INTRODUCTION
Murraya koenigii is a tropical to subtropical plant belonging to the Rutaceae family. This plant is known as “Salam India” in Indonesia and has various regional names such as temurui (Aceh), koro keling (Semarang), sicerek (Minangkabau), and ki becetah (Sunda). In other countries, this plant is referred to as curry (English), garupillai (Malaysia), kerriebladeren (Dutch), feuilles de cari (French), curryblatter (German), fogli de cari (Italian), and hoja (Spanish) [1].
Taxonomy of plant
Kingdom: Plantae
Sub-kingdom: Tracheobionta
Superdivision: Spermatophyta
Division: Magnoliophyta
Class: Magnoliospida
Subclass: Rosidae
Order: Sapindales
Family: Rutaceae
Genus: Murraya
Species: Murraya koenigii
Murraya koenigii is a shrub or small tree, typically growing between 2.5 and 6 m in height [2]. This plant has a short stem with a diameter of 15–40 cm, which is grayish or brown, and lush foliage [3]. Its compound leaves are bipinnately, showcasing 11–21 leaflets, each measuring 2–4 cm in length, and 1–2 cm in width, emitting a distinctive aroma. The bisexual flowers are small, fragrant, and white, while the small egg-shaped fruits have a length of 1.4–1.6 cm and a diameter of 1–1.2 cm, turning purplish–black when ripe [4]. The seeds are approximately 11 mm in length, and 8 mm in diameter, with a weight of about 445 mg [5]. A visual representation of M. koenigii plant is presented in Figure 1.
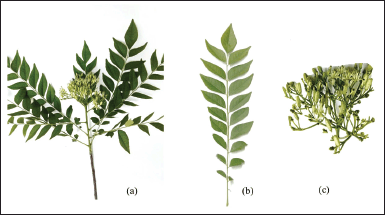 | Figure 1. Murraya koenigii (L.) Spreng. (a) Plant. (b) Leaves. (c) Flower. [Click here to view] |
Murraya koenigii is known for its aromatic leaves used in culinary spice. The most important chemical compounds responsible for its strong distinctive aroma are p-gurjunene, p-caryophyllene, p-elemene, and O-phellandrene. This plant is also high in carbazole alkaloids such as mahanimbine, murrayanine, murrayacine, girinimbine, isomurrayazoline, mahanine, koenine, koenigine, koenidine, koenimbine [6,7], O-methylmahanine, O-methylmurrayamine, isomahanine, bismahanine, and bispyrayafoline [8]. Traditionally, this plant has been used for its stimulant, stomachic, antipyretic, analgesic, and medicinal properties in the treatment of diarrhea, dysentery, and insect bites, as well as its anti-inflammatory and antidepressant effects [9]. Topically applying a paste made from fresh leaves is known to neutralize toxins from animal bites [10].
METHODS
A comprehensive literature search for scientific studies published in electronic databases was conducted. This study reviewed more than 200 scientific papers from various international sources, including PubMed [www.ncbi.nlm.nih.gov/pubmed/], Google Scholar [https://scholar.google.com.pk/], and Scopus [www.scopus.com]. Subsequently, the studies were electronically searched from 1965 to 2023, and only 120 papers were found suitable for this review. The following keywords were used to search the databases: “Murraya koenigii,” “Chemical Composition,” “pharmacological effects,” and “toxicity.” All chemical structures were visualized using ChemDraw Ultra 18.0 software.
RESULTS AND DISCUSSION
Chemical composition
Murraya koenigii is a plant rich in various chemical compounds obtained from extracts using solvents such as petroleum ether, ethyl acetate, chloroform, and ethanol-water. To date, various compounds found including alkaloids, phenylpropanoids, alkanes, sesquiterpenes, flavonoids, and other compound groups.
Alkaloid
Murraya koenigii is rich in alkaloid content, which are the primary components contained in its compounds (summarized in Table 1 and Fig. 2). In recent years, studies have discovered that alkaloids found in this plant play an important role in anti-inflammatory and analgesic effects [11,12], antidiabetic properties [13], anticancer [14], hepatoprotective effects [15], and antimicrobial properties [9].
Fenilpropanoid
Phenylpropanoid compounds isolated are summarized in Table 2 and Figure 3, respectively. Subsequently, Ma et al. [43] successfully isolated five phenylpropanoid compounds, while Srivastava and Srivastava [44] isolated two phenylpropanoid compounds.
Alkanes
Ma et al. [45], successfully isolated four alkane compounds, which include (3S,4E,6E,10R)-2,10-dihydroxy-2-hydroxy-2-methylethyl-6,10-di-methyl-4,6,11-sencolaninic-3-b-D-glucopyranoside, (3R,5S,6E, 8S,10E)-3,7,11-trimethyl-1,6,10-dodecatriene-3,5,8-triol, 5S,6R,7S,8R)-5-amino-(2Z,4Z)-1,2,3-trihydroxybuta 2,4-dienyloxy-pentane-6,7,8,9-tetraol, (3E,6S,7E,9R,10S,11S,17R)-octadeca-3,7-diene-6,9,10,11,17-pentaol (presented in Table 3 and Fig. 4).
Terpenoid
Terpenoids are the most diverse and largest class of chemical compounds found in various plant sources. In addition, they are also known as terpenes or isoprenoids, and most plant-derived terpenoids are used by humans in the pharmaceutical, food, and chemical industries. The terpenoids successfully isolated are summarized in Table 4 and Figure 5.
Flavonoid
Flavonoids are important compounds in natural products. Flavonoids are also found in M. koenigii, such as quercetin, apigenin, and kaempferol, which can be seen in Table 5 and Figure 6.
Other compounds
Furthermore, M. koenigii also contains other compounds such as coumarins (heraclenin, imperatorin), ketones (iso menthone, Z-jasmone), xanthophyll (Lutein), acetate esters (linalyl acetate, lavandulyl acetate, myrtenyl acetate, neryl acetate, and geranyl acetate), alcohols (menthol, tocopherol), and carboxylic acid (nicotinic acid), which are summarized in Table 6 and Figure 7.
Pharmacological effects of M. koenigii
Currently, a study of the pharmacological activities of M. koenigii indicates that its extracts and chemical components exhibit a wide range of biological activities. These activities include anti-inflammatory and analgesic effects, hemostatic coagulation, antibacterial activity, antioxidant effects, anti-tumor effects, and more. Based on this study, pharmacological effects primarily focused on alcohol, water, and methanol extracts, among others. The active compounds isolated from these extracts and their associated pharmacological effects are summarized in Figure 8. The following activities will be detailed.
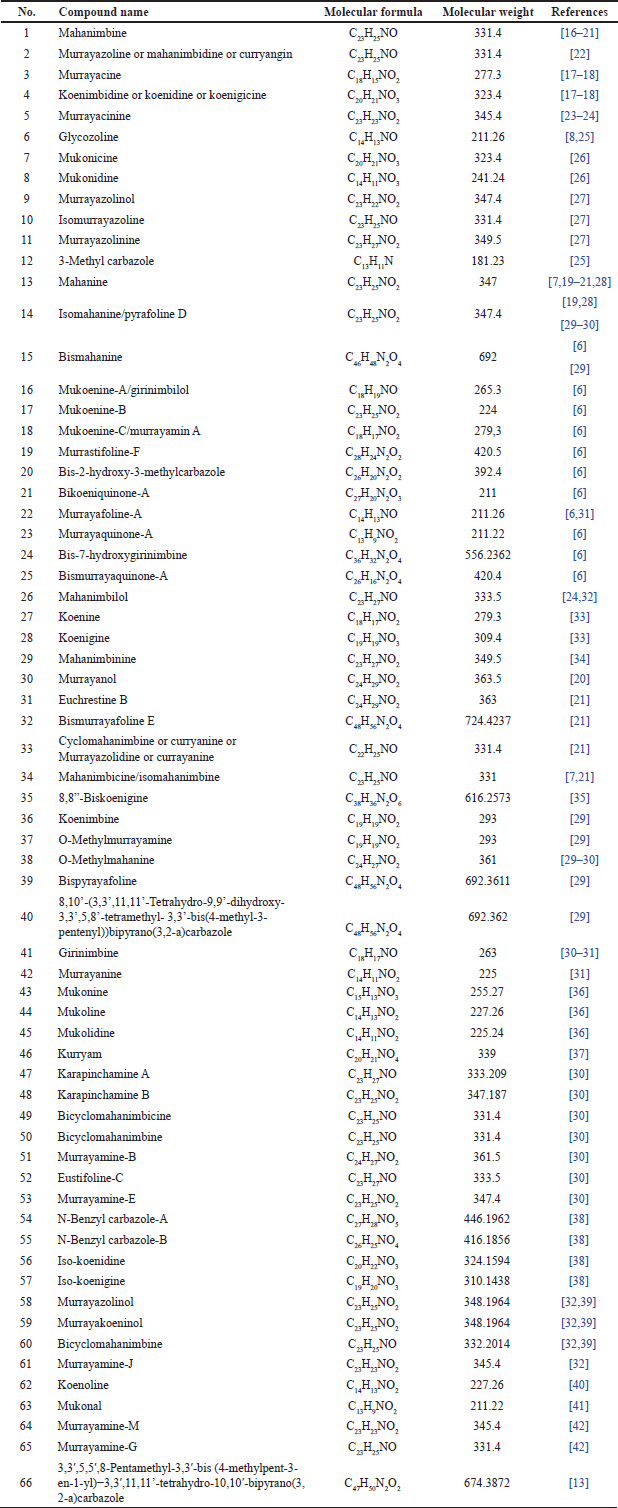 | Table 1. Alkaloids reported from M. koenigii. [Click here to view] |
Anti-inflammatory and analgesic effects
Ethanol extract of M. koenigii leaf has shown significant anti-inflammatory and analgesic activities when tested using the carrageenan-induced paw edema method in rats [62,63], as well as methanol extracts [64], and others. Furthermore, ethanol extracts (300 and 400 mg/kg) also act as antihistamines and can stabilize mast cells [65]. Mani et al. [66] further showed that M. koenigii leaf extracts effectively relieve pain induced by intraperitoneal acetic acid and subplantar formalin injection in rats.
Methanol extracts from M. koenigii have demonstrated the ability to inhibit glutamate-induced pain, with their antinociceptive mechanisms including adenosina trifosfat-sensitive K+ channels [67]. In another study by Nalli et al. [12], compounds such as murrayakonine A, O-methylmurrayamine A, and mukolidine isolated from M. koenigii leaf and stems were found to inhibit the release of inflammatory mediators tumor necrosis factor alpha (TNF-α) and interleukin (IL)-6. Furthermore, girinimbine exhibited anti-inflammatory activity tested on RAW 264.7 cells induced by lipopolysaccharides by reducing nitric oxide (NO) levels and proinflammatory cytokines IL-1β and TNF-α [11]. Husna et al. [68] also proved that ethanol extracts from the leaves pose anti-inflammatory and hepatoprotective activities and play a role in regenerating damaged pancreatic islets.
Antidiabetic
Regarding its antidiabetic properties, studies have shown that feeding rats M. koenigii leaf diets leads to hypoglycemic and antihyperglycemic effects [69]. Ethanol extracts of M. koenigii have also been shown to significantly reduce blood glucose levels [70], with the hypoglycemic effect attributed to their antioxidant properties and insulin-mimetic effects. In addition, M. koenigii exhibits profound antioxidant effects by reducing levels of malondialdehyde, increasing high levels of glutathione, and significantly lowering the homeostatic model assessment-insulin resistance index [71,72].
Oral administration of M. koenigii leaf water extracts has been shown to reduce blood glucose levels in diabetic rats and alloxan-induced diabetic rabbits [73–78]. Chloroform leaf extracts also exhibit antidiabetic activity at doses of 250 and 500 mg/kg body weight in alloxan-induced diabetic rats [79]. In addition, the juice of M. koenigii fruit also lowers blood glucose levels in diabetic rats [80].
Mahanimbine was isolated from M. koenigii using dry petroleum ether extract column chromatography. The antidiabetic activity was conducted on Wistar rats induced with streptozotocin using pure compound at doses of 50 and 100 mg/kg. The results showed the presence of antidiabetic and antilipidemic activities of mahanimbine [47]. In addition, koenidine showed a significant decrease in postprandial blood glucose levels with increased insulin sensitivity in type 2 diabetic rats [81].
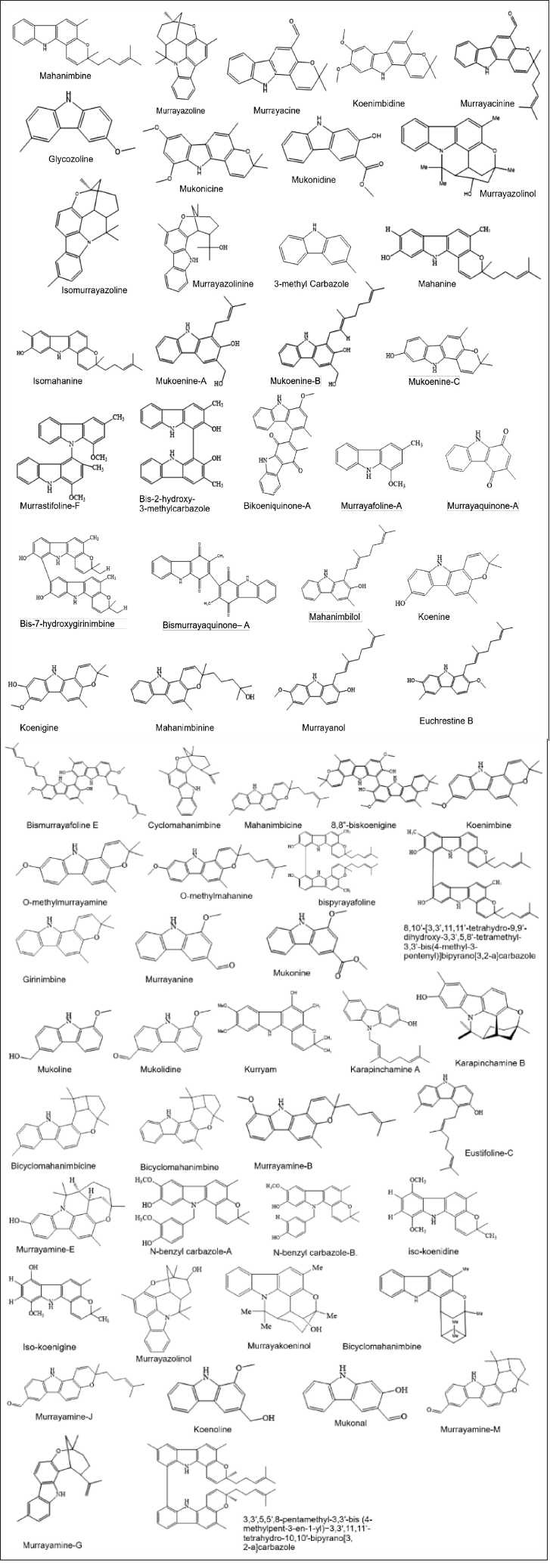 | Figure 2. Structures of alkaloids from M. koenigii (L.) Spreng. [Click here to view] |
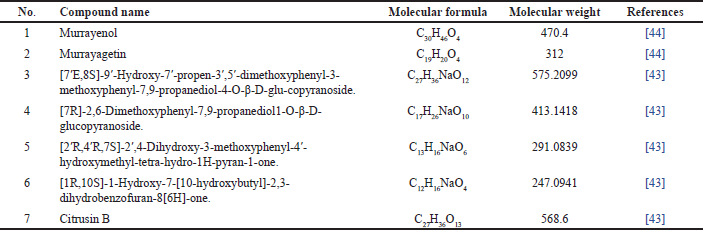 | Table 2. Fenilpropanoid reported from M. koenigii. [Click here to view] |
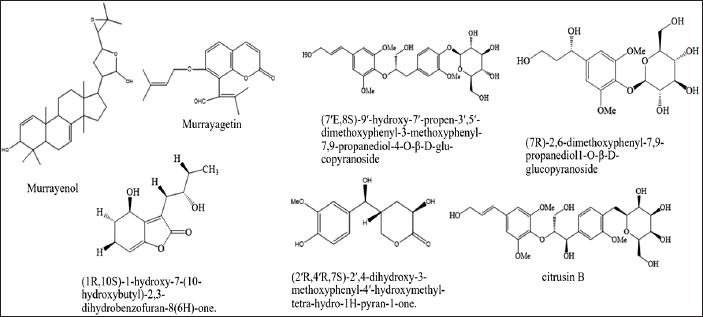 | Figure 3. Structures of fenilpropanoid from M. koenigii (L.) Spreng. [Click here to view] |
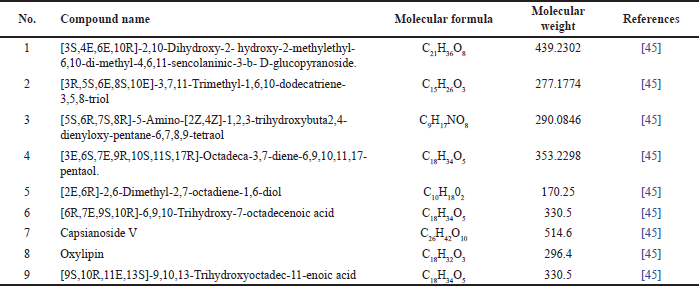 | Table 3. Alkanes reported from M. koenigii (L.) Spreng. [Click here to view] |
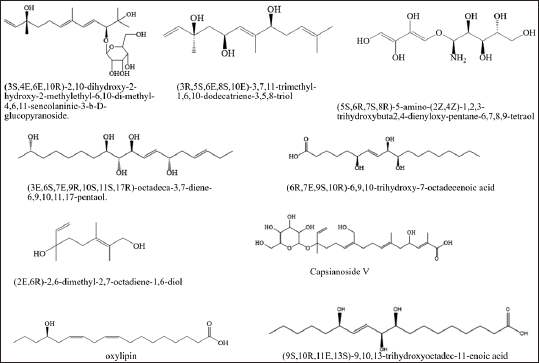 | Figure 4. Structures of alkana from M. koenigii (L.) Spreng. [Click here to view] |
In clinical trials, the administration of M. koenigii leaf powder showed a significant difference in the average fasting and postprandial blood glucose levels in diabetic patients [82]. Sampath et al. [13] demonstrated that a compound derived from M. koenigii, known as 3,3′,5,5′,8-pentamethyl-3,3′-bis(4-methylpent-3-en-1-yl)-3,3′,11,11’-tetrahydro-10,10′-bipyrano (3,2-a) carbazole exhibited in vitro inhibition activity against α-amylase and α-glucosidase, showcasing its potential as an antidiabetic agent. Furthermore, the findings showed that this compound has antidiabetic activity. Its extract exhibited an antihyperglycemic effect by improving key enzymes on carbohydrate metabolism and increased glucose transporter-4 expression against hyperglycemic rats [83]. A 1-month trial of supplementing with 12 g of M. koenigii leaf powder (providing 2.5 g of fiber) was conducted in 30 patients with noninsulin-dependent diabetes mellitus. The findings revealed a temporary decrease in fasting and postprandial blood sugar levels after 15 days, with no significant alterations observed in glycosylated protein levels, glycosylated low-density lipoprotein cholesterol fraction, serum lipids, lipoprotein cholesterol levels, uronic acid, and total amino acids throughout the supplementation period, either at 15 or 30 days [84].
Anticancer effect
Regarding its anticancer properties, M. koenigii leaf has shown potential as an anticancer agent against HeLa cells [85], and colorectal cancer [86]. In addition, the methanol extract of M. koenigii has been reported to have the ability to reduce proliferation in breast cancer cells [87,88]. Alkaloids extracted from the leaves have also been proven to have cytotoxic activity against breast cancer cells with an inhibition concentration (IC50) of 14.4 μg/ml [89].
Ito et al. [90] demonstrated that mahanine, pyrafoline-D, and murrafoline-I exhibited significant cytotoxic activity against HL-60 cells. In addition, girinimbine, a carbazole alkaloid isolated from M. koenigii, showed anticancer activity against human hepatocellular carcinoma and lung cancer [48]. Microtetrazolium assays revealed that girinimbine induced cell death with IC50 19.01 μM [91]. Koenimbin, another compound isolated from M. koenigii exhibited activity in inhibiting MCF7 breast cancer cells [92,93]. Moreover, murrayazoline and O-methylmurrayamine A, obtained from the isolation of these plant leaves showed activity against colorectal cancer through down-regulation of the Akt/mTOR survival pathway and activation of the intrinsic pathway of apoptosis [94]. Mahanimbine, also isolated from M. koenigii leaf, has been shown to inhibit P-glycoprotein involved in lung cancer chemoresistance [14].
Chemoprotective activity
The methanol extract of M. koenigii leaf, administered as a single dose of 100 mg/kg before intraperitoneal injection of 50 mg/kg cyclophosphamide in albino rats, exhibited a protective effect by reducing cyclophosphamide-induced chromosomal damage and enhanced bone protection [95].
Antioxidant effect
In terms of its antioxidant properties, a study by Tachibana et al. [21] indicated that compounds such as mahanimbine and koenigine, derived from leaves, possess antioxidant activity. Antioxidant activity was assessed using the β-carotene-linoleic acid method. Among the various extracts, M. koenigii oleoresin exhibited a maximum activity of 83.2% at 100 ppm, while methanol and water extracts showed activities of 16.7% and 11.3%, respectively. Subsequently, butylhydroxyanisole (a synthetic antioxidant) exhibited 90.2% activity at the same concentration [96]. The ethanol extract obtained from the leaves demonstrated activity against free radical scavenging as tested using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) and NO methods [97]. The ethanol extract also showed high antioxidant activity with an inhibition value of 63.54% in the DPPH assay [98].
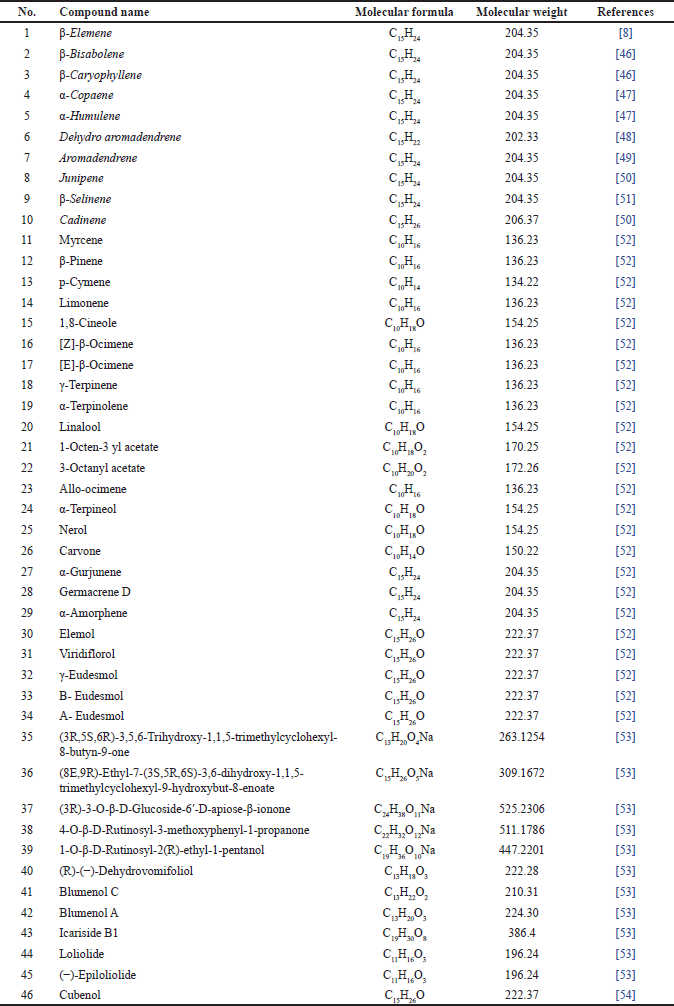 | Table 4. Terpenoid reported from M. koenigii. [Click here to view] |
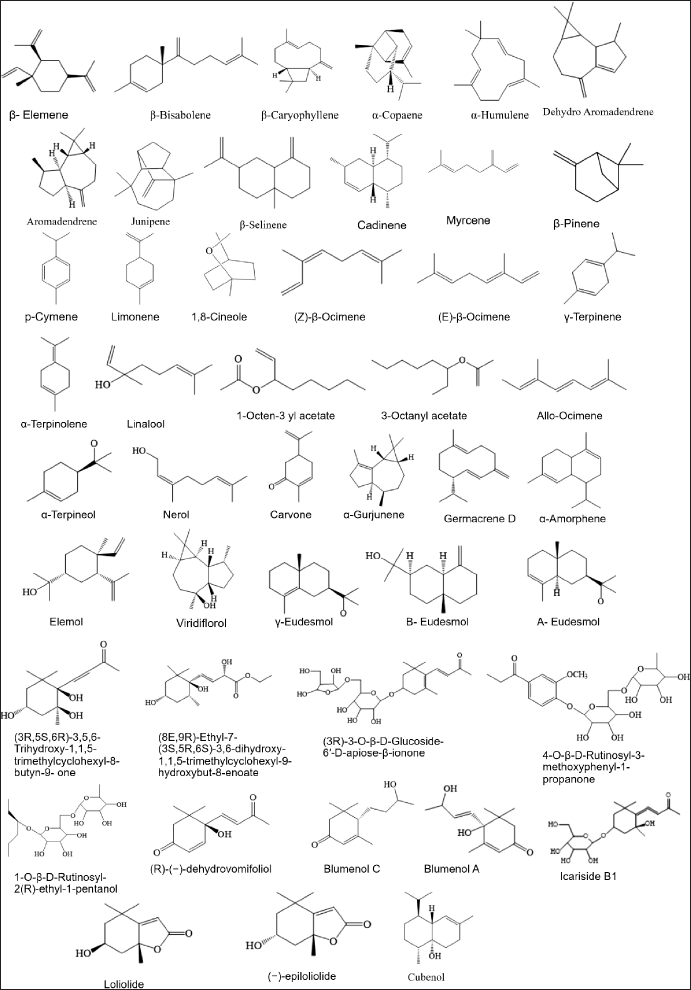 | Figure 5. Structures of terpenoids from M. koenigii (L.) Spreng. [Click here to view] |
Wound healing effect
Murraya koenigii leaf has been found to possess wound-healing activity by reducing epithelialization and supporting collagen synthesis [7]. In addition, the ethanol extract of its leaves exhibited wound-healing activity in excision and incision wound models in male rats [99].
Antipyretic activity
Regarding its antipyretic effects, this study has shown that the ethanol extract derived from M. koenigii leaves has antipyretic activity. This was demonstrated in fever-induced rats using intraperitoneal administration of brewer’s yeast [100] and pyrexia induction methods comparable to paracetamol [101]. Furthermore, the ethanol extract from the leaves also exhibited significant antipyretic activity with the PG1-induced hyperpyrexia method in rabbits [102].
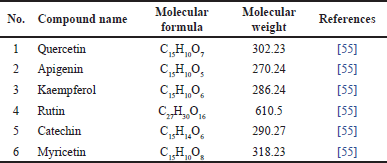 | Table 5. Flavonoid reported from M. koenigii (L.) Spreng. [Click here to view] |
Immunomodulatory activity
The water extract of M. koenigii leaf possesses both specific and nonspecific immunomodulatory activities [103]. The immunomodulatory and anti-inflammatory activities are characterized by the expression of ILs-2, 4, 10, and TNF-α [104].
Hepatoprotective activity
In terms of hepat`oprotective activity, experimental studies have demonstrated that M. koenigii extract offers protection against chronic liver disorders [104]. The hepatoprotective compounds identified in this context include carbazole alkaloids such as mahanimbine, girinimbine, isomahanimbine, murrayazoline, and mahanine [15].
Long-term alcohol consumption is a prevalent and significant factor leading to liver failure and death. Due to the lack of reliable hepatoprotective drugs, the situation becomes more complex. This encourages patients to choose and turn to complementary and alternative medicines to address and handle hepatic complications. The tannins and carbazole alkaloids found in the aqueous extracts showed impressive hepatoprotective effects against ethanol-induced liver toxicity, comparable to the standard drug L-ornithine L-aspartate [105].
Anthelmintic activity
The leaf of M. koenigii has shown anthelmintic effects on the eggs and larvae of Haemonchus contortus [106,107]. In addition, ethanol and water extracts of the leaves exhibit anthelmintic effects comparable to the standard drug piperazine against Pheretima posthuma [108]. Ethanol and water extracts from M. koenigii roots also exhibit anthelmintic activity against Eudrilllus eugeniae [109].
Antimicrobial activity
Regarding its antimicrobial activity, various extracts from M. koenigii have been reported to exhibit antimicrobial activity against Gram-positive bacteria, Gram-negative bacteria, and fungi, assessed through in vitro methods such as agar well diffusion and disk diffusion. Hexane, methanol, and chloroform extracts from M. koenigii roots exhibit antimicrobial activity against Bacillus subtilis, Escherichia coli, Staphylococcus aureus, and Salmonella typhi, as well as fungi such as Aspergillus niger, Candida albicans, and Trichophyton rubrum [110]. The ethanol leaf extract of M. koenigii shows antibacterial activity against Staphylococcus, E. coli, Streptococcus, Proteus, Klebsiella pneumoniae, and Pseudomonas aeruginosa with clear inhibition zones comparable to antibiotics such as amikacin and gentamicin, although not effective against K. pneumoniae and P. aeruginosa [111].
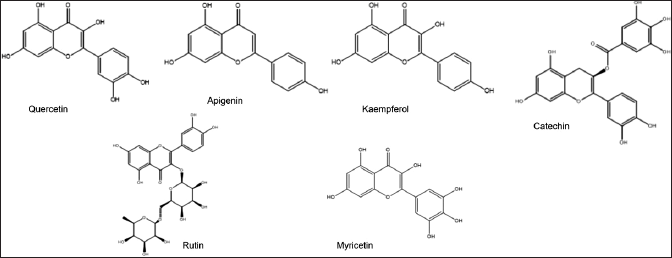 | Figure 6. Structures of flavonoid from M. koenigii (L.) Spreng. [Click here to view] |
Derivatives of benzoisofuranone and carbazole alkaloids obtained from the stem bark of M. koenigii also possess antibacterial and antifungal activities [9]. In addition, the ethanol leaf extract exhibits antifungal activity against Trichophyton mentagrophytes and Microsporum gypseum [112].
Antiulcer activity
The water extract of the leaves at doses of 250 and 400 mg/kg inhibits gastric lesions induced by nonsteroidal anti-inflammatory drugs and pylorus ligation models [113].
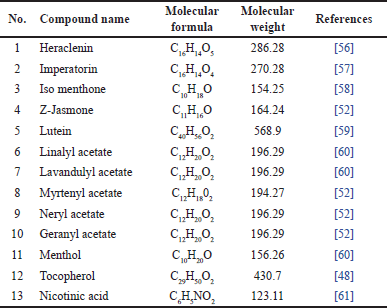 | Table 6. Other compounds reported from M. koenigii. [Click here to view] |
Antidiarrheal activity
The water extract of the leaves exhibits antidiarrheal activity in models of castor oil-induced diarrhea, charcoal meal test, and prostaglandinE2-induced diarrhea [114,115]. Subsequently, koenimbine obtained from M. koenigii seeds exhibits antidiarrheal activity in castor oil-induced diarrhea in rats [37].
Antiobese activity
The ethanol leaf extract of M. koenigii, administered orally to male Wistar rats for 30 days, effectively reduces body weight, cholesterol, triglycerides, and controls glycemic levels [116].
Neuroprotektif activity
The ethanol leaf extract of M. koenigii inhibits brain aging in diabetic rats [117]. The extract also improves memory in rats with chronic partial global cerebral ischemia [118]. In addition, mahanimbine exhibited potential neuroprotective properties against lipopolysaccharide-induced nerve inflammation [119].
Antitrichomonal activity
Carbazole alkaloids and their derivatives from the leaves have activity against Trichomonas gallinae. Girinimbin and girinimbilol are the most active compounds with IC50 values of 1.08 and 1.20 mg/ml [120].
Toxicity test
The methanol extract of M. koenigii leaf exhibited moderate toxicity in rats, with an lethal dose (LD50) value of 316.23 mg/kg body weight, causing liver inflammation at higher doses [121]. However, no signs of death or morbidity were observed in male or female rats given ethanol leaf extract (300 and 500 mg/kg) for 28 days. At a dose of 900 mg/kg, there were no deaths, but congestion, hemorrhage, and lymphocyte infiltration were noted [122]. Other studies have shown that leaf powder and methanolic leaf extract are safe up to 9,000 mg/kg doses in rats [123]. Another study found no signs of death or toxicity at an LD50 value of 200 mg/kg/day for methanol extract of M. koenigii [124].
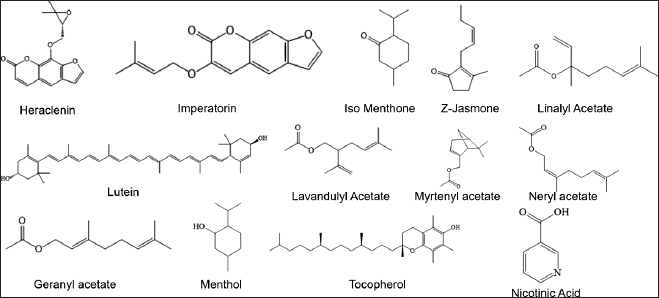 | Figure 7. Structures of another compound from M. koenigii (L.) Spreng. [Click here to view] |
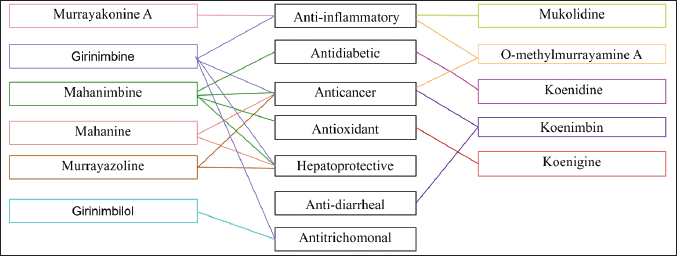 | Figure 8. Relationship between chemical compounds from M. koenigii (L.) Spreng and their pharmacological effects. [Click here to view] |
Ethanol leaf extract at doses of 300 and 500 mg/kg for 28 days did not result in death or morbidity in male or female rats. At a dose of 900 mg/kg, it did not cause death but led to bleeding and lymphocyte infiltration. At a dose of 900 mg/kg, it did not cause death but led to bleeding and lymphocyte infiltration. This study concluded that consumption at a dose of 500 mg/kg is safe and does not cause structural organ damage [120]. Subsequently, rats given a magazine-enriched fraction at a single dose of 5,000 mg/kg body weight, 300–1,500 mg/kg body weight per day for 14 days, and 300 mg/kg body weight for 180 days showed no toxicity, mortality, or significant behavioral changes [124]. Azzubaidi et al. [123] stated that the LD50 of M. koenigii leaf extract is 200 mg/kg/day, and the safest extract dose should not exceed 50 mg/kg/day.
CONCLUSION
In conclusion, M. koenigii has been extensively studied, thereby contributing to the understanding of secondary metabolites and their biological activities in nature. Furthermore, alkaloids were observed to have been the dominant compounds from M. koenigii, followed by terpenoid and fenil propanoid. The literature reports showed that the plant exhibited various biological activities, such as anti-inflammatory, cytotoxic, antidiabetic, and antimicrobial activity. This study showed that most pharmacological activity studies are still limited to in-vitro and in-vivo screenings, with mechanisms of action, bioavailability, and pharmacokinetics not yet explored. Subsequently, further studies should focus on the isolation of phytochemical compounds guided by bioassays, formulation, and drug delivery methods, serving as the basis for drug discovery. This review focuses on scientific studies of the pharmacological effects of M. koenigii.
ACKNOWLEDGMENTS
We would like to thank wholeheartedly Hibah Rekognisi Tugas Akhir of the Universitas Gadjah Mada (Grant number: 5075/UNI.P.II/Dit-Lit/PT.0101/2023) for supporting and providing the research facility for this work.
AUTHOR CONTRIBUTIONS
YDF has equally contributed to conceptualization and writing. NF, and AN reviewed and edited. All authors have read and agreed to the published version of the manuscript.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Gahlawat DK, Dheeraj K, Jakhar S, Dahiya P. Murraya koenigii (L.) Spreng: an ethnobotanical, phytochemical and pharmacological review. J Pharmacogn Phytochem. 2014;3:109–19.
2. Chauhan B, Dedania J, Mashru DRC. Review on Murraya koenigii: versatile role in management of human health. World J Pharm Pharm Sci. 2017;6:476–93.
3. Mhaskar K, Blatter E, Caius JF. Kirtikar and Basu’s illustrated Indian medicinal plants: their usage in Ayurveda and Unani medicines. New Delhi, India: Sri Satguru Publications; 2000. Vol. 1, pp 86–96.
4. Azmin SNHM, Ch’ng HY. Curry leaf (Murraya koenigii): the story of potential miracle plant. Beau Bassin, Mauritius: LAP Lambert Academic Publishing; 2020.
5. Prajapati N, Purohit S, Sharma A, Kumar T. A handbook of medicinal plants. 1st ed. Jodhpur, India: Agrobios India; 2003. 401 p.
6. Ito C, Thoyama Y, Omura M, Kajiura I, Furukawa H. Alkaloidal constituents of Murraya koenigii. Isolation and structural elucidation of novel binary carbazolquinones and carbazole alkaloids. Chem Pharm Bull. 1993;41:2096–100. CrossRef
7. Nagappan T, Segaran TC, Wahid MEA, Ramasamy P, Vairappan CS. Efficacy of carbazole alkaloids, essential oil and extract of Murraya koenigii in enhancing subcutaneous wound healing in rats. Molecules. 2012;17(12):14449–63. CrossRef
8. Narasimhan N, Paradkar M, Chitguppi V, Kelkar S. Alkaloids of Murraya koenigii: structures of mahanimbine, koenimbine, (-)-mahanine, koenine, koenigine, koenidine & (+)-isomahanimbine. Indian J Chem. 1975;13(10):993–9. CrossRef
9. Rahman MM, Gray AI. A benzoisofuranone derivative and carbazole alkaloids from Murraya koenigii and their antimicrobial activity. Phytochemistry. 2005;66(13):1601–6. CrossRef
10. Parrotta, J. Healing plants of Peninsular India. Wallingford, UK; New York, NY: CABI Publishing , 2001. 944 p. CrossRef
11. Iman V, Mohan S, Abdelwahab SI, Karimian H, Nordin N, Fadaeinasab M, et al. Anticancer and anti-inflammatory activities of girinimbine isolated from Murraya koenigii. Drug Des Dev Ther. 2017;11:103–21. CrossRef
12. Nalli Y, Khajuria V, Gupta S, Arora P, Riyaz-Ul-Hassan S, Ahmed Z, et al. Four new carbazole alkaloids from Murraya koenigii that display anti-inflammatory and anti-microbial activities. Org Biomol Chem. 2016;14(12):3322–32. CrossRef
13. Sampath SNTI, Jayasinghe S, Attanayake AP, Karunaratne V, Yaddehige ML, Watkins DL. A new dimeric carbazole alkaloid from Murraya koenigii (L.) leaves with α-amylase and α-glucosidase inhibitory activities. Phytochem Lett. 2022;52:87–91. CrossRef
14. Mondal P, Natesh J, Abdul Salam AA, Meeran SM. Mahanimbine isolated from Murraya koenigii inhibits P-glycoprotein involved in lung cancer chemoresistance. Bioorg Chem. 2022;129:106170. CrossRef
15. Gupta R, Singh D. Protective nature of Murraya koenigii leaves against hepatosuppression through antioxidant status in experimental rats. Pharmacologyonline. 2007 Jan 1;1:232–42.
16. Narasimhan NS, Paradkar MV, Chitguppi VP. Structures of mahanikbin and koenimbin. Tetrahedron Lett. 1968;9(53):5501–4. CrossRef
17. Kureel SP, Kapil RS, Popli SP. Terpenoid alkaloids from Murraya koenigii Spreng. II. The constitution of cyclomahanimbine, bicyclomahanibine, and mahanimbidine. Tetrahedron Lett. 1969 Sep;(44):3857–62. CrossRef
18. Joshi BS, Kamat VN, Gawad DH. On the structures of girinimbine, mahanimbine, isomahanimbine, koenimbidine and murrayacine. Tetrahedron. 1970 Mar;26(6):1475–82. CrossRef
19. Reisch J, Adebajo AC, Aladesanmi AJ, Adesina KS, Bergenthal D, Meve U. Chemotypes of Murraya koenigii growing in Sri Lanka. Planta Med. 1994;60(03):295–6. CrossRef
20. Ramsewak RS, Nair MG, Strasburg GM, DeWitt DL, Nitiss JL. Biologically active carbazole alkaloids from Murraya koenigii. J Agric Food Chem. 1999;47(2):444–7. CrossRef
21. Tachibana Y, Kikuzaki H, Lajis N, Nakatani N. Antioxidative activity of carbazoles from Murraya koenigii leaves. J Agric Food Chem. 2001 Nov 1;49:5589–94. CrossRef
22. Dutta N, Quasim C, Wadia M. Constituents of Murraya koenigii: structure of curryangin. Indian J Chem. 1969;7:1061–2.
23. Chakraborty DP, Bhattacharyya P, Islam A, Roy S. Structure of murrayacinine, a new carbazole alkaloid from Murraya koenigii Spreng. Chem Ind. 1974;4:165–6. CrossRef
24. Reisch J, Adebajo AC, Kumar V, Aladesanmi AJ. Two carbazole alkaloids from Murraya koenigii. Phytochemistry. 1994;36(4):1073–6. CrossRef
25. Adesina SK, Olatunji OA, Bergenthal D, Reisch J. New biogenetically-significant constituents of Clausena anisata and Murraya koenigii. Pharmazie. 1988 Mar;43(3):221–2.
26. Mukherjee M, Mukherjee S, Shaw AK, Ganguly SN. Mukonicine, a carbazole alkaloid from leaves of Murraya koenigii. Phytochemistry. 1983;22(10):2328–9. CrossRef
27. Bhattacharya P, Chowdhury B. 2-Methoxy-3-methyl carbazole from Murraya koenigii. Indian J Chem Sect. 1984;24B(4):452.
28. Reisch J, Goj O, Wickramasinghe A, Bandara Herath HMT, Henkel G. Carbazole alkaloids from seeds of Murraya koenigii. Phytochemistry. 1992;31(8):2877–9. CrossRef
29. Tachibana Y, Kikuzaki H, Lajis NH, Nakatani N. Comparison of antioxidative properties of carbazole alkaloids from Murraya koenigii leaves. J Agric Food Chem. 2003;51(22):6461–7. CrossRef
30. Nakamura S, Nakashima S, Oda Y, Yokota N, Fujimoto K, Matsumoto T, et al. Alkaloids from Sri Lankan curry-leaf (Murraya koenigii) display melanogenesis inhibitory activity: structures of karapinchamines A and B. Bioorg Med Chem. 2013;21(5):1043–9. CrossRef
31. Bakar NHA, Khalid K, Sukari MA, Yusuf UK. Chemical constituents from stem barks and roots of Murraya koenigii (Rutaceae). Malays J Anal Sci. 2007;11(1):173–6.
32. Tan SP, Nafiah MA, Ahmad K. C23-carbazole alkaloids from Malayan Murraya koenigii (L.) Spreng. J Chem Pharm Res. 2014;6(4):1093–8.
33. Saha C, Chowdhury BK. Carbazoloquinones from Murraya koenigii. Phytochemistry. 1998;48(2):363–6.CrossRef
34. Nutan MTH, Hasan CM, Rashid MA. Bismurrayafoline E: a new dimeric carbazole alkaloid from Murraya koenigii. Fitoterapia. 1999;70(2):130–3. CrossRef
35. Wang YS, He HP, Hng X, Zhao Q, Hao XJ. A new binary carbazole alkaloid from Murraya koenigii. Chinese Chem Lett. 2002;13(9):849–50.
36. Bakar NHA, Sukari MA, Rahmani M, Sharif AM, Khalid K, Yusuf UK. Chemical constituents from stem barks and roots of Murraya koenigii (Rutaceae). Malays J Anal Sci. 2007;11(1):173–6.
37. Mandal S, Nayak A, Banerjee SK, Banerji J, Banerji A. A new carbazole alkaloid from Murraya koenigii Spreng (Rutaceae). Nat Prod Commun. 2008;3(10):1679–82. CrossRef
38. Ma Q, Tian J, Yang J, Wang A, Ji T, Wang Y, et al. Bioactive carbazole alkaloids from Murraya koenigii (L.) Spreng. Fitoterapia. 2013;87(1):1–6. CrossRef
39. Ahmad K, Tan SP, Hazni H, Nafiah MA. Cyclic monoterpenoid pyranocarbazole alkaloids from the bark of Murraya koenigii (L.) Spreng. J Teknol. 2015;77(2):73–7. CrossRef
40. Fiebig M, Pezzuto JM, Soejarto DD, Kinghorn AD. Koenoline, a further cytotoxic carbazole alkaloid from Murraya koenigii. Phytochemistry. 1985;24(12):3041–3. CrossRef
41. Wang W, Zhou Z, Zhou X, Chen L, Bie S, Jing Z. Mukonal exerts anticancer effects on the human breast cancer cells by inducing autophagy and apoptosis and inhibits the tumor growth in vivo. AMB Express. 2020;10(1):148. CrossRef
42. Viteritti E, Oliva E, Eugelio F, Fanti F, Palmieri S, Bafile E, et al. Analysis of carbazole alkaloids in Murraya koenigii by means of high performance liquid chromatography coupled to tandem mass spectrometry with a predictive multi experiment approach. J Chromatogr Open. 2022;2:100055. CrossRef
43. Ma Q, Wei R, Yang M, Huang X, Zhong G, Sang Z, et al. Structures and biological evaluation of phenylpropanoid derivatives from Murraya koenigii. Bioorg Chem. 2019;86:159–65. CrossRef
44. Srivastava SK, Srivastava SD. New constituents and biological activity of the roots of Murraya koenigii. J Indian Chem Soc. 1993;70(July):655–9.
45. Ma QG, Xu K, Sang ZP, Wei RR, Liu WM, Su YL, et al. Alkenes with antioxidative activities from Murraya koenigii (L.) Spreng. Bioorg Med Chem Lett. 2016;26(3):799–803. CrossRef
46. Chowdhury JU, Bhuiyan MNI, Yusuf M. Chemical composition of the leaf essential oils of Murraya koenigii (L.) Spreng and Murraya paniculata (L.) Jack. Bangl J Pharmacol. 2008;3(2):59–63. CrossRef
47. Dineshkumar B, Mitra A, Mahadevappa M. Antidiabetic and hypolipidemic effects of mahanimbine (carbazole alkaloid) from Murraya koenigii (rutaceae) leaves. Int J Phytomed. 2010;2(1):22–30.
48. Syam S, Abdul AB, Sukari MA, Mohan S, Abdelwahab SI, Wah TS. The growth suppressing effects of girinimbine on hepg2 involve induction of apoptosis and cell cycle arrest. Molecules. 2011;16(8):7155–70. CrossRef
49. Sindhu R. Phytochemical and pharmacognostical studies on Murraya koenigii L. Spreng roots. Drug Invent Today. 2012;4:325–36.
50. Nguyen TTT, Diep TT, Hoang V, Vo THM, Duus F, Le Thach N. Investigation of curry leaf essential oils of Murraya koenigii Spreng. growing in the South of Vietnam. J Essent Oil Bearing Plants. 2012;15(6):1021–9. CrossRef
51. Nagappan T, Ramasamy P, Vairappan CS. Chemotaxonomical markers in essential oil of Murraya koenigii. Nat Prod Commun. 2012;7(10):1375–8. CrossRef
52. Rajendran MP, Pallaiyan BB, Selvaraj N. Chemical composition, antibacterial and antioxidant profile of essential oil from Murraya koenigii (L.) leaves. Avicenna J Phytomed. 2014;4(3):200–14. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25050318%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC4104627
53. Ma QG, Wang YG, Liu WM, Wei RR, Yang JB, Wang AG, et al. Hepatoprotective sesquiterpenes and rutinosides from Murraya koenigii (L.) Spreng. J Agric Food Chem. 2014;62(18):4145–51. CrossRef
54. Dosoky N, Satyal P, Gautam T, Setzer W. Composition and biological activities of Murraya paniculata (L.) Jack essential oil from Nepal. Medicines. 2016;3(1):7. CrossRef
55. Noolu B, Gogulothu R, Bhat M, Qadri SSYH, Reddy VS, Reddy GB, et al. In vivo inhibition of proteasome activity and tumour growth by Murraya koenigii leaf extract in breast cancer xenografts and by its active flavonoids in breast cancer cells. Anticancer Agents Med Chem. 2016;16(12):1605–14. CrossRef
56. Adebajo A, Reisch J. Minor furocoumarins of Murraya koenigii. Fitoterapia. 2000;71:334–7. CrossRef
57. Rana VS, Juyal JP, Rashmi, Amparo Blazquez M. Chemical constituents of the volatile oil of Murraya koenigii leaves. Int J Aromather. 2004;14(1):23–5. CrossRef
58. Reddy PJM, Shareef MI, Gopinath SM, Dayananda KS, Mandal A. Antimicrobial activity of the essential oil of Murraya. J Biotechnol Biosaf. 2014;2(1):50–6.
59. Shivanna V, Subban N. Carotenoids retention in processed curry leaves (Murraya koenigii L. Spreng). Int J Food Sci Nutr. 2012;64:58–62. CrossRef
60. Tripathi Y, Anjum N, Rana A. Chemical composition and in vitro antifungal and antioxidant activities of essential oil from Murraya koenigii (L.) Spreng. leaves. Asian J Biomed Pharm Sci. 2018;8(65):6–13. CrossRef
61. Widayanti A, Srifiana Y, Efendi K. Antidiabetics activity of salam koja (Murraya koenigii) leaves tea bag. Pharm Sci Res. 2019;6(2):107–10. CrossRef
62. Gupta S, Gerge M, Singhal M, Sharma GN, Garg V. Leaves extract of Murraya koenigii Linn for anti-inflammatory and analgesic activity in animal models. J Adv Pharm Tecnhol Res. 2010;1(1):68–77.
63. Darvekar VM, Patil VR, Choudhari AB, Road D. Anti-inflammatory activity of Murraya koenigii Spreng on experimental animals. J Nat Prod Plant Resour. 2011;1(1):65–9.
64. Mathur A, Prasad G, Dua VK. Anti-inflammatory activity of leaves extracts of Murraya koenigii L. Int J Pharma Bio Sci. 2011;2(1):541–4.
65. Parmar S, Gangwal A, Sheth N. Mast cell membrane stabilization and anti-histaminic actions-possible mechanism of action of anti-inflammatory action of Murraya koenigii. J Curr Pharm Res. 2010;2(1):21–5.
66. Mani V, Ramasamy K, Majeed A. Anti-inflammatory, analgesic and anti-ulcerogenic effect of total alkaloidal extract from Murraya koenigii leaves in animal models. Food Funct. 2013 Jan 29;4:557–67. CrossRef
67. Ani NS, Chakraborty S, Moniruzzaman M. The methanolic extract from Murraya koenigii L. inhibits glutamate-induced pain and involves ATP-sensitive K+ channel as antinociceptive mechanism. Adv Pharmacol Sci. 2016;2016:3790860. CrossRef
68. Husna F, Suyatna FD, Arozal W, Purwaningsih EH, Sani Y. Restoration of pro-inflammatory cytokines and histopathological changes in pancreas and liver of hyperglycemic rats by Murraya koenigii leaves extract. J Appl Pharm Sci. 2020;10(1):8–15. CrossRef
69. Yadav S, Vats V, Dhunnoo Y, Grover JK. Hypoglycemic and antihyperglycemic activity of Murraya koenigii leaves in diabetic rats. J Ethnopharmacol. 2002;82(2):111–6. CrossRef
70. Suman RK, Mohanty IR, Borde MK, Deshmukh YA, Pathak A, Adhikari AK, et al. Evaluation of antidiabetic efficacy of Murraya koenigii on streptozotocin induced diabetes in experimental rats. Int J Basic Clin Pharmacol. 2019;8(8):1906. CrossRef
71. Gul MZ, Attuluri V, Qureshi IA, Ghazi IA. Antioxidant and α-glucosidase inhibitory activities of Murraya koenigii leaf extracts. Pharmacogn J. 2012;4(32):65–72. CrossRef
72. Husna F, Suyatna F, Arozal W, Poerwaningsih E. Anti-diabetic potential of Murraya koenigii (L.) and its antioxidant capacity in nicotinamide-streptozotocin induced diabetic rats. Drug Res (Stuttg). 2018 May 25;68:631–6. CrossRef
73. Vinuthan MK, Girish KV, Ravindra JP, Jayaprakash, Narayana K. Effect of extracts of Murraya koenigii leaves on the levels of blood glucose and plasma insulin in alloxan-induced diabetic rats. Indian J Physiol Pharmacol. 2004;48(3):348–52.
74. Arulselvan P, Senthilkumar GP, Sathish Kumar D, Subramanian S. Anti-diabetic effect of Murraya koenigii leaves on streptozotocin induced diabetic rats. Pharmazie. 2006 Oct;61(10):874–7.
75. Lawal HA, Atiku MK, Khelpai DG, Wannang NN. Hypoglycaemic and hypolipidaemic effect of aqueous leaf extract of Murraya koenigii in normal and alloxan-diabetic rats. Niger J Physiol Sci. 2008;23(1–2):37–40. CrossRef
76. Al-Ani IM, Santosa RI, Yankuzo MH, Saxena AK, Alazzawi KS. The antidiabetic activity of curry leaves “Murraya koenigii” on the glucose levels, kidneys, and islets of langerhans of rats with streptozotocin induced diabetes. Makara J Health Res. 2017;21(2):54–60. CrossRef
77. Desai RR, Khanwelkar CC, Gidamudi S, Phatak RS, Jadhav S, Thorat V, et al. Antidiabetic effect of Murraya koenigii leaves aqueous extract on blood sugar levels in alloxanized diabetic rats. Pravara Med Rev. 2019;11(2):19–24.
78. Kesari AN, Gupta RK, Watal G. Hypoglycemic effects of Murraya koenigii on normal and alloxan-diabetic rabbits. J Ethnopharmacol. 2005;97(2):247–51. CrossRef
79. Ahmed S, Sunil M, Cheekavolu C, Alasyam N. Evaluation of antidiabetic effect of Murraya koenigii leaves chloroform extract (MKLCE) in alloxan induced diabetic albino rats. Pharma Innov J. 2017;6(11):474–7. Available from: https://www.thepharmajournal.com/archives/2017/vol6issue11/PartG/6-11-46-967.pdf
80. Tembhurne S, Sakarkar D. Hypoglycemic effects of fruit juice of Murraya koenigii (L.) in alloxan induced diabetic mice. Int J Pharm Tech Res. 2009;1:1589–93.
81. Patel OPS, Mishra A, Maurya R, Saini D, Pandey J, Taneja I, et al. Naturally occurring carbazole alkaloids from Murraya koenigii as potential antidiabetic agents. J Nat Prod. 2016 May 27;79(5):1276–84. CrossRef
82. Gaikwad V, Ray S. Effectiveness of curry leaves on blood sugar level among diabetic clients. Pharma Innov J. 2018;7(6):457–60.
83. Husna F, Suyatna FD, Arozal W, Purwaningsih EH, Hanafi M, Razali R, et al. Murraya koenigii extract improving rate limiting enzymes on carbohydrate metabolism and GLUT-4 expression of hyperglycemic rats. J Appl Pharm Sci. 2022;12(12):143–9. CrossRef
84. Iyer UM, Mani UV. Studies on the effect of curry leaves supplementation (Murraya koenigii) on lipid profile, glycated proteins and amino acids in non-insulin-dependent diabetic patients. Plant Foods Hum Nutr. 1990 Oct;40(4):275–82. CrossRef
85. Amna U, Halimatussakdiah, Wahyuningsih P, Saidi N, Nasution R. Evaluation of cytotoxic activity from Temurui (Murraya koenigii (Linn.) Spreng) leaf extracts against HeLa cell line using MTT assay. J Adv Pharm Technol Res. 2019;10(2):51–5. CrossRef
86. Khan BA, Abraham A, Leelamma S. Murraya koenigii and Brassica juncea—alterations on lipid profile in 1-2 dimethyl hydrazine induced colon carcinogenesis. Invest New Drugs. 1996;14(4):365–9. CrossRef
87. Noolu B, Ajumeera R, Chauhan A, Nagalla B, Manchala R, Ismail A. Murraya koenigii leaf extract inhibits proteasome activity and induces cell death in breast cancer cells. BMC Complement Altern Med. 2013;13:7. CrossRef
88. Ghasemzadeh A, Jaafar HZE, Rahmat A, Devarajan T. Evaluation of bioactive compounds, pharmaceutical quality, and anticancer activity of curry leaf (Murraya koenigii L.). Evid Based Complement Altern Med. 2014;2014:873803. CrossRef
89. Ismail A, Noolu B, Gogulothu R, Perugu S, Rajanna A, Babu SK. Cytotoxicity and proteasome inhibition by alkaloid extract from Murraya koenigii leaves in breast cancer cells—molecular docking studies. J Med Food. 2016 Dec 1;19(12):1155–65. CrossRef
90. Ito C, Itoigawa M, Nakao K, Murata T, Tsuboi M, Kaneda N, et al. Induction of apoptosis by carbazole alkaloids isolated from Murraya koenigii. Phytomedicine. 2006 May;13(5):359–65. CrossRef
91. Mohan S, Abdelwahab SI, Cheah SC, Sukari MA, Syam S, Shamsuddin N, et al. Apoptosis effect of girinimbine isolated from Murraya koenigii on lung cancer cells in vitro. Evid Based Complement Altern Med. 2013;2013:689865. CrossRef
92. Kok YY, Mooi LY, Ahmad K, Sukari MA, Mat N, Rahmani M, et al. Anti-tumour promoting activity and antioxidant properties of girinimbine isolated from the stem bark of Murraya koenigii S. Molecules. 2012;17(4):4651–60. CrossRef
93. Ahmadipour F, Noordin MI, Mohan S, Arya A, Paydar M, Looi CY, et al. Koenimbin, a natural dietary compound of Murraya koenigii (L.) Spreng: inhibition of MCF7 breast cancer cells and targeting of derived MCF7 breast cancer stem cells (CD44+/CD24-/low): an in vitro study. Drug Des Dev Ther. 2015;9:1193–208. CrossRef
94. Arun A, Patel OPS, Saini D, Yadav PP, Konwar R. Anti-colon cancer activity of Murraya koenigii leaves is due to constituent murrayazoline and O-methylmurrayamine A induced mTOR/AKT downregulation and mitochondrial apoptosis. Biomed Pharmacother. 2017;93:510–21. CrossRef
95. Swati D, Pathak AK, Burande MD. Antigenotoxic effect of Murraya koenigii towards cyclophosphamide induced cytogenetic damage in mouse bone marrow cells. Int J Pharmacogn Phytochem Res. 2012 Jun 1;4:59–63.
96. Rao LJM, Ramalakshmi K, Borse BB, Raghavan B. Antioxidant and radical-scavenging carbazole alkaloids from the oleoresin of curry leaf (Murraya koenigii Spreng.). Food Chem. 2007;100(2):742–7. CrossRef
97. Sindhu RK, Arora S. Evaluation of phenolic contents and antioxidant potential of Murraya koenigii (L.) Spreng roots. J Appl Pharm Sci. 2012;2(11):120–2.
98. Sumalatha D, Nithya TG. Invitro anti-oxidant and anticancer activity of Murraya koenigii against human colon cancer HT-29 cell lines. Asian J Pharm Clin Res. 2014;7(SUPPL. 1):83–6.
99. Anand T, Kalaiselvan A, Gokulakrishnan K. Wound healing activity of Murraya koenigii in male albino rats. Int J Curr Res. 2011;3(2):425–7.
100. Usman M. Phytochemical evaluation and effect of antipyretic activity on Murraya koenigii Spreng. leaves extracts. Int J Pharm Chem Sci. 2012 Jan 1;1:231–6.
101. Patel VR, Patel MG, Patel RK. Anti-pyretic activity of the ethanolic extract of the powdered leaves of Murraya koenigii (L.) Spreng. J Pharm Res. 2009;2(4):731–2. Available from: http://jpronline.info/article/view/296/256
102. Pokala N, Sayeli V, Jayasree T. Evaluation of antipyretic activity of alcoholic extract of Murraya koenigii leaves in rabbits. Int J Basic Clin Pharmacol. 2019;8(7):1577. CrossRef
103. Shah AS, Juvekar AR. Immunostimulatory activity of aqueous extract of Murraya koenigii (Linn.) Spreng. leaves. Indian J Nat Prod Resour. 2010;1(4):450–5.
104. Paul S, Bandyopadhyay TK, Bhattacharyya A. Immunomodulatory effect of leaf extract of Murraya koenigii in diabetic mice. Immunopharmacol Immunotoxicol. 2011 Dec;33(4):691–9. CrossRef
105. Shivashankara AR, Azmidah A, Haniadka R, Rai MP, Arora R, Baliga MS. Dietary agents in the prevention of alcohol-induced hepatotoxicty: preclinical observations. Food Funct. 2012 Feb;3(2):101–9. CrossRef
106. Molla SH, Bandyopadhyay PK. In vitro and in vivo anthelmintic activity of Murraya koenigii against gastro-intestinal nematodes of sheep. J Parasit Dis. 2016;40(2):362–8. CrossRef
107. Sujith S, Priya MN, Deepa CK, Usha PTA. Characterization of the anthelmintic activity of Murraya koenigii (Linn.). Pharmacogn J. 2018;10(6):S100–3. CrossRef
108. Sharma UK, Singh A, Sutar N, Singh PJ. In vitro anthelmintic activity of Murraya koenigii Linn. leaves: extracts. Int J Res Pharm Biomed Sci. 2010 Jan 1;1(3):1–4.
109. Pagariya A, Chatur S, Nawab F. In vitro anthelmintic activity of root extract of Murraya koenigii (Linn.) Spreng. Int J Pharm Innov. 2013;3(1):111–4.
110. Malwal M, Sarin R. Antimicrobial efficacy of Murraya koenigii (Linn.) Spreng. root extracts. Indian J Nat Prod Resour. 2011;2(1):48–51.
111. Al Harbi H, Irfan UM, Ali S. The antibacterial effect of curry leaves (Murraya koenigii). Eur J Pharm Med Res. 2016;3:382–7. Available from: www.ejpmr.com
112. Jayaprakash A, Ebenezer P. Antifungal activity of curry leaf (Murraya koenigii) extract and an imidazole fungicide on two dermatophyte taxa. J Acad Indus Res. 2012;1(3):124.
113. Sharma P, Vidyasagar G, Bhandari A, Singh S, Ghule S, Agrawal A, et al. Antiulcer activity of leaves extract of Murraya koenigii in experimentally induced ulcer in rats. Pharmacologyonline. 2011;2:818–24. CrossRef
114. Ramasamy A, Das S, Mani V, Sengottuvelu S, Vinoth Prabhu V. Evaluation of anti-diarrheal potential of hydro-alcoholic extracts of leaves of Murraya koenigii in experimental animals. J Diet Suppl. 2016;13(4):393–401. CrossRef
115. Sharma P, Vidyasagar G, Bhandari A, Singh S, Bhadoriya U, Ghule S, et al. A pharmacological evaluation of antidiarrhoeal activity of leaves extract of Murraya koenigii in experimentally induced diarrhoea in rats. Asian Pac J Trop Dis. 2012;2(3):230–3. CrossRef
116. Tembhurne SV, Sakarkar DM. Anti-obesity and hypoglycemic effect of ethanolic extract of Murraya koenigii (L.) leaves in high fatty diet rats. Asian Pac J Trop Dis. 2012;2(SUPPL.1):166–8. CrossRef
117. Bhupatiraju L, Bethala K, Wen Goh K, Singh Dhaliwal J, Ching Siang T, Menon S, et al. Influence of Murraya koenigii extract on diabetes induced rat brain aging. J Med Life. 2023;16(2):307–16. CrossRef
118. Azzubaidi MS, Al-Ani IM. Mnemonic and histopathological assessment of the neuroprotective effects of Murraya koenigii leaves extract in rats with partial global cerebral ischaemia. IIUM Med J Malays. 2019;18(2):77–86. CrossRef
119. Azahan NSM, Mani V, Ramasamy K, Lim SM, James RMJ, Alsharidah M, et al. Mahanimbine-induced neuroprotection via cholinergic system and attenuated amyloidogenesis as well as neuroinflammation in lipopolysaccharides-induced mice. Pharmacogn Mag. 2020;16:s57–63. CrossRef
120. Adebajo AC, Ayoola OF, Iwalewa EO, Akindahunsi AA, Omisore NOA, Adewunmi CO, et al. Anti-trichomonal, biochemical and toxicological activities of methanolic extract and some carbazole alkaloids isolated from the leaves of Murraya koenigii growing in Nigeria. Phytomedicine. 2006 Mar;13(4):246–54. CrossRef
121. Sakarkar DM, Tembhume SV MB. 28 days repeated dose toxicity study of ethanolic extract of Murraya koenigii in Wistar rats. Ann Pharmacol Pharm. 2017;2(8):1047.
122. Apostoli P. Elements in environmental and occupational medicine. J Chromatogr B. 2002;778(1):63–97. CrossRef
123. Azzubaidi MS, Saxena AK, Alattraqchi AG, Abdualkader AM. Chronic LD50 vs safest dose for the methanolic extract of curry leaves (Murraya koenigii) cultivated in Malaysia. J Appl Pharm Sci. 2014;4(8):56–8.
124. Satyavarapu EM, Sinha PK, Mandal C. Preclinical development of mahanine-enriched fraction from Indian spice Murraya koenigii for the management of cancer: efficacy, temperature/pH stability, pharmacokinetics, acute and chronic toxicity (14-180 days) studies. Biomed Res Int. 2020;2020:4638132. CrossRef