INTRODUCTION
Glottiphyllum linguiforme (L.) N.E. Br. (Syn. Mesembryanthemum linguiforme), is an ornamental, highly succulent, and rapidly growing plant of the family Aizoaceae. Its name is related to the Greek words “glotta” meaning tongue and “phyllon” meaning leaf, referring to tongue-shaped leaves [1]. It is characterized morphologically with distichous, flat, oval, ascending to erect leaves with narrowing to pustulate base, and small clumps of compact rosette flowers [2]. According to the literature, little is known about its phytochemical constituents: a phytochemical screening expected the presence of alkaloids [2], whereas an LC-MS/MS analysis revealed the occurrence of phenolics, sterols, triterpenes, in addition to miscellaneous compounds [3]. Although several Glottiphyllum plant species have been used in Indian traditional medicine for various bioactivities including anti-inflammatory and antimicrobial activities, these activities lack scientific evidence. Moreover, isolation and identification of metabolites are potential research focuses for further therapeutic studies. Therefore, the present research was designed to identify the phytochemical components as well as to quantify the total phenolic and flavonoid composition of G. linguiforme. The antioxidant, antibacterial, and cytotoxic properties of G. linguiforme ethanolic extract and isolated components were also examined to discover its medical value.
MATERIALS AND METHODS
General experimental procedures
Chromatography procedures were conducted utilizing silica gel 60, Sephadex LH-20, and polyamide-6 (Sigma-Aldrich, Germany). All solvents used were of the analytical grades. Si-TLC plates, Merck (Darmstadt, Germany), were utilized for monitoring the isolation process. A mass spectrometer [LC/Q-TOF, 6530 (Agilent Technologies, Santa Clara, CA)] was used for recording high-resolution mass (HRESIMS) spectra.
Bruker Avance III 400 MHz (Bruker AG, Switzerland) was used for recording NMR spectra and Topspin 3.1 Software (Bruker AG, Fa¨llanden, Switzerland) was used for data analysis.
The details for chromatographic and spectroscopic procedures as well as chemicals and reagents are all included in supplementary materials.
Plant material
Aerial parts of G. linguiforme were picked from the El-Hosary public garden in 6-October City, Egypt in September 2020. The plant identity was confirmed by Eng. Trease Labib, former Head of El-Orman Botanical Garden, Giza, Egypt. A specimen coded as (BUPD-79) was deposited at the Pharmacognosy Department, Faculty of Pharmacy, Beni-Suef University, Beni-Suef, Egypt.
Extraction and isolation
The succulent overground parts of G. linguiforme (15 kg) were macerated in ethanol. The macerate was filtered, and the solvent was removed under negative pressure to obtain 350 g dried extract that was used for chromatographic isolation and quantitative analysis of the metabolite content. For determining the antioxidant efficacy, the total content of the phenolic (TPC) and the flavonoid (TFC) in the total extract, a stock of the plant extract was prepared as 1 mg/ml in methanol/H2O; 80/20, v/v). For chromatographic isolation, the dried extract was suspended in H2O and sequentially partitioned between water and solvents with variable polarities (n-hexane, CH2Cl2, EtOAc, and n-BuOH), and the obtained solvent fractions were dried under reduced pressure. The n-hexane soluble part (2.5 g) was exposed to gradient chromatography on a silica gel column (38 × 3 cm, i.d.) started with n-hexane and followed by 5% increments of EtOAc. The eluate with EtOAc/n-hexane (10:90, v/v) afforded β-sitosterol (1, 5 mg). The dichloromethane fraction (3 g) was similarly chromatographed on a silica gel column (38 × 3 cm, i.d.) using gradient elution started with CH2Cl2, then 5% increments of MeOH, and afforded β-sitosterol-3-O-β-D-glucopyranoside (2, 4 mg) in the MeOH/CH2Cl2 (20:80, v/v) eluate. The n-butanol fraction (5 g) was submitted to a polyamide-6 column (80 × 2.8 cm, i.d.) using MeOH/H2O gradients to obtain two main fractions (B-I and B-II). The B-I (0.6 g), eluted with MeOH/H2O (95:5, v/v), was purified on Sephadex LH-20 column (0.8 × 10 cm, i.d.) using 100% MeOH to obtain 1,1′ biuracil (3, 5 mg). The fraction B-II (1 g), eluted with MeOH/H2O (90:10, v/v), was chromatographed on a silica gel column (46 × 2 cm, i.d.) using a series of CH2Cl2/MeOH gradients, to obtain main sub-fraction (40 mg), eluted with 20:80 v/v MeOH/CH2Cl2). Purification on Sephadex LH-20 column (0.8 × 10 cm, i.d.) with MeOH afforded compound 4 (15 mg).
Spectroscopic data of isolated compounds
β-sitosterol (1)
Key1H NMR (400 MHz, CDCl3) signals; δH: 5.36 (d, J = 8 Hz, H-6), 3.52 (m, H-3), 1.01 (CH3-19), 0.93 (CH3-21), 0.84 (CH3-26), 0.83 (CH3-27), 0.81 (CH3-29), and 0.68 (CH3-18); DEPT-Q NMR (101 MHz, CDCl3) δC: 12.04, 19.5, 18.9, 20.0, 19.2, 23.3, and 12.3 (CH3-18, 19, 21, 26, 27, 28, and 29); δC: 37.2, 31.6, 42.2, 31.9, 36.5, 21.06, 24.4, 28.9, 36.1, 33.9, and 26.2 (CH2-1, 2, 4, 7, 10, 11, 15, 16, 20, 22, and 23); δC: 71.8, 31.9, 50.1, 39.7, 42.19, 56.8, 55.9, 45.8, and 29.3 (CH-3, 8, 9, 12, 13, 14, 17, 24, and 25, respectively); and δC: 140.7 and 121.7, olefinic carbons (CH-5 and 6, respectively).
β-sitosterol-3-O-β-D-glucopyranoside (2)
Key1H NMR (400 MHz, Pyridine-d5) signals δH: 5.37 (t, J = 2.4 Hz, H-6), 5.07 (d, J =7.6 Hz, glucose H-1), 4.6-3.99 (6H, glucose H-2-H2-6), 3.96 (m, H-3), 1.01 (CH3-19), 0.95 (CH3-21), 0.93 (CH3-26), 0.91 (CH3-27), 0.87 (CH3-29), and 0.68 (CH3-18). DEPT-Q NMR (101 MHz, Pyridine-d5) δC: 12.3, 19.5, 19.3, 20.3, 19.7, and 12.5 (CH3-18, 19, 21, 26, 27, and 29, respectively). δC: 37.8, 30.6, 39.7, 32.5, 32.4, 21.6, 40.3, 24.8, 28.9, 36.7, 34.5, 26.7, 23.7, and 63.2 (CH2-1, 2, 4, 7, 8, 11, 12, 15, 16, 20, 22, 23, 28, and 6′, respectively); δC: 78.4, 50.01, 37.2, 42.8, 57.1, 56.6, 46.4, 29.8, 102.9, 75.7, 78.9, 72.0, and 78.8 (CH-3, 9, 10, 13, 14, 17, 24, 25, 1′, 2′, 3′, 4′, and 5´, respectively); and δC: 141.2 and 122.2 olefinic C-5 and C-6, respectively).
2.4.3. 1,1′ Biuracil (3): 1H NMR [400 MHz, dimethyl sulfoxide (DMSO-d6)] δH: 11.00 (2H, br s, H-3/3′), 7.38 (2H, d, J = 7.6 Hz, H-5/5′), 5.44 (2H, d, J = 7.6 Hz, H-6/6′).
Tectoionyl A -3-O- sodium sulfate (4)
Amorphous solid, UV (MeOH) λmax 284 (sh), 221 nm. HRESIMS m/z 307.1192 [M−Na]−, m/z 637.22568 [2M-Na]− (calc. for C26H46O12S2Na), NMR data (Table 1).
Barium chloride test of compound 4
Compound 4 was subjected to acid hydrolysis (mild conditions) using aq. 0.1 N HCl at 100°C for 2 minutes. The aqueous hydrolysate gave a white precipitate upon the addition of a few drops of aqueous BaCl2 [4].
Determination of TPC of G. linguiforme
The total phenolic was determined using the Folin–Ciocalteu’s mixture as reported by Jimoh et al. [5] with brief changes. Briefly, 0.5 ml (1 mg/ml) of the ethanolic extract was poured into 2.25 ml of Folin–Ciocalteu’s reagent (10% aqueous solution) and kept at the ordinary room temperature for 5 minutes. To this mixture, a solution (2.25 ml, 7.5%, w/v) of the sodium carbonate was then added. After 30 minutes at room temperature, absorbance was measured at a wavelength of 725 nm using a UV spectrophotometer. The standard, gallic acid (1 mg/ml in 80% methanol) was prepared and was then diluted to give a series of concentrations (ranging from 0.025 to 0.4 mg/ml). Results were uttered as mg gallic acid equivalents (GAE) in 1 g of dried extract (mg GAE/g) from the calibration curve y = 4.7551x + 0.1645, R² = 0.9575 following the equation C = cV/M, while c is the concentration established from the calibration curve of the stander, V is the extract volume (in ml), and M is the utilized extract mass (in g).
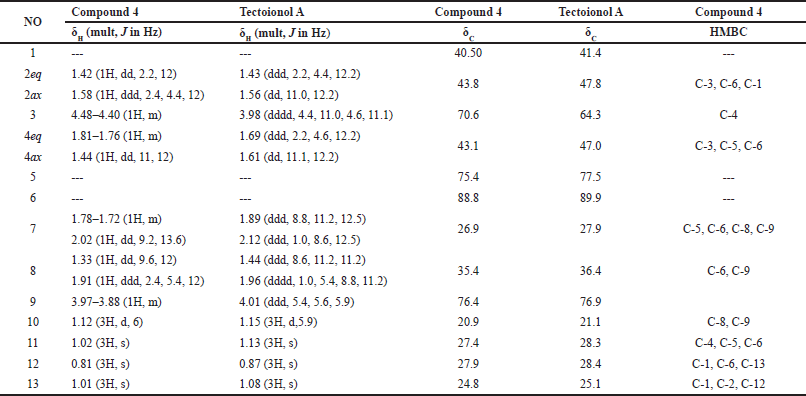 | Table 1. 1H NMR (400 MHz, DMSO-d6), 13C NMR, and HMBC spectroscopic data of compound 4 and tectoionol A. [Click here to view] |
Determination of TFC of G. linguiforme
The flavonoid content was estimated using the modified colorimetric technique described by Bakar et al. [6]. Briefly, 0.5 ml (1 mg/ml) of the extract was added into 2.25 ml of distilled H2O, then 0.15 ml NaNO2 (5%) solution. After 6 minutes, 0.3 ml of the AlCl3.6H2O solution (10%) was added and kept for 5 minutes. Finally, 1.0 ml of a NaOH solution (1 M) was added and mixed well with vortex. The absorbance was measured at 510 nm using a spectrophotometer. The standard used was rutin (initial concentration of 1 mg/ml in 80% methanol), followed by dilution into a series of diluted concentrations (ranging from 0.025 to 0.4 mg/ml). Total flavonoid content was estimated as mg rutin equivalents (REs) in 1 g of dried extract (mg RE/g) from the calibration curve y = 3.9439x + 0.1857, R² = 0.9716 using the formula C = cV/M as mentioned before [6].
Determination of the antioxidant activity of G. linguiforme
The antioxidant activity was estimated using the DPPH free radical scavenging activity following published methods [5,6]. A solution (0.5 mm of 2, 2-diphenyl-1-picrylhydrazyl was prepared in a dark bottle using absolute ethanol. DPPH solution was mixed with tested samples and serially diluted concentrations (0.0125-0.2 mg/ml) of the standard ascorbic acid. The mixture was vortexed and incubated for 30 minutes at the ordinary temperature. The absorbance was read at 517 nm. The antioxidant activity was estimated from the relation: % Scavenging effect = [1 – (sample absorbance/control absorbance)] × 100.
A standard curve including plotting of the % of free radical scavenging activity of ascorbic acid versus its concentration was prepared. The final result was expressed as mg ascorbic acid equivalent antioxidant capacity (AEAC) in 1 g of extract (mg AEAC/g). The calibration curve y = 298.74x + 11.988, R2 = 0.9879 was employed for calculating the activity [5,6].
Antimicrobial activity of the compounds 1–4
The antimicrobial potential of the isolated compounds was determined using the agar well diffusion assay, as described earlier [7,8]. The isolated compounds were examined for their antimicrobial activity against the bacterial species, Enterococcus faecalis (V583), Listeria monocytogenes American Type Culture Collection (ATCC 7644), Salmonella enterica (ATCC 14028), Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), and fungal species, Candida albicans (ATCC 60193). The different microbial strains were grown in sterile saline until they reached a turbidity of 0.5 McFarland. A cotton swab was used to streak the strains on the surface of Mueller–Hinton agar and a sterile borer was used to produce five 10 mm cups in each pre-inoculated plate. Following that, 100 μl of each compound was applied to the wells of different indicator strains at a 2 mg/ml concentration in DMSO. After that, the agar plates were held at 4°C for 100 minutes before being incubated for microbial growth. After 24-hour incubation, the inhibitory zones surrounding each well were measured and the antimicrobial activity of the tested compounds was recorded accordingly. Ciprofloxacin and nystatin were utilized as standard antibacterial and antifungal agents, respectively, while DMSO was used as a negative control.
Cytotoxicity of isolated compounds
Cell culture
Human breast adenocarcinoma (MCF7), human hepatocellular carcinoma (HepG-2) cell lines, and normal African Green Monkey kidney (Vero) cells were purchased from (ATCC, Manassas, USA) and cultivated on Roswell Park Memorial Institute Medium (RPMI 1640) enriched with 1% of 100 mg/ml streptomycin, 100 units/ml of penicillin, and 10% of fetal bovine serum (heat-inactivated) in a humidified atmosphere of 5% (v/v) CO2 at 37°C.
Cytotoxicity assay
In vitro, cytotoxicity was conducted using 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide (MTT). Doxorubicin was used as a standard cytotoxic agent. Cells from different cell lines, in the exponential growth phase, were trypsinized, counted, and seeded into 96-well microtiter plates at a density of 5,000 cells/0.33 cm3. In a humidified atmosphere, the cells were incubated for 24 hours at 37°C. The cytotoxicity of the pure compounds (1-4) was tested at the concentrations (0.01, 0.1, 1, 10, and 100 μg/ml) for 48 hours. The cell viability was determined using the following MTT protocol: After removal of the media, the cells were incubated in 5% MTT solution/well (200 μl) for 2 hours, where the dye was metabolized into colored formazan crystals. The latter crystals were solubilized at room temperature in acidified isopropanol (200 μl) for 30 minutes, where the plate was covered with aluminum foil, and shaken continuously by a Max Q 2000 plate shaker (Thermo Fisher Scientific Inc, MI). An Epoch-2 C plate reader (Bio Tek, Canada) was used for measuring the absorbance (at 570 nm). The cell viability percentage of the control was calculated [9,10].
Statistical analysis
All experiments were conducted in triplicates, and the data were recorded as mean ± SE adopting MS Office Excel 2010.
RESULTS AND DISCUSSION
Structural determination of isolated compounds
Chromatographic fractionation of the n-hexane and CH2Cl2 fractions of G. linguiforme afforded β-sitosterol (1) and its glucoside derivative; β-sitosterol-3-O-β-D-glucopyranoside (2), respectively. Both compounds were identified by comparing their 1H and 13C NMR data (Figure S1–S4) with those reported in the literature [11,12]. Compound 3 was purified from the n-butanol fraction and its structure was identified as 1,1′ biuracil by comparing its 1H NMR spectroscopic data (Figure S5) with the literature [13] as well as by co-TLC with reference standard.
Compound 4 was obtained as an amorphous solid from the n-butanol fraction. Its structure was determined from spectroscopic data and the comparison with literature data. The 13C NMR spectrum showed three quaternary carbons (δC 40.5, 75.4, and 88.8), two tertiary carbons [δC 70.6/(δH 4.44) and 76.4/(δH 3.92)], four methylene carbons (δC 26.9/(δH 1.75, 2.02), 35.4/(δH 1.33, 1.93), 43.1/(δH 1.45, 1.79), and 43.8/(δH 1.42, 1.58)), and four methyl carbons (δC 20.9/(δH 1.12), 24.8/(δH 1.01), 27.4/(δH 1.02), and 27.9/(δH 0.81)) (Figure S7–S12), characteristic of bisnorsesquiterpene with an ionane skeleton [14,15]. Reviewing literature spectral data revealed that compound 4 shares the same planar structure with tectoionol A (Table 1), which was isolated from Tectona grandis [14,15]; however, proton and carbon chemical shifts at position 3 (δH 4.44 ppm, 70.6 ppm) were significantly shifted to lower field compared to tectoionol A [δH 4.480–4.399 (1H, m), δC 64.3, Table 1). The negative mode HRESIMS spectrum of 4 exhibited a molecular ion peak at m/z 307.1192, which is an 80 mass units, corresponds to SO3, more than the deprotonated molecular ion peak of tectoionol A (m/z 227.1649) [14]. Thus, compound 4 was suggested as a 3-O-sulphate ester of tectoionol A, which also explains the low field shift of the proton and carbon signal of position 3. The sulfate group was further confirmed by the appearance of a white precipitate when acid hydrolysate of 4 reacted with BaCl2 test solution (experimental section). The negative mode HRESIMS spectrum of 4 (Figure S6) also exhibited an ion peak (m/z 637.22568) equivalent to a dimer of the sulfated form of 4 in addition to a Na atom, which indicates the occurrence of 4 as sodium sulfate rather than hydrogen sulfate. Consistently, the negative ion is thus produced due to a loss of sodium atom from one molecule (found for C26H46O12S2Na m/z 637.22568 [2M–Na]−, calc. for C26H46O12S2Na, m/z 637.2328). The planer structure of 4 was further confirmed by the HMBC correlations (Figure 1B and Table 1). Finally, the relative configuration of compound 4 was confirmed based on key NOESY correlations among H-3/H3-13/H3-11/H3-10, in addition to other NOESY correlation (Figures 1C and S13), which are also the same NOESY correlations reported for tectoionol A [14]. Therefore, compound 4 was concluded as tectoionyl A 3-O-sodium sulfate (Figure 1A), which is isolated herein for the first time.
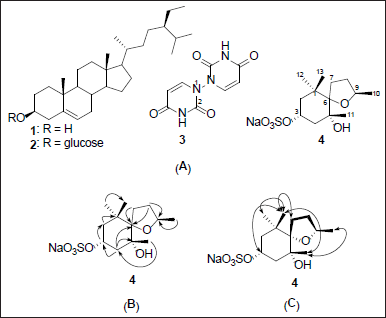 | Figure 1. Structures of compounds 1–4 isolated from G. linguiforme (A), key HMBC correlations (B), and key NOESY correlations (C) of compound 4. [Click here to view] |
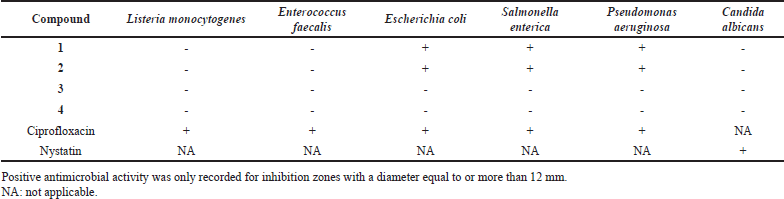 | Table 2. Result of the antimicrobial activity screening of the compounds 1–4. [Click here to view] |
Glottiphyllum linguiforme metabolite analysis by LC-MS indicated the presence of further sulfate derivatives, suggesting its importance as a chemical marker for G. linguiforme [3].
Total phenolic and flavonoid contents, and antioxidant activity
Folin–Ciocalteu’s reagent and AlCl3 were used to determine TPC and TFC, respectively, in the ethanolic extract of G. linguiforme. The TPC and TFC were quantified as 18.25 ± 1.1 mg GAE/g dry extract and 25 ± 0.1 mg RE/g dry extract, respectively.
The ability of the ethanolic extract to scavenge DPPH free radicals was assessed. The results revealed a moderate antioxidant capacity of the extract calculated as 0.026 ± 0.0001 mg AEAC/g dry extract (ascorbic acid IC50 = 0.127 mg/ml).
According to previous reports, Mesembryanthemum species, an old genus name of many plants recently listed under the Glottiphyllum genus [16], showed great inter-species variability in TPC, TFC, and antioxidant capacities. Mesembryanthemum forsskaolii, Mesembryanthemum crystallinum, and Mesembryanthemum nodiflorum showed low TPC and TFC contents, and low antioxidant activity [17–22]. However, M. edule exhibited higher TPC and TFC contents, and a higher antioxidant activity [23,24]. TPC and TFC findings of G. linguiforme may not strongly support plant use as a source of functional phenolics, flavonoids, or as a significant antioxidant. Although, these findings are useful in plant scientific validation.
Antimicrobial activity
In vitro investigation of the antibacterial and antifungal activity of the isolated compounds showed that β-sitosterol (1) and β-sitosterol-3-O-β-D-glucopyranoside (2) displayed antibacterial activity against the Gram-negative organisms: E. coli, S. enterica, and P. aeruginosa with obvious inhibitory zones (~12–23 mm, Table 2). The Clinical and Laboratory Standards Institute determines the minimum diameter of the zone of inhibition that is deemed clinically significant (the cut-off value) based on the type of microorganism being tested and the antimicrobial agent being used. For example, the recommended cut-off value for the zone of inhibition of antibiotics used to treat urinary tract infections caused by Escherichia coli is 12 mm [25]. As a result, the cut-off value for the compound to be regarded as significantly active was set at the 12 mm inhibition zone. These results were consistent with previous reports for β-sitosterol-3-O-β-D-glucopyranoside [26,27]. Furthermore, the results of the antimicrobial of a combination of the β-sitosterol-3-O-D-glucopyranoside with quinolones, cephalosporins, and aminoglycosides against P. aeruginosa and E. coli strains showed a significant decrease in minimum inhibitory concentrations and a significant increase in the activity of these antibiotics [28]. β-Sitosterol (1) isolated from Momordica charantia showed high antibacterial activity against E. coli and P. aeruginosa at lower concentrations [29]. Compounds 3 and 4 did not show any antimicrobial activity up to 2 mg/ml against different indicator strains.
Cytotoxic activity
The compounds 1–4 cytotoxicity against MCF7 and HepG2 cancer cell lines and the normal Vero cells showed low effects (15%–30% growth inhibition against both cancer cell lines and normal (Vero) cells at the examined concentration (100 μg/ml) (Figure 2).
These results come in agreement with the previously published data for compound β-sitosterol-3-O-β-D-glucopyranoside (2), which showed low cytotoxicity on HepG2 cell line (IC50 = 251 μg ml−1) [30] and compound β-sitosterol (1) that showed moderate inhibition of MCF-7 cells and no cytotoxicity was observed on Vero normal cells [31]. In addition, tectoionol C, structurally related to compound 4, did not show a growth inhibitory effect against the human hepatoma cell line (BEL-7402), human gastric cell line (SGC-7901), and human myeloid leukemia cell line (K562) [32]. In light of the published data, this is the first report on the cytotoxic activity of compound 1,1′-biuracil (3) on the selected cell lines that showed no cytotoxicity. Noteworthy, 1,1′-biuracil was also reported to be safe on Hs68 human fibroblasts at 10 ppm. However, these low cytotoxicity results of 3 are encouraging and shell motivate researchers to carry out more research to develop 3 as a skin protector from the UV-radiation-induced aging effects, as shown in an earlier study [13].
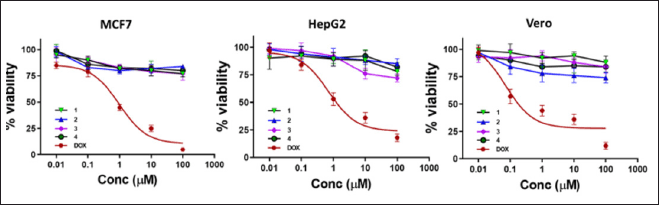 | Figure 2. Effect of compounds (1-4) on cell viability of the MCF7 and HepG2 cancer cell lines and the normal Vero cells at concentrations ranging from 0.01 to 100 μg/ml. Doxorubicin was used as a standard anticancer agent. [Click here to view] |
CONCLUSION
Our phytochemical study has led to the isolation of one new and three known compounds for the first time from leaves of G. linguiforme. The isolated compound β-sitosterol-3-O-β-D-glucopyranoside displayed selective antibacterial activity against E. coli, S. enterica, and P. aeruginosa. The compounds 1–4 showed mild cytotoxicity against MCF7 and HepG2 cancer cell lines, and the normal Vero cells at the test concentration (100 μg/ml). The compounds such as 1,1′-biuracil (3), a derivative of the RNA nitrogenous base uracil, and the sulfate derivative, tectoionyl A-3-O-sodium sulfate (4), might be important chemotaxonomic markers for verifying the identity of the plant.
ACKNOWLEDGMENTS
The authors are thankful to the Deanship of Scientific Research at Najran University, Saudi Arabia for funding this work, under the Research Groups Funding Program (Grant Code (NU/RG/MRC/12/2).
AUTHOR CONTRIBUTIONS
Conceptualization: NA, AIO, HSA, DEA, Formal analysis: NA, HAS, and HMHM investigation: NA, HSA, MS, TMA, and MAAO; resources: EA, NA, HSA, DEA, and HMHM; writing original data: NA, HSA, MS, TMA, HMHM and MAAO, writing–review and editing: EA, NA, HSA, MS, TMA, AIO, DEA, AHE, and MAAO. All authors have read and agreed to the published version of the manuscript.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article and in supporting materials.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Abd El-Raouf HS. Taxonomic significance of leaves in family Aizoaceae. Saudi J Biol Sci. 2021;28(1):512–22. CrossRef
2. Bint P. The genus Glottiphyllum, often vilified. CactusWorld. 2018;36(1):15–23.
3. Hamed AR, El-Hawary SS, Ibrahim RM, Abdelmohsen UR, El-Halawany AM. Identification of chemopreventive components from halophytes belonging to Aizoaceae and Cactaceae through LC/MS-bioassay guided approach. J Chromatogr Sci. 2021;59(7):618–26. CrossRef
4. Hussein S. Phenolic sodium sulphates of Frankenia laevis L. Pharmazie. 2004;59(4):304–8.
5. Jimoh MO, Afolayan AJ, Lewu FB. Antioxidant and phytochemical activities of Amaranthus caudatus L. harvested from different soils at various growth stages. Sci Rep. 2019;9(1):12965. CrossRef
6. Bakar MFA, Mohamed M, Rahmat A, Fry J. Phytochemicals and antioxidant activity of different parts of bambangan (Mangifera pajang) and tarap (Artocarpus odoratissimus). Food Chem. 2009;113(2):479–83. CrossRef
7. Gavin JJ. Analytical microbiology: II. The diffusion methods. Appl Microbiol. 1957;5(1):25–33. CrossRef
8. Sebak M, Saafan AE, AbdelGhani S, Bakeer W, El-Gendy AO, Espriu LC, et al. Bioassay-and metabolomics-guided screening of bioactive soil actinomycetes from the ancient city of Ihnasia, Egypt. PLoS One. 2019;14(12):e0226959. CrossRef
9. Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, et al. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48(17):4827–30.
10. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. CrossRef
11. Elwekeel AH, Amin E, Khairallah A, Moawad AS. Terminalia arjuna flowers: secondary metabolites and antifungal activity. Pharm Sci Asia. 2022;49(3):249–56. CrossRef
12. Afifi NI, Moawad AS, Hetta MH, Mohammed RM. Phytochemical composition and antioxidant activity of two species related to family Arecaceae. Pharm Sci Asia. 2022;49(1):43–50. CrossRef
13. Lee YG, Lee DG, Gwag JE, Kim M, Kim M, Kim HG, et al. A 1, 1′-biuracil from Epidermidibacterium keratini EPI-7 shows anti-aging effects on human dermal fibroblasts. Appl Biol Chem. 2019;62(1):1–6. CrossRef
14. Macías FA, Lacret R, Varela RM, Nogueiras C, Molinillo JM. Bioactive apocarotenoids from Tectona grandis. Phytochmistry. 2008;69(15):2708–15. CrossRef
15. Abe F, Yamauchi T. Megastigmanes and flavonoids from the leaves of Scorodocarpus borneensis. Phytochemistry. 1993;33(6):1499–501. CrossRef
16. Chesselet P, Cole D, Cole N, Gerbaulet M, Groen L, Ihlenfeldt HD, et al. Illustrated handbook of succulent plants: Aizoaceae FZ. Berlin, Germany: Springer Science & Business Media; 2002.
17. El-Amier YA, Al-hadithy O, Fahmy A, El-Zayat M, Suliman M. Alghanem. Phytochemical analysis and biological activities of three wild Mesembryanthemum species growing in heterogeneous habitats. J Phytol. 2021;13:1–8. CrossRef
18. Bakr, RA. Comprehensive review of the Aizoaceae family: phytochemical and biological studies. Nat Prod J. 2021;11(3):288–304. CrossRef
19. Ibtissem B, Abdelly C, Sfar S. Antioxidant and antibacterial properties of Mesembryanthemum crystallinum and Carpobrotus edulis extracts. Adv Chem Eng Sci. 2012;2(3):359–65. CrossRef
20. Arena R, Manuguerra S, Collins E, Mahdhi A, Renda G, Messina CM, et al. A. antioxidant properties of a supercritical fluid extract of the halophyte Mesembryanthemum nodiflorum L. from sicilian coasts: nutraceutical and cosmeceutical applications. Appl Sci. 2020;10(7):2374. CrossRef
21. Calvo MM, Martín-Diana AB, Rico D, López-Caballero ME, Martínez-Álvarez O. Antioxidant, antihypertensive, hypoglycaemic and nootropic activity of a polyphenolic extract from the halophyte ice plant (Mesembryanthemum crystallinum). Foods. 2021;11(11):1581. CrossRef
22. Hanen F, Riadh K, Samia O, Sylvain G, Christian M, Chedly A. Interspecific variability of antioxidant activities and phenolic composition in Mesembryanthemum genus. Food Chem Toxicol. 2009;47(9):2308–13. CrossRef
23. Falleh H, Ksouri R, Medini F, Guyot S, Abdelly C, Magné C. Antioxidant activity and phenolic composition of the medicinal and edible halophyte Mesembryanthemum edule L. Ind Crops Prod. 2011;34(1):1066–71. CrossRef
24. Falleh H, Trabelsi N, Bonenfant-Magné M, Le Floch G, Abdelly C, Magné C, et al. Polyphenol content and biological activities of Mesembryanthemum edule organs after fractionation. Ind Crops Prod. 2013;42:145–52. CrossRef
25. Piddock LJ. Techniques used for the determination of antimicrobial resistance and sensitivity in bacteria antimicrobial agents research group. J Appl Bacteriol. 1990;68(4):307–18. CrossRef
26. Marcel TB, Stephen YG, Kofi A. The antimicrobial activity of Croton membranaceus, a species used in formulations for measles in Ghana. J Pharmacogn Phytother. 2009;1(4):47–51.
27. Subramaniam,S, Keerthiraja M, Sivasubramanian A. Synergistic antibacterial action of β-sitosterol-D-glucopyranoside isolated from Desmostachya bipinnata leaves with antibiotics against common human pathogens. Rev Bras Farmacogn. 2014;24(1):44–50. CrossRef
28. de Silva JPR, da Silva Policarpo I, Chaves TP, Coutinho HDM, da Silva Alves H. A glycosylated β-Sitosterol, isolated from Tacinga inamoena (Cactaceae), enhances the antibacterial activity of conventional antibiotics. S Afr J Bot. 2020;133:193–200. CrossRef
29. Sen A, Dhavan P, Shukla KK, Singh S, Tejovathi G. Analysis of IR, NMR and antimicrobial activity of β-sitosterol isolated from Momordica charantia. Sci Secure J Biotechnol. 2012;1(1):9–13.
30. Maiyoa F, Moodley R, Singh M. Phytochemistry, cytotoxicity and apoptosis studies of β-sitosterol-3-oglucoside and β-amyrin from Prunus africana. Afr J Tradit Complement Altern Med. 2016;13(4):105–12. CrossRef
31. Park S, Hwang K, Na JR, Lee K, Jeong ES, Kim S. Triterpenoids from the leaves of Dendropanax morbifera Léveille and its cytotoxic activity toward breast MCF-7 and lung A549 cancer cells. Korean J Food Preserv. 2018;25(4):471–81. CrossRef
32. Zeng YB, Ma SS, Guo ZK, Jiang B, Mei WL, Dai HF. A new degraded sesquiterpene from the twigs of Trigonostemon lutescens. Nat Prod Commun. 2016;11(3):369–70. CrossRef
SUPPLEMENTARY MATERIAL
Supplementary data can be downloaded from the journal’s website