycle arrest. Oncol Rep. 2021;45(2):630–40. CrossRef
167. Shylla A, Roy B. Phytochemical screening and toxicological assessment of crude extract of Aesculus assamica Griff. and Gaultheria fragrantissima Wall. in Swiss albino mice. Gorteria J. 2021;34(8):140–51. CrossRef
168. Yan-Ling X, Du XY, Yi-Rong L, Lu L. The complete chloroplast genome of Gaultheria fragrantissima Wall. (Ericaceae) from Yunnan, China, an aromatic medicinal plant in the wintergreens. Mitochondrial DNA B Resour. 2021;6(6):1761–2. CrossRef
169. Narayanan DP, Rexliene MJ, Suresh S. Assessment of carrageenan-induced anti-inflammatory activity of Gaultheria fr agrantissima Wall. and Byttneria herbaceae Roxb. collected from Idukki district. Int J Pharmacogn Phytochem Res. 2020;12(3):138–2. CrossRef
170. Kumar M, Sarma P, Dkhar MS, Kayang H, Raghuvanshi R, Dubey NK. Assessment of chemically characterised Gaultheria fragrantissima Wall. essential oil and its major component as safe plant-based preservative for millets against fungal, aflatoxin contamination and lipid peroxidation during storage. J Food Sci Technol. 2018;55(1):111–9. CrossRef
171. Pandey BP, Thapa R, Upreti A. Chemical composition, antioxidant and antibacterial activities of essential oil and methanol extract of Artemisia vulgaris and Gaultheria fragrantissima collected from Nepal. Asian Pac J Trop Med. 2017;10(10):952–9. CrossRef
172. Goel B, Dey B, Chatterjee E, Tripathi N, Bhardwaj N, Kumar S, et al. Antiproliferative potential of gloriosine: a lead for anticancer drug development. ACS Omega. 2022;7(33):28994–9001. CrossRef
173. Ionkova I, Shkondrov A, Zarev Y, Kozuharova E, Krasteva I. Anticancer secondary metabolites: from ethnopharmacology and identification in native complexes to biotechnological studies in species of genus Astragalus L. and Gloriosa L. Curr Issues Mol Biol. 2022;44(9):3884–904. CrossRef
174. Pandey DK, Kaur P, Kumar V, Banik RM, Malik T, Dey A. Screening the elite chemotypes of Gloriosa superba L. in India for the production of anticancer colchicine: simultaneous microwave-assisted extraction and HPTLC studies. BMC Plant Biol. 2021;21(1):77. CrossRef
175. Balkrishna A, Das SK, Pokhrel S, Joshi A, Laxmi, Verma S, et al. Colchicine: isolation, LC–MS QT of screening, and anticancer activity study of Gloriosa superba seeds. Molecules. 2019;24(2772):2772. CrossRef
176. Teja PK, Patel P, Bhavsar D, Bindusri C, Jadhav K, Chauthe SK. Traditional uses, phytochemistry, pharmacology, toxicology and formulation aspects of Glycosmis species: a systematic review. Phytochemistry. 2021;190:112865. CrossRef
177. Amutha S, Sridhar S. Green synthesis of magnetic iron oxide nanoparticle using leaves of Glycosmis. J Innov Pharm Biol Sci. 2018;5(2):22–6.
178. Shoja MH, Reddy ND, Nayak PG, Biswas S, Srinivasan KK, Rao CM. In vitro mechanistic and in vivo anti-tumor studies of Glycosmis pentaphylla (Retz.) DC against breast cancer. J Ethnopharmacol. 2016;186:159–68. CrossRef
179. Ghosh AR, Alsayari A, Habib AH, Wahab S, Nadig APR, Rafeeq MM, et al. Anti-tumor potential of Gymnema sylvestre saponin rich fraction on in vitro breast cancer cell lines and in vivo tumor-bearing mouse models. Antioxidants (Basel). 2023;12(1):134. CrossRef
180. Packialakshmi B, Raga Sowndriya S. Anti-cancer effect of Gymnema sylvestre leaf extract against MG63, human osteosarcoma cell line—an in vitro analysis. Int J Curr Res Rev. 2019;11(11):18–24. CrossRef
181. Chakraborty D, Ghosh S, Bishayee K, Mukherjee A, Sikdar S, Khuda-Bukhsh AR. Antihyperglycemic drug Gymnema sylvestre also shows anticancer potentials in human melanoma A375 cells via reactive oxygen species generation and mitochondria-dependent caspase pathway. Integr Cancer Ther. 2013;12(5):433–41. CrossRef
182. Yasukawa K, Okuda S, Nobushi Y. Inhibitory effects of gymnema (Gymnema sylvestre) leaves on tumour promotion in two-stage mouse skin carcinogenesis. Evid Based Complement Altern Med. 2014;2014:328684. CrossRef
183. Arunachalam KD, Arun LB, Annamalai SK, Arunachalam AM. Potential anticancer properties of bioactive compounds of Gymnema sylvestre and its biofunctionalized silver nanoparticles. Int J Nanomed. 2014;10:31–41. CrossRef
184. Tummala PK, Nannapaneni S, Durvasula SP, Chadalavada S, Venigandla S, Vemuru S, et al. Evaluation of Hemidesmus indicus plant compounds for anti-cancer studies—an in-silico approach. J Pharm Res Int. 2021;33:64–9. CrossRef
185. Nandy S, Mukherjee A, Pandey DK, Ray P, Dey A. Indian Sarsaparilla (Hemidesmus indicus): recent progress in research on ethnobotany, phytochemistry and pharmacology. J Ethnopharmacol. 2020;254:112609. CrossRef
186. Turrini E, Catanzaro E, Ferruzzi L, Guerrini A, Tacchini M, Sacchetti G, et al. Hemidesmus indicus induces apoptosis via proteasome inhibition and generation of reactive oxygen species. Sci Rep. 2019;9(1):7199. CrossRef
187. Joshi A, Lad H, Sharma H, Bhatnagar D. Evaluation of phytochemical composition and antioxidative, hypoglycaemic and hypolipidaemic properties of methanolic extract of Hemidesmus indicus roots in streptozotocin-induced diabetic mice. Clin Phytosci. 2018;4:7. CrossRef
188. Statti G, Marrelli M, Conforti F, Spagnoletti A, Tacchini M, Fimognari C, et al. Inhibition of cancer cell proliferation and antiradical effects of decoction, hydroalcoholic extract, and principal constituents of Hemidesmus indicus R. Br. Phyther Res. 2015;29(6):857–63. CrossRef
189. ?liwi?ski T, Kowalczyk T, Sitarek P, Kolanowska M. Orchidaceae-derived anticancer agents: a review. Cancers (Basel). 2022;14(3):754. CrossRef
190. Satish B, Vishwanatha D. Screening for cytotoxic activity of Habenaria longicorniculata J graham tubers- an in-vitro study. J Phytopharm. 2020;9(5):367–70. CrossRef
191. Shana KM, Vishnupriya VV, Fahmeeda PP, Prajna PP, Reshmi R, Jothi ET. A review on the phytochemistry and pharmacology of Hemigraphis colorata. World J Biol Pharm Heal Sci. 2022;12(2):105–9. CrossRef
192. Hallier H, Mathew F. Phytochemical screening of Hemigraphis colorata (Blume) H.G. Hallier. J Pharmacogn Phytochem. 2021;10(6):360–3.
193. Sasidharan S, Pottail L. Anti-bacterial and skin-cancer activity of AuNP, rGO and AuNP-rGO composite using Hemigraphis alternata (Burm.F.) T. Anderson. Biocatal Agric Biotechnol. 2020;25:101596. CrossRef
194. Zahara K, Panda SK, Swain SS, Luyten W. Metabolic diversity and therapeutic potential of Holarrhena pubescens: an important ethnomedicinal plant. Biomolecules. 2020;10(9):1341. CrossRef
195. Cheenpracha S, Boapun P, Limtharakul Née Ritthiwigrom T, Laphookhieo S, Pyne SG. Antimalarial and cytotoxic activities of pregnene-type steroidal alkaloids from Holarrhena pubescens roots. Nat Prod Res. 2019;33(6):782–8. CrossRef
196. Yoon H, Park J, Park KK, Kim J, Bandara NC, Bandara BMR, et al. Methanol extract of Holarrhena antidysenterica inhibits the growth of human oral squamous cell carcinoma cells and osteoclastogenesis of bone marrow macrophages. Evid Based Complement Alternat Med. 2017;2017:7272947. CrossRef
197. Yende A, Rama Bhat P, Zainab A, Acharya S, Padyana S. Evaluation of antioxidant and antimicrobial activities of Holigarna arnottiana Hook. f. Photon. 2013;139:278–88.
198. Manilal A, Idhayadhulla A. Potential in vitro antimicrobial efficacy of Holigarna arnottiana (Hook F). Asian Pac J Trop Biomed. 2014;4(1):25–9. CrossRef
199. Ravi A, Saj OP. Antioxidant and cytotoxic potential of the plant Holigarna arnottiana hook.f. bark ethanolic extract. World J Pharm Res. 2013;2(5):1685–703.
200. Du Q, Chan LY, Gilding EK, Henriques ST, Condon ND, Ravipati AS, et al. Discovery and mechanistic studies of cytotoxic cyclotides from the medicinal herb Hybanthus enneaspermus. J Biol Chem. 2020;295(32):10911–25. CrossRef
201. Murugan M, Kamaraj M, Naidu T. Green synthesis of CeO2 nanoparticles using Hybanthus enneaspermus and their cytotoxic effects against human breast cancer cell line (MCF-7). J Emerg Technol Innov Res. 2018;5(11):316–5. CrossRef
202. Jaikumar K, Sheik Noor Mohamed M, Marimuthu S, Anantha Padmanabhan S, Anand D, Saravanan P. In vitro anticancer activity of ethanolic leaf extract of Acampe praemorsa (Roxb.). Indo Am J Pharm Res. 2018;7(7):1020–5. CrossRef
203. Beaula Stary BL, Uma Devi S, Johnsi Cristobel G, Beena L. Phytochemical screening of Hybanthus enneaspermus (Linn) F. Muell. Int J Innov Sci Eng Technol. 2008;1(5):111–22.
204. Kato K, Nagane M, Aihara N, Kamiie J, Miyanabe M, Hiraki S, et al. Lipid-soluble polyphenols from sweet potato exert antitumor activity and enhance chemosensitivity in breast cancer. J Clin Biochem Nutr. 2021;68(1):193–200. CrossRef
205. Silva-Correa CR, Vargas JH, Torre VEVL, Calderon-Pena AA, Gonzalez-Siccha AD, Aspajo-Villalaz CL, et al. Potential anticancer activity of bioactive compounds from Ipomoea batatas. Pharmacogn J. 2022;14(3):650–9. CrossRef
206. Lin HH, Lin KH, Wu KF, Chen YC. Identification of Ipomoea batatas anti-cancer peptide (IbACP)-responsive genes in sweet potato leaves. Plant Sci. 2021;305:110849. CrossRef
207. Budiman MR, Wiraswati HL, Rezano A. Purple sweet potato phytochemicals: potential chemo-preventive and anticancer activities. Open Access Maced J Med Sci. 2021;9(F):288–98. CrossRef
208. Sun Y, Pan Z, Yang C, Jia Z, Guo X. Comparative assessment of phenolic profiles, cellular antioxidant and antiproliferative activities in ten varieties of sweet potato (Ipomoea batatas) storage roots. Molecules. 2019;24(24):4476. CrossRef
209. Das G, Patra JK, Basavegowda N, Vishnuprasad CN, Shin HS. Comparative study on antidiabetic, cytotoxicity, antioxidant and antibacterial properties of biosynthesized silver nanoparticles using outer peels of two varieties of Ipomoea batatas (L.) lam. Int J Nanomed. 2019;14:4741–54. CrossRef
210. Kang HG, Jeong SH, Cho JH. Antimutagenic and anticarcinogenic effect of methanol extracts of sweet potato (Ipomea batata) leaves. Toxicol Res. 2010;26(1):29–35. CrossRef
211. Ashraf VKM, Kalaichelvan VK, Venkatachalam VV, Ragunathan R. In vitro anticancer potential of aerial parts of Ipomoea horsfalliae hook in different human cancer cell lines. Ind Crops Prod. 2020;155:112746. CrossRef
212. Babu K, Dharishini P, Austin A. Studies on anatomy and phytochemical analysis of Ipomoea pes-tigridis L. J Pharmacogn Phytochem. 2018;7(1):791–4.
213. Venkateshan N, Subramaniyam M, Santhanakumar M. Review of Ipomoea pes-tigridis L.: ethnobotanical characteristics, pharmacological activities. Int J Curr Pharm Res. 2018;10(6):1–4. CrossRef
214. Begum SS, Aruna A, Sivakumar T, Premanand C. In vitro cytotoxic activity on ethanolic extracts of leaves of Ipomoea pes-tigridis (Convolulaceae) against liver Hepg2 cell line. Int J Ayu Her Med. 2015;5(3):1778–84.
215. Carneiro MRB, Sallum LO, Martins JLR, Peixoto JC, Napolitano HB, Rosseto LP. Overview of the Justicia genus: insights into its chemical diversity and biological potential. Molecules. 2023;28(3):1190. CrossRef
216. Kumar S, Singh R, Dutta D, Chandel S, Bhattacharya A, Ravichandiran V, et al. In vitro anticancer activity of methanolic extract of Justicia adhatoda leaves with special emphasis on human breast cancer cell line. Molecules. 2022;27(23):8222. CrossRef
217. Sudevan S, Parasivam R, Sundar S, Velauthan H, Ramasamy V. Investigation of anti-inflammatory and anti-cancer activity of Justicia adathoda metabolites. Pak J Pharm Sci. 2019;32(4):1555–61.
218. Jiju V. Assessment of in vivo anticancer activity of Justicia adathoda using Dal cell lines. East Afr J Med Sci. 2019;2(7):438–42. CrossRef
219. Mangai AS. A cytotoxic approach of Justica gendarussa Burm.F against human cancer cell lines. Int Res J Pharm. 2018;8(12):34–7. CrossRef
220. Chandra S, Lo D. A review on the bioactivities of Justicia gendarussa. IOP Conf Ser Earth Environ Sci. 2021;794(1):012137. CrossRef
221. Ayob Z, Mohd Bohari SP, Abd Samad A, Jamil S. Cytotoxic activities against breast cancer cells of local Justicia gendarussa crude extracts. Evid Based Complement Alternat Med. 2014;2014:732980. CrossRef
222. Joseph L, Ranjani JM, Pai KSR, Srinivasan KK. Promising anticancer activities of Justicia simplex D. Don. in cellular and animal models. J Ethnopharmacol. 2017;199:231–9. CrossRef
223. Eswari MG, Rathi RL, Harini J, Aruna R. Phytochemical screening of Justicia simplex D. Don a valuable medicinal plant extract against dental pathogens. Int Lett Nat Sci. 2014;21:10–21. CrossRef
224. Ravi L, Sreenivas BKA, Kumari GRS, Archana O. Anticancer cytotoxicity and antifungal abilities of green-synthesized cobalt hydroxide (Co (OH)2) nanoparticles using Lantana camara L. Beni-Suef Univ J Basic Appl Sci. 2022;11:124. CrossRef
225. Al-Hakeim HK, Al-Zabibah RS, Alzihari HF, Almensoori AK, Al-Zubaidi HA. Anticancer and antiangiogenic activities of alkaloids isolated from Lantana camara by adsorption on the magnetic nanoparticles. Karbala Int J Mod Sci. 2021;7(1):11. CrossRef
226. Bhaskar D, Mashrea DS, Amresh N, Sathyamurthy B. In vitro studies on the effect of Lantana camara Linn. in liver. Eur J Pharm Med Res. 2017;4(9):539–45.
227. Han EB, Chang BY, Jung YS, Kim SY. Lantana camara induces apoptosis by Bcl-2 family and caspases activation. Pathol Oncol Res. 2015;21(2):325–31. CrossRef
228. Radhakrishnan R, Liakath F, Khan A, Muthu A. Green synthesis of copper oxide nanoparticles mediated by aqueous leaf extracts of Leucas aspera and Morinda tinctoria. Lett Appl Nano Biosci. 2021;10(4):2706–14. CrossRef
229. Madhu GC, Kannaiyan J, Paulraj B, Veeramani V. Anti-diabetic, anti-cancer activity and associated toxicity of Leucas aspera extract in Wistar albino rats. Int J Pharm Sci Drug Res. 2019;11(06):387–92. CrossRef
230. Mohan A, Nair SV, Lakshmanan VK. Leucas aspera nanomedicine shows superior toxicity and cell migration retarded in prostate cancer cells. Appl Biochem Biotechnol. 2017;181(4):1388–400. CrossRef
231. Rasul A, Riaz A, Wei W, Sarfraz I, Hassan M, Li J, et al. Mangifera indica extracts as novel PKM2 inhibitors for treatment of triple negative breast cancer. Biomed Re Int. 2021;2021:5514669. CrossRef
232. Yap KM, Sekar M, Seow LJ, Gan SH, Bonam SR, Mat Rani NNI, et al. Mangifera indica (Mango): a promising medicinal plant for breast cancer therapy and understanding its potential mechanisms of action. Breast Cancer (Dove Med Press). 2021;13:471–503. CrossRef
233. Morozkina SN, Nhung Vu TH, Generalova YE, Snetkov PP, Uspenskaya MV. Mangiferin as new potential anti-cancer agent and Mangiferin-integrated polymer systems-a novel research direction. Biomolecules. 2021;11(1):79. CrossRef
234. Kumar M, Saurabh V, Tomar M, Hasan M, Changan S, Sasi M, et al. Mango (Mangifera indica L.) leaves: nutritional composition, phytochemical profile, and health-promoting bioactivities. Antioxidants. 2021;10(2):299. CrossRef
235. Kemegne GA, Bettache N, Nyegue MA, Etoa FX, Menut C. Cytotoxic activities of Psidium guajava and Mangifera indica plant extracts on human healthy skin fibroblasts and human hepatocellular carcinoma. Issues Biol Sci Pharma Res. 2020;8(4):58–64. CrossRef
236. Chaudhary MK, Misra A, Srivastava S. Comparative pharmacognostical studies of three Mahonia species: exploring the possibilities as a substitute for the Ayurvedic drug ‘Daruharidra’. Indian J Tradit Knowl. 2022;21(4):774–81. CrossRef
237. Tuzimski T, Petruczynik A, Kapro? B, Makuch-Kocka A, Szultka-M?y?ska M, Misiurek J, et al. Determination of cytotoxic activity of selected isoquinoline alkaloids and plant extracts obtained from various parts of Mahonia aquifolium collected in various vegetation seasons. Molecules. 2021;26(4):816. CrossRef
238. Latha R, Rajanathan TM, Khusro A, Chidambaranathan N, Agastian P, Nagarajan S. Anticancer activity of Mahonia leschenaultii methanolic root extract and berberine on Dalton’s ascitic lymphoma in mice. Asian Pac J Trop Med. 2019;12(6):264–71. CrossRef
239. He JM, Mu Q. The medicinal uses of the genus Mahonia in traditional Chinese medicine: an ethnopharmacological, phytochemical and pharmacological review. J Ethnopharmacol. 2015;175:668–3. CrossRef
240. Lestari OA, Palupi NS, Setiyono A, Kusnandar F, Yuliana ND. In vitro antioxidant potential and phytochemical profiling of Melastoma malabathricum leaf water extract. Food Sci Technol Campinas. 2022;42:e92021. CrossRef
241. Kumar V, Sachan R, Rahman M, Rub RA, Patel DK, Sharma K, et al. Chemopreventive effects of Melastoma malabathricum L. extract in mammary tumor model via inhibition of oxidative stress and inflammatory cytokines. Biomed Pharmacother. 2021;137:111298. CrossRef
242. Idris A, Ahmad S. Melastoma malabathricum ethyl acetate fraction induces secondary necrosis in human breast and lung cancer cell lines. Pharmacogn Mag. 2017;13(Suppl 4):179–88. CrossRef
243. Adib JC, Yunos N. Anti-cancer, antimicrobial, and antioxidative potentials of Mesua ferrea L. and its phytochemical constituents: a review. Asian J Pharmacogn. 2019;3(3):5–19.
244. Asif M, Shafaei A, Abdul Majid AS, Ezzat MO, Dahham SS, Ahamed MBK, et al. Mesua ferrea stem bark extract induces apoptosis and inhibits metastasis in human colorectal carcinoma HCT 116 cells, through modulation of multiple cells signalling pathways. Chin J Nat Med. 2017;15(7):505–14. CrossRef
245. Rajendran K, Reddy EV, Khanna A. Anticancer effect of Mesua ferrea extracts on human pancreatic cancer cell line. Int J Life Sci Res. 2016;2(2):198–205.
246. Yesmin R, Das PK, Belal H, Aktar S, Siddika MA, Asha SY, et al. Anticancer potential of Michelia champaca Linn. bark against Ehrlich Ascites carcinoma (EAC) cells in Swiss albino mice. Nat Prod J. 2021;11(1):85–96. CrossRef
247. Sinha R, Varma R. Antioxidant activity in leaf extracts of Michelia champaca L. J Adv Pharm Educ Res. 2017;7(2):86–8.
248. Zuhrotun A, Suganda AG, Wirasutisna KR, Wibowo MS. Isolation of bioactive compound of Michelia champaca L. bark and its activity test using mechanism-based yeast bioassay. Asian J Pharm Clin Res. 2016;9(5):158–61. CrossRef
249. Ginting B, Mustanir, Nurdin, Maulidna, Murniana, Safrina. Evaluation of antioxidant and anticancer activity of Myristica fragrans houtt. bark. Pharmacogn J. 2021;13(3):780–6. doi: 10.5530/pj.2021.13.99
250. Ginting B, Saidi N, Murniana, Mustanir, Maulidna, Simanjuntak P. Lignan compound isolated from n-hexane extract Myristica fragrans Houtt root as antioxidant and antitumor activities against MCF-7 cell lines data. Data Brief. 2020;31:105997. CrossRef
251. Le TV, Nguyen PH, Choi HS, Yang J, Kang KW, Ahn S, et al. Diarylbutane-type lignans from Myristica fragrans (Nutmeg) show the cytotoxicity against breast cancer cells through activation of AMP-activated protein kinase. Nat Prod Sci. 2017;23(1):21–8. CrossRef
252. Rengasamy G, Venkataraman A, Veeraraghavan VP, Jainu M. Cytotoxic and apoptotic potential of Myristica fragrans Houtt. (mace) extract on human oral epidermal carcinoma KB cell lines. Braz J Pharm Sci. 2018;54(3):e18028. CrossRef
253. Prakash E, Gupta DK. Cytotoxic activity of ethanolic extract of Myristica fragrans (Houtt) against seven human cancer cell lines. Univers J Food Nutr Sci. 2013;1(1):1–3. CrossRef
254. Vallinayagam S, Rajendran K, Sekar V. Pro-apoptotic property of phytocompounds from Naringi crenulata in HER2+ breast cancer cells in vitro. Biotechnol Equip. 2021;35(1):354–65. CrossRef
255. Manjula V, Norman TSJ, Senthilnathan S. Naringi crenulata (Roxb.)—a potential drug for the future. Pharma Innov J. 2017;6(10):261–3.
256. Pratheeba T, Vivekanandhan P, Nur Faeza AK, Natarajan D. Chemical constituents and larvicidal efficacy of Naringi crenulata (Rutaceae) plant extracts and bioassay guided fractions against Culex quinquefasciatus mosquito (Diptera: Culicidae). Biocatal Agric Biotechnol. 2019;19:101137. CrossRef
257. Rashan LJ, Özenver N, Boulos JC, Dawood M, Roos WP, Franke K, et al. Molecular modes of action of an aqueous Nerium oleander extract in cancer cells in vitro and in vivo. Molecules. 2023;28(4):1871. CrossRef
258. Barai AC, Paul K, Dey A, Manna S, Roy S, Bag BG, et al. Green synthesis of Nerium oleander-conjugated gold nanoparticles and study of its in vitro anticancer activity on MCF-7 cell lines and catalytic activity. Nano Converg. 2018;5(1):10. CrossRef
259. Hamad O, Obaidi SA. Studies on antibacterial and anticancer activity of Nerium oleander extracts. Eur Chem Bull. 2014;3(3):259–62.
260. Ahmed SS, Rahman MO, Algahtani AS, Sultana N, Almarfadi OM, Ali MA, et al. Anticancer potential of phytochemicals from Oroxylum indicum targeting lactate dehydrogenase a through bioinformatic approach. Toxicol Rep. 2023;10:56–75. CrossRef
261. Menon S, Albaqami JJ, Hamdi H, Lawrence L, Padikkala J, Mathew SE, et al. Oroxylum indicum vent root bark extract inhibits the proliferation of cancer cells and induce apoptotic cell death. Processes. 2023;11(1):188. CrossRef
262. Rai D, Aswatha Ram HN, Neeraj Patel K, Babu UV, Sharath Kumar LM, Kannan R. In vitro immuno-stimulatory and anticancer activities of Oroxylum indicum (L.) Kurz.: evidence for substitution of aerial parts for conservation. J Ayurveda Integr Med. 2022;13(2):100523. CrossRef
263. Sharmila KP, Shetty SS, Kumari S, Harishkumar M, Prabhu A, Satheesh Kumar Bhandary B. Oroxylum indicum stem bark extract exerts antitumor potential against Ehrlich’s ascites carcinoma in Swiss albino mice. Biomedicine. 2022;42(4):686–92. CrossRef
264. Rishu K, Nutan K. Review on Oroxylum indicum: unfathomed source of anticancer drug. Scope Phytochem Unexplored Med Plants. 2017;2017:99–109.
265. Saahene RO, Agbo E, Barnes P, Yahaya ES, Amoani B, Nuvor SV, et al. A review: mechanism of Phyllanthus urinaria in cancers—NF-κB, P13K/AKT, and MAPKs signaling activation. Evid Based Complement Altern Med. 2021;2021:4514342. CrossRef
266. Pammi S, Giri A. In vitro cytotoxic activity of Phyllanthus amarus Schum. & Thonn. World J Biol Pharm Heal Sci. 2021;6(2):34–42. CrossRef
267. Omoregie FO, Eriyamremu GE, Kapur S. Therapeutic effects of aqueous and ethanolic extracts of Phyllanthus amarus on 1, 2 dimethylhydrazine induced colon carcinogenesis in Balb/c mice. Int J Biochem Res Rev. 2020;29(7):36–43. CrossRef
268. Ahmad MS, Bano S, Anwar S. Cancer ameliorating potential of Phyllanthus amarus: in vivo and in vitro studies against aflatoxin B1 toxicity. Egypt J Med Hum Genet. 2015;16(4):343–53. CrossRef
269. Tang YQ, Jaganath I, Manikam R, Sekaran SD. Phyllanthus suppresses prostate cancer cell, PC-3, proliferation and induces apoptosis through multiple signalling pathways (MAPKs, PI3K/Akt, NFκB). Evid Based Complement Alternat Med. 2013; 2013:609581. CrossRef
270. Mitra S, Anand U, Jha NK, Shekhawat MS, Saha SC, Nongdam P, et al. Anticancer applications and pharmacological properties of piperidine and piperine: a comprehensive review on molecular mechanisms and therapeutic perspectives. Front Pharmacol. 2022;12:772418. CrossRef
271. Turrini E, Sestili P, Fimognari C. Overview of the anticancer potential of the ‘King of spices’ Piper nigrum and its main constituent piperine. Toxins (Basel). 2020;12(12):747. CrossRef
272. Ngo QMT, Cao TQ, Hoang LS, Ha MT, Woo MH, Min BS. Cytotoxic activity of alkaloids from the fruits of Piper nigrum. Nat. Prod. Commun. 2018;13(11):1467–9. CrossRef
273. Prashant A, Rangaswamy C, Yadav AK, Reddy V, Sowmya MN, Madhunapantula S. In vitro anticancer activity of ethanolic extracts of Piper nigrum against colorectal carcinoma cell lines. Int J Appl Basic Med Res. 2017;7(1):67–72. CrossRef
274. Krishnan V, Bupesh G, Manikandan E, Thanigai Arul K, Mangesh S, Kalyanaraman R, et al. green synthesis of silver nanoparticles using Piper nigrum concoction and its anticancer activity against MCF-7 and Hep-2 Cell lines. J Antimicrob Agents. 2016;2(3):1000123. CrossRef
275. Chen YC, Chia YC, Huang BM. Phytochemicals from Polyalthia species: potential and implication on anti-oxidant, anti-inflammatory, anti-cancer, and chemoprevention activities. Molecules. 2021;26(17):5369. CrossRef
276. Afolabi SO, Olorundare OE, Babatunde A, Albrecht RM, Koketsu M, Syed DN, et al. Polyalthia longifolia extract triggers ER stress in prostate cancer cells concomitant with induction of apoptosis: insights from in vitro and in vivo studies. Oxid Med Cell Longev. 2019;2019:6726312. CrossRef
277. Vijayarathna S, Chen Y, Kanwar JR, Sasidharan S. Standardized Polyalthia longifolia leaf extract (PLME) inhibits cell proliferation and promotes apoptosis: the anti-cancer study with various microscopy methods. Biomed Pharmacother. 2017;91:366–77. CrossRef
278. Luna PYM, Limbo CA, Jacinto SD. Cytotoxicity of fractions from Quisqualis indica Linn. against selected human cancer cell lines. Int J Biosci. 2019;15(5):518–26. CrossRef
279. Ub Wijerathne C, Park HS, Jeong HY, Song JW, Moon OS, Seo YW, et al. Quisqualis indica improves benign prostatic hyperplasia by regulating prostate cell proliferation and apoptosis. Biol Pharm Bull. 2017;40(12):2125–33. CrossRef
280. Mukhopadhyay R, Kazi J, Debnath MC. Synthesis and characterization of copper nanoparticles stabilized with Quisqualis indica extract: evaluation of its cytotoxicity and apoptosis in B16F10 melanoma cells. Biomed Pharmacother. 2018;97:1373–85. CrossRef
281. Abraham S, Selvaraj J, Gayatri R, Dilipan E. In-vitro anticancer activity of Rauvolfia tetraphylla extract on mcf-7 breast cancer cell lines. Bioinformation. 2023;19(1):43–7. CrossRef
282. Vanjari K, Bangar S, Thorve A, Wagh S. Medicinal plant Rauvolfia tetraphylla l its medicinal uses and pharmacological activities. J Med Plants Stud. 2022;10(5):119–21.
283. Das S, Langbang L, Haque M, Belwal VK, Aguan K, Singha Roy A. Biocompatible silver nanoparticles: an investigation into their protein binding efficacies, anti-bacterial effects and cell cytotoxicity studies. J Pharm Anal. 2021;11(4):422–34. CrossRef
284. Iqbal AAM, Khan FAK, Khan M. Ethno-phyto-pharmacological overview on Rauwolfia tetraphylla L. Int J Pharm Phytopharm Res. 2013;2(4):247–51.
285. Arora S, Meena S. Bio-activity in flowers of Sarcostemma viminale (L.) R.Br.- an endangered medicinal plant from Thar Desert of Rajasthan (India). Pharmacogn J. 2018;10(5):871–4. CrossRef
286. Brestovac B, Coghlan O, Jackaman C, Nelson D, Townsend D. Sarcostemma viminale activates macrophages to a pro-inflammatory phenotype. Comp Clin Path. 2015;24:817–26. CrossRef
287. Brian B, Jessica S, Gaywin E, Alexander P, David T. Sarcostemma viminale: a potential anticancer therapy. Comp Clin Path. 2015;24:9-–17. CrossRef
288. Girme AS, Bhalke RD, Nirmal SA, Chavan MJ. Chromatographic and chemical analysis of Sarcostemma viminale R. Br. Pharm Exp Med. 2014;14:279–84. CrossRef
289. Sulaiman CT, Deepak M, Praveen TK, Lijini KR, Anandan EM, Salman M, et al. Purification of Bhallathaka (Semecarpus anacardium L.f.) enhanced anti-cancer activity. Regul Toxicol Pharmacol. 2021;122:104898. CrossRef
290. Joseph JP, Raval SK, Sadariya KA, Jhala M, Kumar P. Anti-cancerous efficacy of ayurvedic milk extract of Semecarpus anacardium nuts on hepatocellular carcinoma in Wistar rats. Afr J Tradit Compliment Altern Med. 2013;10(5):299–304.
291. Premalatha B, Muthulakshmi V, Sachdanandam P. Anticancer potency of the milk extract of Semecarpus anacardium Linn. nuts against aflatoxin B1 mediated hepatocellular carcinoma bearing Wistar rats with reference to tumour marker enzymes. Phyther Res. 1999;13(3):183–7. CrossRef
292. Kowalczyk T, Merecz-Sadowska A, Rijo P, Mori M, Hatziantoniou S, Górski K, et al. Hidden in plants-a review of the anticancer potential of the Solanaceae family in in vitro and in vivo studies. Cancers (Basel). 2022;14(6):1455. CrossRef
293. Helilusiatiningsih N, Yunianta A, Harijono A, Wijanarko SB. Cytotoxic activity and selectivity index of Solanum torvum fruit on T47D breast cancer cells. Indian J Public Heal Res Dev. 2020;11(2):1592. CrossRef
294. Balachandran C, Emi N, Arun Y, Yamamoto Y, Ahilan B, Sangeetha B, et al. In vitro anticancer activity of methyl caffeate isolated from Solanum torvum Swartz. fruit. Chem Biol Interact. 2015;242:81–90. CrossRef
295. Panigrahi S, Muthuraman MS, Natesan R, Pemiah B. Anticancer activity of ethanolic extract of Solanum torvum sw. Int J Pharm Sci. 2014;6(1):93–8.
296. Li R, Yang JJ, Song XZ, Wang YF, Corlett RT, Xu YK, et al. Chemical composition and the cytotoxic, antimicrobial, and anti-inflammatory activities of the fruit peel essential oil from Spondias pinnata (Anacardiaceae) in Xishuangbanna, Southwest China. Molecules. 2020;25(2):343. CrossRef
297. Patathananone S, Daduang J, Koraneekij A, Li CY. Tyrosinase inhibitory effect, antioxidant and anticancer activities of bioactive compounds in ripe hog plum (Spondias pinnata) fruit extracts. Orient J Chem. 2019;35(3):916–26. CrossRef
298. Chaudhuri D, Ghate NB, Singh SS, Mandal N. Methyl gallate isolated from Spondias pinnata exhibits anticancer activity against human glioblastoma by induction of apoptosis and sustained extracellular signal-regulated kinase 1/2 activation. Pharmacogn Mag. 2015;11(42):269–76. CrossRef
299. Zhu X, Xu Y, Sun D, Li H, Chen L. The genus Strobilanthes: phytochemistry and pharmacology. TMR Mod Herb Med. 2022;5(3):15. CrossRef
300. Senniyappan V, Subban R, Kaveri S, Ramasamy K. Acetylcholinesterase inhibitory and cytotoxic activity of extracts and isolated compounds from Strobilanthes ciliatus Nees. Int J Pharm Sci Rev Res. 2022;77(25):155–9. CrossRef
301. Farzana H, Aysha OS. Phytochemical, antioxidant, antibacterial and mutagenic property (AMES) in vitro analysis of Strobilanthes barbatus. Int J Innov Res Technol. 2021;7(11):728–35.
302. Puranik SI, Hiremath MB, Nerli RB, Ghagane SC. Evaluation of in vitro antioxidant and anticancer activity of Tabernaemontana divaricata leaf extracts against T-24 human bladder cancer cell lines. Int J Cancer Res. 2018;14(2):100–8. CrossRef
303. Selvakumar S, Kumar A. Antiproliferative efficacy of Tabernaemontana divaricata against HEP2 cell line and Vero cell line. Pharmacogn Mag. 2015;11(Suppl 1):S46–52. CrossRef
304. Poornima K, Gopalakrishnan VK. Anticancer activity of Tabernaemontana coronaria against carcinogen-induced clear cell renal cell carcinoma. Chin J Biol. 2014;2014:584074. CrossRef
305. Akhila SD, Shankar Guru P, Ramya Devi D, Vedha Hari BN. Evaluation of in vitro anticancer activity of hydroalcoholic extract of Tabernaemontana divaricata. Asian J Pharm Clin Res. 2012;5(4):59–61.
306. Chandrasekaran C, Ramar K. Molecular docking studies of potential anticancer agents from Thunbergia fragrans against colorectal cancer mutant genes through in-silico study. Int J Ayurvedic Med. 2021;12(4):792–5. CrossRef
307. Kumar PY, Subramaniyan M. Phytochemical evaluation and pharmacological activities of Thunbergia species—a comprehensive review. Int J Biol Pharm Allied Sci. 2021;10(11):233–43. CrossRef
308. Pal P, Gupta N, Jain S. Preparation, characterization and evaluation of silver nanoparticles of Thunbergia grandiflora and its antimicrobial activity. J Drug Deliv Ther. 2019;9(s):229–35.
309. El-Hakim A, Kinasih A, Putri R, Putri SU, Kurniawan FY, Semiarti E. In silico study of secondary metabolite in Vanilla planifolia to inhibit nudt5 as breast cancer target. 1st Bioinformatics and Biodiversity Conference; 2021; Jawa Timur, Indonesia: NST Proceedings.
310. Kaliappan V, Kumaravelu P. Antiproliferative effects of Vanilla planifolia leaf extract against breast cancer MCF-7 cells. Int J Basic Clin Pharmacol. 2019;8(1):51–5.
311. Vijaybabu K, Punnagai K. In-vitro anti-proliferative effects of ethanolic extract of Vanilla planifolia leaf extract against A431 human epidermoid carcinoma cells. Biomed Pharmacol J. 2019;12(3):1141–6. CrossRef
312. D’Souza JN, Nagaraja GK, Prabhu A, Navada KM, Kouser S, Manasa DJ. AgVI and Ag/ZnOVI nanostructures from Vateria indica (L.) exert antioxidant, antidiabetic, anti-inflammatory and cytotoxic efficacy on triple negative breast cancer cells in vitro. Int J Pharm. 2022;615:121450. CrossRef
313. Siddiqui A, Tabassum K, Anjum A. Pharmacological activities of Kahruba (Vateria indica Linn.)-a literary review. Int J Adv Res Dev. 2019;4(2):6–9.
314. Mishima S, Matsumoto K, Futamura Y, Araki Y, Ito T, Tanaka T, et al. Antitumor effect of stilbenoids from Vateria indica against allografted sarcoma S-180 in animal model. J Exp Ther Oncol. 2003;3(5):283–88. CrossRef
315. Pakpisutkul J, Suwapraphan J, Sripaya N, Sitkhuntod N, Loyrat S, Yahayo W, et al. The effects of Vernonia cinerea less extracts on antioxidant gene expression in colorectal cancer cells. Asian Pac J Cancer Prev. 2022;23(11):3923–30. CrossRef
316. Amuthan A, Devi V, Shreedhara CS, Rao V, Jasphin S, Kumar N. Vernonia cinerea regenerates tubular epithelial cells in cisplatin induced nephrotoxicity in cancer-bearing mice without affecting antitumor activity. J Tradit Complement Med. 2020;11(3):279–86. CrossRef
317. Chandra S, Das A, Ray T, Mukherjee L, Samanta J. Putative role of Moringa oleifera in prophylaxis of chemotherapy-induced neuropathic pain in mice. J Drug Deliv Ther. 2019;9(3Suppl.):615–20.
318. Khay M, Toeng P, Mahiou-Leddet V, Mabrouki F, Sothea K, Ollivier E, et al. HPLC analysis and cytotoxic activity of Vernonia cinerea. Nat Prod Commun. 2012;7(10):1259–62. CrossRef
319. Vo GV, Nguyen TH, Nguyen TP, Do TH, Tran NM, Nguyen HT, et al. In silico and in vitro studies on the anti-cancer activity of Artemetin, Vitexicarpin and Penduletin compounds from Vitex negundo. Saudi Pharm J. 2022;30(9):1301–14. CrossRef
320. Gouthami K, Veeraraghavan V, Nagaraja P. In-silico characterization of phytochemicals identified from Vitex negundo (L) extract as potential therapy for Wnt-signaling proteins. Egypt J Med Hum Genet. 2022;3:23. CrossRef
321. Edwin Jose B, Manikandan S, Jebaseelan S, Meera DR. Phytochemical investigation and anti-cancer activity of Vitex negundo. Int J Pharm Sci Rev Res. 2021;66(1):65–9. CrossRef
322. Winarno EK, Susanto, Winarno H. Antiproliferative activity against cancer cell lines of gamma irradiated ‘legundi’ (Vitex trifolia L.) leaves and its chromatogram profiles. AIP Conf Proc. 2020;2296:020068. CrossRef
323. Lacombe J, Cretignier T, Meli L, Wijeratne EMK, Veuthey JL, Cuendet M, et al. Withanolide D enhances radiosensitivity of human cancer cells by inhibiting DNA damage non-homologous end-joining repair pathway. Front Oncol. 2020;9:1468. CrossRef
324. Aziz A, Azhar MF. Antioxidant and phytochemical composition of leaves, stem and root extracts of Withania coagulans and Withania somnifera. J Med Spice Plants. 2020;24(1):27–30.
325. Akhtar N, Baig MW, Haq IU, Rajeev V, Cutillas PR. Withanolide metabolites inhibit PI3K/AKT and MAPK pro-survival pathways and induce apoptosis. Biomedicines. 2020;8(9):333. CrossRef
326. Saleem S, Muhammad G, Hussain MA, Altaf M, Bukhari SNA. Withania somnifera L.: insights into the phytochemical profile, therapeutic potential, clinical trials, and future prospective. Iran J Basic Med Sci. 2020;23(12):1501–26. CrossRef
327. Thakur S, Kaurav H, Chaudhary G. A Review on Woodfordia fruticosa Kurz (Dhatki): ayurvedic, folk and modern uses. J Drug Deliv Ther. 2021;11(3):126–31. CrossRef
328. Raji NR, Roshni P, Latha MS. Induction of apoptosis by the ethyl acetate fraction of Woodfordia fruticosa Kurz. flowers through NFκB mediation in hepatocellular carcinoma. Int J Adv Res. 2017;5(8):885–93. CrossRef
329. Kumar D, Sharma M, Sorout A, Saroha K, Verma S. Woodfordia fruticosa Kurz.: a review on its botany, chemistry and biological activities. J Pharmacogn Phytochem. 2016;5(3):293–8.
330. Rao BG, Devarakonda R, Battu H. Phytochemical and pharmacological studies on Wrightia tinctoria. World J Pharm Sci. 2019;7(4):562–85. CrossRef
331. Jose B. Evaluation of anticancer potential of the medicinal plant Wrightia tinctoria (Roxb) R. Br., from South India. J Forensic Res. 2016;7(5Suppl):74.
332. Fatima N, Ahmad MK. Ansari JA, Ali Z, Khan AR, Mahdi AA. Anticancer, antioxidant potential and profiling of polyphenolic compounds of Wrightia tinctoria Roxb. (R.Br.) bark. J Adv Pharm Technol Res. 2016;7(4):159–65. CrossRef
333. Wang Y, Chinnathambi A, Nasif O, Alharbi SA. Green synthesis and chemical characterization of a novel anti-human pancreatic cancer supplement by silver nanoparticles containing Zingiber officinale leaf aqueous extract. Arab J Chem. 2021;14(4):103081. CrossRef
334. Qian S, Fang H, Zheng L, Liu M. Zingerone suppresses cell proliferation via inducing cellular apoptosis and inhibition of the PI3K/AKT/mTOR signaling pathway in human prostate cancer PC-3 cells. J Biochem Mol Toxicol. 2021;35(1):e22611. CrossRef
335. Abbas A, Ali A, Seidmostafa N, Elaheh A, Rezvan A, Elham M, et al. Apoptotic effects of ginger extract (Zingiber officinale) on esophageal cancer cells ESO26: an in vitro study. J Reports Pharm Sci. 2020;9(2):183–8. CrossRef
336. Mathiyazhagan J, Kodiveri Muthukaliannan G. Combined Zingiber officinale and Terminalia chebulic induces apoptosis and modulates mTOR and hTERT gene expressions in MCF-7 cell line. Nutr Cancer. 2020;73(7):1207–16. CrossRef
337. Zhao L, Rupji M, Choudhary I, Osan R, Kapoor S, Zhang HJ, et al. Efficacy based ginger fingerprinting reveals potential antiproliferative analytes for triple negative breast cancer. Sci Rep. 2020;10:19182. CrossRef
338. Sarmoko S, Solihati I, Setyono J, Ekowati H, Fadlan A. Zingiber officinale Var. rubrum extract increases the cytotoxic activity of 5-fluorouracil in colon adenocarcinoma widr cells. Indonesian J Pharm. 2020;31(4):266–72. CrossRef
339. Dissanayake K, Waliwita W, Liyanage R. A review on medicinal uses of Zingiber officinale (Ginger). Int J Heal Sci Res. 2020;10(6):142–8.
340. Maslikah SI, Lestari SR, Amin M, Amalia A. Anticancer activity of phenolic leaves of Bidara (Ziziphus mauritiana) against breast cancer by in silico. AIP Conf Proc. 2021;2353:030042. CrossRef
341. Beg MA, Teotia UVS, Farooq S. In vitro antibacterial and anticancer activity of Ziziphus. JMPS. 2016;4(5):230–3. doi: 10.1088/1742-6596/1341/3/032042
342. Bhatia A, Mishra T, Khullar M. Anticancer potential of aqueous ethanol seed extract of Ziziphus mauritiana against cancer cell lines and Ehrlich ascites carcinoma. J Evid Based Complement Alternat Med. 2011;2011:765029. CrossRef
SUPPLEMENTARY MATERIALS
 | Supplementary Table S1. RR for common effect and random effect models. [Click here to view] |
 | Supplementary Table S2. Test of heterogeneity. [Click here to view] |
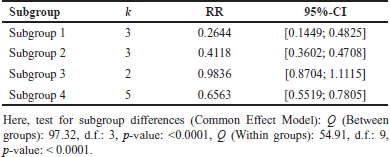 | Supplementary Table S3. Subgroup analysis (Common effects model). [Click here to view] |
 | Supplementary Table S4. Subgroup analysis (Random effects model). [Click here to view] |
 | Supplementary Table S5. Publication bias analysis. [Click here to view] |
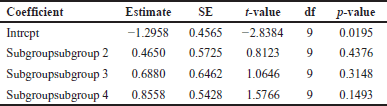 | Supplementary Table S6. Meta regression analysis of subgroups. [Click here to view] |
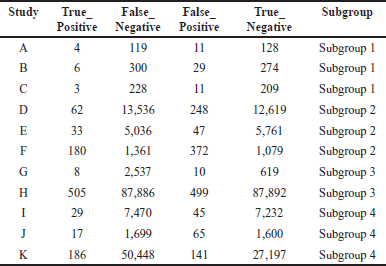 | Supplementary Table S7. Publication bias analysis. [Click here to view] |
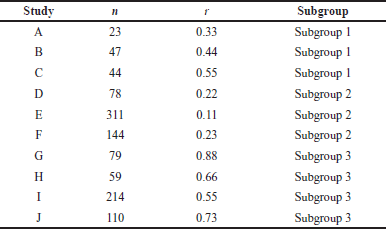 | Supplementary Table S8. Publication correlation analysis. [Click here to view] |