INTRODUCTION
Oral bioavailability represents the proportion of an orally administered drug that successfully enters the systemic circulation, playing a crucial role in assessing its efficacy. However, achieving optimal oral bioavailability poses considerable challenges due to various factors, including inadequate solubility, limited permeability across the intestinal epithelium, extensive first-pass metabolism, and the presence of efflux transporters [1]. A notable obstacle in oral drug delivery lies in circumventing the impact of first-pass metabolism, which can be overcome by redirecting drug delivery toward the intestinal lymphatic system [2,3]. An effective strategy for enhancing drug solubility in lipids and thereby improving oral bioavailability involves the design of lipophilic prodrugs, leveraging the enhanced transport offered by the lymphatic system.
The lymphatic system, an intricate network of vessels, nodes, and organs, harmoniously upholds fluid balance, supports immune function, and facilitates lipid absorption. With its ability to bypass the liver and evade first-pass metabolism, the lymphatic system assumes a significant role in drug delivery. Comprising lymph nodes, lymphatic vessels, and widely distributed lymphoid organs throughout the body, this intricate system plays a vital role in the transportation of drugs, particularly large molecules and lipophilic compounds that encounter challenges in penetrating blood capillaries. A comprehensive comprehension of the lymphatic system’s anatomy and physiology serves as a crucial foundation for devising targeted strategies for drug delivery along this distinctive route.
This review aims to delve into uncharted territories in drug delivery by centering on the delivery of drugs to the lymphatic system. The study will intricately explore the physiological and pharmaceutical barriers that influence drug bioavailability in the context of oral administration. Furthermore, it will provide an in-depth investigation of diverse pharmaceutical technologies and drug delivery systems, encompassing nanocarriers, micelles, cyclodextrins, and lipid-based carriers, which have been extensively studied to enhance oral drug absorption. In addition, the review will shed light on the impact of variability in the gastrointestinal tract on oral drug absorption and pharmacokinetics [4–6].
By meticulously examining the challenges associated with oral bioavailability and thoroughly scrutinizing the anatomical features of the lymphatic system, we intend to illuminate the inherent advantages, strategic approaches, and recent advancements in lymphatic drug delivery. This comprehensive analysis of existing literature will offer valuable insights into the promising prospects of this often overlooked and underutilized route for improving drug delivery and optimizing therapeutic outcomes [7].
APPROACHES TO IMPROVE ORAL BIOAVAILABILITY
Prodrugs
Prodrugs, inactive derivatives of active drug moieties, undergo enzymatic biotransformation in the body to release the active parent drug. This chemical modification strategy is widely employed to enhance drug properties such as aqueous solubility, lipophilicity, stability, mucosal membrane permeability, and therapeutic index. By improving water solubility and gastrointestinal permeability and overcoming first-pass metabolism, prodrug design can effectively enhance the oral bioavailability of drugs. Various prodrugs exist, including those utilizing ester, amide, carbonate, carbamate, azo, glucuronidic, and glycosidic bonds.
Prodrug development aims to optimize drug solubility, permeability, stability, or target selectivity, ultimately leading to improved oral bioavailability. Prodrugs are designed to release the active pharmacological agent at the target site through specific enzymatic or chemical reactions in the body. For instance, the prodrug enalapril undergoes conversion to its active form, enalaprilat, in the liver, resulting in enhanced oral bioavailability and prolonged therapeutic effects. The strategic utilization of prodrugs offers excellent potential for overcoming oral bioavailability challenges and achieving improved drug delivery outcomes.
Prodrugs offer excellent potential for enhancing oral bioavailability; however, challenges such as enzyme specificity (Prodrug activation relies on specific enzymatic pathways, and variations in enzyme expression among individuals may impact the predictability of prodrug conversion), variable absorption rates (The rate of prodrug activation and subsequent release of the active drug may vary among patients, affecting the consistency of therapeutic outcomes), and chemical stability (Some prodrugs may exhibit limited chemical stability, leading to premature activation or degradation before reaching the target site) need careful consideration in their design to ensure consistent and reliable drug delivery [8].
Nanoparticles
Nanoparticles, submicron-sized particles, provide a versatile and practical platform for enhancing the oral bioavailability of drugs. These particles can encapsulate drugs and protect them from degradation within the gastrointestinal tract while improving drug absorption through increased surface area and extended residence time in the gut. Nanoparticles can be fabricated from diverse materials such as lipids, polymers, and metals. Various nanoparticle-based drug delivery systems, including liposomes, solid lipid nanoparticles, polymeric nanoparticles, and gold nanoparticles, have been explored to improve oral bioavailability.
By formulating nanoparticles with different materials, such as polymers, lipids, or inorganic substances, drug solubility can be enhanced, degradation can be prevented, and targeted delivery can be achieved. Nanoparticles can incorporate drugs into their structure or use drug-loaded coatings on their surface. This protective capability ensures drug stability within the gastrointestinal tract, facilitates efficient drug absorption, and assists drug transport across the intestinal epithelium. Promising examples of nanoparticle-based approaches encompass polymeric nanoparticles, lipid nanoparticles (liposomes and solid lipid nanoparticles), and inorganic nanoparticles like gold nanoparticles.
While nanoparticles provide a versatile platform for improving oral bioavailability, challenges such as biocompatibility concerns (Certain materials used in nanoparticle formulations may raise biocompatibility issues, necessitating thorough biocompatibility studies), manufacturing issues (The scalability and reproducibility of nanoparticle manufacturing processes can be challenging, impacting their widespread application), and limited targeting precision (Achieving precise targeting with nanoparticles remains a challenge, as unintended interactions with off-target tissues may occur) warrant careful consideration in their application [9].
Permeation enhancers
Permeation enhancers play a crucial role in improving drug absorption by increasing the permeability of the intestinal epithelium. These compounds can exert their effects through various mechanisms, such as disrupting the lipid bilayer of cell membranes, promoting paracellular transport, or inhibiting efflux transporters. Surfactants, bile salts, fatty acids, and chelating agents are permeation enhancers in drug delivery. However, it is essential to note that using permeation enhancers may pose challenges, including potential toxicity and irritation in the gastrointestinal tract.
Permeation enhancers modify the properties of biological barriers, like the intestinal epithelium, to enhance drug absorption. They can improve drug solubility, disrupt tight junctions between epithelial cells, alter the activity of efflux transporters, or increase membrane permeability. Typical permeation enhancers encompass surfactants, bile salts, fatty acids, and chelating agents. For instance, sodium caprate, a surfactant, has been demonstrated to enhance paracellular transport and improve the oral bioavailability of specific drugs.
While permeation enhancers play a crucial role in improving drug absorption, concerns such as potential toxicity (Some of the permeation enhancers, particularly at higher concentrations, may exhibit toxicity and pose risks of irritation in the gastrointestinal tract), non-targeted effects (The action of permeation enhancers may not be specific to the intended drug, potentially affecting the absorption of co-administered substances), and the need for long-term safety assessments (The long-term safety of using permeation enhancers, especially with chronic administration, requires thorough investigation) underscore the importance of careful consideration in their application.
In summary, various approaches, including prodrugs, nanoparticles, and permeation enhancers, can enhance the oral bioavailability of drugs. These approaches offer different strategies to improve drug solubility, protect against degradation, and enhance absorption within the gastrointestinal tract. It is crucial to consider the advantages and limitations of each approach, as the optimal choice depends on the specific characteristics of the drug being developed or administered. Researchers and pharmaceutical scientists can optimize drug delivery and improve therapeutic outcomes by carefully selecting the appropriate approach [10].
THE LYMPHATIC SYSTEM AS A DRUG DELIVERY ROUTE
Anatomy and physiology of the lymphatic system
The lymphatic system is a complex network of lymphatic capillaries, lymph nodes, and lymphatic vessels that plays a crucial role in maintaining tissue fluid balance, immune function, and lipid absorption Figure 1.
Lymphatic capillaries
Lymphatic capillaries, the smallest vessels of the lymphatic system, play a crucial role in the uptake of interstitial fluid, macromolecules, immune cells, and drugs from the tissues. Composed of a single layer of overlapping endothelial cells, they form a one-way valve system that allows for the entry of substances into the lymphatic system. These thin-walled capillaries are distributed throughout the body, collecting excess interstitial fluid and dissolved substances like drugs. As the capillaries merge, they give rise to larger lymphatic vessels, facilitating the transport of the collected lymph fluid through lymph nodes and ultimately returning it into the bloodstream. This intricate network of lymphatic capillaries and vessels forms the foundation for drug delivery via the lymphatic system [4] Figure 2.
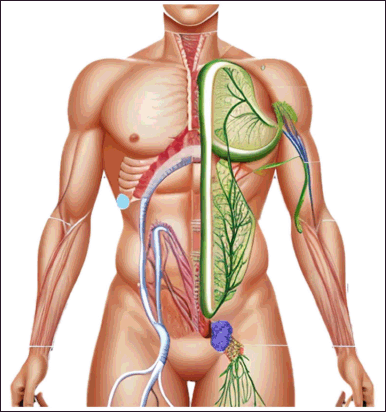 | Figure 1. Illustrates the lymphatic system as a circulatory system in the body. [Click here to view] |
Lymphatic nodes
Lymph nodes are small, bean-shaped structures distributed along the body’s lymphatic vessels. These organs filter lymphatic fluid and eliminate foreign particles, including bacteria, viruses, and cancer cells. In addition, lymph nodes are vital in the immune response, activating lymphocytes and producing antibodies to combat infections.
Lymph nodes serve as specialized organs along the lymphatic network, hosting various immune cells that filter and capture foreign particles, pathogens, and antigens in the lymph fluid. They also play a pivotal role in initiating immune responses and generating immune memory. Knowledge of the anatomy and distribution of lymph nodes is critical for targeted drug delivery, as these nodes can serve as potential sites for drug accumulation and interaction with the immune system [11] Figure 3.
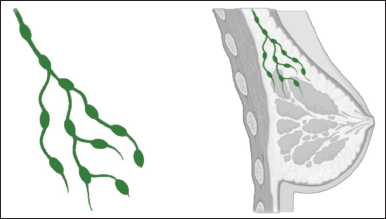 | Figure 2. Lymphatic capillaries in the breast. [Click here to view] |
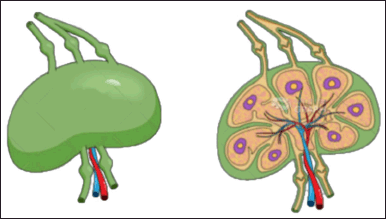 | Figure 3. Represents the cross-sectional view of a lymphatic node. [Click here to view] |
Lymphatic vessels
Lymphatic vessels, larger than lymphatic capillaries, consist of multiple layers of smooth muscle cells and serve as conduits for lymphatic fluid and immune cells. These vessels transport lymph from the tissues to the lymph nodes and ultimately to the bloodstream. In addition, they play a crucial role in maintaining fluid balance and lipid metabolism within the body.
Similar in structure to blood vessels but with thinner walls and valves that prevent backward flow, lymphatic vessels facilitate the movement of lymph fluid and its contents, including absorbed drugs and particles. The lymphatic vessels propel the collected lymph and any substances toward larger lymphatic trunks, eventually reaching the venous circulation and bypassing the liver’s first-pass effect. The unique characteristics of lymphatic vessels make them an attractive avenue for drug delivery, particularly for substances that face challenges crossing blood capillaries [3] Figure 4.
Mechanisms of drug delivery to the lymphatic system
Lymphatic uptake
Lymphatic uptake refers to how drugs or particles are absorbed from the administration site into the lymphatic capillaries. This uptake can occur through various mechanisms, including diffusion, convection, and active transport. The unique structure of lymphatic capillaries, characterized by discontinuous endothelial cells and loose intercellular junctions, allows for the easy entry of macromolecules and lipophilic compounds. Targeting lymphatic uptake holds significant promise in drug delivery strategies as it offers several advantages. Utilizing the lymphatic system as a drug delivery route can improve drug bioavailability, particularly for substances susceptible to first-pass metabolism. Furthermore, bypassing the liver’s first-pass effect through lymphatic uptake can enhance therapeutic outcomes. Therefore, understanding and harnessing the mechanisms of lymphatic uptake plays a crucial role in developing effective strategies for delivering drugs to the lymphatic system [12].
Lymphatic transport
Lymphatic transport is the process by which drugs or particles, once absorbed into the lymphatic capillaries, are transported through the lymphatic vessels toward the lymph nodes and eventually reach the bloodstream. This transport is facilitated by the contraction of lymphatic smooth muscles and the rhythmic propulsion of lymphatic valves, ensuring unidirectional flow and preventing the backflow of lymphatic fluid. Various factors, including particle size, charge, hydrophobicity, and surface properties, can influence the efficiency of lymphatic transport. Understanding the mechanisms underlying lymphatic transport is crucial for designing and developing drug-delivery systems that can effectively target the lymphatic system. By harnessing lymphatic transport, drug delivery to the lymphatic system can be enhanced, offering opportunities for improved therapeutic outcomes and bypassing first-pass metabolism [13].
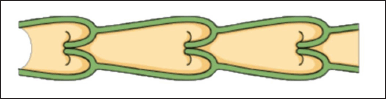 | Figure 4. Illustrates the lymphatic vessels. [Click here to view] |
Lymphatic drainage
Lymphatic drainage refers to the process by which the lymph fluid and any absorbed drugs or particles are drained from the lymphatic vessels back into the bloodstream. This vital process involves larger lymphatic trunks that eventually connect to the thoracic or right lymphatic duct, leading to the subclavian veins. By bypassing the liver, drugs delivered via the lymphatic system can avoid first-pass metabolism, resulting in increased systemic circulation of the active drug form and improved therapeutic outcomes.
The lymphatic drainage process is facilitated by the rhythmic contraction of lymphatic smooth muscles and the presence of one-way valves that prevent the backflow of lymphatic fluid. These mechanisms ensure the efficient movement of lymph fluid and its contents, including drugs, toward the bloodstream. It is worth noting that understanding the specific anatomical pathways, such as the thoracic duct and right lymphatic duct, is crucial for targeted drug delivery to the lymphatic system.
Using the lymphatic drainage pathway, drugs can bypass the liver’s first-pass effect, significantly impacting their bioavailability. This advantage allows for a higher concentration of the active drug to reach systemic circulation, increasing its therapeutic potential. Moreover, avoiding first-pass metabolism reduces the potential for drug degradation and inactivation, further enhancing drug efficacy.
Lymphatic drainage plays a vital role in drug delivery by ensuring the return of lymphatic fluid and absorbed substances to the bloodstream. By harnessing this pathway, drugs can bypass hepatic metabolism, improving bioavailability and therapeutic outcomes. Understanding the mechanisms and anatomical pathways of lymphatic drainage is essential for designing effective drug delivery systems that target the lymphatic system.
In summary, the lymphatic system is an important route for drug delivery as it can bypass the liver and avoid first-pass metabolism. The lymphatic system consists of lymphatic capillaries, lymph nodes, and vessels that work together to transport lymphatic fluid and immune cells throughout the body [2].
DRUG DELIVERY SYSTEMS THAT DELIVER DRUGS VIA THE LYMPHATIC SYSTEM
Lipid-based formulations
Lipid-based formulations are a common approach for delivering drugs to the lymphatic system. Lipids are naturally transported via the lymphatic system, and lipid-based formulations can enhance drug solubility and stability and protect drugs from degradation in the gastrointestinal tract. Lipid-based formulations include liposomes, solid lipid nanoparticles, and self-emulsifying drug delivery systems (SEDDSs).
Liposomes are lipid-based vesicles composed of a lipid bilayer that can encapsulate hydrophilic and hydrophobic drugs within their aqueous cores or lipid bilayers. These spherical vesicles offer controlled release, improved stability, and enhanced drug solubility. Liposomes can facilitate lymphatic drug transport by utilizing the lymphatic system as a drug delivery route, enhancing drug bioavailability. An illustrative example of a liposomal formulation is Doxil (doxorubicin liposome), commonly employed in cancer treatment. Although Doxil is administered intravenously, it serves as an example to highlight the potential of liposomal formulations in enhancing drug delivery and improving therapeutic efficacy. This example underscores the versatility of liposomal formulations, even if the specific administration route differs from the context discussed in this article [9,14–17].
Solid lipid nanoparticles are submicron-sized particles with a solid lipid core that can encapsulate hydrophobic drugs. They can protect drugs from degradation and enhance drug absorption by increasing their residence time in the gut [18,19].
Lipid-indinavir nanoparticles have emerged as groundbreaking strategies, showcasing a substantial enhancement in drug delivery to lymph nodes compared to traditional methods [20,21]. Simulations further suggest the potential for less frequent, yet potent dosing, offering improved control over viral replication in critical lymphoid tissues [22]. The adaptability of lipid-based formulations is exemplified by a three-in-one nanoformulation, holding the potential for sustained drug concentrations in lymph node cells [23]. These advancements underscore the transformative impact of lipid-based drug delivery on optimizing therapeutic efficacy for lymphatic targeting.
SEDDS, or self-emulsifying drug delivery systems, are lipid-based formulations that can spontaneously form fine oil-in-water emulsions in the gastrointestinal tract when exposed to aqueous fluids. These formulations offer advantages such as increased drug solubility, enhanced absorption, and improved lymphatic uptake and transport. An example of a SEDDS is Sandimmune (cyclosporine), used to improve lymphatic delivery and exert immunosuppressive effects. By leveraging the self-emulsifying properties of SEDDS, drug solubility, and lymphatic drug delivery can be significantly improved.
Nanoemulsions are oil-in-water or water-in-oil emulsions with droplet sizes in the nanometer range. They can incorporate lipophilic drugs and improve their solubility, absorption, and lymphatic transport. One example is Intralipid, a nanoemulsion formulation that delivers fat-soluble vitamins and nutrients [20–23].
Examples of drugs delivered via lipid-based formulations include methotrexate, paclitaxel, and cyclosporine. These drugs have been shown to have improved bioavailability and therapeutic efficacy when delivered via the lymphatic system using lipid-based formulations.
Protein-based formulations
Protein-based formulations present an alternative strategy for drug delivery to the lymphatic system. By engineering proteins, it becomes possible to target specific receptors on lymphatic endothelial cells, facilitating the uptake of drugs into the lymphatic system. Various protein-based formulations have been developed, including albumin-based nanoparticles, immunoglobulin G (IgG)-based nanoparticles, and lymphatic-targeting peptides. These formulations offer improved stability, controlled release, and enhanced targeting capabilities. For instance, BIND-014 is a protein-based nanoparticle formulation of docetaxel that demonstrates effective lymphatic delivery and is used in cancer treatment.
Albumin-based nanoparticles are submicron-sized particles composed of human serum albumin, which can encapsulate hydrophobic drugs. These nanoparticles offer several advantages, including enhanced drug solubility, improved stability, and facilitation of lymphatic drug transport. Utilizing albumin as a drug carrier makes it possible to harness its binding properties for efficient transport through the lymphatic system. An example of an albumin-based formulation is Abraxane, which consists of paclitaxel albumin-bound nanoparticles. This formulation has shown efficacy in treating breast, pancreatic, and lung cancers [24–26].
IgG-based nanoparticles are submicron-sized particles composed of IgG that can target the neonatal Fc receptor on lymphatic endothelial cells, facilitating their uptake into the lymphatic system. Lymphatic-targeting peptides are short peptides that can bind to lymphatic endothelial cells and promote the uptake of drugs into the lymphatic system.
Conjugating drugs to carrier proteins can enhance their lymphatic delivery and improve pharmacokinetic properties. The protein carrier facilitates the interaction with lymphatic transporters and receptors, enhancing drug accumulation in lymphatic tissues. Kadcyla (ado-trastuzumab emtansine) is a protein-drug conjugate used to treat HER2-positive breast cancer [27].
Examples of drugs delivered via protein-based formulations include paclitaxel, doxorubicin, and siRNA. These drugs have been shown to have improved bioavailability and therapeutic efficacy when delivered via the lymphatic system using protein-based formulations.
In summary, lipid-based and protein-based formulations are two approaches for delivering drugs to the lymphatic system. Lipid-based formulations can enhance drug solubility and stability and protect drugs from degradation in the gastrointestinal tract, while protein-based formulations can target specific receptors on lymphatic endothelial cells, facilitating their uptake into the lymphatic system. Examples of drugs delivered via these formulations include methotrexate, paclitaxel, and doxorubicin.
ADVANTAGES AND LIMITATIONS OF DRUG DELIVERY TO THE LYMPHATIC SYSTEM
Advantages
Enhancing bioavailability
Drug delivery to the lymphatic system offers several advantages for enhancing drug efficacy and bioavailability. First, it improves bioavailability by bypassing the liver’s first-pass metabolism and delivering drugs directly to the systemic circulation. This is particularly beneficial for drugs with low solubility or poor absorption through other routes. For example, the liposomal formulation of Doxil has demonstrated enhanced bioavailability by utilizing the lymphatic system as a drug delivery route. Second, delivering drugs to the lymphatic system allows for targeted delivery to lymphatic tissues and lymph nodes, where diseases often originate or spread. This can be achieved using targeted protein-based nanoparticles or lymphatic-targeting peptides [3].
Targeted delivery
The lymphatic system provides a targeted delivery route for drugs to effectively treat diseases that spread through this system, including cancer and infectious diseases. By harnessing the lymphatic transport mechanism, drugs can be selectively directed toward specific lymphatic tissues or lymph nodes, allowing for precise and efficient treatment. This targeted approach enhances therapeutic efficacy, particularly in the case of diseases like cancer where lymphatic metastases are involved [1].
Reduce the first pass metabolism
The lymphatic system can reduce the first-pass metabolism of drugs by bypassing the liver and delivering drugs directly to the systemic circulation. Drugs delivered via the lymphatic system can bypass the liver’s first-pass effect, where drugs undergo metabolism and degradation before reaching systemic circulation. This is achieved through the anatomical route of drug transport through the lymphatic vessels, such as the thoracic duct, which drains directly into the systemic circulation, thus avoiding metabolism in the liver. By avoiding first-pass metabolism, drugs delivered through the lymphatic system can maintain their pharmacological activity and achieve higher systemic concentrations, improving therapeutic efficacy [1,4,28].
Limitations
Toxicity
Drug delivery via the lymphatic system can increase the risk of lymphatic system toxicity, including lymphedema, fibrosis, and inflammation. Directly targeting the lymphatic system for drug delivery may carry the risk of lymphatic system toxicity, which can occur due to the accumulation of drugs or drug carriers in the lymphatic tissues. This accumulation can cause local tissue damage or systemic toxicity, leading to adverse effects. For example, in certain studies involving liposomal formulations, the accumulation of liposomes in the lymph nodes has been associated with lymph node fibrosis and inflammation. To minimize these risks, it is crucial to carefully consider factors such as dosage, formulation, and toxicity profiles. Understanding the mechanisms through which lymphatic system toxicity can occur is essential for developing safe and effective drug delivery strategies [29].
Drug delivery via the lymphatic system can induce an inflammatory response, leading to tissue damage and impaired lymphatic function. When drugs are delivered to the lymphatic system, they can interact with lymphatic cells and trigger immune activation, releasing pro-inflammatory cytokines. This inflammatory response within the lymphatic tissues should be carefully monitored and controlled to prevent adverse effects and maintain proper lymphatic function. Monitoring the inflammatory response can help assess the safety and efficacy of drug delivery systems targeting the lymphatic system [13].
Limited number of drugs
There currently needs to be a limited number of drugs approved for lymphatic system drug delivery, which can restrict the widespread clinical application of this approach. Developing lymphatic-targeted formulations necessitates extensive research, including rigorous clinical trials, to establish their safety, efficacy, and regulatory approval. This limitation poses challenges and limits the availability of lymphatic drug delivery options for numerous therapeutic agents. However, ongoing research and advancements in drug delivery technologies continue to expand the potential of this approach, offering hope for developing more approved drugs designed explicitly for lymphatic system delivery in the future.
In summary, drug delivery to the lymphatic system offers several advantages, including improved bioavailability, targeted delivery, and reduced first-pass metabolism. However, there are also limitations to this approach, such as the risk of lymphatic system toxicity, the potential for an inflammatory response, and the limited number of drugs approved for lymphatic system drug delivery.
Overall, drug delivery via the lymphatic system provides significant benefits, including enhanced bioavailability, targeted delivery, and reduced first-pass metabolism. However, it is essential to be aware of the potential limitations, such as the risk of lymphatic system toxicity, the potential for an inflammatory response, and the limited number of approved drugs. These factors should be carefully considered and addressed during the development and evaluation of lymphatic drug delivery systems to ensure their safety and effectiveness.
FUTURE DIRECTIONS AND CHALLENGES
Future directions
Developing novel formulations
Developing novel formulations is a promising future direction for drug delivery to the lymphatic system. Recent advancements in formulation design have paved the way for improved drug solubility, stability, controlled release, and targeting capabilities. Novel formulations can enhance drug solubility, stability, and lymphatic transport and target specific vessels. Such formulations include lipid-based nanoparticles, protein-based nanoparticles, and lymphatic-targeting peptides. These innovative formulations hold great potential for improving drug delivery to the lymphatic system [30,31].
Exploration of new drug delivery systems and technologies, such as nanoparticles, liposomes, micelles, and hydrogels, is another aspect of future research. These systems can enhance lymphatic drug delivery by improving drug encapsulation, release kinetics, and targeting capabilities. Advancements in formulation design are vital to enhancing drug stability, controlled release, and targeting capabilities. Investigating novel materials and carriers can also optimize drug solubility, lymphatic transport, and lymph node targeting. These advancements in drug delivery systems and technologies offer promising opportunities for more effective and targeted drug delivery to the lymphatic system.
In summary, developing novel formulations and exploring new drug delivery systems and technologies represent an exciting future direction for drug delivery to the lymphatic system. These approaches can improve drug solubility, stability, lymphatic transport, and targeting capabilities, enhancing therapeutic outcomes. Continued research and development in these areas will contribute to the advancement of drug delivery strategies tailored to the unique challenges and opportunities presented by the lymphatic system.
Targeting lymphatic vessels
Targeting specific lymphatic vessels is another promising future direction for drug delivery to the lymphatic system. Specific lymphatic vessels can be targeted to treat diseases that spread through the lymphatic system, such as cancer and infectious diseases. By selectively targeting certain lymphatic vessels, drug delivery can be tailored to specific disease sites or regions, enabling more precise and targeted therapy.
• Development of strategies to target specific lymphatic vessels and lymph nodes for improved drug delivery.
• Utilizing lymphatic-targeting ligands, such as antibodies or peptides, enhances the selectivity and specificity of drug delivery to specific lymphatic sites.
• Research on identifying specific lymphatic markers or receptors that can be targeted for enhanced lymphatic drug delivery.
Recent advancements in imaging techniques and molecular targeting strategies have opened opportunities for identifying and selectively targeting lymphatic vessels associated with specific diseases. For example, targeted ligands such as antibodies or peptides can enhance the selectivity and specificity of drug delivery to specific lymphatic sites. Furthermore, ongoing research focuses on identifying specific lymphatic markers or receptors that can be targeted for enhanced lymphatic drug delivery. These advancements offer promising avenues for improving the efficacy and specificity of drug delivery to the lymphatic system.
Drug delivery can be optimized by targeting specific lymphatic vessels, allowing for increased therapeutic efficacy and reduced toxicity. This approach holds great promise for developing more personalized and effective therapies in the field of lymphatic drug delivery [3,6,7,32,33].
Combination therapy
Combination therapy is a promising future direction for drug delivery to the lymphatic system. By targeting multiple pathways involved in disease progression, combination therapy can enhance drug efficacy and reduce the risk of drug resistance. One example of combination therapy is the simultaneous use of chemotherapy and immunotherapy for cancer treatment.
• Exploration of combination therapies involving lymphatic drug delivery to enhance treatment efficacy.
• Investigation of synergistic drug combinations that can target multiple pathways involved in lymphatic diseases or metastatic cancers.
• Study of combination therapies that can promote lymphangiogenesis or modulate lymphatic function for therapeutic purposes.
Combining different therapeutic approaches or drugs synergistically presents an exciting strategy for enhancing lymphatic drug delivery. Combination therapy can address multiple disease mechanisms simultaneously and improve drug delivery to target sites. For instance, combining chemotherapy agents with immunomodulatory drugs or immune checkpoint inhibitors can enhance the immune response within the lymphatic system and improve treatment outcomes. Recent advancements in combination therapy have demonstrated promising results in preclinical and clinical studies. Further exploration of combination therapy in lymphatic drug delivery holds great promise for optimizing treatment strategies and improving patient outcomes.
In addition, it is essential to emphasize the significance of preclinical and clinical studies in validating the safety and efficacy of these novel formulations. Rigorous evaluation through preclinical models and clinical trials is crucial to ensuring the translation of promising drug delivery strategies into clinical practice. These studies play a vital role in understanding the developed formulations’ pharmacokinetics, biodistribution, toxicity, and therapeutic potential. These innovative approaches can only pave the way for practical and effective drug delivery to the lymphatic system through comprehensive evaluation and validation.
Overall, these future directions hold immense potential for advancing drug delivery to the lymphatic system, improving treatment outcomes, and addressing the challenges associated with current approaches. Continued research and collaborative efforts in these areas will contribute to the development of safe, targeted, and efficient strategies for delivering drugs to the lymphatic system [34,35].
Challenges
Regulatory bodies
Regulatory hurdles are a significant challenge for drug delivery to the lymphatic system. The regulatory approval process for lymphatic system drug delivery is complex and time-consuming, which can limit the clinical translation of this approach. To address these challenges, regulatory agencies must establish guidelines and frameworks to approve lymphatic drug delivery systems. Robust preclinical and clinical studies are required to demonstrate lymphatic delivery formulations’ safety, efficacy, and pharmacokinetic profiles. Standardized protocols and guidelines should also be developed to evaluate the performance and quality of lymphatic drug delivery systems. Overcoming these regulatory hurdles is essential for ensuring the successful advancement and implementation of lymphatic drug delivery strategies, ultimately benefiting patients and healthcare providers [36].
Safety concerns
Safety concerns pose significant challenges for drug delivery to the lymphatic system. Delivering drugs via the lymphatic system increases the risk of adverse effects, including lymphatic system toxicity and inflammatory responses, which can result in tissue damage and impaired lymphatic function.
To address these safety concerns, thorough assessments and mitigation strategies are essential. It is crucial to evaluate potential adverse effects of lymphatic drug delivery, such as lymphatic system toxicity, inflammatory responses, and tissue damage. Long-term safety profiles and potential complications from sustained drug exposure in the lymphatic system should be carefully examined. Understanding the biodistribution and clearance mechanisms of drug carriers or nanoparticles can also help minimize systemic toxicity and accumulation.
By systematically assessing and mitigating safety concerns, researchers and clinicians can enhance the safety profile of lymphatic drug delivery and minimize the risk of adverse effects. This approach is vital for successfully translating lymphatic drug delivery strategies into clinical practice [37,38].
Cost and scalability
Cost and scalability pose significant challenges for drug delivery to the lymphatic system. Developing and producing novel formulations for lymphatic drug delivery can be expensive and time-consuming, impeding their widespread clinical application. To address these challenges, it is crucial to focus on the following aspects:
• Development of cost-effective and scalable manufacturing processes tailored explicitly for lymphatic drug delivery systems.
• Consideration of the economic feasibility and accessibility of lymphatic drug delivery technologies to ensure their widespread implementation in clinical settings.
• Exploration of strategies aimed at streamlining production, reducing manufacturing costs, and enhancing scalability without compromising the quality and efficacy of the delivery systems.
Efforts to optimize cost and scalability factors are essential to enhancing the feasibility and practicality of lymphatic drug delivery and ensuring that innovative therapies can reach a broader patient population. Translating lymphatic drug delivery systems from the laboratory to clinical practice can be accelerated by prioritizing cost-effectiveness and scalability, benefiting patients who need targeted and effective treatments.
In summary, the development of novel formulations, such as lipid-based nanoparticles, protein-based nanoparticles, and lymphatic-targeting peptides, targeting specific lymphatic vessels, and combination therapy, such as combining chemotherapy with immunotherapy, are promising future directions for drug delivery to the lymphatic system.
The exploration of new drug delivery systems and technologies, such as nanoparticles, liposomes, micelles, and hydrogels, has shown potential for improving lymphatic drug delivery. Advancements in formulation design, such as enhanced drug stability, controlled release, and targeting capabilities, have been achieved. In addition, research on the utilization of lymphatic-targeting ligands, identifying specific lymphatic markers or receptors, and studying synergistic drug combinations for lymphatic drug delivery have been conducted [39].
However, regulatory hurdles remain significant challenges, including the need for specific guidelines and frameworks for the approval of lymphatic drug delivery systems and safety concerns such as assessing and mitigating potential adverse effects on the lymphatic system. Furthermore, the cost and scalability of developing and manufacturing novel formulations for lymphatic drug delivery must be addressed to facilitate widespread clinical implementation [40].
To overcome these challenges, ongoing research focuses on developing cost-effective and scalable manufacturing processes for lymphatic drug delivery systems. In addition, the economic feasibility and accessibility of lymphatic drug delivery technologies are being considered to ensure their clinical application. Streamlining production, reducing manufacturing costs, and enhancing scalability without compromising quality or efficacy are essential areas of exploration.
CONCLUSION
Drug delivery to the lymphatic system offers promising avenues for improving drug bioavailability and efficacy. Developing novel formulations, such as lipid and protein-based formulations, shows great potential for enhancing drug solubility, stability, and lymphatic transport. In addition, targeting specific lymphatic vessels can improve drug efficacy and minimize toxicity. Combination therapy, involving synergistic drug combinations, further enhances treatment outcomes by addressing multiple disease pathways.
However, the clinical translation of drug delivery to the lymphatic system faces challenges that must be addressed. Regulatory hurdles, safety concerns, and cost and scalability issues pose significant barriers to the widespread adoption of lymphatic drug delivery approaches. To overcome these challenges, future research should focus on developing cost-effective and scalable manufacturing processes, robust preclinical and clinical studies to assess safety and efficacy, and establishing standardized protocols and guidelines for evaluating lymphatic drug delivery systems.
Drug delivery to the lymphatic system has the potential to revolutionize drug therapy and improve patient outcomes. Researchers can unlock the full potential of lymphatic drug delivery by advancing novel formulations, targeting specific lymphatic vessels, and addressing regulatory and safety challenges. Continued research, collaboration, and innovation in this field will drive the progress of personalized medicine and transform the landscape of disease treatment. The future of drug delivery to the lymphatic system is bright, and its impact on improving therapeutic efficacy and patient well-being cannot be underestimated.
ACKNOWLEDGMENT
The authors express gratitude to the Manipal Academy of Higher Education for their unwavering support throughout the duration of this study.
AUTHORS CONTRIBUTIONS
All authors of this manuscript have made substantial contributions to the research process. They collectively participated in the conception and design of the study, the acquisition of data, and the analysis and interpretation of the results. Each author actively contributed to drafting the article and revising it critically for important intellectual content. Furthermore, all authors have agreed to submit this work to the current journal, provided their final approval for the version to be published, and have committed to being accountable for all aspects of the work. This authorship conforms to the requirements and guidelines set forth by the International Committee of Medical Journal Editors (ICMJE). Each author has met the criteria for authorship as outlined by the ICMJE, ensuring that they have played a significant role in the development and completion of this research.
FINANCIAL SUPPORT
The authors thank the Manipal Academy of Higher Education for the funding support via the Intra-Mural Fund.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest related to the publication.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All information and data discussed in this review have been sourced from the existing literature. The references cited in this article provide a comprehensive overview of the studies and findings included in our analysis.
PUBLISHERS NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Alqahtani MS, Kazi M, Alsenaidy MA, Ahmad MZ. Advances in oral drug delivery. Front Pharmacol. 2021;12:618411. CrossRef
2. Ahn H, Park JH. Liposomal delivery systems for intestinal lymphatic drug transport. Biomater Res. 2016;20(1):36. CrossRef
3. He R, Zang J, Zhao Y, Dong H, Li Y. Nanotechnology-based approaches to promote lymph node targeted delivery of cancer vaccines. ACS Biomater Sci Eng. 2022 Jan 10;8(2):406–23. CrossRef
4. Zhang Z, Lu Y, Qi J, Wu W. An update on oral drug delivery via intestinal lymphatic transport. Acta Pharm Sin B. 2021;11(8):2449–68. CrossRef
5. Darwis Y, Ali Khan A, Mudassir J, Mohtar N. Advanced drug delivery to the lymphatic system: lipid-based nanoformulations. Int J Nanomed [Internet]. 2013 Jul;8:2733. CrossRef
6. Han S, Trevaskis N, Gershkovich P, Kagan L. Lymphatic delivery and targeting of drugs, vaccines, and imaging agents. Front Pharmacol. 2022;13:1011778. CrossRef
7. Managuli RS, Raut SY, Reddy MS, Mutalik S. Targeting the intestinal lymphatic system: a versatile path for enhanced oral bioavailability of drugs. Expert Opin Drug Deliv. 2018;15(8):787–804. CrossRef
8. Dahan A, Zimmermann EM, Ben-Shabat S. Modern prodrug design for targeted oral drug delivery. Molecules. 2014;19(10):16489–505. CrossRef
9. Malam Y, Loizidou M, Seifalian AM. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci. 2009;30(11):592–9. CrossRef
10. Eleraky NE, Swarnakar NK, Mohamed DF, Attia MA, Pauletti GM. Permeation-enhancing nanoparticle formulation to enable oral absorption of enoxaparin. AAPS PharmSciTech. 2020;21(3):1–1. CrossRef
11. Boyd M, Risovic V, Jull P, Choo E, Wasan KM. A stepwise surgical procedure to investigate the lymphatic transport of lipid-based oral drug formulations: cannulation of the mesenteric and thoracic lymph ducts within the rat. J Pharmacol Toxicol Methods. 2004;49(2):115–20. CrossRef
12. Banan B, Wei Y, Simo O, Tso P, Abumrad NN, Flynn CR, et al. Intestinal lymph collection via cannulation of the mesenteric lymphatic duct in mice. J Surg Res. 2021;260:399–408. CrossRef
13. Trevaskis NL, Hu L, Caliph SM, Han S, Porter CJH. The mesenteric lymph duct cannulated rat model: application to the assessment of intestinal lymphatic drug transport. J Vis Exp. 2015;6(97):e52389. CrossRef
14. Laouini A, Jaafar-Maalej C, Limayem-Blouza I, Sfar S, Charcosset C, Fessi H. Preparation, characterization and applications of liposomes: state of the art. J Colloid Sci Biotechnol. 2012;1(2):147–68. CrossRef
15. Buboltz JT, Feigenson GW. A novel strategy for the preparation of liposomes: rapid solvent exchange. Biochim Biophys Acta (BBA) Biomembr [Internet]. 1999 Mar;1417(2):232–45. CrossRef
16. Ghule MM and Bhoyar GS. Formulation and evaluation of modified liposome for transdermal drug. J Dev Drugs. 2018;07(1):1–3. Available from: https://www.omicsonline.org/open-access/formulation-and-evaluation-of-modified-liposome-for-transdermal-drug-2329-6631-1000186-102364.html
17. Jain S, Kumar D, Swarnakar NK, Thanki K. Polyelectrolyte stabilized multilayered liposomes for oral delivery of paclitaxel. Biomaterials. 2012;33(28):6758–68. CrossRef
18. Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47(2–3):165–96. CrossRef
19. Abdel-Mageed HM, Abd El Aziz AE, Mohamed SA, AbuelEzz NZ. The tiny big world of solid lipid nanoparticles and nanostructured lipid carriers: an updated review. J Microencapsul. 2022;39(1):72–94. CrossRef
20. Kinman L, Brodie SJ, Tsai CC, Bui T, Larsen K, Schmidt A, et al. Lipid–drug association enhanced HIV-1 protease inhibitor indinavir localization in lymphoid tissues and viral load reduction: a proof of concept study in HIV-2287-infected macaques. J Acquir Immune Defic Syndr. 2003;34(4):387–97. CrossRef
21. Khanlou H, Louie S, Farthing C. Response to: lack of interaction between atazanavir and proton pump inhibitors in HIV-infected patients treated with ritonavir-boosted atazanavir. J Acquir Immune Defic Syndr. 2006;41(3):394. CrossRef
22. Snedecor SJ, Sullivan SM, Ho RJY. Feasibility of weekly HIV drug delivery to enhance drug localization in lymphoid tissues based on pharmacokinetic models of lipid-associated indinavir. Pharm Res. 2006;23(8):1750–5. CrossRef
23. Koehn J, Iwamoto JF, Kraft JC, McConnachie LA, Collier AC, Ho RJY. Extended cell and plasma drug levels after one dose of a three-in-one nanosuspension containing lopinavir, efavirenz, and tenofovir in nonhuman primates. AIDS. 2018;32(17):2463–7. CrossRef
24. Timur SS, Gürsoy RN. Design and in vitro evaluation of solid SEDDS for breast cancer therapy. J Drug Deliv Sci Technol. 2020;60:102023. CrossRef
25. Bhargavi P, Naha A, Kannan S, Rama A. Development, evaluation and optimization of solid self emulsifying drug delivery system (s-sedds) of lercanidipine hydrochloride. Res J Pharm Technol. 2020;13(10):4931–40. CrossRef
26. Chamieh J, Tarrat AD, Doudou C, Jannin V, Demarne F, Cottet H. Peptide release from SEDDS containing hydrophobic ion pair therapeutic peptides measured by Taylor dispersion analysis. Int J Pharm. 2019;559:228–34. CrossRef
27. Morgen M, Saxena A, Chen XQ, Miller W, Nkansah R, Goodwin A, et al. Lipophilic salts of poorly soluble compounds to enable high-dose lipidic SEDDS formulations in drug discovery. Eur J Pharm Biopharm. 2017;117:212–23. CrossRef
28. Liu J, Liu K, Zhang L, Zhong M, Hong T, Zhang R, et al. Heat/pH-boosted release of 5-fluorouracil and albumin-bound paclitaxel from Cu-doped layered double hydroxide nanomedicine for synergistical chemo-photo-therapy of breast cancer. J Control Release. 2021;335:49–58. CrossRef
29. Khalili L, Dehghan G, Hosseinpour Feizi MA, Sheibani N, Hamishekar H. Development of an albumin decorated lipid-polymer hybrid nanoparticle for simultaneous delivery of methotrexate and conferone to cancer cells. Int J Pharm. 2021;599:120421. CrossRef
30. Raval N, Mistry T, Acharya N, Acharya S. Development of glutathione-conjugated asiatic acid-loaded bovine serum albumin nanoparticles for brain-targeted drug delivery. J Pharm Pharmacol. 2015;67(11):1503–11. CrossRef
31. Yoo HS, Choi HK, Park TG. Protein–fatty acid complex for enhanced loading and stability within biodegradable nanoparticles. J Pharm Sci. 2001;90(2):194–201. CrossRef
32. Zhang L, Wang S, Zhang M, Sun J. J Drug Target. [cited 2002 Aug 12]:1–13. Available from: http://informahealthcare.com/
33. Edwards GA, Porter CJH, Caliph SM, Khoo SM, Charman WN. Animal models for the study of intestinal lymphatic drug transport. Adv Drug Deliv Rev. 2001;50(1–2):45–60. CrossRef
34. Wadhwa A, Mathura V, Lewis SA. Emerging novel nanopharmaceuticals for drug delivery. Asian J Pharm Clin Res. 2018;11(7):35–42. CrossRef
35. Kumar S, Dilbaghi N, Rani R, Bhanjana G, Umar A. Novel approaches for enhancement of drug bioavailability. Rev Adv Sci Eng. 2013;2(2):133–54. CrossRef
36. Zhang XY, Lu WY. Recent advances in lymphatic targeted drug delivery system for tumor metastasis. Cancer Biol Med. 2014;11(4):247–54.
37. Trevaskis NL, Kaminskas LM, Porter CJH. From sewer to saviour—targeting the lymphatic system to promote drug exposure and activity. Nat Rev Drug Discov. 2015;14(11):781–803. CrossRef
38. Kwiatkowski S, Knap B, Przystupski D, Saczko J, Kedzierska E, Knap-Czop K, et al. Photodynamic therapy–mechanisms, photosensitizers and combinations. Biomed Pharmacother. 2018;106:1098–107. CrossRef
39. Fu D, Li C, Huang Y. Lipid–polymer hybrid nanoparticle-based combination treatment with cisplatin and EGFR/HER2 receptor-targeting Afatinib to enhance the treatment of nasopharyngeal carcinoma. Onco Targets Ther. 2021;14:2449–61. CrossRef
40. Vashisth S, Singh G, Nanda A. A comparative study of regulatory trends of pharmaceuticals in Brazil, Russia, India and China (BRIC) countries. J Generic Med. 2012;9(3):128–43. CrossRef