INTRODUCTION
Berberine (BERB) is an isoquinoline alkaloid obtained from Rhizome coptidis, cortex phellodendri, and other plants from Beridaceae, Ranunculaceae, and Rutaceaefamily [1–3]. In traditional Ayurvedic and Chinese medicine, it has been employed as an antidiarrheal. BERB exhibits multiple therapeutic properties namely antidiabetic, antitumor, anti-inflammatory, antirheumatic, antiplatelet coagulation, hepatoprotective, anti-hyperlipidemic, and neuroprotective properties. It also exerts a wide spectrum of anti-bacterial and anti-leishmaniatic activity [4–8]. Numerous research reports in recent years indicated that the diverse therapeutic applications of BERB have been linked to its antioxidant capability [9–12].
BERB is a biopharmaceutical classification system class IV drug that has significantly affected the therapeutic use of the drug. In addition, BERB has a low rate of absorption in the intestine due to active excretion by P-gp and multidrug resistance protein-1 [13,14]. Due to its low bioavailability, when a high dose of BERB (0.9%–1.5%) is given to humans to produce the required therapeutic effect, it may lead to gastrointestinal side effects [15].
To increase solubility and permeability, multiple approaches were used in the past, including solid dispersion, inclusion complexes, pro-drugs, microspheres, nanoemulsion, liposomes, and solid lipid nanoparticles. Lipid-based nanosystems have demonstrated a noteworthy enhancement in the solubility and permeability of BERB. However, these systems encounter drawbacks such as insufficient drug loading, limited long-term stability, and potential toxicity [16,17]. Therefore, it was essential to design new techniques to improve BERB’s solubility and permeability. Lipid polymeric nanomicelles have become highly popular among nanocarriers due to their various advantages as drug carriers. These advantages include their small size, ability to biodegrade, cost-effectiveness, easy preparation, good permeability, enhanced solubility, and protection against enzymatic degradation [18–20].
Hence, the utilization of nanomicelles containing lipid-based BERB represents a promising approach for enhancing both solubility and permeability concerns limiting its oral bioavailability and subsequent therapeutic effects. In addition, the BERB-loaded nanomicelles can be lyophilized to reduce the stability issues related to the earlier-developed nanosystems of BERB.
In the present study, a thin-film hydration technique was adopted to develop BERB-loaded nanomicelles using ethanol as a solvent and Gelucire 44/14 and 50/13 were screened as a lipid polymer stabilizer. A 3-level, 2-factor, and 09-run experiment design was adopted to investigate the influence of independent factors. To verify ideal levels of these independent factors, a numerical optimization function was utilized. The optimized batch of BERB nanomicelles formulation was also further lyophilized to enhance the stability of the prepared formulation.
MATERIALS AND METHODS
Materials
BERB procured from Yucca Enterprises, Mumbai. Lipid excipients were provided as gift samples by Gattefosse, Mumbai, India.
Methods
Preparation of BERB-loaded nanomicelles formulation
Thin-film hydration procedure was adopted to develop BERB-loaded nanomicelles using a rotary vacuum evaporator (Superfit Rota Vap, Mumbai) [21–23]. Ethanol was used as a solvent and Gelucire 44/14 and 50/13 were screened as a polymer stabilizer. Initially, 500 mg of the drug was dissolved into a sufficient quantity of ethanol (5 ml) followed by the addition of a predetermined amount of polymer in different weight ratios (Table 2) in the round bottom flask (RBF). The RBF connected with a rotary vacuum evaporator under specific conditions for 15 minutes. After the ethanol has evaporated from the solution, a thin film begins to form around the RBF. The obtained thin film was mixed with 10 ml of deionized water with regular shaking on a heating water bath. On a regulated speed magnetic stirrer, the mixture was constantly agitated (20 minutes) at 200 to 500 rpm and further ultrasonicated for 30 minutes to obtain nanomicelles. Initially, gelucire 44/14 and 50/13 were screened as polymer stabilizers for the preparation of nanomicelles. In preliminary studies, it was found that in batches prepared with gelucire 50/13 after the rotary vacuum evaporation process, the obtained film was too sticky and difficult to process further. Hence, gelucire 44/14 was selected for the preparation of further batches. An investigation was conducted to analyze the impact of the ratio between polymer and drug (referred to as X1) as well as the stirring speed (referred to as X2) on both the size of particles and the efficiency of entrapment. This investigation utilized a 32, 09-run experiment factorial design, employing Design Expert v10.0.8 by Stat-Ease. Tables 1 and 2. The desirability approach was employed to identify the optimum formulation batch-keeping constraint of the lowest size of the particle and highest entrapment efficiency (EE). The optimized batch of BERB nanomicelles formulation was lyophilized using mannitol (10% w/v) as a cryoprotectant by Lyophilizer (α Martin Christ 2-4 LSC basic, Germany). The sample was frozen in a deep freezer at −80°C (Make-Remi, Model-ULT-185) before lyophilization to solidify any remaining liquid such as solvent moisture contained in the samples. After overnight freezing, the sample is quickly lyophilized to prevent the frozen liquid in the sample from melting. During the lyophilization process temperature kept was at −85°C, pressure-0.005 mbar (4 hours) and 0.1 mbar (4 hours) in primary and secondary drying, respectively (α 2-4 LSC, Martin Christ).
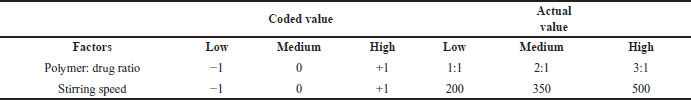 | Table 1. Coded and actual value of independent variables. [Click here to view] |
Evaluations
Fourier transform infra-red (FTIR) spectroscopy
FTIR scans of samples were determined on FTIR (Shimadzu, 8400S, Japan) to determine the drug-polymer compatibility [24].
Determination of critical micellar concentration (CMC)
Using the commonly known iodine UV-visible spectroscopy technique, the polymers’ CMC value was determined. UV absorbance analysis was performed on multiple polymer dilutions (ranging from 1 to 10 mg/ml) at a wavelength of 225 nm. The dilutions included a standard KI/I2 solution of 25 μl. To determine the CMC value, absorbance measurements were plotted against log polymer concentration [21].
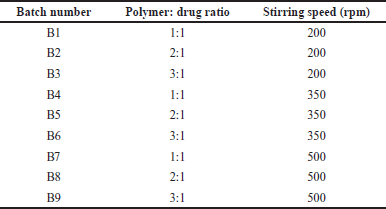 | Table 2. Formulation composition of BERB loaded nanomicelles. [Click here to view] |
Particle size, polydispersity index (PDI), and zeta potential
Size of the micelle (z-average), uniformity, and zeta value of BERB loaded nanomicelle formulation determined in triplicate using Horiba particle size analyzer (Nano ZS, Malvern Co., UK) [25,26].
Entrapment efficiency
To assess the EE, the micellar solution was rotated at 15,000 rpm for 20 minutes. Subsequently, the solution was filtered through 44 microns syringe filter. BERB concentration in samples analyzed on 347 nm (measured by UV-160, SHIMADZU, Japan) [27–29]. The following equation was used to calculate the drug entrapment:
Statistical optimization
Size of particle and EE data were acquired and analyzed by Design-expert® software. All responses were transformed into quadratic models. Statistical parameters were further analyzed by ANOVA to identify relevant model terms. Response surface plots and regression equations were also generated. The desirability approach is employed to determine the optimal level of independent variables [24].
Saturated solubility and in-vitro dissolution study
To determine enhancement in solubility of the optimized batch BERB nanomicelles (lyophilized batch B6) compared to pure drug saturated solubility experiments were performed. Solubility experiments were performed using distilled water, 0.1 N HCl with a pH of 1.2, and PO43−with pH 6.8 buffer at 37°C ± 0.5°C using an orbital water bath shaker (Remi Cis-18 Plus, Mumbai) [24]. The USP I (Basket type) apparatus was used to conduct in-vitro dissolution tests for pure drug and lyophilized optimized nanomicelles formulation [30–33]. Capsules filled with 100 mg of the equivalent drug were taken into 900 ml 0.1 N HCL at a temperature of 37°C ± 0.5°C on 100 rpm. 5 ml samples were taken at preset times and estimated by UV visible spectroscopy at 347 nm to determine cumulative drug release [22].
Transmission electron microscopy (TEM)
Under TEM (JEM-1200EX, Japan) on 20 kV increasing voltage, the morphology of optimized nanomicelles formulation was investigated.
Powder X-ray diffraction (PXRD)
PXRD pattern of samples acquired by CuKα source at 1.5406 Å λ on Philips 1830 diffractometer, Netherlands.
Ex-vivo absorption study
Ex-vivo absorption studies of optimized lyophilized BERB nanomicelles formulation and the pure drug were determined by perfusion apparatus using everted gut sac of a rat intestine [34]. The study was approved by IAEC and in accordance with CPCSEA, India. The everted segment was mounted on the perfusion apparatus and 25 ml of tyroid solution was taken into it. The assembly was introduced into a beaker holding 1,000 ml PO43−pH 6.8 buffer with 10 ml of optimized nanoformulation at 37°C ± 0.5°C under continuous aeration. The internal part of the perfusion apparatus tube served as the serosal side whereas the buffer solution in the beaker side would serve as the mucosal side. The samples from the serosal side were taken at predetermined intervals and spectrophotometrically analyzed at 347 nm.
Anti-oxidant activity
The anti-peroxidation and antiradical activity of BERB nanomicelles (batch, B- 6) was assessed to compare their enhanced antioxidant effect to that of the pure drug. The determination of the anti-peroxidation effects was accomplished by assessing the extent of reduction in lipid peroxidation. The 2,2-diphenyl-1-picrylhydrazyl (DPPH) inhibition technique was used to measure the antiradical activity. For DPPH inhibition activity, sample (BERB and BERB nanomicelles) stock (1.0 mg/ml) serial dilutions are prepared in methanol. To conduct the experiment, 0.3 mM DPPH solution was introduced, in the quantity of 1 ml, into the prepared sample solutions. The sample was incubated for 30 minutes and analyzed at 515 nm. In the lipid peroxidation assay, 0.2 ml of samples were combined with 0.2 ml 8.1% sodium dodecyl sulfate and 1.5 ml 20% acetic acid solution at pH 3.5. Further mixture added in 1.5 ml of a 0.8% thiobarbituric acid solution, and 0.6 ml distilled water. The mixture was then heated at 95°C, for 60 minutes. After cooling, 1 ml distilled water with a 5.0 ml mixture of n-butanol and pyridine at a ratio of 15:1 (v/v) was added and vigorously shaken. Following centrifugation at 4,000 rpm for 10 minutes, analysis was performed at a wavelength of 532 nm, using a Shimadzu1700 instrument in Japan [35–37].
Stability study
Accelerated stability study of nano micelle formulation was done by using ICH Q1 A-R2 guidelines. The lyophilized formulation was filled in the capsule and then placed at 40°C ± 2°C and 75% RH. The size of the particles, zeta value, PDI, and % release were examined at specific time intervals over a period of 6 months [24].
RESULTS AND DISCUSSION
FTIR spectroscopy
BERB FTIR spectra displayed distinct peaks on 700–1,300 cm−1, representing skeletal C-C vibrations. In addition, on 11,251.84 cm−1 (C-O-C stretching), 12,033.88 cm−1 (C-O), and 1,033.88 cm−1 (C-H bending), as depicted in Figure 1. Gelucire 44/14, on the other hand, exhibited typical peaks on 875.71 cm−1 (C-H bending), 1,159.26 cm−1 (C-O), 1,734.06 cm−1 (C=O stretching). FTIR analysis of physical combination, the typical peaks of both the drug and lipid were observed, indicating minimal variation in wave numbers. Based on the findings of the FTIR studies, it can be concluded that the drug is compatible with gelucire.
Critical micellar concentration
The concentration of polymers used is critical for the formation of micelles. To determine the CMC values of gelucire 44/14 and 50/13, the absorbance intensity of I2 was closely examined. The significant increase in absorbance intensity implies the presence of micelle formation. As depicted in Figure 2, the CMC values for gelucire 44/14 and 50/13 are 5.88 ± 0.02 and 7.99 ± 0.01 mg/ml, respectively. During the preparation of micelles, the amount of polymer used should be above its CMC value. According to reports, the stability of micellar solutions is improved after dilution by utilizing polymers that possess low CMC values [26,38]. Hence, gelucire 44/14 and 50/13 in the concentration above their CMC value were screened in the current investigation for micelle formulation.
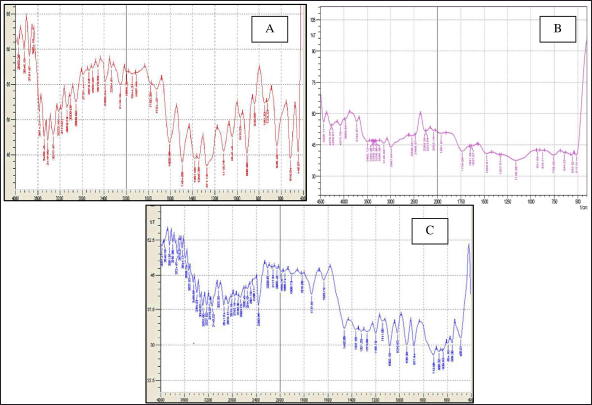 | Figure 1. FTIR scans of A. BERB, B. Gelucire 44/14, C. Physical mixture of drug and gelucire. [Click here to view] |
Particle size and poly dispersity index
Developed BERB nanomicelles size are found from 69 ± 1.57 to 576 ± 1.73 nm indicated in Table 3. Micelle size was observed to be decreased at high gelucire concentrations and stirring speed indicated a strong effect of polymer concentration on prepared nanomicelles. The compaction of micelles and the decrease in surface free energy may be attributed to the hydrophobic segment of gelucire residing within the micellar core [39,40]. PDI values range from 0.1 ± 0.001 to 0.25 ± 0.005 indicating homogenous nano micelle size distributions. Colloidal systems should preferably have a zeta value from −30 to +30 mV. Zeta value for formulations of BERB-loaded nanomicelles ranges from −12.72 ± 0.05 to 10.20 ± 0.03 mV indicating the stability of the prepared system.
Entrapment efficiency
EE is the ratio of actual drug mass included in nanomicelles to original drug loading. The EE of prepared batches ranged from 61.84% ± 1.96% to 87.34% ± 1.88 %, implying that the response is greatly influenced by the amount of polymer used in the current study. A higher gelucire 44/14 amount is thought to tip the balance between the micellar system’s attractive hydrophobic forces and repulsive forces resulting in an enhancement in EE with an increasing proportion of lipid polymer.
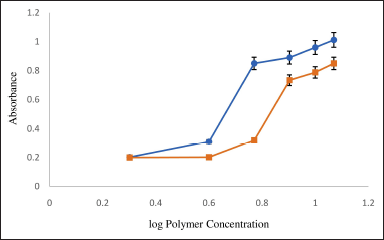 | Figure 2. Critical micellar concentration. Mean ± S.D.; n = 3. [Click here to view] |
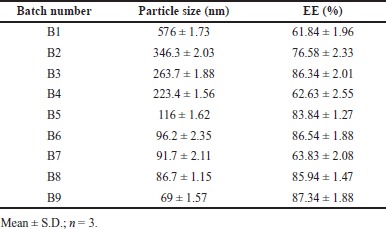 | Table 3. DOE response of particle size and EE. [Click here to view] |
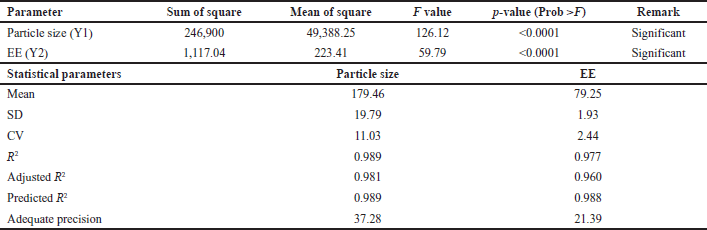 | Table 4. ANOVA data for response particle size and % EE. [Click here to view] |
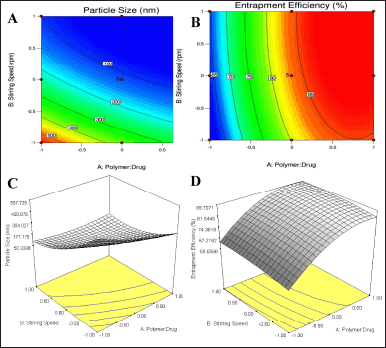 | Figure 3. Response plots showing the effect of polymer: drug ratio and stirring speed A) Contour plot particle size, B) Contour plot EE, C) 3-D surface plot particle size D) 3-D surface plot EE. [Click here to view] |
Statistical optimization
Utilizing design expert software, the quadratic models suggested in Table 4 were employed to analyze micelle size (Y1) and EE (Y2) of nine batches (B1–B9). Results indicated p < 0.0001, indicating the model’s statistical significance. The adjusted and predicted R2 were closely aligned, as presented in Table 4. Therefore, this model can effectively guide the design process as the precision attained a value greater than 4, ensuring ample signal strength. The obtained effect is fitted into the model to generate regression equations (A: Polymer: Drug ratio; B: Stirring Speed). The effects of polymer and stirring speed on particle size were found to be negative, while the regression coefficients of EE indicated a positive impact from the independent variables.
Particle size (nm) (Y1) = +117.41−77.03*A−156.43*B−38.88* A2 + 95.58*B2 + 72.40*AB
EE (%) (Y2) = +83.45 + 11.99*A + 2.06*B−7.89*A2 −1.21B2 −0.25*AB
The obtained response and contour plots of micelle size and EE were indicated in Figure 3. The obtained contour plots showed a significant effect of the independent variable on responses. A decrease in BERB nanomicelles size is observed with a higher amount of polymer: drug and stirring speed. A rise in the amount of polymer and stirring speed from level (−1) to (1), increases the EE. The obtained response plots are also correlated with the generated regression equations. When gelucire concentrations and stirring speed were high, it was found that the size of the micelles reduced, indicating the significant influence of independent variables on the prepared nanomicelles. This may be due to the compression of the micelles and the decrease in surface free energy produced by the hydrophobic component of gelucire in the micellar core with increasing stirring speed. Increased amounts of gelucire 44/14 and higher stirring speed are anticipated to bring about an equilibrium between the appealing hydrophobic forces and the repulsive forces within the micellar system. Consequently, this equilibrium is expected to enhance the EE when higher proportions of lipid polymers are utilized. The comparison between the predicted and actual values also demonstrated a significant concordance in experimental predicted values. This indicates that the utilized design space is well-suited for effective navigation.
After applying constraints to both the independent variables and dependent variables, the optimal formula was determined. The independent factors were kept in range, while the desired micelle size and EE were set to minimum and maximum values, respectively. Through the design expert software, 10 solutions were generated, 1 with a greater desirability value selected. This chosen solution is presented in Table 5. The results of numerical optimization studies indicated that the best combination was +1 level of X1 and 0 level of X2 for batch B6, illustrated in Table 5. Experimental values of formulation B6 closely aligned with the theoretical values obtained through numerical optimization, confirming it as the optimized batch in terms of micelle size and EE. Optimized BERB nanomicelles formulation (batch B6) was lyophilized using mannitol (10% w/v) as cryoprotectant by Lyophilizer (Martin Christ 2–4 LSC basic, Germany).
Saturated solubility and in-vitro dissolution study
The saturation solubility of BERB and lyophilized nanomicelles was determined in different media illustrated in Figure 4A. BERB solubility was compared to that of optimized nanomicelles batch B6. In distilled water, the solubility obtained was 0.008 mg/ml for BERB and 0.036 mg/ml for the nanomicelles. 0.1 N HCl, the solubility was 0.01 mg/ml for the pure drug and 0.051 mg/ml for the nanomicelles. PO4-3 buffer, the solubility was 0.014 mg/ml for the pure drug and 0.055 mg/ml for the nanomicelles. The release behavior of the pure drug and lyophilized BERB nanomicelles powder (batch-6) in 0.1 N HCl was assessed through an in-vitro study, as illustrated in Figure 4B. In 2 hours, the pure drug and BERB nanomicelles formulation released about 29.14% ± 1.11% and 88.17% ± 1.25%, respectively. The rate at which the pure drug dissolved was slow owing to its low inherent solubility. The solubility of the optimized formulation was found to be around 05 times greater than that of the pure drug. The employed lipid polymer’s solubilization and micellization actions may be responsible for the improvement in dissolution rate.
Transmission electron microscopy
The surface characteristics of batch B6 nanomicelles were examined using TEM as illustrated in Figure 5. TEM analysis revealed that BERB nanomicelles exhibited a spherical morphology and displayed uniform size distribution when dispersed in an aqueous solution.
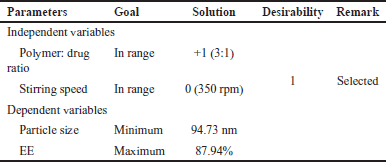 | Table 5. Summary of numerical optimization of optimized formulation (B6). [Click here to view] |
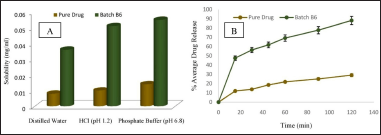 | Figure 4. A. Solubility and B. In-vitro release profile of pure drug and BERB loaded nanomicelles (Batch–B6). Mean ± S.D.; n = 3. [Click here to view] |
XRD
The BERB drug displays distinct crystalline peaks on 2θ values of 14.73°, 18.79°, 21.28°, 23.48°, 26.06°, 29.60°, and 26.06°, indicating its crystalline nature. Gelucire 44/14 also exhibits some crystallinity with typical peaks observed on 2θ values of 18.55°, 24.81°, and 26.31°. However, in the prepared nanomicelles formulation (Fig. 6), typical crystalline peaks of the drug vanish. This suggests that the drug has transformed into amorphous material within nanomicelles formulation. The XRD results further indicate that the drug is molecularly dispersed in the formulated product.
Ex-vivo absorption studies
The major objective of the development of any formulation is to achieve maximum therapeutic benefit. For the assessment of intestinal permeability, different in-vitro models have been developed. The results of in-vitro dissolution showed significant enhancement in a release from optimized nanomicelles product. Ex-vivo absorption studies of the pure drug and the optimized lyophilized BERB nanomicelles formulation were conducted to further evaluate any enhancements in permeability. In the current study, Ex-vivo absorption studies were performed by perfusion apparatus using the everted gut sac of a rat intestine. In 2 hours, the pure drug and optimized nanomicelles formulation showed 27.55% ± 1.33% and 65.72% ± 1.65% drug permeation, respectively, as shown in Figure 7A. The micellization effects of the employed lipid polymer can be linked to a 2.38 times improvement in ex-vivo drug permeability.
Anti-oxidant activity
The anti-peroxidation and antiradical activity of BERB nanomicelles (batch, B-6) was assessed to compare their enhanced antioxidant effect to that of the pure drug. The BERB nanomicelles formulation showed the highest percentage peroxidation reduction and DPPH inhibition (81.12 ± 1.21; 73.12 ± 2.07) compared to the pure drug (31.99 ± 1.32; 38.71 ± 2.07) (Fig. 7B and C). However, above a concentration of 90 μg/ml, a decline in the pure drug’s anti-peroxidation action was seen, probably as a result of a pro-oxidant mechanism. BERB nanomicelles formulation has strong dose-dependent antioxidant ability, as evidenced by their high level of DPPH radical scavenging activity and % reduction in peroxidation. The likely reason for the BERB nanomicelles formulation’s higher antioxidant capacity may be the enhancement in its overall bioavailability due to improved solubility and permeability.
 | Figure 5. TEM image of optimized nanomicelles formulation (batch B-6). [Click here to view] |
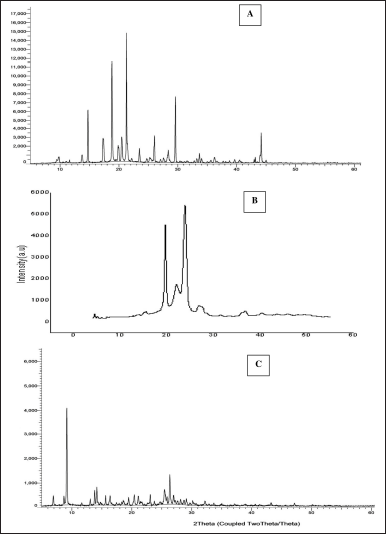 | Figure 6. X-ray diffractograms of A) Pure BERB B) Gelucire 44/14, C) BERB nanomicelles formulation. [Click here to view] |
Stability studies
The micelle size, uniformity, zeta potential, and % release analysis on 40°C/75% RH for 6 months duration are illustrated in Table 6. The prepared drug lyophilized nanomicelle formulation (batch B6) was stable since no considerable changes in the size of the micelle, PDI, zeta value, and percent drug release were found during storage.
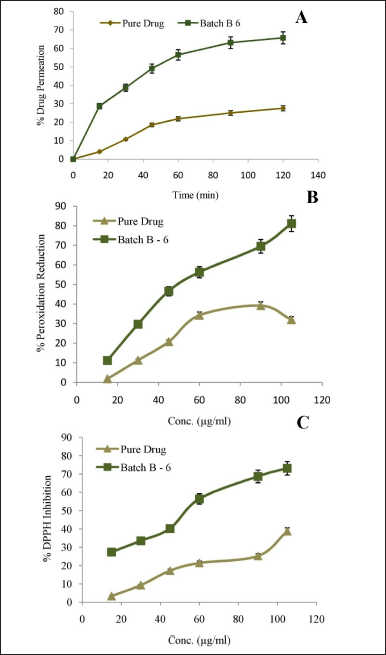 | Figure 7. A. Ex-vivo drug permeation B. % Peroxidation reduction; C. % DPPH inhibition, mean ± S.D.; n = 6. [Click here to view] |
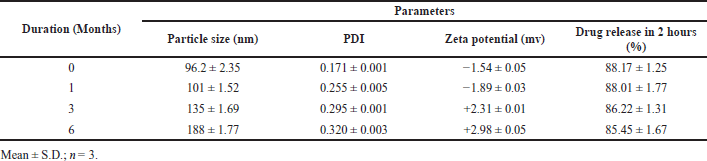 | Table 6. Accelerated stability studies batch B-6. [Click here to view] |
CONCLUSION
BERB-loaded lyophilized nanomicelles successfully prepared by thin film hydration technique along with Gelucire 44/14 as lipid polymer. Micelle size and EE of optimum BERB nanomicelles formulation ranged from 96.2 ± 2.35 nm and 86.54% ± 1.88%. The designed optimized nanomicelles system showed nearly five-fold enhancement in the solubility and three-fold enhancement in the percent release compared to pure BERB. Due to the micellization effects of the employed lipid polymer, in 2 hours the pure drug and optimized nanomicelles formulation showed 27.55% ± 1.33% and 65.72% ± 1.65% ex-vivo drug permeation, respectively. The BERB nanomicelles formulation showed 2.61 and 1.92 fold increased percentage peroxidation reduction and DPPH inhibition effect compared to pure drug indicated enhancement in antioxidant potential. The optimized batch of BERB nanomicelles exhibited a consistent and even size distribution, as observed through TEM images, which showed a spherical shape. The results of accelerated stability studies indicated the stability of prepared lyophilized nano micelle formulation. The developed method helps increase the BERB antioxidant effect by improving its solubility and permeability issues and could be used to develop formulations of other phytochemicals suffering from drawbacks of poor bioavailability.
ACKNOWLEDGMENT
The authors acknowledge the institute for their generous support in conducting this research and Gattefosse India Pvt. Ltd., Mumbai, for graciously providing a gift sample of lipid-based excipients.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJEs) requirements/guidelines.
FINANCIAL SUPPORT
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by the Institutional Animal Ethics Committee of Dr. D. Y. Patil Institute of Pharmaceutical Sciences and Research, Pune (Registration No. 198/PO/Re/S/2000/CPCSEA).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Kosalec I, Gregurek B, Kremer D, Zovko M, Sankovic K, Karlovic K. Croatian barberry (Berberis croatica Horvat): a new source of BERB—analysis and antimicrobial activity. World J Microbiol Biotechnol. 2009;25(1):145–50. CrossRef
2. Kettmann V, Kostalova D, Jantova S, Cernakova M. In-vitro cytotoxicity of BERB against HeLa and L1210 cancer cell lines. Pharmazie. 2004;59(7):548–51.
3. Jantova S, Cipak LU, Cernakova M, Alova KD. Effect of BERB on proliferation, cell cycle and apoptosis in HeLa and L1210 cells. J Pharm Pharmacol. 2003;55(8):1143–9. CrossRef
4. Shen N, Li X, Zhou T, Bilal MU, Du N, Hu Y, et al. ShensongYangxin capsule prevents diabetic myocardial fibrosis by inhibiting TGF-β1/Smadsignaling. J Ethnopharmacol. 2014;157:161–70. CrossRef
5. Rauf A, Abu-Izneid T, Khalil AA, Imran M, Shah ZA, Emran TB, et al. Berberine as a potential anticancer agent: a comprehensive review. Molecules. 2021 Dec 4;26(23):7368.
6. Babalghith AO, Al-Kuraishy HM, Al-Gareeb AI, De Waard M, Al-Hamash SM, Jean-Marc S, et al. The role of berberine in COVID-19: potential adjunct therapy. Inflammopharmacology. 2022;30(6):2003–16.
7. Lv T, Zhang C, Hu L, Wang C, Li S, Wang H, et al. Berberine in sepsis: effects, mechanisms, and therapeutic strategies. J Immunol Res. 2023;25(1):1–8. CrossRef
8. Tian CX, Li MY, Shuai XX, Jiang F, Dong YL, Gui Y, et al. Berberine plays a cardioprotective role by inhibiting macrophage Wnt5a/β-catenin pathway in the myocardium of mice after myocardial infarction. Phytother Res. 2023 Jan;37(1):50–61.
9. Behl T, Singh S, Sharma N, Zahoor I, Albarrati A, Albratty M, et al. Expatiating the pharmacological and nanotechnological aspects of the alkaloidal drug berberine: current and future trends. Molecules. 2022 Jun 9;27(12):3705.
10. Mushtaq Z, Imran M, Saeed F, Imran A, Ali SW, Shahbaz M, et al. Berberine: a comprehensive approach to combat human maladies. Int J Food Prop. 2023 Dec 31;26(1):787–807.
11. Li Z, Geng YN, Jiang JD, Kong WJ. Antioxidant and anti-inflammatory activities of berberine in the treatment of diabetes mellitus. Evid Based Complement Alternat Med. 2014;2014:289264.
12. Imenshahidi M, Hosseinzadeh H. Berberine neuroprotection and antioxidant activity. In: Martin CR, Preedy VR, editors. Oxidative stress and dietary antioxidants in neurological diseases. Cambridge, MA: Academic Press; 2020. pp. 199–216.
13. Prajwala B, Raghu N, Gopenath TS, Shanmukhappa B, Karthikeyan M, Ashok G, et al. Berberine and its pharmacology potential: a review. Eur J Biomed. 2020;7(5):115–23.
14. Cheng H, Liu J, Tan Y, Feng W, Peng C. Interactions between gut microbiota and berberine, a necessary procedure to understand the mechanisms of berberine. J Pharm Anal. 2022;12(4):541–55.
15. Maeng HJ, Yoo HJ, Kim IW, Song IS, Chung SJ, Shim CK. P-glycoprotein–mediated transport of BERB across Caco-2 cell monolayers. J Pharm Sci. 2002;91(12):2614–21. CrossRef
16. Ke Z, Zhu Z, Xu Z, Fang C, Hu S. Formulation design and in vitro evaluation of BERB-loaded self-nanoemulsifying drug delivery system. Trop J Pharm Res. 2015;14(5):747–52. CrossRef
17. Sailor G, Seth AK, Parmar G, Chauhan S, Javia A. Formulation and in vitro evaluation of BERB containing liposome optimized by 32 full factorial designs. J Appl Pharm Sci. 2015;5(7):023–8. CrossRef
18. Xu W, Ling P, Zhang T. Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water-soluble drugs. J Drug Deliv. 2013;(1):1–15. CrossRef
19. Pawar YB, Purohit H, Valicherla GR, Munjal B, Lale SV, Patel SB, et al. Novel lipid based oral formulation of curcumin: development and optimization by design of experiments approach. Int J Pharm. 2012;436(1–2):617–23. CrossRef
20. Toljan K, Ashok A, Labhasetwar V, Hussain MS. Nanotechnology in stroke: new trails with smaller scales. Biomedicines. 2023 Mar 4;11(3):780.
21. Hatamipour M, Sahebkar A, Alavizadeh SH, Dorri M, Jaafari MR. Novel nanomicelle formulation to enhance bioavailability and stability of curcuminoids. Iran J Basic Med Sci. 2019;22(3):282.CrossRef
22. Ai X, Zhong L, Niu H, He Z. Thin-film hydration preparation method and stability test of DOX-loaded disulfide-linked polyethylene glycol 5000-lysine-di-tocopherol succinate nanomicelles. Asian J Pharm Sci. 2014;9(5):244–50. CrossRef
23. Amano A, Sanjo N, Araki W, Anraku Y, Nakakido M, Matsubara E, et al. Peripheral administration of nanomicelle-encapsulated anti-Aβ oligomer fragment antibody reduces various toxic Aβ species in the brain. J Nanobiotec. 2023;21(1):36.
24. Mishra R, Dhole S. Lipid-based floating multiparticulate delivery system for bioavailability enhancement of berberine hydrochloride. J Appl Pharm Sci. 2019;9(11):036–47. CrossRef
25. GökçeKocabay Ö, Ismail O. Preparation and optimization of biodegradable self-assembled PCL-PEG-PCL nano-sized micelles for drug delivery systems. Int J Polym Mater. 2021;70(5):328–37. CrossRef
26. Prasad Kushwaha J, Baidya D, Patil S. Harmine-loaded galactosylated pluronic F68-gelucire 44/14 mixed micelles for liver targeting. Drug Dev Ind Pharm. 2019;45(8):1361–8. CrossRef
27. Fritz HF, Ortiz AC, Velaga SP, Morales JO. Preparation of a novel lipid-core micelle using a low-energy emulsification method. Drug Deliv Transl Res. 2018;8(6):1807–14. CrossRef
28. Yordanov Y, Stefanova D, Spassova I, Kovacheva D, Tzankova V, Konstantinov S, et al. Formulation of nanomicelles loaded with cannabidiol as a platform for neuroprotective therapy. Pharmaceutics. 2022;14(12):2625.
29. Devi KR, Paulraj SJ, Shah Y, Kumar SR, Kejamurthy P. Chitosan-tripolyphosphate nanoparticles encapsulated rutin targeting bacterial growth inhibition and its cytotoxicity on PANC-1 pancreatic adenocarcinoma cell. J Appl Pharm Sci. 2023;13(4):141–8. CrossRef
30. Fares AR, ElMeshad AN, Kassem MA. Enhancement of dissolution and oral bioavailability of lacidipine via pluronic P123/F127 mixed polymeric micelles: formulation, optimization using central composite design and in vivo bioavailability study. Drug Deliv. 2018;25(1):132–42. CrossRef
31. Sahibzada MU, Sadiq A, Faidah HS, Khurram M, Amin MU, Haseeb A, et al. Berberine nanoparticles with enhanced in vitro bioavailability: characterization and antimicrobial activity. Drug Des Devel Ther. 2018;12:303. CrossRef
32. Kamath PP, Rajeevan R, Maity S, Nayak Y, Narayan R, Mehta CH, et al. Development of nanostructured lipid carriers loaded caffeic acid topical cream for prevention of inflammation in wistar rat model. J Appl Pharm Sci. 2023;13(1):064–75. CrossRef
33. Kuna SK, Choppala AD. Biocompatible osimertinib nanoliposomes coated with PEGylated hyaluronic acid enhanced tumor selectivity and cytotoxicity via CD44-mediated NSCLC targeting: development, characterization and in-vitro biochemical studies. J Appl Pharm Sci. 2023;13(7):204–13. CrossRef
34. Dhome AG, Deshkar SS, Shirolkar SV. Gliclazide solid lipid nanoparticles: formulation, optimization and in vitro characterization. Pharm Reson. 2018;1(1):8–16.
35. Pryor WA, Stanley JP. Suggested mechanism for the production of malonaldehyde during the autoxidation of polyunsaturated fatty acids. Nonenzymic production of prostaglandin endoperoxides during autoxidation. J Org Chem. 1975;40:3615–617.
36. Mensor LL, Menezes FS, Leitace GG, Reis AS, Dos Santos TC, Coube CS, et al. Screening of Braziliam plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res. 2001;15:127–30.
37. Mishra RV, Dhole SN. Enhanced pharmacological efficacy of berberine hydrochloride loaded lipid based pellets for the treatment of metabolic diseases. Biomed Pharmacol J. 2021;14(2):993–1005.
38. Li G, Fan Y, Li X, Wang X, Li Y, Liu Y, et al. Preparation and evaluation of novel mixed micelles as nanocarriers for intravenous delivery of propofol. Nanoscale Res Lett. 2011;6:275.
39. Patel S, Patel A. The influence of polymers on physicochemical properties and in vitro release of erlotinib loaded nanomicelles: development and characterization. PUJHSR. 2022;1(1):5–13.
40. Zhang Q, Bao J, Duan T, Hu M, He Y, Wang J, et al. Nanomicelle-microsphere composite as a drug carrier to improve lung-targeting specificity for lung cancer. Pharmaceutics. 2022;14(3):510.