INTRODUCTION
The amount of agricultural waste generated worldwide has surged astronomically in recent years due to the fast-growing population, resulting in the increasing demand for food [1]. This should not come as a surprise, seeing that the world population has grown from 3.7 billion recorded in 1970 to 7.9 billion in 2021, which implies an increasing demand for food and the dire need for food security if everyone is to survive [1,2]. Thus, to ensure the sustenance of life, the demand for food and to meet the ever growing human population, there has been a significant increase in crop and livestock production, leading to a continuous rise in agricultural wastes [1,3]. In the quest toward maintaining a sustainable food production process and combating the issues of food security challenges, the accompanying agricultural waste has become a significant environmental concern to life. For instance, India and China, with about 3 billion population, account for solid waste (predominantly agricultural waste) of about 350–990 million and about 130 million tons per year, respectively [4]. This is a worrying amount of waste by just two nations, most of which are not properly managed and disposed of. Most of these agricultural wastes are usually disposed of by burning, resulting in the generation of harmful chemicals that pose severe threats to the environment and humans, such as greenhouse gases which include CO2, CH4, and N2O [5]. Thus, the improper and limited management of these agricultural wastes require urgent attention for agricultural sustainability and human food and health security [1]. Although culturally, some of these wastes have been repurposed as a roofing material, soil mulching, compositing, animal fodder, combustion material, and in paper production, the vast majority are usually managed by burning or burying in soil, which causes soil, air, and water pollution and ultimately causes global warming [1]. Therefore, repurposing these materials to generate value-added products that can provide job opportunities and improve the farmer’s livelihood while maintaining a sustainable environmental practice is in dire need [6,7].
There are different agricultural waste sources, and the primary sources of significant concern include [8–10]:
• Livestock: waste feed, urine, wash water, dung, and residual milk.
• Crop-residues: husks, stalks, weeds, leaf litter, seed pods, peels, stems, and straws.
• Aquaculture: fecal waste and uneaten feed.
• Agro-industrial waste: peels, molasses, bagasse, pulps, and oil-seed cakes.
Among the listed waste sources, agro-industrial waste, mainly from food processing industries, has been a significant source of agricultural waste. For instance, about 180.73 million metric tons of bagasse from sugarcane are produced yearly worldwide, and it has been forecasted to increase to 221 million metric tons by 2024 [11,12]. The material found as waste in this class includes bagasse and molasses from sugarcane, fruit skin and pomace from fruits (such as apple, mango, amla, orange, guava, litchi, tomato, cabbage, lettuce, and so on), rice husk, vegetable, starch residue, eggshells, poultry, and farm animal skin and meat from respective industries. Also, horticultural wastes belong to this class. They have been reported to contribute a significant amount of waste to ago-industrial waste and are usually produced during the preparation and processing of food items such as fruit juice, cider, jams, ketchup, jellies, sauces, and pickles [1]. Examples of these wastes include fruit seeds, peel, and pomace (mango, citrus, litchi, watermelon, pawpaw, musk melon, banana, apple, pineapple, and so on). Citrus fruits have received the most attention in this class due to their abundance and unique taste [13]. They have become a stable dietary component of most food consumed due to their enormous health benefits and their anti-infective, anti-inflammatory, antioxidative, anticancer, and neuroprotective properties [14]. The industrial processing of citrus into different dietary materials has led to the enormous generation of waste materials from almost every part of the plant. It has been reported that citrus accounts for nearly a fifth of the total cultivars industrially processed into food materials, leading to a significant agricultural waste of about 120 million tons per year worldwide [14–16]. Nevertheless, only 45% of the total fruit weight is being harnessed in this process which leads to the production of waste materials such as peel (flavedo; 27%), pulp (albedo and endocarp; 26%), and seeds (2%) [17]. Even though some of the citrus bye-products amassed from the industrial processing plants have been reused or repurposed, a significant amount of these materials is released into the environment, causing environmental concerns. Nevertheless, these byproducts have been identified as an excellent economical and renewable source of valuable compounds, which can be used in pharmaceutical, nutraceutical, food, and cosmetic industries [18].
Citrus peels are useful in providing low-cost, high-energy cattle feed [18]. The oil from citrus peels is known for its pleasant odor, used to flavor confectionaries and beverages, and as a fragrance in cosmetic and perfumery industries. They are a good source of many bio-resource materials, which have been useful as reducing agents in synthesizing many nanoparticles [18]. This review thus seeks to identify the possible usage of citrus waste materials as potential agents for various biological applications, such as their usage as anti-inflammatory, antimicrobial, antioxidant, and anticancer agents.
MATERIALS AND METHODS
The study was conducted by an extensive literature search for published articles, using relevant scientific databases, such as Google Scholar, Mendeley, PubMed, ScienceDirect, Scopus, and Web of Science. Searches were made for relevant publications on the subject matter in the last 25 years (1999–2023). The major search keywords included “citrus wastes,” “citrus raw materials,” and “biological applications of citrus wastes.” The inclusion criteria for the literature search included “citrus plants,” “orange plants,” “citrus fruits,” “citrus peels,” “citrus leaves,” “citrus seeds,” “phytochemical contents of citrus peels, leaves, and seeds,” “chemistry of citrus leaves, peels, and seeds,” “antioxidant properties of citrus,” “anti-inflammatory properties of citrus,” “antimicrobial properties of citrus,” “prebiotic activity of citrus,” “cytotoxic properties of citrus,” “insecticidal properties of citrus,” “antidiabetic properties of citrus,” and “usefulness of citrus wastes.” Exclusion criteria included “phytochemical contents of citrus juice,” biological applications of citrus juice” and “biological applications of plant waste other than those from citrus.” The molecular structures of compounds identified in citrus peels, leaves, and seeds were drawn with the ChemDraw Ultra® 7.0 software package, CambridgeSoft Corporation (Cambridge, MA). Figure illustrations and pie charts were drawn with Microsoft 365 Publisher and Microsoft 365 Excel (Microsoft Corporation, Redmond, DC), respectively.
THE GENUS CITRUS: PRODUCTION AND ECONOMIC ANALYSIS OF CITRUS
The genus of citrus has been identified as one of the most widely grown subunits of the family Rutaceae worldwide [19]. This is due to their enormous nutritional and pharmaceutical properties, making them highly valuable to man [20]. In addition, citrus fruits have been reported to be a rich source of minerals, vitamins, and dietary fibers, which are responsible for the general well-being of the human body [21]. Although citrus has been reported to originate from Northern India, Northern Myanmar, Southern China, and Southeast Asia [22], their cultivation has spread to other parts of the world due to their enormous economic value, making them one of the most sought-after fruits in the world [23]. The citrus genus has been found to possess about 1,400 genera and 1,300 species. Notable examples are the Citrus aurantifolia (Limes), Citrus sinensis (Orange), Citrus reticulata (Mandarin), Citrus paradisi (Grapefruit), Citrus limon (Lemon), Citrus junos (Yuzu), Citrus bergamia (Bergamot), and Citrus japonica (Kumquat) [24]. Citrus sinensis (sweet orange) accounts for about 70% of this genus’s total production and consumption [19]. These evergreen plants usually occur as a shrub or a tree with a height that ranges between 3 and 15 m, with elliptical-shaped leathery leaves and stemmy spikes, which produce flowers that grow individually in the axils of the leaves [20]. The various types and their respective byproducts are presented in Figure 1.
The fruits of citrus are rich in a wide range of phytochemicals and bioactive compounds, which possess several health properties. These chemicals include folic acid, vitamin C, pectin, and potassium [25]. The interest in this group of crops has continued to increase over the years because of its high polyphenols, mainly flavonoids, and antioxidant potential. For instance, in the peels and tissues of citrus fruit, different phytochemicals like flavones such as naringin and hesperidin have been found, which indicates their therapeutic potential as anti-inflammatory, antioxidative, and anti-carcinogenic agents [26]. The ever rising interest in their fruit is due to their fragrance, appealing taste, and nutritional benefits [27]. Generally, all species of citrus have similar anatomical features, as shown in Figure 2 [27].
In the past, the various varieties of their fruit were only used and traded as fresh fruits, even in places that did not support their growth due to extraordinary post-harvest stability, promoting global trade [27]. Their constant demand has encouraged the industrialization of citrus fruits because they are not only consumed unprocessed but have been used in the production of various items such as jams, jellies, marmalades, and essential oils (EOs) [23,27]. About 18% of these fruits are utilized in the industries, mainly for juice production [28]. Citrus fruits have been reported to account for about 98% of the total industrialized crops, and oranges have been said to account for 82% of the whole citrus fruits produced [29,30]. In 2020, about 158.49 million metric tons were produced globally [27]. Among these, Asia, Africa, America, Europe, and Oceania accounted for 47.7%, 43.7%, 8.1%, 0.4%, and 0.1% of the total citrus fruit production, respectively [27]. China alone is responsible for about 28.16% of the world’s citrus fruit production in 2020, approximately 44.63 million metric tons of global citrus fruit production. Other major producing nations that account for over 5% include India, Brazil, and Mexico. Furthermore, it has been reported that about 10.07 million hectares of land, mainly in nations such as China, India, Nigeria, Brazil, and Mexico, are currently being used to produce citrus fruits [27]. Thus, the geographical production distribution of the major types has been concisely presented in Figure 3, according to Sharma et al. [19].
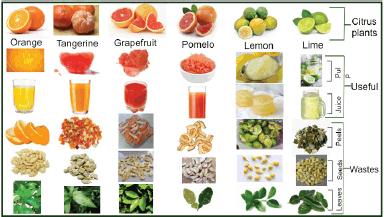 | Figure 1. Some types of citrus fruits and their wastes [19]. [Click here to view] |
The continued interest in citrus production has, in turn, resulted in the continued generation of waste which has become a severe environmental burden [27]. The unhygienic disposal of citrus waste has been termed dangerous if not treated before disposal [31]. The citrus wastes include peels (which account for about 50%–55% of the total fruit mass), seeds (which account for 20%–40% of total fruit mass, pomace), and wastewater (from spoiled fruit, seeds, pulp, and peels) [27]. Yearly, about 10 million citrus wastes are reported globally, just from the fruits alone, causing a severe ecological issue [16]. The genuine concern is that citrus waste is problematic because it contains 80% water, thus inviting microbes, flies, and mold. Therefore, they rot quickly and produce harmful mycotoxins [32]. Hence, their proper disposal and management are vital, as their indiscriminate disposal can lead to water and soil pollution and destroy the aquatic ecosystem [16].
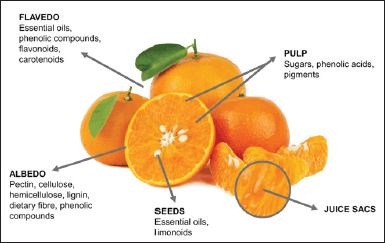 | Figure 2. The general anatomical characteristics of citrus fruits [27]. [Click here to view] |
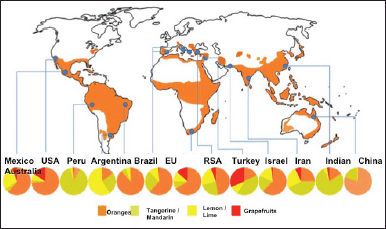 | Figure 3. Map showing the geographical distribution of the major types of citrus fruits production from 2007–2016 across the globe [19]. [Click here to view] |
Nevertheless, citrus leaves, seeds, fruit peels, and pomace, have been identified as a powerhouse for many bioresource materials that have found application as biological agents due to the enormous health properties of the available bioactive compounds found in these materials [27]. They have already been used in food industries as baking additives and as a source of flavoring, colorant, and pectin. Furthermore, many applications have been found for these materials in the pharmaceutical industries and research areas, such as biofuel, bio-absorbent, biofertilizer, activated charcoal, and packaging materials [19]. A large volume of EOs within these waste materials has led to their use as fragrances in cosmetic products [27].
BIOLOGICAL APPLICATIONS OF CITRUS WASTES
The application of plant byproducts for health needs has increased tremendously in the last few decades due to their availability, biological potentials, minimal side effects, and low cost of sourcing byproducts, unlike what is obtainable in the production of modern therapeutic drugs and other healthcare products [33]. Citrus wastes are among such popular plant byproducts, which can act as medicinal raw materials because of their bioactive phytochemicals, which include volatile (EOs and limonoids) and nonvolatile (phenolics and flavonoids) compounds [34]. Their peels and leaves are implicated along with many aromatic plants in aromatherapy—an alternative or supportive therapy that uses EO-bearing plants and their oils for disease management [35]. Citrus wastes have found medicinal applications across many cultures. For instance, in traditional Chinese medicine, dried lemon peel oils and extracts are used as remedies for coughs and to reduce phlegm in the upper respiratory tract [36]. The Xhosa tribe of Amathole District from Eastern Cape, South Africa, also uses lemon peels to manage respiratory and skin diseases [37]. The fiber portion of citrus peels is involved in improving intestinal and physiological functions. It is often associated with a lower risk of life-threatening chronic diseases such as diabetes, cardiovascular disease, obesity, and cancer [38].
Over the years, some ethnomedicinal claims have been validated to be effective, with reports of considerable inhibitory effects of the extracts and EOs of citrus peels on inducible nitric oxide synthase (iNOS), a pro-inflammatory enzyme involved in many respiratory tract diseases [39]. This unique biological property of citrus wastes (peels) has been linked to their rich flavonoid contents, especially nobiletin, and tangeretin, which are reported to inhibit the deoxyribonucleic acid (DNA)-binding activity of pro-inflammatory cytokines, nuclear factor kappa B (NF-κB) and reactive oxygen species production in lipopolysaccharide (LPS)-activated RAW 264.7 macrophages [40]. Extracts and oils from citrus wastes (leaves and seeds) have also been shown to exhibit free radical scavenging, antiperoxidase, and antipolyphenol oxidase activities [41,42]. Also, the waste material from C. junos is implicated in the Asian traditional medicine for treating asthma, coughs, and chronic inflammatory diseases, while coumarin compounds, notably isogosferol, are reported to be responsible for the anti-inflammatory properties observed in the seed shell extract [43]. Isogosferol potently attenuated the production of nitric oxide (NO) in LPS-induced RAW 264.7 cells and inhibited the expression of iNOS and cyclooxygenase-2 (COX-2) in LPS-stimulated macrophages; thus, suggesting it as a drug candidate for the treatment of inflammatory diseases [43].
In recent times, the limonene content of citrus wastes (peels and leaves) is extracted for use in household products as a sweetener in drugs and foods, and as an antiseptic and odorant in the cosmetics industry, to produce sanitizers, soaps, body creams, and perfumes [44]. The pectin-rich dry peels of citrus have gelation and emulsion stabilization properties, making them useful in manufacturing drugs, cosmetics, and food products [45]. The fragrance and biological properties of EOs from citrus wastes make them a valuable raw material in cosmetic products [46]. Citrus reticulata peel extracts have shown anti-collagenase and anti-elastase activities, and this byproduct is a potential anti-aging agent in cosmetic products [47]. Also, the EO component of the peels has been reported to show strong antibacterial and anti-inflammatory activities [48].
The peels of Citrus medica, C. sinensis, C. limon, C. reticulata, and C. maxima are now known to be important cancer preventive food additives based on their remarkable cytotoxic and cancer preventive properties, which are attributed to their volatile (EOs and limonoids) and nonvolatile (polymethoxy flavones) components [49]. Dry citrus peels are rich in cellulose, hemicellulose, and pectin, which can be utilized as fermentation substrates and processed into cattle feeds and molasses [50]. They are also used as sources of fiber for food enrichment [38] and as natural antioxidants such as polyphenols in processed foods and beverages [51]. Citrus wastes are also processed for their limonene content to produce solvents and resins, as a wetting and dispersing agent, and in insect control [44].
Citrus limon peels can be hydrolyzed by enzymes into sugars, which can be successively fermented to produce bioethanol and used as fuel for industrial processing plants [52]. Citrus wastes can also be used as substrates in the industrial production of biofertilizers, biofuel, and biosorbents [19]. They can also serve as natural biosorbents for removing dyes and other contaminants in water and wastewater treatments [45]. They have also found usefulness in the production of polystyrene, a common thermoplastic polymer used in the production of packaging materials and household and consumer goods [33].
Most recently, citrus wastes, like many natural product wastes, are utilized in nanoparticle synthesis as alternative, eco-friendly, and biocompatible waste-derived nanoformulations for many applications [53]. The citrus waste material can act as a capping and reducing agent to control the aggregation of the nanoparticles [54]. Nowadays, the waste material (peels) from C. sinensis, C. limon, and Citrus limetta can serve as a bio-reductant in the biogenic synthesis of metal or metal-oxide nanoparticles for optimal biological functions, such as antioxidant, antimicrobial, and cytotoxic activities, to name a few [55]. Evidence has emerged on the utilization of citrus wastes as part of nanomaterials used for the preparation of nano-insecticides (nano-emulsions) for food pest control, citrus EO nano-emulsion for disease vector mosquito control, nano-formulated bio-insecticides, aerosol and fumigant for home use, and as antimicrobial EO nano-formulations [56]. The cellulosic component of citrus waste can also be formulated into nano-cellulose for water and waste treatments, thus, offering an alternative to zeolites and activated carbon, which are conventional adsorbents [57,58]. Based on the above evidence, it may not be far-fetched to describe citrus wastes as a valuable raw material for biological applications, such as in pharmaceutical, cosmeceutical, food, industrial, and agricultural productions (Fig. 4).
Antioxidant activity
Antioxidants are chemical substances, that when present at low concentrations compared to those of an oxidizable substrate significantly delay or prevent oxidation of that substrate [59]. There has been increasing scientific evidence of the considerable antioxidant properties of citrus fruits and their waste materials [60,61]. This biological function has been attributed to their rich phenolic and polyphenolic contents, such as phenolic acids, flavonoids, and their derivatives [62]. The peel EO of citrus fruits such as C. reticulata and C. sinensis has been reported to be of considerable antioxidant potentials, while that of Citrus aurantium, C. limon, and Citrus paradisii showed remarkable free radical scavenging activities [63–66]. The leaf EO of Citrus limettioides and Citrus pseudolimon of Nainital District showed considerable antioxidant properties, while the leaf methanolic and aqueous extracts of Citrus clementina and C. limon showed remarkable antioxidant activities owing to their high phenolic and flavonoid contents [42,67]. The antioxidant constituents of citrus play an important role in delaying or inhibiting the process leading to inflammation, microbial infections, cancer, diabetes, chronic respiratory diseases, and cardiovascular diseases among others [62,68].
Anti-inflammatory activity
Inflammation sets in when the biological system is immune compromised, a scenario triggered by damaged cells, toxic compounds and materials, pathogenic microorganisms, and a host of other factors [69]. The inflammatory process sets off oxidative stress and lowers cellular antioxidant capacity to prevent microbial infections, cancer, and a variety of age-related diseases, including arthritis, diabetes, cardiovascular, and autoimmune diseases [70]. Therefore, anti-inflammatory agents are biologically active substances that can interfere in the pathophysiological process of inflammation to inhibit the release of chemicals and migrating cells, such as bradykinin, histamine, leukotrienes, prostaglandins, phospholipase, COX-2, lipoxygenase (LOX), cytokines, interleukin-1β (IL-1β), interferon-γ, tumor-necrosis factor-α (TNF-α), NF-κB, and platelet-activating factor [71]. There have been several reports alluding to the considerable anti-inflammatory potential of citrus fruits and their waste products, which include peels, leaves, and seeds. The EOs obtained from the peels of four Citrus species, C. limon, C. latifolia, C. aurantifolia, and C. limonia exhibited significant anti-inflammatory properties at a dose range of 10–100 mg/kg, p.o. in mice by reducing cell migration, cytokine production, and protein extravasation caused by carrageenan [72]. The EO obtained from the fresh blossom (leaves and flowers) of C. aurantium showed considerable anti-inflammatory activity at a dose of 40 mg/kg, by significantly reducing carrageenan-induced paw edema in rats, and when compared to 50 mg/kg of diclofenac sodium (standard drug), they exhibited a comparable activity [73]. The aqueous leaf extract of C. reticulata exhibited a dose-dependent in vivo anti-inflammatory activity between 100 and 500 mg/kg b.w. by a significant dose-dependent ear section weight reduction in mice [74], while the leaf EOs of C. limon var Meyer and C. aurantifolia var Sans epines showed considerable in vitro anti-inflammatory properties by exhibiting inhibitory effects against the oxidation of linoleic acid in an LPS-treated cell at IC50 values of 46.5 and 49.35 ppm, respectively [75]. Methanolic extract from the young fruit peel of Citrus unshiu showed considerable anti-inflammatory activity by inhibiting iNOS in rat primary astrocytes [76], while the extract from the peel of C. reticulata peel acted as an in vivo anti-inflammatory agent by suppressing NO production and inhibiting NF-κB in macrophage RAW 264.7 cells [74]. Also, the crude methanolic extract of C. aurantium peel demonstrated considerable anti-inflammatory properties by modulating the expression of COX-2 iNOS protein, TNF-α, and COX-2 messenger ribonucleic acid (mRNA), in LPS-stimulated macrophage RAW 264.7 cells via the NF-κB pathway in a dose-dependent manner [77], as illustrated in Figure 5.
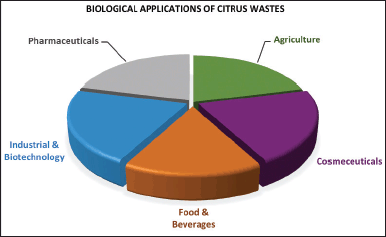 | Figure 4. Biological applications of citrus wastes. [Click here to view] |
Prebiotic activity
Prebiotics are nondigestible food ingredient that favorably affects the host by particularly stimulating the growth and activity of one or a limited number of bacteria in the colon, to improve host health [78]. They are mostly nondigestible dietary fibers that resist digestion in the small intestine until they reach the colon to get fermented by the gut microorganisms, producing short-chain fatty acids, which help to improve digestion, immune function, metabolic health, and other health benefits [79]. Prebiotic substances include fructan, galacto-oligosaccharide, starch and glucose-derived oligosaccharide, and pectin [78,80]. Some natural sources of prebiotic foods are apples, asparagus, bananas, barley, chicory root, citrus peels, cocoa, dandelion greens, flaxseeds, garlic, jerusalem artichoke, leek, oats, onions, and seaweeds [81,82].
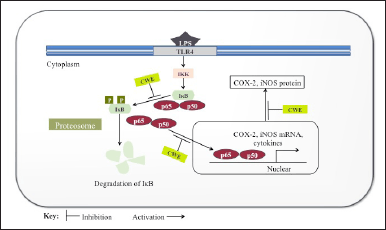 | Figure 5. Anti-inflammatory response of citrus waste extract (CWE) by its inhibitory effects on LOX, LPS-induced inflammation. An illustration of CWE blocking NF-κB signaling pathway via inhibition of (i) IκB phosphorylation, (ii) subunits (p65/p50) of NF-κB translocation in nuclear, and (iii) proinflammatory mediators (COX-2, iNOS) transcription. Black arrows indicate the NF-κB signal pathway and target gene that DNA binding site of NF-κB. CWE: Citrus waste extract (methanol extract of C. aurantium peel); IKK: IκB kinase; IκB: Inhibitor of κB in the cytoplasm; p50/p65: subunits of NF-κB; COX-2: cyclooxygenase-2; iNOS: inducible nitric oxide synthase. The figure was redrawn according to Kim et al. [77], with copyright permission from ©Hindawi Publishing Corporation. [Click here to view] |
Citrus peel wastes are an important source of prebiotic dietary fibers, such as pectins, which are known to play a significant role as nutraceuticals [83]. Structurally, pectins are heteropolysaccharides that contain galacturonic acid units, linked at positions 1 and 4 [84]. Pectins as prebiotics can promote anti-inflammatory commensal bacteria in the colon, such as Eubacterium eligens and Faecalibacterium prausnitzii, to produce anti-inflammatory cytokines and modulate chronic inflammation [85].
In a recent study, the pectins obtained from citrus peel wastes demonstrated greater prebiotic activity than other commercially available prebiotics, such as inulin and fructo-oligosaccharides [81]. In addition, those pectins obtained through enzymatic treatment and membrane separation processes were shown to be more effective as prebiotic and antimicrobial agents [81]. Pectic oligosaccharides (pectin derivatives) extracted from C. limon peel wastes have also been shown to exert considerable prebiotic properties, with the joint populations of bacteria in the genera bifidobacteria and lactobacilli increasing from 19% to 29% [86].
Furthermore, pectic oligosaccharides obtained through enzymatic depolymerization of citrus peel wastes were reported to cause high prebiotic activity in Bifidobacterium infantis, Lactobacillus acidophilus, Clostridium perfringens, and Bacteroides fragilis, with considerable antibacterial activity against Staphylococcus aureus, Bacillus subtilis, and Escherichia coli as well as inhibitory activity against the invasion Caco-2 cells by Campylobacter species [87,88]. Pectin oligosaccharide obtained from citrus peel pectin by controlled chemical degradation showed a significantly high prebiotic score of 0.41 for Lactobacillus paracasei LPC-37 and 0.92 for Bifidobacterium bifidum ATCC 29521 [89]. Also, pectin-derived oligosaccharides obtained through an enzymatic fermentation process from lemon peel wastes resulted in similar shifts in the elderly microbiota compared to fructo-oligosaccharides, the standard prebiotic [90]. Thus, this result shows the potential of pectins as food ingredients for the improvement of the profiles and metabolic activities of gut microbiota in the elderly [90]. Based on the various prebiotic activities of pectins among other biological properties, they have been found useful in the pharmaceutical industry, for drug delivery, tissue engineering, and wound healing, while they are used as gelling, thickening, and stabilizing agents in the confectionery industry [84,91].
Antimicrobial activity
Citrus is an important fruit that has been widely known across many cultures to be used for the management of microbial-related infections such as cholera, dysentery, diarrhea, wound infections, chronic inflammatory diseases, and urinary tract infections [62,92]. Citrus sinensis peel and leaf EOs are known to demonstrate considerable inhibitory activities against the growth of several pathogenic bacteria, including S. aureus, Listeria monocytogenes, Vibrio parahaemolyticus, Salmonella typhimurium, E. coli, and Pseudomonas aeruginosa [93]. The peel and leaf EOs of C. reticulata inhibited the growth of several bacteria including E. coli, B. subtilis, P. aeruginosa, and S. aureus [94]. Citrus paradisii EO has also been reported to inhibit the growth of wild food-borne spoilage and pathogenic bacterial strains [95]. The EO of C. unshiu peel demonstrated antibacterial activity against Bacillus cereus, B. subtilis, and S. aureus [96]. The ethanolic extract of C. sinensis peels demonstrated antibacterial activity against E. coli and S. aureus, while the EO of C. limon peel demonstrated both minimum inhibitory concentration (MIC) and minimum bactericidal concentration, which were the antibacterial activities of 1.25 and 5.0 mg/ml against S. aureus typed strain that has been implicated in food poisoning [97]. The EOs of C. limon and C. aurantium remarkably inhibited the mycelial growths of Aspergillus niger and Geotrichum candidum at the MIC values of 5 and 10 ppm, respectively [98]. The aqueous seed extract of C. paradisii has also been reported to exhibit higher antifungal activities against A. niger, Candida albicans, Cladosporium cucumerinum, Penicillium digitatum, Penicillium italicum, and Penicillium chrysogenum than its ethanol extract, thus, making it to be a suitable raw material to produce natural disinfectants or antiseptics [99].
Antidiabetic activity
Diabetes, also known as diabetes mellitus (is a chronic heterogenous metabolic disease that is characterized by unsuitably elevated blood glucose levels (BGLs) because of defects in insulin secretion, insulin action, or both [100,101]. Antidiabetic agents can act by stimulating insulin secretion, reducing hepatic glucose production, improving insulin action, or delaying digestion and absorption of starch hydrolyzing enzymes such as α-glucosidase and α-amylase inhibitors [102]. For decades, citrus plants have been used in traditional medicine and as an important dietary aromatic ingredient for the regulation of human BGL [103,104]. The extract of C. sinensis peel at a dose of 500 mg/kg b.w. showed the highest (61.36% ± 5.57%) in vivo antidiabetic and 55% antihypercholesterolemic activities [105]. It was found that the n-hexane extract of C. limon peel at a dose of 10 mg/kg b.w. in rats showed 44.57%, 75.96%, and 95.43% anti-hyperglycaemic effects after 24, 48, and 72 hours of drug administration, respectively, while the hydroethanolic extract of C. reticulata peel exhibited considerable antidiabetic activity [106,107]. The ethanol extract of C. junos Tanaka (Yuzu) peel exerted antidiabetic activity by increasing glucose uptake in C2C12 myotubes and by modulating the adenosine-5-monophosphate, AMP-activated protein kinase, and peroxisome proliferator-activated receptor gamma signaling pathways in a dose-dependent manner, thus, improving insulin resistance in mice that were fed a high-fat diet [108]. The methanol extract of C. limetta peels at 200 and 400 mg/kg b.w. demonstrated significant anti-hyperglycaemic effects over a 15-day period, which may be attributed to its remarkable antioxidant properties [109]. The aqueous extract of fresh Citrus hystrix leaf alone and in combination with two other extracts, Eugenia polyantha leaf and Pandanus amaryllifolius leaf showed considerable in vitro antidiabetic properties by their inhibitory effects on starch hydrolyzing enzymes, α-amylase and α-glucosidase, partly due to their rich phenolic composition and antioxidant properties [110]. The seed hydro-ethanolic extract of Citrullus colocynthis showed both in vitro and in vivo antidiabetic properties by α-glucosidase enzyme inhibition and by showing a marked time-dependent decrease in BGLs in streptozotocin-induced diabetic rat model, respectively [111]. Also, in an in vivo study, using a streptozotocin-induced diabetic rat model, the petroleum ether extract of Citrus medica seed (200 and 400 mg/kg, p.o.) induced a significant reduction in fasting blood glucose, serum cholesterol, serum triglycerides, high-density lipoprotein, low-density lipoprotein, and very-low-density lipoprotein in a dose-dependent manner [112].
Cytotoxic (anticancer) activity
Cancer can be described as a group of noncommunicable diseases characterized by uncontrolled or rapid growth and spread of abnormal cells, with a high propensity to cause death in affected individuals [113]. There is much to be desired about the potency and safety of cytotoxic agents and the currently available cancer drugs to date. Nevertheless, considerable experimental studies have shown the bio-functionality of many dietary natural products including citrus fruits and their by-products to inhibit or delay the development and progression of cancer [114].
The EOs obtained from the peel of C. limon grown in four different geographical locations have been reported to demonstrate 80% colorectal cancer cells (LIM1863) reduction, with an IC50 ranging between 5.75 and 7.92 μg/ml, while doxorubicin (standard drug) caused a 92% reduction in the cancer cell viability [115]. Likewise, an investigation of the Iranian C. limon peel EO revealed considerable levels of cytotoxic against the human breast (MCF-7) and cervical (HeLa) cancer cell lines at IC50 values of 10 and 17 μg/ml, respectively [116]. The EOs of four Citrus species, C. limon, C. reticulata, and C. paradisii were reported for their anticancer property, by showing their cytotoxicity against lung cancer cell lines (BV2) microglial cells at an IC50 ranging between 321.37 and 1,558.87 μg/ml [117]. The cytotoxicity was shown to be by an induction of a G0/G1 cell cycle arrest [117]. At 200 and 400 mg/kg b.w. the methanolic extract of C. limetta peel showed a considerable in vivo antitumor property [118]. The EO of C. aurantifolia peel showed 78% inhibition against human colon cancer cell lines (SW-480) at 100 μg/ml within a 48-hour period in vitro, while the aqueous extract of C. aurantifolia has been reported for its cytoprotective action against aflatoxin B1 induced liver injury in vivo [119]. The peel powders obtained from C. sinensis, C. reticulata, and C. limon have been shown in a recent study, to exhibit considerable cytotoxicity against the human colon carcinoma cell line (HCT116) [120]. The n-hexane extract of C. hystrix leaf has been reported to show considerable cytotoxicity against triple-negative breast cancer cells (MDA-MB-231), with an IC50 value of 317.63 ± 2.00 μg/ml [121], while the hydro-methanol extract of C. limon seed demonstrated 29.1% inhibition against human breast adenocarcinoma (MCF-7) at 100 μg/ml within 48 and 72 hours via apoptosis [122].
Insecticidal activity
According to the United States Environmental Protection Agency, insecticides are chemicals used for the control of insects and/or insect pests by killing them or preventing them from engaging in undesirable or destructive activities [123]. Insecticides are produced to help minimize disease burden or at best eradicate them, with the ultimate goals of attaining good health and wellbeing, food security, and economic stability [123]. Commercially available synthetic insecticides, such as organophosphates, carbamates, dinitrophenols, and some of the older, less costly ones can remain for years in soil and water thereby constituting a lot of hazards to the ecosystem [124]. Although many of these chemicals have been banned from agricultural use in developed countries, they are still used in many developing countries [125]. Thus, there is a need to exploit medicinal plants for natural insecticides, which are known to be easily biodegradable, biocompatible, eco-friendly, and less toxic [126].
The limonene-rich citrus waste (peel) is one such natural raw material that is now being repurposed as natural insecticides for preservation against food pests [127]. EOs from the peel of C. reticulata have been reported to have contact and fumigant toxicities on various insects that attack stored food products [128]. The peel EO extracted from C. reticulata has been reported to show remarkable insecticidal and growth inhibition activities against two strains of Rhyzopertha dominica (Fabricius) found in wheat [129]. The C. limon peel EO has been reported to possess fumigant activity, with dose-response relationship and repellent activity against Sitophilus granaries and Sitophilus oryzae [130,131]. The C. aurantifolia peel EO has shown a considerable level of insecticidal activity (contact, fumigation, and feeding deterrent activities) against the maize weevil, Sitophilus zeamais [132]. Also, C. sinensis EO has shown significant insecticidal activity against Tribolium confusum, Callosobruchus maculatus, and S. oryzae [133]. The EO of C. paradisii peel has also been reported to show insecticidal activity against Ceratitis capitata, while the petroleum ether extract of C. aurantium peel showed insecticidal activities against the males and females of olive fruit fly, Bactrocera oleae (Gmelin) and medfly, C. capitata (Wiedemann) adults at LD50 values of 44.8 and 40.1 μg/insect against the former and 38.8 and 67.8 μg/insect, respectively, against the latter insect [134].
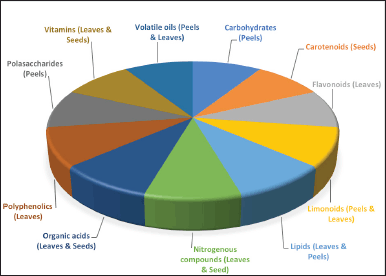 | Figure 6. Class of compounds found in citrus wastes (peels, leaves, and seeds), according to Sharma et al. [19]. [Click here to view] |
PHYTOCHEMICAL OUTLOOK OF CITRUS WASTES
Source of bioactive compounds
Phytochemicals are chemical substances stored in grains, vegetables, fruits, and other food products for desirable health benefits beyond basic nutritional needs, including reducing the risk of major cardiovascular and chronic inflammatory diseases [135]. They are primarily secondary metabolites, such as flavonoids, alkaloids, terpenoids, steroids, saponins, and phenolic compounds, with unique chemical structures and demonstrable biological properties [136]. These bioactive compounds are known to be present in both the edible and nonedible parts of grains, vegetables, and fruits [137]. Most parts of the citrus plants generally possess rich phytochemical contents such as carbohydrates, carotenoids, flavonoids, limonoids, lipids, nitrogenous compounds, organic acids, polyphenolics, polysaccharides, vitamins, and volatile oils [19], which are highlighted in Figure 6. These useful phytochemicals are often not wholly expended after consumption but find their way as the constituent of citrus waste [103]. The biological activities and mechanisms of action of some compounds identified in citrus wastes are presented in Table 1. Furthermore, the chemical structures of some of the implicated compounds are shown in Figure 7.
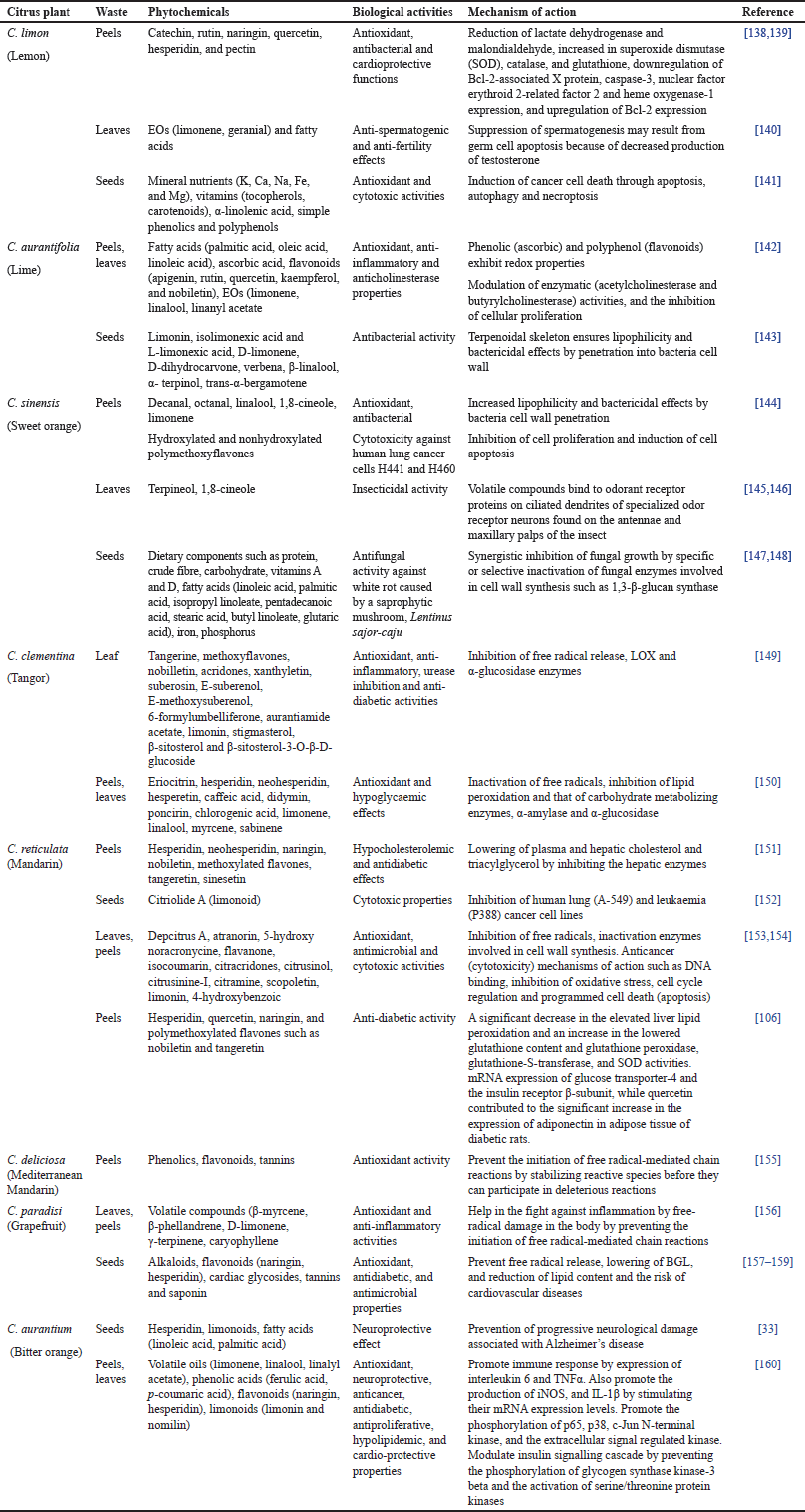 | Table 1. Reported phytochemical contents and biological activities of some citrus wastes. [Click here to view] |
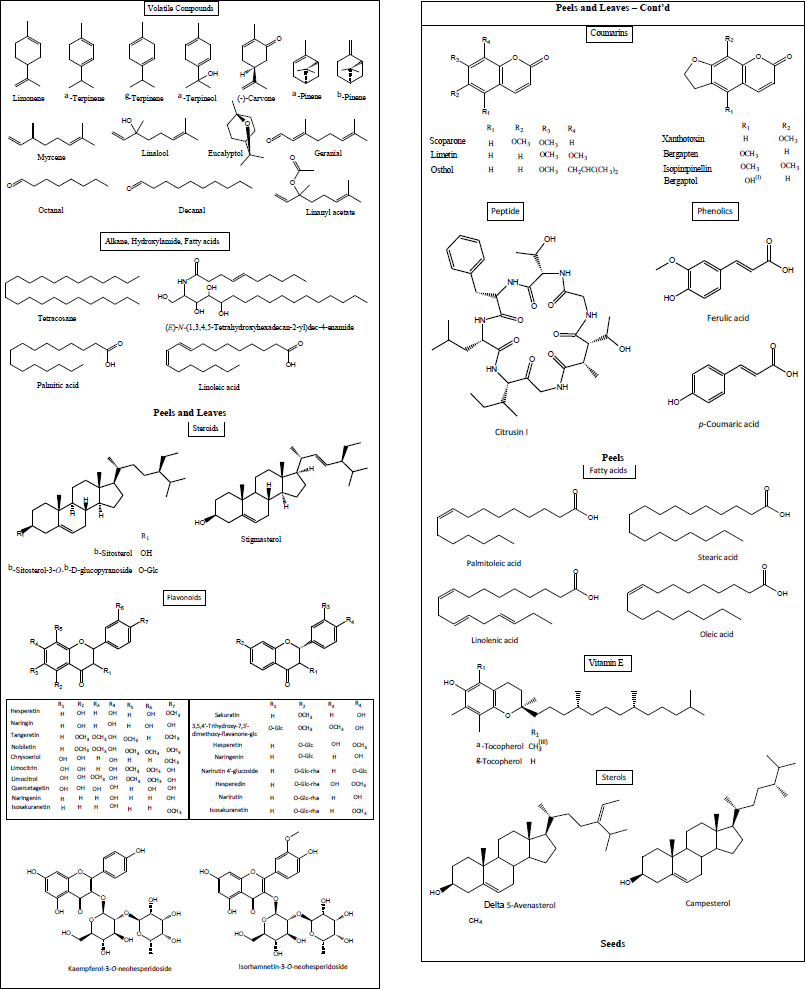 | Figure 7. Some phytochemical constituents of citrus wastes (peels, leaves, and seeds). [Click here to view] |
CONCLUSION
This study has shown that citrus byproducts (peels, leaves, and seeds), though may portend serious environmental hazards if not well managed but can be repurposed as a useful raw material because of their unique chemical entities, with novel biological functions, such as antioxidants, anti-inflammatory, prebiotic, antimicrobials, cytotoxic, antidiabetic, and insecticidal activities. Flavonoids (hesperidin, nobiletin, quercetin, and tangeretin), coumarins (isogosferol and isocoumarin), volatile oils (limonene, linalool, myrcene, and sabinene), fatty acids (palmitoleic, linolenic, and stearic acid), phenolic acids (p-coumaric acid, ferulic acid, and chlorogenic acid), alkaloids (citracridones, citramine, and citrusinine), and pectins are among the bioactive compounds of citrus wastes highlighted in this report.
Many of these bioactive constituents from citrus wastes are now sourced as useful ingredients for pharmaceutical (antioxidants and antibiotics), cosmeceutical (body cream, perfumes, and soaps), agricultural (biofertilizers and pesticides), food and beverages (fibers, sweeteners, and food additives), and industrial (biofuel and biosorbent) productions. Citrus byproducts are fast becoming the biogenic raw materials of choice, as alternatives to conventional adsorbents (e.g., zeolites and activated carbon), and to produce nano-cellulose for water and waste treatments.
In light of the above-mentioned applications, it has become apparent that the useful applications of citrus wastes would help to mitigate possible environmental hazards that are associated with their indiscriminate disposal. It would also help to improve health and social status and promote food security and industrialization for sustainable development. Nevertheless, there is a need to further exploit citrus wastes for their pharmacological potentials, as most studies were done to evaluate their biological properties at the in vitro and in vivo preclinical level. Thus, the bioavailability and tangible benefits of these byproducts can be fully harnessed by obtaining relevant clinical data on them.
ACKNOWLEDGEMENTS
The authors are grateful to the National Research Foundation (NRF), South Africa, for funding support through the grant number, Citrus NRF UID-135455.
LIST OF ABBREVIATIONS
BGL: blood glucose level; CWE: citrus waste extract; COX-2: cyclooxygenase-2; DNA: deoxyribonucleic acid; EO: essential oil; IC50: concentration at 50% inhibition; IκB: IkappaB kinase or IKK; IL-1β: Interleukin-1beta; iNOS: inducible nitric oxide synthase; LD50: lethal dose; LPS: lipopolysaccharide; MIC: minimum inhibitory concentration; mRNA: messenger ribonucleic acid; NF-κB: nuclear factor kappa B; NO: nitric oxide; p50/p65: NF-κB heterodimer; RAW 264.7: a semi-adherent macrophage-like cell line derived from BALB/c mice; SOD: superoxide dismutase; TNF-α: tumor necrosis factor-alpha.
AUTHOR CONTRIBUTIONS
Conceptualization and design—AOO (Adebola O. Oyedeji), YSH, OOO, SKK, GMM, AOO (Ayodeji O. Oriola); manuscript draft—DT and AOO (Ayodeji O. Oriola); revision of manuscript—AOO (Adebola O. Oyedeji), YSH, OOO, SKK. All authors approved the final version of the manuscript to be published.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used AI-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Koul B, Yakoob M, Shah MP. Agricultural waste management strategies for environmental sustainability. Environ Res. 2022;206:112285. CrossRef
2. Godfray HCJ, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, et al. Food security: the challenge of feeding 9 billion people. Science (1979). 2010;327:812–8. CrossRef
3. Adejumo IO, Adebiyi OA. Agricultural solid wastes: causes, effects, and effective management. In: Saleh HM, editor. Strategies of sustainable waste management. London, UK: IntechOpen; 2020 [cited 2023 Feb 25]. CrossRef
4. Singh Y, Sidhu HS. Management of cereal crop residues for sustainable rice-wheat production system in the Indo-Gangetic plains of India. Proc Indian Nat Sci Acad. 2014;80:95–114. CrossRef
5. Lynch J, Cain M, Frame D, Pierrehumbert R. Agriculture’s contribution to climate change and role in mitigation is distinct from predominantly fossil CO2-emitting sectors. Front Sustain Food Syst. 2021;4:518039. CrossRef
6. Agamuthu P. Challenges and opportunities in agro-waste management: an Asian perspective. Inaugural Meeting of First Regional 3R Forum in Asia, Tokyo, Japan; 2009 [cited 2023 Feb 25]. vol. 11, pp 4153–8. Available from: https://www.uncrd.or.jp/content/documents/Session2_Agamuthu.pdf
7. Bracco S, Calicioglu O, Gomez San Juan M, Flammini A. Assessing the contribution of bioeconomy to the total economy: a review of national frameworks. Sustainability. 2018;10:1698. CrossRef
8. Duque-Acevedo M, Belmonte-Ureña LJ, Cortés-García FJ, Camacho-Ferre F. Agricultural waste: review of the evolution, approaches and perspectives on alternative uses. Glob Ecol Conserv. 2020;22:e00902. CrossRef
9. Seidavi A, Zaker-Esteghamati H, Scanes CG. Poultry byproducts. Byproducts from agriculture and fisheries. Oxford, UK: John Wiley & Sons; 2019. pp 123–46. CrossRef
10. Tripathi N, Hills CD, Singh RS, Atkinson CJ. Biomass Waste utilisation in low-carbon products: harnessing a major potential resource. NPJ Clim Atmos Sci. 2019;2:35. CrossRef
11. Pattanaik L, Pattnaik F, Saxena DK, Naik SN. Chapter 5–Biofuels from agricultural wastes. In: Basile A, Dalena F, editors. Second and third generation of feedstocks. The evolution of biofuels. Amsterdam, The Netherlands: Elsevier; 2019. pp 103–42. CrossRef
12. Saini JK, Saini R, Tewari L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: concepts and recent developments. 3 Biotech. 2015;5:337–53. CrossRef
13. Riaz S, Ahmad A, Farooq R, Hussain N, Riaz T, Hussain K, et al. Citrus: an overview of food uses and health benefits. Advances in citrus production and research. London, UK: IntechOpen; 2022 [cited 2023 Feb 25]. CrossRef
14. Russo C, Maugeri A, Lombardo GE, Musumeci L, Barreca D, Rapisarda A, et al. The second life of citrus fruit waste: a valuable source of bioactive compounds. Molecules. 2021;26:1–20. CrossRef
15. Chavan P, Singh AK, Kaur G. Recent progress in the utilization of industrial waste and by-products of citrus fruits: a review. J Food Process Eng. 2018;41:e12895. CrossRef
16. Zema DA, Calabrò PS, Folino A, Tamburino V, Zappia G, Zimbone SM. Valorisation of citrus processing waste: a review. Waste Manag. 2018;80:252–73. CrossRef
17. Leporini M, Tundis R, Sicari V, Loizzo MR. Citrus species: modern functional food and nutraceutical-based product ingredient. Ital J Food Sci. 2021;33:63–107. CrossRef
18. Mahato N, Sharma K, Sinha M, Cho MH. Citrus waste derived nutra-/pharmaceuticals for health benefits: current trends and future perspectives. J Funct Foods. 2018;40:307–16. CrossRef
19. Sharma K, Mahato N, Cho MH, Lee YR. Converting citrus wastes into value-added products: economic and environmental friendly approaches. Nutrition. 2017;34:29–46. CrossRef
20. Klimek-Szczykutowicz M, Szopa A, Ekiert H. Citrus limon (lemon) phenomenon–a review of the chemistry, pharmacological properties, applications in the modern pharmaceutical, food, and cosmetics industries, and biotechnological studies. Plants. 2020;9:119. CrossRef
21. Ahmed M, Saeid A. Citrus fruits: nutritive value and value-added products. Citrus—research development and biotechnology. London, UK: IntechOpen; 2021 [cited 2023 Feb 26]. CrossRef
22. Rao MJ, Zuo H, Xu Q. Genomic insights into citrus domestication and its important agronomic traits. Plant Commun. 2020;2:100138. CrossRef
23. Izquierdo L, Sendra JM. Citrus fruits: composition and characterization. Encyclopedia of food sciences and nutrition. San Diego, CA: Academic Press; 2003 [cited 2023 Feb 26]. CrossRef
24. Bora H, Kamle M, Mahato DK, Tiwari P, Kumar P. Citrus essential oils (CEOs) and their applications in food: an overview. Plants. 2020;9:357. CrossRef
25. Rafiq S, Kaul R, Sofi SA, Bashir N, Nazir F, Ahmad Nayik G. Citrus peel as a source of functional ingredient: a review. J Saudi Soc Agric Sci. 2018;17:351–8. CrossRef
26. Espinosa-Pardo FA, Nakajima VM, Macedo GA, Macedo JA, Martínez J. Extraction of phenolic compounds from dry and fermented orange pomace using supercritical CO2 and cosolvents. Food Bioprod Proc. 2017;101:1–10. CrossRef
27. Suri S, Singh A, Nema PK. current applications of citrus fruit processing waste: a scientific outlook. Appl Food Res. 2022;2:100050. CrossRef
28. United Nations–Food and Agriculture Organization. Citrus fruit. Fresh and processed—statistical bulletin. Geneva, Switzerland: UN FAO; 2016 [cited 2023 Feb 26]. p 47. Available from: https://www.fao.org/3/i8092e/i8092e.pdf
29. Kuna A, Sowmya M, Sahoo MR, Mayengbam PD, Dasgupta M, Sreedhar M. Value addition and sensory evaluation of products made from underutilized Kachai lemon (Citrus jambhiri) Lush. fruits. J Pharmacogn Phytochem. 2018;7:3032–6.
30. Marín FR, Soler-Rivas C, Benavente-García O, Castillo J, Pérez-Alvarez JA. By-products from different citrus processes as a source of customized functional fibres. Food Chem. 2007;100:736–41. CrossRef
31. Mahawar MK, Jalgaonkar K, Bibwe B, Bhushan B, Meena VS, Sonkar RK. Post-harvest processing and valorization of Kinnow Mandarin (Citrus reticulata L.): a review. J Food Sci Technol. 2020;57:799–815. CrossRef
32. Berk Z. Chapter 10—By-products of the citrus processing industry. In: Berk Z, editor. Citrus processing industry. San Diego, CA: Academic Press; 2016. pp 219–33. CrossRef
33. Abou Baker DH, Ibrahim EA, Salama ZAE. Citrus peels as a source of bioactive compounds with industrial and therapeutic applications. In: Badria FA, editor. Phenolic compounds—chemistry, synthesis, diversity, non-conventional industrial, pharmaceutical and therapeutic applications. London, UK: IntechOpen; 2021 [cited 2023 Feb 27]. CrossRef
34. Dimou C, Karantonis HC, Skalkos D, Koutelidakis AE. Valorization of fruits by-products to unconventional sources of additives, oil, biomolecules and innovative functional foods. Curr Pharm Biotechnol. 2019;20:776–86. CrossRef
35. Reis D, Jones T. Aromatherapy: using essential oils as a supportive therapy. Clin J Oncol Nurs. 2017;21:16–9. CrossRef
36. Ho SC, Lin CC. Investigation of heat-treating conditions for enhancing the anti-inflammatory activity of citrus fruit (Citrus reticulata) peels. J Agric Food Chem. 2008;56:7976–82. CrossRef
37. Otang WM, Afolayan AJ. Antimicrobial and antioxidant efficacy of Citrus limon L. peel extracts used for skin diseases by Xhosa tribe of Amathole District, Eastern Cape, South Africa. S Afr J Bot. 2016;102:46–9. CrossRef
38. Figuerola F, Hurtado ML, Estévez AM, Chiffelle I, Asenjo F. Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem. 2005;91:395–401. CrossRef
39. Choi SY, Ko HC, Ko SY, Hwang JH, Park JG, Kang SH, et al. Correlation between flavonoid content and the NO production inhibitory activity of peel extracts from various citrus fruits. Biol Pharm Bull. 2007;30:772–8. CrossRef
40. Choi SY, Hwang JH, Ko HC, Park JG, Kim SJ. Nobiletin from citrus fruit peel inhibits the dna-binding activity of NF-κB and ROS production in LPS-activated RAW 264.7 cells. J Ethnopharmacol. 2007;113:149–55. CrossRef
41. Inan Ö, Özcan MM, Aljuhaimi F. Effect of location and Citrus species on total phenolic, antioxidant, and radical scavenging activities of some citrus seed and oils. J Food Process Preserv. 2018;42:e13555. CrossRef
42. Khettal B, Kadri N, Tighilet K, Adjebli A, Dahmoune F, Maiza-Benabdeslam F. Phenolic compounds from Citrus leaves: antioxidant activity and enzymatic browning inhibition. J Complement Integr Med. 2017;14:1–13. CrossRef
43. Song HY, Jo A, Shin J, Lim EH, Lee YE, Jeong DE, et al. Anti-inflammatory activities of isogosferol, a furanocoumarin isolated from Citrus junos seed shells through bioactivity-guided fractionation. Molecules. 2019;24:4088. CrossRef
44. Nikfar S, Behboudi AF. Limonene. Encyclopedia of toxicology. 3rd edition. Amsterdam, The Netherlands: Elsevier Inc; 2014. pp 78–82. CrossRef
45. Mahato N, Agarwal P, Mohapatra D, Sinha M, Dhyani A, Pathak B, et al. Biotransformation of citrus waste-II: bio-sorbent materials for removal of dyes, heavy metals and toxic chemicals from polluted water. Processes. 2021;9:1544. CrossRef
46. Pinto D, Cádiz-Gurrea MDLL, Silva AM, Delerue-Matos C, Rodrigues F. Cosmetics–food waste recovery. Processing technologies, industrial techniques, and applications. San Diego, CA: Academic Press; 2021. pp 503–28. CrossRef
47. Apraj VD, Pandita NS. Evaluation of skin anti-aging potential of Citrus reticulata Blanco Peel. Pharmacogn Res. 2016;8:160. CrossRef
48. Murakami A. Chemoprevention with phytochemicals targeting inducible nitric oxide synthase. Forum Nutr. 2009;61:193–203. CrossRef
49. Nair SA, Sr RK, Nair AS, Baby S. Citrus peels prevent cancer. Phytomedicine. 2018;50:231–7. CrossRef
50. Grohmann K, Manthey JA, Cameron RG, Buslig BS. Purification of citrus peel juice and molasses. J Agric Food Chem. 1999;47:4859–67. CrossRef
51. Khan MK, Abert-Vian M, Fabiano-Tixier AS, Dangles O, Chemat F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010;119:851–8. CrossRef
52. Boluda-Aguilar M, López-Gómez A. Production of bioethanol by fermentation of lemon (Citrus limon L.) peel wastes pretreated with steam explosion. J Ind Crops Prod. 2013;41:188–97. CrossRef
53. Abdelbasir SM, McCourt KM, Lee CM, Vanegas DC. Waste-derived nanoparticles: synthesis approaches, environmental applications, and sustainability considerations. Front Chem. 2020;8:782. CrossRef
54. Khabeeri OM, Al-Thabaiti SA, Khan Z. Citrus sinensis peel waste assisted synthesis of AgNPs: effect of surfactant on the nucleation and morphology. SN Appl Sci. 2020;2:2038. CrossRef
55. Annu, Ahmed S, Kaur G, Sharma P, Singh S, Ikram S. Fruit waste (peel) as bio-reductant to synthesize silver nanoparticles with antimicrobial, antioxidant and cytotoxic activities. J Appl Biomed. 2018;16:221–31. CrossRef.
56. Hamdy AS, Gomaa M, Ali AM. Fundamentals of waste recycling for nanomaterial manufacturing. In: Topics in mining, metallurgy and materials engineering waste recycling technologies for nanomaterials manufacturing. Switzerland AG: Springer Nature; 2021 [cited 2023 Mar 01]. pp 3–24. Available from: https://link.springer.com/book/10.1007/978-3-030-68031-2
57. Hof F, Kampioti K, Huang K, Jaillet C, Derré A, Poulin P, et al. Conductive inks of graphitic nanoparticles from a sustainable carbon feedstock. Carbon NY. 2017;111:142–9. CrossRef
58. Teo EYL, Ali GAM, Algarni H, Cheewasedtham W, Rujiralai T, Chong KF. One-step production of pyrene-1-boronic acid functionalized graphene for dopamine detection. Mater Chem Phys. 2019;231:286–91. CrossRef
59. Veskoukis AS, Tsatsakis AM, Kouretas D. Dietary oxidative stress and antioxidant defense with an emphasis on plant extract administration. Cell Stress Chaperones. 2012;17:11. CrossRef
60. Costanzo G, Vitale E, Iesce MR, Naviglio D, Amoresano A, Fontanarosa C, et al. Antioxidant properties of pulp, peel and seeds of Phlegrean Mandarin (Citrus reticulata Blanco) at different stages of fruit ripening. Antioxidants (Basel). 2022;11:187. CrossRef
61. Zou Z, Xi W, Hu Y, Nie C, Zhou Z. Antioxidant activity of citrus fruits. Food Chem. 2016;196:885–96. CrossRef
62. Lv X, Zhao S, Ning Z, Zeng H, Shu Y, Tao O, et al. Citrus fruits as a treasure trove of active natural metabolites that potentially provide benefits for human health. Chem Cent J. 2015;9:68. CrossRef
63. Anwar F, Abbas A, Alkharfy KM, Gilani AH. Cardamom (Elettaria cardamomum Maton) oils. Essential oils in food preservation, flavor and safety. San Diego, CA: Academic Press; 2016. pp 295–301. CrossRef
64. Castro-Vazquez L, Alañón ME, Rodríguez-Robledo V, Pérez-Coello MS, Hermosín-Gutierrez I, Díaz-Maroto MC, et al. Bioactive flavonoids, antioxidant behaviour, and cytoprotective effects of dried grapefruit peels (Citrus paradisi Macf.). Oxid Med Cell Longev. 2016;2016:8915729. CrossRef
65. Kamal GM, Yasin Ashraf M, Hussain AI, Shahzadi A, Chughtai MI. Antioxidant potential of peel essential oils of three Pakistani Citrus species: Citrus reticulata, Citrus sinensis and Citrus paradisii. Pak J Bot. 2013;45:1449–54.
66. Moosavy MH, Hassanzadeh P, Mohammadzadeh E, Mahmoudi R, Khatibi SA, Mardani K. Antioxidant and antimicrobial activities of essential oil of lemon (Citrus limon) peel in vitro and in a food model. J Food Qual Hazards Control. 2017;4:42–8.
67. Janoti DS, Rana M, Rawat AKS. Comparative antioxidant activity of essential oil of leaves of Citrus limettioides and Citrus pseudolimon of Nainital district. J Pharmacogn Phytochem. 2014;2:24–6.
68. Zhang YJ, Gan RY, Li S, Zhou Y, Li AN, Xu DP, et al. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules. 2015;20:21138–56. CrossRef
69. Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9:7204. CrossRef
70. Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov. 2009;3:73–80. CrossRef
71. Branco ACCC, Yoshikawa FSY, Pietrobon AJ, Sato MN. Role of histamine in modulating the immune response and inflammation. Med Inflamm. 2018;2018:9524075. CrossRef
72. Amorim JL, Simas DLR, Pinheiro MMG, Moreno DSA, Alviano CS, da Silva AJR, et al. Anti-inflammatory properties and chemical characterization of the essential oils of four Citrus species. PLoS One. 2016;11:e0153643. CrossRef
73. Khodabakhsh P, Shafaroodi H, Asgarpanah J. Analgesic and anti-inflammatory activities of Citrus aurantium L. blossoms essential oil (Neroli): involvement of the nitric oxide/cyclic-guanosine monophosphate pathway. J Nat Med. 2015;69:324–31. CrossRef
74. Nasri M, Bedjou F, Porras D, Martínez-Flórez S. Activités antioxydantes, anti-inflammatoires et analgésiques des extraits des meuilles de Citrus reticulata Blanco: etude in vivo et in vitro. Phytotherapie. 2017;2017:1–13. CrossRef
75. Michel P, Dongmo J, Tchoumbougnang F, Boyom FF, Sonwa ET, Henri P, et al. Antiradical, antioxidant activities and anti-inflammatory potential of the essential oils of the varieties of Citrus limon and Citrus aurantifolia growing in Cameroon. J Asian Sci Res. 2013;3:1046–57.
76. Ihara H, Yamamoto H, Ida T, Tsutsuki H, Sakamoto T, Fujita T, et al. Inhibition of nitric oxide production and inducible nitric oxide synthase expression by a polymethoxyflavone from young fruits of Citrus unshiu in rat primary astrocytes. Biosci Biotechnol Biochem. 2012;76:1843–8. CrossRef
77. Kim GS, Kang SR, Han DY, Park K, Park HS, Cho YB, et al. Suppressive effect on lipopolysaccharide-induced proinflammatory mediators by Citrus aurantium L. in macrophage RAW 264.7 cells via NF-κB signal pathway. Evid Based Complement Alternat Med. 2011;2011:248592. CrossRef
78. Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, et al. Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods. 2019;8(3):92. CrossRef
79. Gibson GR, Scott KP, Rastall RA, Tuohy KM, Hotchkiss A, Dubert-Ferrandon A, et al. Dietary prebiotics: current status and new definition. Food Sci Technol Bull Funct Foods. 2010;7:1–19. CrossRef
80. Bamigbade GB, Subhash AJ, Kamal-Eldin A, Nystrom L, Ayyash M. An updated review on prebiotics: insights on potentials of food seeds wastes as source of potential prebiotics. Molecules. 2022;18:5947. CrossRef
81. Foti P, Ballistreri G, Timpanaro N, Rapisarda P, Romeo FV. Prebiotic effects of citrus pectic oligosaccharides. Nat Prod Res. 2022;36:3173–6.
82. Swaroopa C, Kashmira L, Vikas G, Rajan W. Assessment of the prebiotic potential of seed coats from green gram (Vigna radiata) and black gram (Vigna mungo) J Food Sci Technol. 2022;59:583–8. CrossRef
83. Fallico B, Ballistreri G, Arena E, Brighina S, Rapisarda P. Bioactive compounds in blood oranges (Citrus sinensis (L.) Osbeck): level and intake. Food Chem. 2017;215:67–75.
84. Dranca F, Oroian M. Extraction, purification and characterization of pectin from alternative sources with potential technological applications. Food Res Intern. 2018;113:327–50. CrossRef
85. Chung WSH, Meijerink M, Zeuner B, Holck J, Louis P, Meyer AS, et al. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiol Ecol. 2017;93:fix127. CrossRef
86. Gómez B, Gullón B, Yáñez R, Schols H, Alonso JL. Prebiotic potential of pectins and pectic oligosaccharides derived from lemon peel wastes and sugar beet pulp: a comparative evaluation. J Funct Foods. 2016;20:108–21. CrossRef
87. Ho YY, Lin CM, Wu MC. Evaluation of the prebiotic effects of citrus pectin hydrolysate. J Food Drug Anal. 2017;25:550–8.
88. Li P-J, Xia J-L, Nie Z-Y, Shan Y. Pectic oligosaccharides hydrolyzed from orange peel by fungal multi-enzyme complexes and their prebiotic and antibacterial potentials. LWT Food Sci Tech. 2016;69:203–10.
89. Zhang S, Hu H, Wang L, Liu F, Pan S. Preparation and prebiotic potential of pectin oligosaccharides obtained from citrus peel pectin. Food Chem. 2018;244:232–7. CrossRef
90. Míguez B, Vila C, Venema K, Parajó JC, Alonso JL. Prebiotic effects of pectooligosaccharides obtained from lemon peel on the microbiota from elderly donors using an in vitro continuous colon model (TIM-2). Food Funct. 2020;11:9984–99. CrossRef
91. Ciriminna R, Fidalgo A, Delisi R. Pectin production and global market. Food Ind Hi Tech. 2016;27(5):17–20.
92. Ladaniya MS. Nutritive and medicinal values of citrus fruits. Citrus fruit. San Diego, CA: Academic Press; 2008. pp 501–14. CrossRef
93. Franco-Vega A, Reyes-Jurado F, Cardoso-Ugarte GA, Sosa-Morales ME, Palou E, López-Malo A. Sweet orange (Citrus sinensis) oils. Essential oils in food preservation, flavor and safety. San Diego, CA: Academic Press; 2016. pp 783–90. CrossRef
94. Yabalak E, Erdogan Eliuz EA, Nazli MD. Evaluation of Citrus reticulata essential oil: chemical composition and antibacterial effectiveness incorporated gelatin on E. coli and S. aureus. Int J Environ Health Res. 2022;32:1261–70.
95. Vasek OM, Cáceres LM, Chamorro ER, Velasco GA. Antibacterial activity of Citrus paradisii essential oil. J Nat Prod. 2015;8:16–26.
96. Xiao Nan Y, Sun Chul K. Chemical composition, antioxidant and antibacterial activities of essential oil from Korean Citrus unshiu peel. J Agric Chem Environ. 2013;2013:42–9. CrossRef
97. Guo Y, Baschieri A, Amorati R, Valgimigli L. Synergic antioxidant activity of γ-terpinene with phenols and polyphenols enabled by hydroperoxyl radicals. Food Chem. 2021;345:128468. CrossRef
98. Kumar VR, Chaurasia L, Verma RK, Kumar M. Antifungal activity of essential oils against selected building fungi. Indian J Nat Prod Resour. 2011;2:448–51.
99. Al–Âni W, Tawfik N, Shehab E. Antimicrobial activity of grapefruit seeds extracts (in vitro study). Al-Rafidain Dent J. 2011;11:341–5. CrossRef
100. Banday MZ, Sameer AS, Nissar S. Pathophysiology of diabetes: an overview. Avicenna J Med. 2020;10:174–88. CrossRef.
101. Schuster DP, Duvuuri V. Diabetes mellitus. Clin Podiatr Med Surg. 2002;19:79–107. CrossRef
102. Krentz AJ, Bailey CJ. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs. 2005;65:385–411. CrossRef
103. Khan UM, Sameen A, Aadil RM, Shahid M, Sezen S, Zarrabi A, et al. Citrus genus and its waste utilization: a review on health-promoting activities and industrial application. Evid Based Complement Alternat Med. 2021;2021:2488804. CrossRef
104. Oguntibeju OO. Hypoglycaemic and anti-diabetic activity of selected African medicinal plants. Int J Physiol Pathophysiol Pharmacol. 2019;11:224.
105. Azizah T, Suhendi A, Heng Yen K. Antidiabetic and antihypercholesterolemic activities of Citrus sinensis peel: in vivo study. Nat J Phys Pharm Pharmacol. 2015;5:382–5. CrossRef
106. Ali AM, Gabbar MA, Abdel-Twab SM, Fahmy EM, Ebaid H, Alhazza IM, et al. Antidiabetic potency, antioxidant effects, and mode of actions of Citrus reticulata fruit peel hydroethanolic extract, hesperidin, and quercetin in nicotinamide/streptozotocin-induced Wistar diabetic rats. Oxid Med Cell Longev. 2020;2020:1730492. CrossRef
107. Naim M, Mohammad Amjad F, Sultana S, Nazrul Isalm S, Amjad Hossain M, Begum R, et al. A comparative study of antidiabetic activity of hexane-extract of lemon peel (Citrus limon) and glimepiride in alloxan-induced diabetic rats. Bangladesh Pharm J. 2012;15:131–4.
108. Kim SH, Hur HJ, Yang HJ, Kim HJ, Kim MJ, Park JH, et al. Citrus junos tanaka peel extract exerts antidiabetic effects via AMPK and PPAR-γ both in vitro and in vivo in mice fed a high-fat diet. Evid Based Complement Alternat Med. 2013;2013:921012. CrossRef
109. Kundusen S, Haldar PK, Gupta M, Mazumder UK, Saha P, Bala A, et al. Evaluation of antihyperglycemic activity of Citrus limetta fruit peel in streptozotocin-induced diabetic rats. ISRN Endocrinol. 2011;2011:1–6. CrossRef
110. Nurdin SU, Sabarina D, Subeki, Astuti S. Antidiabetic and antioxidant activities of bay, pandan, Citrus leaves and their combination in vitro. Biomed Pharmacol J. 2019;12:833–41. CrossRef
111. Ghauri AO, Ahmad S, Rehman T. In vitro and in vivo anti-diabetic activity of Citrullus colocynthis pulpy flesh with seeds hydro-ethanolic extract. J Complement Integr Med. 2020;17: 1–9.. CrossRef
112. Sah AN, Joshi A, Juyal V, Kumar T. Antidiabetic and hypolipidemic activity of Citrus medica Linn. seed extract in streptozotocin induced diabetic rats. Pharmacogn J. 2011;3:80–4. CrossRef
113. Cooper GM. The development and causes of cancer. In: The cell: a molecular approach. 2nd edition. Sunderland, MA: Sinauer Associates; 2000.
114. Li Y, Li S, Meng X, Gan RY, Zhang JJ, Li H. Dietary natural products for prevention and treatment of breast cancer. Nutrients. 2017;9:728. CrossRef
115. Jomaa S, Rahmo A, Alnori AS, Chatty ME. The cytotoxic effect of essential oil of Syrian Citrus limon peel on human colorectal carcinoma cell line (Lim1863). Middle East J Cancer. 2012;3:15–21.
116. Monajemi R, Oryan S, Haeri-Roohani A, Ghannadi A, Jafarian A. Cytotoxic effects of essential oils of some Iranian citrus peels. Iran J Pharm Res. 2005;3:183–7. CrossRef
117. Li C, Cai Q, Wu X, Tan Z, Huang S, Wei C, et al. Variation in compositions and biological activities of essential oils from four Citrus species: Citrus limon, Citrus sinensis, Citrus paradisii, and Citrus reticulata. Chem Biodivers. 2022;19:e202100910. CrossRef
118. Kundusen S, Bala A, Kar B, Bhattacharya S, Mazumder UK, Gupta M, et al. Antitumor potential of Citrus limetta fruit peel in ehrlich ascites carcinoma bearing Swiss albino mice. Alternat Med Studies. 2012;2:e10. CrossRef
119. Ruknuddin G, Prajapati P, Chaudhari SY. Ethno-medicinal values of Citrus genus: a review. Med J Dr DY Patil Univ. 2016;9:560. CrossRef
120. Zaki NL, Naeem MMM. Antioxidant, antimicrobial and anticancer activities of citrus peels to improve the shelf life of yoghurt drink. Egypt J Food Sci. 2021;49:249–65. CrossRef
121. Ho Y, Suphrom N, Daowtak K, Potup P, Thongsri Y, Usuwanthim K. Anticancer effect of Citrus hystrix DC. leaf extract and its bioactive constituents citronellol and citronellal on the triple negative breast cancer MDA-MB-231 cell line. Pharmaceuticals. 2020;13:476. CrossRef
122. Kim J, Jayaprakasha GK, Uckoo RM, Patil BS. Evaluation of chemopreventive and cytotoxic effect of lemon seed extracts on human breast cancer (MCF-7) cells. Food Chem Toxicol. 2012;50:423–30. CrossRef
123. United States Environmental Protection Agency (US EPA). Insecticides. Washington, DC: US EPA, CADDIS Vol. 2 [updated 2023 May 18; cited 2023 May 25]. Available from: https://www.epa.gov/caddis-vol2/insecticides
124. Damalas CA, Eleftherohorinos IG. Pesticide exposure, safety issues, and risk assessment indicators. Int J Environ Res Public Health. 2011;8:1402. CrossRef
125. World Health Organization. Pesticide residues in food. Geneva, Switzerland: WHO; [updated 2022 Sep 15; cited 2023 May 25]. Available from: https://www.who.int/news-room/fact-sheets/detail/pesticide-residues-in-food
126. Ahmed N, Alam M, Saeed M, Ullah H, Iqbal T, Al-Mutairi KA, et al. Botanical insecticides are a non-toxic alternative to conventional pesticides in the control of insects and pests. Global decline of insects. London, UK: IntechOpen; 2021. CrossRef
127. Hollingsworth RG. Limonene, a citrus extract, for control of mealybugs and scale insects. J Econ Entomol. 2005;98:772–9. CrossRef
128. Musa AK, Sulyman A. Bioefficacy of single and mixed applications of Citrus paradisii Maef and Citrus aurantifolia Swingle peel extracts against seed beetle, Callosobruchus maculatus (F.) (Coleoptera: Chrysomelidae). Discourse J Agric Food Sci. 2014;2:85–90.
129. Author C, Kosar Abbas S, Ahmad F, Sagheer M, Yasir M, Ahmad S, et al. Insecticidal and growth inhibition activities of Citrus paradisii and Citrus reticulata essential oils against lesser grain borer, Rhyzopertha dominica (F.) (Coleoptera: Bostrichidae). World J Zoo. 2012;7:289–94. CrossRef
130. Akhtar M, Iram N. Repellent effects of certain plant extract against rice weevil Sitophilus oryzae L. (Coleoptera: Curculionidae). Int J Agric Appl Sci. 2013;5:69–73.
131. Guettal S, Tine S, Tine-Djebbar F, Soltani N. Evaluation of Citrus limonum (Sapindales: Rutaceae) L. essential oil as protectant against the granary weevil, Sitophilus granarius (L.) (Coleoptera: Curculionidae). Allelo J. 2020;51:79–92. CrossRef
132. Fouad HA, da Camara CAG. Chemical composition and bioactivity of peel oils from Citrus aurantiifolia and Citrus reticulata and enantiomers of their major constituent against Sitophilus zeamais (Coleoptera: Curculionidae). J Stored Prod Res. 2017;73:30–6. CrossRef
133. Oboh G, Ademosun AO, Olumuyiwa TA, Olasehinde TA, Ademiluyi AO, Adeyemo AC. Insecticidal activity of essential oil from orange peels (Citrus sinensis) against Tribolium confusum, Callosobruchus maculatus and Sitophilus oryzae and its inhibitory effects on acetylcholinesterase and Na+/K+-ATPase activities. Phytoparasitica. 2017;45:501–8. CrossRef
134. Siskos EP, Konstantopoulou MA, Mazomenos BE. Insecticidal activity of Citrus aurantium peel extract against Bactrocera oleae and Ceratitis capitata adults (Diptera: Tephritidae). J Appl Entomol. 2009;133:108–16. CrossRef
135. Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr. 2004;134:3479S–85S. CrossRef
136. Kennedy DO, Wightman EL. Herbal extracts and phytochemicals: plant secondary metabolites and the enhancement of human brain function. Adv Nutr. 2011;2:32. CrossRef
137. Oz AT, Kafkas E, Oz AT, Kafkas E. Phytochemicals in fruits and vegetables. Superfood and functional food–an overview of their processing and utilization. London, UK: IntechOpen; 2017 [cited 2023 May 26]. CrossRef
138. Presentato A, Scurria A, Albanese L, Lino C, Sciortino M, Pagliaro M, et al. Superior antibacterial activity of integral lemon pectin extracted via hydrodynamic cavitation. Chem Open. 2020;9:628–30. CrossRef
139. Wang J, Zhai Y, Ou M, Bian Y, Tang C, Zhang W, et al. Protective effect of lemon peel extract on oxidative stress in H9c2 rat heart cell injury. Drug Des Devel Ther. 2021;15:2047–58. CrossRef.
140. Singh N, Singh SK. Citrus limon extract: possible inhibitory mechanisms affecting testicular functions and fertility in male mice. Syst Biol Reprod Med. 2016;62:39–48. CrossRef
141. Rahman MM, Jahan FI, Mim SA. A brief phytochemical investigation and pharmacological uses of citrus seed: a review. PhOL. 2019;1:94–103.
142. Loizzo MR, Tundis R, Bonesi M, Menichini F, De Luca D, Colica C, et al. Evaluation of Citrus aurantifolia peel and leaves extracts for their chemical composition, antioxidant and anti-cholinesterase activities. J Sci Food Agric. 2012;92:2960–7. CrossRef.
143. Bahaa Al-Rubai A, Hind Hussein OL, Tahrrer Hadi SA. Antimicrobial activity for crude watery extract of seeds of Citrus aurantifolia (lime fruit) against Gram positive and negative bacteria in vitro. J Al-Ma’moon Coll. 2016;27:404–16.
144. Favela-Hernández JMJ, González-Santiago O, Ramírez-Cabrera MA, Esquivel-Ferriño PC, Camacho-Corona MDR. Chemistry and pharmacology of Citrus sinensis. Molecules. 2016;21:247. CrossRef
145. Maia MF, Moore SJ. Plant-based insect repellents: a review of their efficacy, development and testing. Malaria J. 2011;10:1–15. CrossRef
146. Traboulsi AF, El-Haj S, Tueni M, Taoubi K, Nader NA, Mrad A. Repellency and toxicity of aromatic plant extracts against the mosquito Culex pipiens Molestus (Diptera: Culicidae). Pest Manag Sci. 2005;61:597–604. CrossRef
147. Aldholmi M, Marchand P, Ourliac-Garnier I, le Pape P, Ganesan A. A decade of antifungal leads from natural products: 2010–2019. Pharmaceuticals. 2019;12:182. CrossRef
148. Yekeen MO, Ajala OO, Alarape AB. Antifungal activities of Citrus sinensis seed oil against Lentinus sajor-caju. Pelagia Res Library Adv Appl Sci Res. 2014;5:109–13.
149. Bissim SM, Kenmogne SB, Lobe JS, Atangana AF, Bissoue AN, Langat MK, et al. The chemistry and biological activities of Citrus clementina Hort. Ex Tanaka (Rutaceae), a vegetatively propagated species. Nat Prod Res. 2021;35:4839–42. CrossRef
150. Leporini M, Loizzo MR, Sicari V, Pellicanò TM, Reitano A, Dugay A, et al. Citrus × clementina hort. juice enriched with its by-products (peels and leaves): chemical composition, in vitro bioactivity, and impact of processing. Antioxidants. 2020;9:298. CrossRef
151. Fayek NM, El-Shazly AH, Abdel-Monem AR, Moussa MY, Abd-Elwahab SM, El-Tanbouly ND. Comparative study of the hypocholesterolemic, antidiabetic effects of four agro-waste citrus peels cultivars and their HPLC standardization. Rev Bras Farmacogn. 2017;27:488–94. CrossRef
152. Liao J, Xu T, Liu YH, Wang SZ. A new limonoid from the seeds of Citrus reticulata Blanco. Nat Prod Res. 2012;26:756–61. CrossRef
153. Kowalski S, Wyrzykowski D, Inkielewicz-Stepniak I. Molecular and cellular mechanisms of cytotoxic activity of vanadium compounds against cancer cells. Molecules. 2020;25:1757. CrossRef
154. Phetkul U, Phongpaichit S, Watanapokasin R, Mahabusarakam W. New depside from Citrus reticulata Blanco. Nat Prod Res. 2014;28:945–51. CrossRef
155. Siahpoosh A, Javedani F. Antioxidative capacity of Iranian Citrus deliciosa peels. Free Rad Antioxidants. 2012;2(2):62–7. CrossRef
156. Miya G, Nyalambisa M, Oyedeji O, Gondwe M, Oyedeji A. Chemical profiling, toxicity and anti-inflammatory activities of essential oils from three grapefruit cultivars from Kwazulu-Natal in South Africa. Molecules. 2021;26:3387. CrossRef
157. Adeneye AA. Methanol seed extract of Citrus paradisii Macfad lowers blood glucose, lipids and cardiovascular disease risk indices in normal Wistar rats. Nig Q J Hosp Med. 2008;18:16–20. CrossRef
158. Adeneye AA. Hypoglycemic and hypolipidemic effects of methanol seed extract of Citrus paradisii Macfad (Rutaceae) in alloxan-induced diabetic Wistar rats. Nig Q J Hosp Med. 2008;18:211–5. CrossRef
159. Cvetnic Z and V-KS. Antimicrobial activity of grapefruit seed and pulp ethanolic extract. Acta Pharm. 2004;54:243–50.
160. Maksoud S, Abdel-Massih RM, Rajha HN, Louka N, Chemat F, Barba FJ, et al. Citrus aurantium L. active constituents, biological effects and extraction methods. An updated review. Molecules. 2021;26:5832. CrossRef