INTRODUCTION
Celtis tournefortii Lam. (Celtis aetnensis) is a flowering plant of the Cannabaceae family that is native to East Asia. It is commonly known as the Japanese Hackberry. The bark and leaves of the tree are commonly used in Korean medicine. The bark is used for treating shortness of breath, swollen feet, dyspepsia, loss of appetite, and so on [1]. Apart from these properties, the plant is known for healing stomach pain, inflammation of teeth, diarrhea, and low back pain. Such qualities are brought about by the presence of active substances such as flavonoids, tannins, and saponins. To date, C. tournefortii Lam. has not been investigated much when compared to the other species.
Phytochemical constituents of other species of Celtis have been previously investigated and reported. The presence of vitamin C, phenols, carotenoids, and flavonoids was reported in C. tournefortii species [2]. These constituents add to the antioxidant properties of the species. Celtis australis is used as a natural remedy for amenorrhea, colds, ulcers, and stomach disorders. It was reported to have a range of phytochemicals, such as flavonoids, terpenoids, and anthocyanins, which exert various biological effects [3]. Celtis integrifolia, which commonly grows in temperate regions of Africa and Asia, was found to contain saponins, tannins, flavonoids, and cardiac glycosides. The leaf extracts showed excellent antidiarrheal activity [4]. Phenolic profiling of the C. tournefortii species identified fumaric acid, gentisic acid, vanillic acid, and scutellarin in the twigs, leaves, and fruits. According to the literature [5], it demonstrated considerable antioxidant action. In addition to phenols, phytochemicals such as alkaloids, flavonoids, terpenoids, coumarins, coumaroyl tyramines, lignan glycosides, steroids, and phenolics have also been reported [6]. Celtis africana leaves and stems were found to have antioxidant properties.
Compounds with antitumor properties were identified in the twigs of Celtis sinensis. They primarily belong to the steroid and terpenoid groups [7]. Celtis aetnensis was found to be effective against human colon carcinoma [8]. Silver nanoparticles synthesized from Celtis leaves showed antimicrobial effects against pathogenic strains and significant antitumor activity [9]. The bioactivities of C. aetnensis were examined in another study, as well as any prospective effects on cancer treatment and human health. Through the induction of apoptosis and necrosis, the extract was found to decrease the viability of Caco2 cells [8]. The present study focuses on evaluating the anticancer properties of C. aetnensis in human liver cancer cells (HepG2).
MATERIALS AND METHODS
Sample collection and identification
The bark of C. tournefortii Lam. (C. aetnensis) was collected in the tea estates of Ooty (India) and authenticated by Dr. Ravikumar, Botanist at FRLHT (The Foundation of Revitalization of Local Health Traditions, Bangalore).
Reagents for in vitro cell culture
Human hepatocellular carcinoma (HCC) (HepG2 cells) was obtained from NCCS Pune, India. Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and antibiotic-antimycotic solution were purchased from Thermoacoustic, and the (3-(4, 5-dimethylthiazolyl-2)-2, 5- diphenyltetrazolium bromide) (MTT) reagent was purchased from Sigma Aldrich, USA. The culture medium was prepared as per the manufacturer’s instructions and the filter was sterilized, followed by the addition of 10 ml of antibiotics (penicillin?streptomycin—100 U/ml−100 μg/ml). The medium was stored at 4°C in a sterile container. Growth medium (DMEM) was prepared by adding 10 ml FBS to 90 ml DMEM. It was stored in a sterile container at 4°C. Phosphate buffered saline (PBS; pH 7.4) was prepared [sodium phosphate monobasic (NaH2PO4)—0.63 g, sodium phosphate dibasic (Na2HPO4)—0.17 g, sodium chloride (NaCl)—4.5 g; sterile double distilled water—500 ml], and the pH was adjusted to 7.4, filter sterilized, and stored in the refrigerator.
Preparation of the bark extract
Celtis tournefortii Lam. bark was extracted using water and chloroform at a ratio of 9:1. Initially, the plant bark powder was incubated for 3 days in the solvent, followed by filtration using a muslin cloth, and the semisolid residue was collected. The residue was then vacuum dried followed by freeze-drying. The dry weight was then recorded. The stock solution of the bark extract was prepared at a concentration of 50 mg/ml media. The test solutions were prepared from the stock solution at 10, 25, 50, 100, 250, and 500 μg/ml concentrations.
Cell culture, treatment, and cytotoxicity assessment using the MTT assay
HepG2 cells were allowed to proliferate to form a monolayer. To initiate treatment, the spent media from the monolayers was removed, and the monolayers were washed with PBS. The cells were trypsinized, and 3 ml of the growth media was added to the culture flasks. The cells were counted, and from the stock cell suspension, 1 × 105 viable cells/ml suspended in media were seeded in a 25 cm2 tissue culture flask containing approximately 4 ml of fresh media and incubated until the cells reached 60%–70% confluency. The cells were trypsinized, counted, and seeded for further experimentation. Culture plates were seeded at 5 × 103 cells/well and incubated for 24 hours. Following incubation, cells were treated with 100 μl of the various concentrations of the bark extract and incubated for 48 hours. Control wells received 0.5% or 0.25% Dimethyl Sulfoxide (DMSO). Triplicates were maintained for all treatments and controls. After 48 hours, 30 μl of 5 mg/ml MTT solution was added to each well and incubated at 37°C for 3 hours. The plates were covered with aluminum foil during incubation with MTT solution. After 3 hours of incubation, the MTT-containing media was carefully aspirated, and 100 μl of DMSO was added to each well to dissolve the formed formazan crystals. The plate was shaken on a gyratory shaker for 5 minutes. The optical density was measured using an Enzyme Linked Immunosorbent Assay (ELISA) reader at 540 nm.
Lactate dehydrogenase (LDH) assay
For the LDH assay, 50, 100, 250, and 500 μg/ml were used. The treatment protocol was the same as mentioned for the MTT assay except for the conditioned medium, which was used for the LDH leakage assay. Then, 0.1 ml of conditioned medium was added to 1 ml of the buffered substrate and maintained at 37°C in a water bath. After that, 0.2 ml of NAD+ solution was added, carefully mixed, and incubated for 15 minutes at 37°C. The Dinitrophenylhydrazine (DNPH) reagent was then added, and the mixture was incubated for an additional 15 minutes. After adding 10 ml of sodium hydroxide (0.4 N), the absorbance was measured at 440 nm after 1–5 minutes. To create the standard graph, standards were performed concurrently and treated with sodium pyruvate as in the assays. The ratio of the number of lysed cells to the color produced is linear.
ROS determination
The 2’,7’-Dichlorodihydrofluorescein diacetate (DCFDA) assay was carried out as per [10]. The test solutions were used at 100, 250, and 500 μg/ml concentrations. The results are expressed as the mean ± SE value of fold change in fluorescence intensity plotted against the tested drug concentration.
FACS analysis
An apoptosis assay was used to evaluate the mechanism of drug-induced HepG2 cell death. By employing a flow cytometer and the PI/FITC Annexin V apoptosis detectiosn kit from BD Biosciences, apoptosis/necrosis experiments were carried out. HepG2 cells were treated with 100 and 200 g/ml of the extract for 24 hours. According to the manufacturer’s instructions, flow cytometry was used to determine the number of apoptotic/necrotic cells.
AKT expression by PCR
Gene expression profiling was performed by Real time Polymerase Chain Reaction (RT?PCR) using a specific primer against the AKT gene. Freshly grown mid-log phase HepG2 cells were treated with drugs (100 and 200 μg/ml). Cells were processed for RNA isolation using the TRIZOL technique after 12 hours of incubation. For subsequent processing, the extracted RNA pellet was dissolved in double-distilled water free of RNase and quantified using a NanoDrop (Thermo Scientific, USA). For the RT?PCR, cDNA was created using a cDNA synthesis kit. Each cycle of the 35-cycle PCR lasted 45 seconds of extension at 72°C, 30 seconds of annealing between 5°C and 8°C, and 30 seconds of denaturation at 95°C. Samples were warmed at 95°C for 5 minutes before PCR.
RESULTS AND DISCUSSION
HCC is a common and aggressive form of liver cancer with limited treatment options. Natural products, such as plant extracts, have gained significant interest as potential sources of novel anticancer agents. In this study, we investigated the in vitro anticancer activity of C. aetnensis bark extract against HepG2 cell lines, a widely used model for studying HCC. Our findings provide valuable insights into the potential of C. aetnensis bark extract as a therapeutic candidate for HCC treatment.
The results of our study revealed significant anticancer activity of C. aetnensis bark extracts against HepG2 cell lines in vitro. We employed multiple assays to evaluate the extract’s effects on cell viability, proliferation, and apoptosis.
Extraction
After the extraction process, approximately 1.54% of the yield was obtained.
MTT assay
To assess the cytotoxic potential of the plant extract, we performed the MTT assay, which measures the cell viability. A total of six drug concentrations were tested on the cell line for their ability to induce cell death. Among the six concentrations, 500 μg/ml showed maximum cytotoxicity (Figure 1). The lowest concentration (10 μg/ml) showed low cancer cell death and a higher cell survival percentage, and as the concentration increased, cell death increased, and the highest concentration (500 μg/ml) showed a low cell survival percentage, implying increased cell death.
Our results demonstrated a dose-dependent decrease in cell viability following treatment with various concentrations of C. aetnensis bark extract. The results obtained suggest that plant extract effectively inhibits the growth of HepG2 cells, indicating its potential as an anticancer agent for HCC treatment.
The results are expressed as the mean ± SE. OD values (proportional to cell survival) plotted against the tested drug concentration.
LDH assay
The results are expressed as the mean ± SE. OD values (Figure 2 % LDH release plotted against the tested drug concentration).
LDH only exists in the cytoplasm and leaves the cell through ruptured membranes [11]. An early sign of increased necrotic cell death is the rise in LDH release, which is consistent with increased cell damage [12]. In the present study, a dose-dependent increase in LDH was documented. This observation is consistent with the reported literature where plant extracts caused necrosis of HepG2 cells which was measured by LDH leakage [13–15].
ROS determination
The production of ROS is elevated in tumor cells because of increased metabolic rate, gene mutation, and relative hypoxia, and excess ROS are quenched by increased antioxidant enzymatic and non-enzymatic pathways in the same cells. Increasing the ROS concentration can act as a cytotoxic factor and cause cell death. As depicted in the graph (Figure 3), tumor cells (control group) showed low ROS levels, and treatment with H2O2 (hydrogen peroxide) caused higher ROS levels. In the case of extract, treatment with 100 μg/ml significantly increased the ROS levels when compared to a control group. Higher treatment doses further increased ROS generation, indicating that treatment with extract can effectively increase the ROS levels in tumor cells in a dose-dependent manner to cause cytotoxicity.
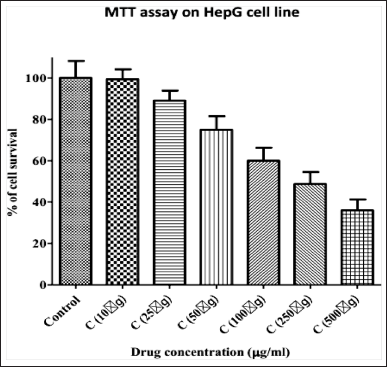 | Figure 1. Cytotoxicity Assay of Bark Extract of C. tournefortii Lam. (C. aetnensis) at different concentrations. [Click here to view] |
ROS are highly reactive molecules that play a crucial role in cell signaling and apoptosis. A significant increase in ROS generation upon treatment with the plant extract suggests that the extracts induce oxidative stress in cancer cells, which can lead to DNA damage, lipid peroxidation, and ultimately cell death.
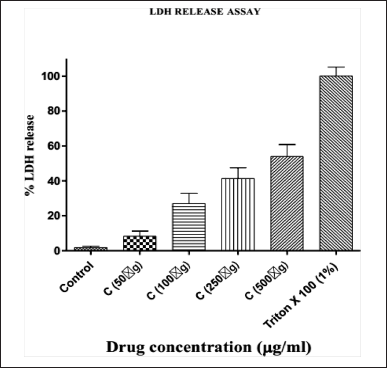 | Figure 2. LDH release Assay of Bark Extract of C. tournefortii Lam. (C. aetnensis) at different concentrations. [Click here to view] |
The LDH release and ROS generation assays provide complementary information about the anticancer activity of the plant extract. LDH release reflects the overall cytotoxic effects on the cancer cells, whereas ROS generation indicates the involvement of oxidative stress-mediated pathways in the cytotoxic mechanism. The combination of these assays provides a more comprehensive understanding of the potential mechanisms of action of the bark extract of C. tournefortii Lam.
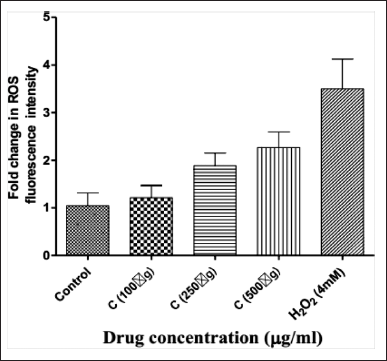 | Figure 3. Fold Change in ROS with bark extract of C.tournefortii Lam at different concentrations. [Click here to view] |
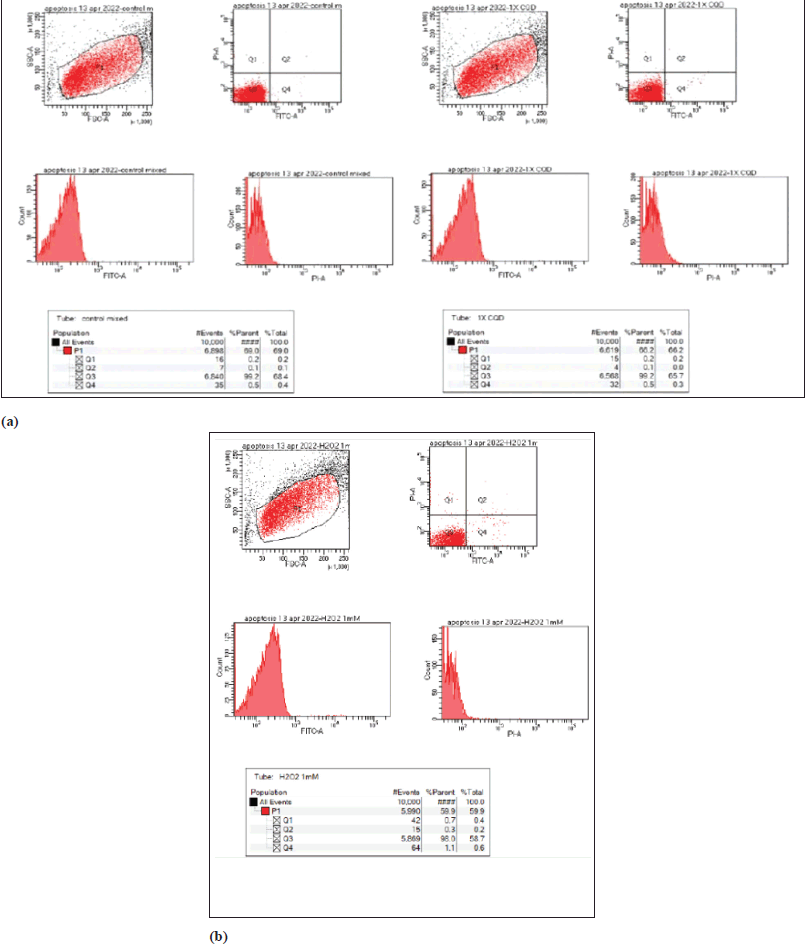 | Figure 4. Apoptosis assay using FACS Analysis. [Click here to view] |
FACS analysis
The induction of apoptosis or necrosis was evaluated in drug-treated cells (Figure 4). The lower left quadrant (Q3) shows living cells (both FITC and PI negative). The lower right quadrant (Q4) represents cells in early apoptosis (FITC+/PI−) and cells in the upper right quadrant (Q2) are cells in late apoptosis due to PI entering the cells through leaky membranes. The upper left quadrant (Q1) shows necrotic cells (FITC−/PI+). The results showed that there was no significant apoptosis or necrosis when compared to control.
A KT gene expression
The fold change was calculated from the Cq values, and a significant decrease in the expression of the AKT gene in cells treated with 1× and 2× concentrations was observed (Figure 5).
Existing evidence suggests that ERKs and AKT genes play a role in cell proliferation [16,17]. The AKT gene coding for the protein kinase B is impaired in liver cancers. Several natural products and plant extracts have been reported to reduce the expression of this gene, thereby causing cell death [18,19]. Butt et al. [20] reported that treatment with taxifolin significantly reduces the expression of the AKT gene along with HIF1-α and Vascular Endothelial Growth Factor (VEGF) in a dose-dependent manner. In the present study, treatment with bark extract of C. tournefortii Lam. significantly reduced the AKT gene expression in a dose-dependent manner. To the best of our knowledge, this is the first report documenting the effect of C. tournefortii Lam. on the AKT gene expression in HepG2 cells. Suppression of the AKT gene has been associated with apoptosis in cancer cells [21] and therefore, deregulating the AKT signaling pathway has become one of the therapeutic targets for cancer treatment. On that note, the result suggests that the AKT gene could be the potential primary target for the bioactive components in the bark extract of C. aetnensis, encouraging its use in the treatment of liver cancer, pending further investigation on narrowing down to the active ingredient responsible for the therapeutic activity and its safety/toxicity in preclinical and clinical studies.
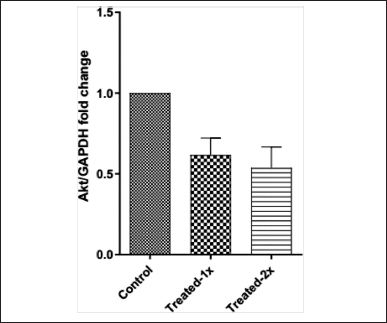 | Figure 5. AKT-gene expression analysis of Bark Extract of C. tournefortii Lam. (C. aetnensis) at different concentrations. [Click here to view] |
CONCLUSION
HCC is the fifth most prevalent cancer in the world and third in terms of cancer-related mortality. Despite advancements in therapy, numerous side effects have been reported. Traditional medicine has been considered as a safer therapeutic alternative to minimize if not eliminate adverse effects. This study provides compelling evidence for the in vitro anticancer activity of bark extract of C. tournefortii Lam. The results suggest that the plant extract exerts its cytotoxic effects by inhibiting cell viability, and inducing and promoting apoptosis in HepG2 cells. These findings highlight the potential of the phytochemicals of C. tournefortii Lam. as a valuable source of novel anticancer agents for HCC treatment. Further investigations, including in vivo studies and identification of active compounds are necessary to fully explore the therapeutic potential of the bark extract of C. tournefortii Lam. and its suitability for clinical applications in HCC patients.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Duke JA, Ayensu ES. Medicinal plants of China. 1–5th ed. Algonac, MI: Reference Publications; 1985.
2. Y?ld?r?m I, Ugur Y, Kutlu T. Investigation of antioxidant activity and phytochemical compositions of Celtis tournefortii. Free Radic Antioxid. 2017 Mar 1;7(2):160–5. CrossRef
3. Benamar K, Koraichi SI, Fikri-Benbrahim K. Ethnobotany, phytochemistry and pharmacological activities of Celtis australis: a review. J Herbmed Pharmacol. 2023 Jan;12(1):54–72. CrossRef
4. Nao A. Acute toxicity, phytochemistry and anti-diarrheal effects of Celtis integrifolia Lam. aqueous leaf extract in Wistar Albino rats. Br J Pharm Res. 2016;14(5):1–7. CrossRef
5. Gecibesler IH. Antioxidant activity and phenolic profile of Turkish Celtis tournefortii. Chem Nat Compd. 2019 Jul 1;55(4):738–43. CrossRef
6. Adedapo AA, Jimoh FO, Afolayan AJ, Masika PJ. Antioxidant properties of the methanol extracts of the leaves and stems of Celtis africana. Rec Nat Prod. 2009 Jan 1;3(1):23–31. CrossRef
7. Kim DK, Lim JP, Kim JW, Park HW, Eun JS. Antitumour and anti-inflammatory constituents from Celtis sinensis. Arch Pharm Res. 2005 Jan;28(1):39–43. CrossRef
8. Acquaviva R, Sorrenti V, Santangelo R, Cardile V, Tomasello B, Malfa G, et al. Effects of an extract of Celtis aetnensis (Tornab.) Strobl twigs on human colon cancer cell cultures. Oncol Rep. 2016 Oct 1;36(4):2298–304. CrossRef
9. Baran A, Keskin C, Kandemir S?. Rapid biosynthesis of silver nanoparticles by Celtis tournefortii Lam. leaf extract; investigation of antimicrobial and anticancer activities. Kahramanmara? Sütçü ?mam Üniv Tar?m Do?a Derg. 2022 Dec 30;25(1):72–84. CrossRef
10. Eruslanov E, Kusmartsev S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol Biol. 2010;594:57–72. CrossRef
11. Watanabe W, Sudo K, Asawa S, Konno K, Yokota T, Shigeta S. Use of lactate dehydrogenase to evaluate the anti-viral activity against influenza A virus. J Virol Methods. 1995;51(2–3):185–91. CrossRef
12. Nagiah S, Phulukdaree A, Chuturgoon A. Inverse association between microRNA-124a and ABCC4 in HepG2 cells treated with antiretroviral drugs. Xenobiotica. 2016;46(9):825–30. CrossRef
13. Yurdakök B, Baydan E. Cytotoxic effects of Eryngium kotschyi and Eryngium maritimum on Hep2, HepG2, Vero and U138 MG cell lines. Pharm Biol. 2013;51(12):1579–85. CrossRef
14. Tamboli AM, Wadkar KA. Comparative cytotoxic activity of Convolvulus pluricaulis against human hepatoma cell line (HepG2) and normal cell line (L929) via apoptosis pathways by flow cytometry analysis. Bull Natl Res Cent. 2022;46:145. CrossRef
15. Sibiya T, Ghazi T, Mohan J, Nagiah S, Chuturgoon AA. Spirulina platensis mitigates the inhibition of selected miRNAs that promote inflammation in HAART-treated HepG2 cells. Plants. 2023;12(1):119. CrossRef
16. Singh N, Zaidi D, Shyam H, Sharma R, Balapure AK. Polyphenols sensitization potentiates susceptibility of MCF-7 and MDA MB-231 cells to centchroman. PLoS One. 2012;7(6):e37736. CrossRef
17. Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35(6):600–4. CrossRef
18. Tan BL, Norhaizan ME, Chan LC. Manilkara zapota (L) P. Royen leaf water extract induces apoptosis in human hepatocellular carcinoma (HepG2) cells via ERK1/2/Akt1/JNK1 signaling pathways. Evid Based Complement Alternat Med. 2018;2018:7826576. CrossRef
19. Lim E, Kim G, Kim B, Kim E, Kim SY, Kim Y. Ethanol extract from Cnidium monnieri (L.) Cusson induces cell cycle arrest and apoptosis via regulation of the p53?independent pathway in HepG2 and Hep3B hepatocellular carcinoma cells. Mol Med Rep. 2018;17(2):2572–80. CrossRef
20. Butt SS, Khan K, Badshah Y, Rafiq M, Shabbir M. Evaluation of pro-apoptotic potential of taxifolin against liver cancer. PeerJ. 2021 May 25;9:e11276. CrossRef
21. Huang KF, Zhang GD, Huang YQ, Diao Y. Wogonin induces apoptosis and down-regulates survivin in human breast cancer MCF-7 cells by modulating PI3K-AKT pathway. Int Immunopharmacol. 2012;12(2):334–41. CrossRef