INTRODUCTION
Ferrous fumarate is a source of iron, an essential micronutrient in the body [1]. However, ferrous fumarate has poor flow properties, which often cause compression problems and unpleasant taste and smell [2,3]. On the other hand, ferrous fumarate is a very effective source of iron but has a high frequency of side effects compared to other sources of iron [4]. Ferrous fumarate is insoluble in neutral pH but soluble in acidic solutions (such as stomach acid) [3]. Ferrous fumarate’s low bioavailability is related to its slow dissolution [5]. Ferrous fumarate has limited solubility in water, which causes the absorption of iron from ferrous fumarate into the digestive system to be limited. Iron in the ferrous form (Fe2+) is more easily absorbed by the body than iron in the ferric form (Fe3+). However, a high stomach acid environment can oxidize ferrous iron to ferric iron, which is less absorbed by the body [2]. Other substances in foods or supplements taken together with ferrous fumarate, such as polyphenols in tea, coffee, or red wine, may form complexes with iron. These complexes may inhibit the body’s absorption of iron, reducing the bioavailability of ferrous fumarate. Ferrous fumarate may cause side effects such as gastrointestinal disorders, such as diarrhea, nausea, or constipation [2,6].
Technology in formulation, especially pharmaceutical preparations and drug delivery systems, focuses on increasing the effectiveness of drug delivery at the right amount [7]. Liposomes with special designs can be used to protect active ingredients from biological degradation [7,8].
Liposomes have received particular attention in nanopharmaceuticals. The non-toxicity property of phospholipids and the ability to encapsulate different compounds, such as hydrophilic, lipophilic, and amphiphilic, make liposomes a promising option for better skin drug delivery [9,10]. However, most reports on conventional liposomes describe the local effect as an accumulation of vesicles in the stratum corneum or upper layers of the epidermis [11,12]. To overcome these limitations, nanoparticle technology can be used to push the absorption beyond the stratum corneum or epidermis. It can improve their bioavailability by using new lipid vesicles with pronounced membrane elasticity, such as flexible and elastic deformable liposomes [13,14]. This liposome exhibits superior skin penetration ability and can transport active substances to the deeper layers of the skin for the transdermal delivery system.
The aim of this study is to prepare and characterize ferrous fumarate chitosan liposome nanoparticle preparations for later application via transdermal delivery as an alternative for drug substances that have low bioavailability. Cholesterol and synthetic or natural phospholipids were combined to improve the stability of the nanoliposome. To reduce and uniform the size of liposome to the nanometer scale, ultrasonic and size extruders were employed. This study could give new insight into the delivery design of ferrous fumarate to improve its bioavailability and efficacy.
MATERIALS AND METHODS
Materials
Ferrous fumarate was purchased from FerroPharma Chemicals Ltd. 13, Erdosor Street H-6766 Doc, Hungary. Lipoid Dipalmitoyl phosphatidyl choline (DPPC) and Lipoid S100 (Lipoid GMBH) were purchased from Germany. Deionized water was purchased from OneMed (Sidoarjo, Indonesia). Ascorbic acid, Hydrochloric acid, Chloroform, Methanol Pa, 2,2bypyridine (Emerck), Cholesterol (Sigma-Aldrich), Tube 3 ml Solutions within (SUPELCO) (Sigma-Aldrich), and Strat-M® Membrane were purchased from Merck (Darmstadt, Germany). Phosphate-buffered saline (PBS) (Oxoid) pH 7.4 was purchased from ThermoFisher Scientific (USA).
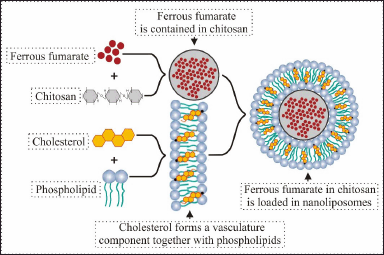 | Figure 1. Design of the nanoliposomes prepared in the current study. [Click here to view] |
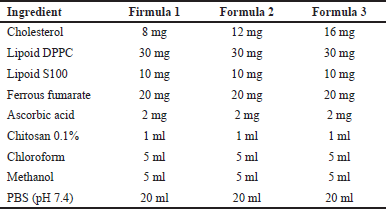 | Table 1. Formulation of ferrous fumarate nanoliposomes. [Click here to view] |
Preparation of ferrous fumarate liposomes into nanoliposomes
The thin film hydration method is the simplest in the manufacture of liposomes. This method uses volatile solvents such as chloroform, ether, and methanol to dissolve lipids (Table 1) [15]. The manufacture of nanoliposomes is based on two phases, namely phase 1 (non-polar) and phase 2 (polar) (Fig. 2A).
Liposomes were produced by the thin-layer hydration method with slight modification. Phase 1 ingredients consisting of DPPC lipoid, S100 lipoid, and cholesterol in various amounts (30:10:8, 30:10:12, 30:10:16 w/w) were mixed in chloroform and methanol (1:1 v/v) and then homogenized using a vortex for 3 minutes. The mixture was then evaporated using a rotary evaporator with a vacuum pressure of 200 mBar, a temperature of 50°C at 125 rpm for ±5 minutes. After the sample is seen to form a thin layer on the wall of the round bottom flask, the sample is incubated at room temperature to remove all the solvent that still remains in the sample for 30 minutes. The sample is back in the rotary evaporator with the same mechanism without being conditioned using a vacuum and adding the ingredients contained in phase 2, which have been homogenized using a vortex for 1–3 minutes. The preparation is again in the rotary evaporator with the same conditions for 30 minutes; after the liposome preparation is formed, it is put in the refrigerator at 2°C–8°C for 10–15 minutes Figure 1.
The liposome preparation method using thin film hydration has a drawback; namely, it is difficult to produce nano-sized liposomes. Therefore, additional procedures, such as sonication and extrusion, were used to obtain nanometer-sized vesicles (Fig. 2B) [16]. Suspensions of the formed liposomes were down-sized using ultrasonic (130 W, 20 kHz, USA) at 70% power in an ice bath for three stages (one stage consists of 5 minutes of which 2 minutes ultrasonic treatment and 3 minutes rest to allow cooling of the sample), and finally particle size homogenized using a mini extruder. One milliliter of nanoliposome suspension was passed through a membrane filter at 40°C. A water bath was used to maintain the desired liposome temperature. The extrusion process was performed with a 0.2 μm polycarbonate membrane filter (three cycles). The formed nanoliposome preparation was purified using solid phase extraction (SPE). Samples taken as much as 3 ml were placed in the tube solutions within and then flowed through the membrane assisted by the SPE push, and further characterization was carried out.
Evaluation of the physical characteristics of various prepared ferrous fumarate nanoliposomal formulations
An organoleptic test is performed to visually observe the sample from the sense of sight and smell based on the model’s color, shape, and smell. Particle size, polydispersity index value, and zeta potential value were observed using particle size analyser (PSA); 1 ml of the sample was put into the cuvette and then inserted into the cuvette holder to measure the particles and polydispersity index values. Zeta potential was analyzed using a zeta sizer. The sample is placed into a special zeta cuvette in the holder PSA to measure the zeta potential value. Morphological observations of ferrous fumarate nanoliposomes were performed using transmission electron microscopy (TEM) by placing approximately 500 μl of sample solution on a grid in the form of an electric grid and absorbing using filter paper with the help of a vacuum.
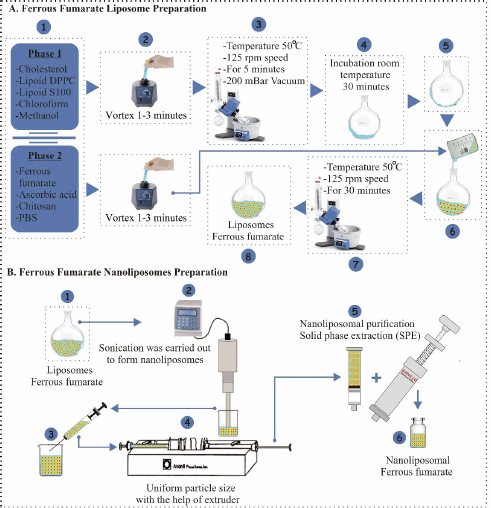 | Figure 2. Preparation of ferrous fumarate nanoliposomes in the current study. (A) Liposome preparation with thin film hydration method and (B) size reduction of liposome to nanoliposome. The sonication method followed by the extrusion process was used to reduce the size of liposomes to the nanometer scale. [Click here to view] |
Encapsulation efficiency evaluation
Two-milliliter aliquots of the sample were centrifuged at 5,000 rpm for 30 minutes at 4°C to separate the supernatant from the precipitated nanoliposomes. Four hundred microliters of sample/supernatant into a 10 ml volumetric flask, add 0.1 N HCl to the limit. Then, take the equivalent of 400 μl of a sample, put it into a 10 ml volumetric flask, add 400 μl of 0.5% bipyridine, and add to mark limits using a pH 4.6 list [17]. The analysis was performed using a Ultra Violet and Visible (UV/VIS) spectrophotometer, which had previously been validated to measure the amount of encapsulated and non-encapsulated ferrous fumarate. The absorption spectrum is 450–650 nm, and the maximum wavelength is 517.8 nm [18]. The encapsulation efficiency is determined as the percentage of ferrous fumarate encapsulated in nanoliposomes, which is calculated using the following equation [19]:
Stability test
The stability test of the preparation is seen on the physical stability, which is based on research by Gupta et al. [20] by looking at the material changes of shape, color, odor, particle size, and potential zeta value in environmental conditioning with a freeze-thaw cycle and a dilution resistance test. Moreover, a storage test of 2°C–8°C for 30 days by placing nanoliposomes in a vial and then storing it in a refrigerator at a temperature of 2°C–8°C. Samples were taken on day 30. Then, the preparation’s particle size and polydispersity index were tested [21,22].
In-vitro penetration test using Franz diffusion cells
Penetration test of ferrous fumarate nanoliposomes was performed with a Strat-M membrane using a Franz diffusion cell (diffusion area 1.77 cm2, compartment volume 12.0 ml using phosphate buffer solution pH 7.4 in the receptor compartment and temperature 37°C + 0.5°C). We weighed 1 g of ferrous fumarate nanoliposomes, and then applied them to the surface of the Strat-M membrane in the donor compartment. A total of 1 ml of sample was taken periodically for 2 hours from the receptor compartment using a one cc syringe and replaced with the same amount of solution, namely phosphate buffer pH 7.4. The sample was homogenized and put in a 10 ml volumetric flask with 0.1 N HCl up to the mark. Then, take the equivalent of 400 μl of a sample, put it in a 10 ml volumetric flask, add 400 μl of 0.5% bypiridine solution, and add it to the mark using a pH list of 4.6 [17]. Analysis was performed using a UV/VIS spectrophotometer with an absorption spectrum of 450–650 nm and a maximum wavelength of 517.8 nm.
RESULTS AND DISCUSSION
Evaluation of the physical characteristics of various prepared ferrous fumarate nanoliposomal formulations
The organoleptic test of ferrous fumarate nanoliposomes showed that the color was clear milky white, with a slight smell of fat, in the form of a suspension (Fig. 3). The milky white color of the dispersion indicates the formation of nanoliposomes [23,24].
The results of particle measurements in Table 2 show that the greater the cholesterol concentration, the smaller the particle size. Cholesterol is an essential component in cell membranes; it does not form double layers by itself, but will readily dissolve in the phospholipid bilayer [25]. The hydrophobic portion of cholesterol easily interacts with the core of the membrane. The addition of cholesterol to the liposome bilayer can increase its stability; because it becomes more stable, the preparation will not be easily affected by environmental influences to form uniform and more complex particles. Therefore, the results show a higher concentration of cholesterol results in a smaller size (Fig. 4A) [13].
 | Figure 3. Nanoliposomes ferrous fumarate after ultrasonic treatment and extrusion using a mini extruder. [Click here to view] |
The polydispersity index values are shown in Table 2, indicating that the greater the cholesterol concentration in each formula used, the smaller the polydispersity index value obtained. From the results, it can be seen that the value of the polydispersity index is a good value; it can also be seen in the distribution strain produced by each formulation on the particle size taken in Figure 5. The polydispersity index shows the particle size distribution in which the polydispersity index ranges from 0 (for samples that are perfectly uniform concerning particle size) to 1 (for highly polydisperse representatives with multiple particle size populations) [26]. A good polydispersity index value indicates long-term stability and particle size distribution in a formula [26]. The results of the polydispersity index of ferrous fumarate nanoliposomes have met the requirements for a good polydispersity index value by showing that the variations of the three formulations have relatively different average polydispersity index values (Fig. 4B).
The zeta potential value is shown in Table 2. This value is considered the optimal potential to ensure the stability of the particles that display a sufficient charge to inhibit vesicle aggregation [18]. The resulting zeta potential value shows that the variations from the three optimizations have sizes with an average that is not significantly different (Fig. 4C), but these results show that a higher concentration of cholesterol produces a more excellent potential zeta value; it is known that cholesterol can increase the stability of nanoliposome particle size by increasing the bending modulus and absolute zeta potential of nanoliposomes [27]. The results of the zeta potential evaluation showed that the addition of cholesterol increased the absolute zeta potential of ferrous fumarate nanoliposomes following the theory presented [28]. The lipids’ composition of lipids primarily determines the surface properties of nanoliposomes, such as lipids with a positive, negative, or neutral charge [14]. The resulting zeta potential is positively charged due to the binding of metal ions. Nanoliposomes can carry positively charged metal ions around their surface, which can cause the zeta potential to be positive [29]. In ferrous fumarate, the iron (II) ion (Fe2+) has twice the positive charge (+2).
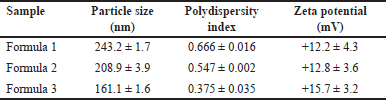 | Table 2. Values of particle size and polydispersity index and zeta potential of ferrous fumarate nanoliposomes. [Click here to view] |
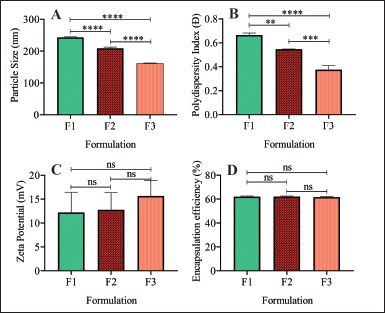 | Figure 4. Physical characteristic of ferrous fumarates nanoliposome. (A) Particle size, (B) polydispersity index, (C) zeta potential, and (D) encapsulation efficiency of the ferrous fumarate nanoliposome prepared. Samples were analyzed with one-way Analysis of variance (ANOVA) at a 95% confidence interval, followed by the post hoc comparison test. n: 3; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. [Click here to view] |
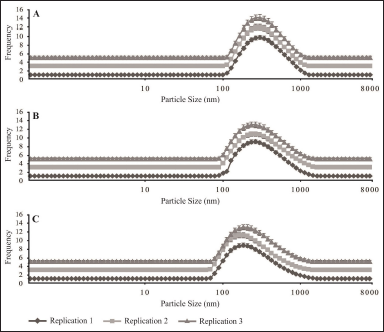 | Figure 5. Particle size distribution. (A) Formula 1, (B) formula 2, and (C) formula 3. [Click here to view] |
The morphology of nanoliposomes is spherical with multilamellar vesicles consisting of vesicles indicated by arrows, with each vesicle generally having five or more concentric lamellae with a relatively dense matrix structure with an even distribution among the particles (Fig. 6) [30].
The structure of the nanoliposome matrix is relatively dense due to the presence of a large number of matrix constituents, namely DPPC, which is solid. No drug crystals are visible on the TEM image; this may be due to the preparation technique or the drug loaded. Cholesterol is often used in liposome formulations due to its important role in improving membrane stability. Cholesterol helps to support the organization of the lipid layer, prevents excessive aggregation, and reduces membrane permeability. As a result of this stabilizing effect, liposomes tend to remain multilayered after sonication and extrusion processes [31].
Encapsulation efficiency evaluation
The results of the percent encapsulation efficiency of ferrous fumarate nanoliposome preparations can be seen in Table 3. The encapsulation is acceptable as the value is more than 50%. Statistical analysis showed that the ferrous fumarate nanoliposomes of the three formulations had relatively the same average encapsulation efficiency (Fig. 4D). Encapsulation efficiency indicates the amount of active substance that can be entrapped in the liposome nanoparticle matrix [32]. The higher value of encapsulation efficiency means that more active substances are trapped in the liposome nanoparticle preparation [33].
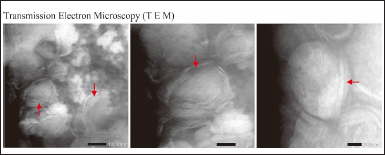 | Figure 6. Morphological observations of ferrous fumarate nanoliposomes were performed using TEM. [Click here to view] |
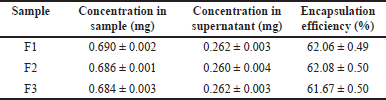 | Table 3. Encapsulation efficiency of ferrous fumarate nanoliposome. [Click here to view] |
Drug release from nanoliposomes is influenced by the synergistic effect of phospholipids and cholesterol, respectively. At lower amounts of cholesterol, liposomes showed faster drug release time than higher amounts of cholesterol liposomes [34]. The number of phospholipids and cholesterol can affect the percent encapsulation efficiency and particle size. The lower amount of cholesterol allows maximum internal water volume of the liposome vesicle bilayer, leading to more encapsulation. In comparison, higher cholesterol concentrations limit the entry of the internal vesicle core volume and cause a decrease in percent encapsulation efficiency [35]. Higher cholesterol concentration can improve the stability of nanoliposomes and prevent leakage or degradation of active ingredients during storage and shipment, which can help strengthen the lipid layer in the nanoliposome membrane, which is more stable and well-structured tends to result in more uniform and consistent nanoliposome particle size [36,37].
Cholesterol can affect the particle size regulation of nanoliposomes. Increasing cholesterol concentration tends to reduce the adhesion between lipid layers of nanoliposomes, prevent excessive aggregation, and strengthen the membrane. This leads to the formation of nanoliposome particles that are smaller and homogeneous in size [38]. Cholesterol may affect the flexibility of nanoliposome membranes. Higher cholesterol concentrations help to increase membrane flexibility, allowing nanoliposomes to better adjust in shape and size. A more flexible membrane enhances the ability of nanoliposomes to adapt to the environment and interact with the skin, thereby supporting better penetration and gradual release of active ingredients so as to improve the efficiency of penetration through the skin and uptake of active ingredients by target cells to enhance the transdermal therapeutic effect [39,40]. Nanoliposomes with higher cholesterol concentrations tend to have slower release rates of active ingredients. A more stable and flexible membrane slows the diffusion of active ingredients through the liposome membrane, thereby promoting a longer and sustained release of the active ingredients as the liposomes interact with the skin [34,41]. However, in this case, it is important to conduct a careful evaluation of costs and benefits when considering the use of higher cholesterol concentrations in nanoliposome formulations. Factors such as delivery effectiveness, product stability, formulation cost, and specific application needs must be considered to make an informed decision.
Stability test
The overall results of the stability test treatment show that the particle size obtained still meets the range of particle size values that are good for penetration into the dermis or through the route into the hair follicle to the systemic circulation system with a size of 100–300 nm in all stability tests [42,43]. The 250 times dilution level already shows a particle size distribution that tends to be significant (Table 4). This shows that small changes in nanoliposome preparation solubility can change the practice’s particle size. The greater the dilution factor, the more significant the change in the particle size of the preparation. This shows that the blisters tend to agglomerate under excessive dilution conditions [44].
While most of the polydispersity index values still show good polydispersity index values, which describe long-term stability and particle size distribution in a formula in all stability tests [26]. The 100 and 250 dilutions showed higher polydispersity index values (Table 4). This may be due to the higher dilution factor causing the preparation particles to be aggregated [26]. The characteristics of ferrous fumarate nanoliposomes at 100 and 250 times dilution are thought to form sedimentation, thereby affecting the particle size distribution of the preparation [45].
The zeta potential values resulting from all the stability test parameters showed no significant changes from the three formulations compared to the samples before the stability test (Table 4). This indicates that there is no significant effect on particle size stability that usually affects the zeta potential at the particle surface [18,46,47].
The encapsulation efficiency test showed that the percent encapsulation value of the three formulations for stability test parameters obtained values that were still acceptable (more than 50%) [35]. The resistance test to dilution of 50, 100, and 250 times results in encapsulation efficiency values below 50% (Table 4). This can happen because a higher dilution factor causes suppression of the maximum internal water volume of the liposome vesicle bilayer, which leads to more encapsulation that can affect the bonding of the phospholipid and cholesterol membranes, causing liposomes to leak [35].
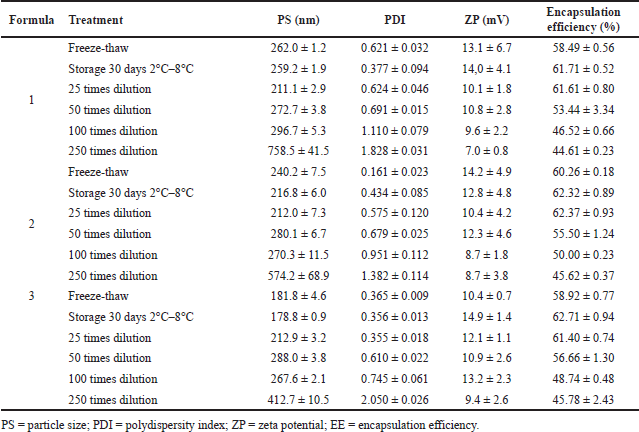 | Table 4. The stability profile of ferrous fumarate nanoparticles was examined with freeze-thaw, storage, and dilution tests. [Click here to view] |
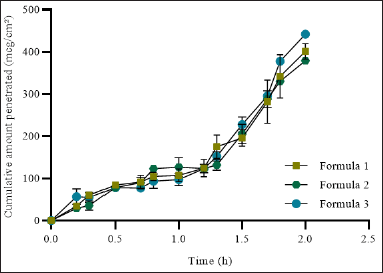 | Figure 7. In vitro skin permeation of ferrous fumarate nanoliposomes examined with Franz diffusion cell and Strat-M® Membrane as an in vitro skin model. [Click here to view] |
In-vitro penetration test using Franz diffusion cells
Synthetic membranes for in-vitro permeation studies were initially developed for use as an alternative to the use of human skin models [48]. Determination of permeation of drug formulations using ex-vivo human skin methods has several drawbacks that hamper the reproducibility of drug candidate screening data, including variation in skin thickness of the skin donor, diseased skin state, skin storage conditions, complexity of membrane preparation, hair follicle density, donor age, and high laboratory costs [49,50]. Some advantages of using synthetic membranes are controlled membrane thickness, faster membrane preparation time, low storage space, and relatively low cost [48].
Strat-M® membranes were designed with the intent to share similar structural and chemical characteristics found in the human epidermis with the goal of being a cost-effective membrane for testing and optimizing pharmaceutical formulations with good reproducibility to increase confidence during early-stage drug/formula development [48]. Strat-M® has been used extensively to study transdermal penetration to reduce the use of animals engineered to mimic human skin’s layered structure and lipid chemistry [48]. In this study, Start-M was used to asses skin penetration of the ferrous fumarate nanoliposime using Franz diffusion cells. The results of penetration testing through Strat-M® membrane showed the cumulative amount of ferrous fumarate nanoliposome diffusion per unit area at the second hour of each formula showed a cumulative value of formula 1 with an average of 401.048 mcg/cm² ± 32.364, formula 2 with an average of 378.854 mcg/cm² ± 10.124, and formulation 3 with an average of 442.077 mcg/cm² ± 17.270. The results showed that higher cholesterol concentration showed a higher cumulative diffusion rate of ferrous fumarate nanoliposomes per unit area compared to formulations with lower cholesterol concentration (Fig. 7).
The values obtained describe the levels of ferrous fumarate nanoliposomes in the receptor medium. This suggested that the number of phospholipids and cholesterol can affect the percent encapsulation efficiency, particle size, and the speed and penetration of the drug being delivered. The lower amount of cholesterol allows maximum internal water volume of bilayer vesicle liposomes, leading to more encapsulation; hence, liposomes show faster drug release time. Whereas a higher concentration of cholesterol restricts the influx of internal core volume of vesicles and leads to a decrease in percent encapsulation efficiency and shows relatively slower drug release [35].
The penetration rate (Flux) of ferrous fumarate nanoliposomes can be calculated from the data of the cumulative amount of diffusion of ferrous fumarate nanoliposomes per unit area by drawing a linear line to get a slope that shows the flux value of the preparation. Formula 1 had a flux value of 13,838 mcg/cm−2/hour−1 with a log time of 1 hour. Formula 2 has a flux value of 12,572 mcg/cm−2/hour−1 with a log time of 0.9 hours. Formula 3 has a flux value of 14,575 mcg/cm−2/hour−1 with a log time of 1 hour. The penetration rate (flux) of ferrous fumarate nanoliposomes is directly proportional to the cumulative amount of ferrous fumarate nanoliposome diffusion per unit area according to Fick’s first law. Therefore, the factors that affect the cumulative amount of diffusion of the preparation also affect the penetration rate (flux) through the Strat-M® membrane.
To deliver active drug ingredients to the skin by passive diffusion penetration through the stratum corneum. Penetration may also occur in hair follicles or sweat glands to a lesser extent [51]. Nanoliposomes have many advantages, such as increasing the absorption effect, preventing skin hydration, and increasing the penetration of active substances because of the ability of nanoliposomes to form an occlusive layer on the skin surface so that they can achieve the desired dosage and release interval [20,39,40,47]. According to research by Aguilar-Pérez et al. [52], nanoliposomes show deeper penetration ability in the skin. This proves that nanoliposomes’ penetration is a good option for drug delivery because of their similarity to biomembranes [53]. When nanoliposomes are applied to the surface of the skin, the water contained in the preparation will evaporate and leave an adhesive layer covering the skin, thereby reducing transepidermal water loss, which facilitates the drug to penetrate the deepest layers of the skin, reducing the corneocyte density and widening the inter-corneocyte gap [20,54]. This occlusive effect is related to the size of the nanoliposome. The size of the nanoparticles will provide an occlusive impact 15 times that of microparticles [26]. Based on research conducted by Tampucci et al. [55], it is known that the size of nanoparticles suitable for forming an occlusive layer on the skin is below 300 nm [55].
CONCLUSION
Preparation of ferrous fumarate nanoliposomes with higher concentrations of cholesterol using the thin layer hydration method, which was proceeded with an ultrasonic and mini extruder for the size reduction, could result in good physical characteristics and acceptable encapsulation efficiency. The nanoliposome was stable in freeze-thaw cycle environmental conditions, 30 days storage at 2°C–8°C, and 25 and 50 times dilution. Higher cholesterol concentrations are associated with a higher ferrous fumarate permeation in the in vitro model of the transdermal delivery system.
ACKNOWLEDGMENTS
The author would like to express his deepest gratitude to the Laboratory of Pharmacy, Master of Pharmacy Study Program, Universitas Gajah Mada, and those who supported the publication of this paper.
AUTHOR CONTRIBUTIONS
The authors would like to express their deepest gratitude to the Pharmaceutics Laboratory, Master of Pharmacy Study Program, Gadjah Mada University, and those who have helped support the publication of this paper. The authors would like to express the gratitude to Dr. Lutfi Chabib and Dr. Yandi Syukri for discussion and critical input. There is no conflict of interest.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Zhang Q, Lu XM, Zhang M, Yang CY, Lv SY, Li SF, et al. Adverse effects of iron deficiency anemia on pregnancy outcome and offspring development and intervention of three iron supplements. Sci Rep. 2021;11(1):1347. CrossRef
2. Harrington M, Hotz C, Zeder C, Polvo GO, Villalpando S, Zimmermann MB, et al. A comparison of the bioavailability of ferrous fumarate and ferrous sulfate in non-anemic Mexican women and children consuming a sweetened maize and milk drink. Eur J Clin Nutr. 2011;65(1):20–5. CrossRef
3. Sultana K. Formulation of ferrous fumarate (combination) tablets by using a direct-compression method. Indian J Sci Technol. 2010;3(9):994–1000. CrossRef
4. Tolkien Z, Stecher L, Mander AP, Pereira DIA, Powell JJ. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLOS ONE. 2015;10(2):e0117383. CrossRef
5. Geisser P, Burckhardt S. The pharmacokinetics and pharmacodynamics of iron preparations. Pharmaceutics. 2011;3(1):12–33. CrossRef
6. Bloor SR, Schutte R, Hobson AR. Oral iron supplementation—gastrointestinal side effects and the impact on the gut microbiota. Microbiol Res. 2021;12(2):491–502. CrossRef
7. Martien R., Adhyatmika A, Irianto IDK, Farida V, Sari P. Perkembangan Teknologi Nanopartikel Sebagai Sistem Penghantaran Obat. Majalah Farmaseutik. 2012;8:133–44. CrossRef
8. Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. PLGA-based nanoparticles: an overview of biomedical applications. J Controlled Release. 2012;161(2):505–22. CrossRef
9. He H, Lu Y, Qi J, Zhu Q, Chen Z, Wu W. Adapting liposomes for oral drug delivery. Acta Pharm Sin B. 2019;9(1):36–48. CrossRef
10. Gharib A, Faezizadeh Z, Godarzee M. In vitro and in vivo activities of ticarcillin-loaded nanoliposomes with different surface charges against Pseudomonas aeruginosa (ATCC 29248). DARU J Pharm Sci. 2012;20(1):41. CrossRef
11. Palac Z, Engesland A, Flaten GE, Škalko-Basnet N, Filipovi?-Gr?i? J, Vani? Ž. Liposomes for (trans)dermal drug delivery: the skin-PVPA as a novel in vitro stratum corneum model in formulation development. J Liposome Res. 2014;24(4):313–22. CrossRef
12. Müller S, Gruhle K, Meister A, Hause G, Drescher S. Bolalipid-doped liposomes: can bolalipids increase the integrity of liposomes exposed to gastrointestinal fluids? Pharmaceutics. 2019;11(12):646. CrossRef
13. Hosta-Rigau L, Zhang Y, Teo BM, Postma A, Städler B. Cholesterol – a biological compound as a building block in bionanotechnology. Nanoscale. 2013;5(1):89–109. CrossRef
14. Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;10:975. CrossRef
15. Danaei M, Kalantari M, Raji M, Fekri HS, Saber R, Asnani GP, et al. Probing nanoliposomes using single particle analytical techniques: effect of excipients, solvents, phase transition and zeta potential. Heliyon. 2018;4(12):e01088. CrossRef
16. Monteiro N, Martins A, Reis RL, Neves NM. Liposomes in tissue engineering and regenerative medicine. J R Soc Interface. 2014;11(101):20140459. CrossRef
17. Ismet R, Raeis BK, Sriwulan S. Penetapan Kadar Fe Gluconate dengan Metode Fe Fumarate Secara Spektrofotometri UV-Vis. J Ilm Tek Kim. 2022;6(1):39. CrossRef
18. Bochicchio S, Dalmoro A, Lamberti G, Barba AA. Advances in nanoliposomes production for ferrous sulfate delivery. Pharmaceutics. 2020;12(5):445. CrossRef
19. Verawaty V, Halim A, Febriyenti F. Efektivitas Sistem Penghantaran Liposom pada Katekin Sebagai Antioksidan. J Sains Farm Klin. 2016;2(2):176. CrossRef
20. Gupta S, Chavhan S, Sawant KK. Self-nanoemulsifying drug delivery system for adefovir dipivoxil: design, characterization, in vitro and ex vivo evaluation. Colloids Surf Physicochem Eng Asp. 2011;392(1):145–55. CrossRef
21. Haeri A, Alinaghian B, Daeihamed M, Dadashzadeh S. Preparation and characterization of stable nanoliposomal formulation of fluoxetine as a potential adjuvant therapy for drug-resistant tumors. Iran J Pharm Res. 2014;13:12.
22. Isalomboto Nkanga C, Murhimalika Bapolisi A, Ikemefuna Okafor N, Werner Maçedo Krause R. General perception of liposomes: formation, manufacturing and applications. In: Catala A, editor. Liposomes – advances and perspectives. London, England: IntechOpen; 2019. CrossRef
23. Anwekar H, Patel S, Singhai AK. Liposome – as drug carriers. Life Sci. 2011;2(7):7.
24. Popovska O, Simonovska J, Kavrakovski Z, Rafajlovska V. An overview: methods for preparation and characterization of liposomes as drug delivery systems. Int J Pharm Phytopharmacol Res 2013;3(3):182–9.
25. Melzak K, Melzak S, Gizeli E, Toca-Herrera J. Cholesterol organization in phosphatidylcholine liposomes: a surface plasmon resonance study. Materials. 2012;5(11):2306–25. CrossRef
26. Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10(2):57. CrossRef
27. Kondratowicz A, Weiss M, Juzwa W, Majchrzycki ?, Lewandowicz G. Characteristics of liposomes derived from egg yolk. Open Chem. 2019;17(1):763–78. CrossRef
28. Choudhury A. Liposome: a carrier for effective drug delivery. J Appl Pharm Res. 2020;8(1):22–8. CrossRef
29. Yoo JW, Irvine DJ, Discher DE, Mitragotri S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat Rev Drug Discov. 2011;10(7):521–35. CrossRef
30. Trevisan JE, Cavalcanti LP, Oliveira CLP, de La Torre LG, Santana MHA. Technological aspects of scalable processes for the production of functional liposomes for gene therapy. In: Yuan X, editor. Non-viral gene therapy. InTech; 2011. CrossRef
31. Cagdas FM, Ertugral N, Bucak S, Atay NZ. Effect of preparation method and cholesterol on drug encapsulation studies by phospholipid liposomes. Pharm Dev Technol. 2011;16(4):408–14. CrossRef
32. Derman S. Caffeic acid phenethyl ester loaded PLGA nanoparticles: effect of various process parameters on reaction yield, encapsulation efficiency, and particle size. J Nanomater. 2015;2015:1–12. CrossRef
33. Mouffok M, Mesli A, Abdelmalek I, Gontier E. Effect of the formulation parameters on the encapsulation efficiency and release behavior of p-aminobenzoic acid-loaded ethylcellulose microspheres. J Serbian Chem Soc. 2016;81(10):1183–98. CrossRef
34. Ruwizhi N, Aderibigbe BA. The efficacy of cholesterol-based carriers in drug delivery. Molecules. 2020;25(18):4330. CrossRef
35. Jabbar ASA, Ashour SM. Formulation and evaluation of antiretroviral drug loaded unsaturated phospholipid based stealth liposome. Syst Rev Pharm. 2021;12(1):7. CrossRef
36. Lombardo D, Kiselev MA. Methods of liposomes preparation: formation and control factors of versatile nanocarriers for biomedical and nanomedicine application. Pharmaceutics. 2022;14(3):543. CrossRef
37. Xia H, Tang Y, Huang R, Liang J, Ma S, Chen D, et al. Nanoliposome use to improve the stability of phenylethyl resorcinol and serve as a skin penetration enhancer for skin whitening. Coatings. 2022;12(3):362. CrossRef
38. Nsairat H, Khater D, Sayed U, Odeh F, Al Bawab A, Alshaer W. Liposomes: structure, composition, types, and clinical applications. Heliyon. 2022;8(5):e09394. CrossRef
39. Ghanbarzadeh S, Arami S. Enhanced transdermal delivery of diclofenac sodium via conventional liposomes, ethosomes, and transfersomes. BioMed Res Int. 2013;2013:1–7. CrossRef
40. Nakhaei P, Margiana R, Bokov DO, Abdelbasset WK, Jadidi Kouhbanani MA, Varma RS, et al. Liposomes: structure, biomedical applications, and stability parameters with emphasis on cholesterol. Front Bioeng Biotechnol. 2021;9:705886. CrossRef
41. Nikolova MP, Kumar EM, Chavali MS. Updates on responsive drug delivery based on liposome vehicles for cancer treatment. Pharmaceutics. 2022;14(10):2195. CrossRef
42. Nyerovwo DT. Effect of nanoliposomes on the stabilization of incorporated retinol. Afr J Biotechnol. 2010;9(37):6158–61. CrossRef
43. Fang CL, Aljuffali IA, Li YC, Fang JY. Delivery and targeting of nanoparticles into hair follicles. Ther Deliv. 2014;5(9):991–1006. CrossRef
44. Hurler J, Sørensen KK, Fallarero A, Vuorela P, Škalko-Basnet N. Liposomes-in-hydrogel delivery system with mupirocin: in vitro antibiofilm studies and in vivo evaluation in mice burn model. BioMed Res Int. 2013;2013:1–8. CrossRef
45. Jumaryatno P, Chabib L, Hayati F, Awaluddin R. Stability study of ipomoea reptans extract self-nanoemulsifying drug delivery system (SNEDDS) as anti-diabetic therapy. J Appl Pharm Sci. 2018;8(9):11–14. CrossRef
46. Lee JM, Lim DS, Jeon SH, Hur DH. Zeta potentials of magnetite particles and alloy 690 surfaces in alkaline solutions. Materials. 2020;13(18):3999. CrossRef
47. Rasmussen MK, Pedersen JN, Marie R. Size and surface charge characterization of nanoparticles with a salt gradient. Nat Commun. 2020;11(1):2337. CrossRef
48. Haq A, Goodyear B, Ameen D, Joshi V, Michniak-Kohn B. Strat-M® synthetic membrane: permeability comparison to human cadaver skin. Int J Pharm. 2018;547(1–2):432–7. CrossRef
49. Semlin L, Schäfer-Korting M, Borelli C, Korting HC. In vitro models for human skin disease. Drug Discov Today. 2011;16(3–4):132–9. CrossRef
50. Mathes SH, Ruffner H, Graf-Hausner U. The use of skin models in drug development. Adv Drug Deliv Rev. 2014;69–70:81–102. CrossRef
51. Alkilani A, McCrudden MT, Donnelly R. Transdermal drug delivery: innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics. 2015;7(4):438–70. CrossRef
52. Aguilar-Pérez KM, Avilés-Castrillo JI, Medina DI, Parra-Saldivar R, Iqbal HMN. Insight into nanoliposomes as smart nanocarriers for greening the twenty-first century biomedical settings. Front Bioeng Biotechnol. 2020;8:579536. CrossRef
53. Bianchi A, Velot É, Kempf H, Elkhoury K, Sanchez-Gonzalez L, Linder M, et al. Nanoliposomes from agro-resources as promising delivery systems for chondrocytes. Int J Mol Sci. 2020;21(10):3436. CrossRef
54. Schneider M, Stracke F, Hansen S, Schaefer UF. Nanoparticles and their interactions with the dermal barrier. Dermatoendocrinol. 2009;1(4):197–206. CrossRef
55. Tampucci S, Paganini V, Burgalassi S, Chetoni P, Monti D. Nanostructured drug delivery systems for targeting 5-α-reductase inhibitors to the hair follicle. Pharmaceutics. 2022;14(2):286. CrossRef