INTRODUCTION
A person who experiences hair loss every day can be considered normal if the amount of hair that falls out is still in the 50–100 strands daily. Hair loss in this amount is part of the normal hair growth cycle, where hair that falls out will be replaced with new hair [1]. However, if hair loss is more than 100 strands per day, it can indicate a disruption in the hair growth cycle, such as telogen effluvium, which stress, hormonal changes, or other factors can cause [2]. The hair growth cycle occurs in three phases, namely, the anagen phase (growth), the catagen phase (transition), and the telogen phase (hair loss) [3]. Hair growth medication can be used to treat baldness. However, some people are reluctant to use hair growth drugs because they are worried about the side effects of the drugs or cannot wait for the results [4].
Peppermint essential oil topical application can quickly stimulate and induce hair growth in the telogen phase [5]. This could be the answer to the problem of side effects or environmental impact of drugs that function as accelerating hair growth with synthetic active ingredients that are increasingly limited due to their toxicity and health risks. However, using essential oils, when applied directly, has disadvantages. It is difficult to penetrate the stratum corneum skin layer because it is volatile, has low adhesion, and is readily decomposed, and penetration into the skin is less effective [6].
Nanotechnology-based drug delivery systems can encapsulate various drugs in a nanocarrier system so that solubility and stability can be improved and the pharmacokinetic profile of the molecule can be improved [7,8]. The nontoxicity of phospholipids and the ability to encapsulate different compounds, such as hydrophilic, lipophilic, and amphiphilic, make liposomes a promising option for better skin drug delivery [9]. Most reports on conventional liposomes describe localized effects due to the accumulation of vesicles in the stratum corneum or upper epidermis layer [7]. To overcome this limitation, nanoparticle technology can enhance absorption past the epidermis, especially the stratum corneum. This can improve penetration and help increase drug bioavailability by using novel lipid vesicles with membrane elasticity, such as flexible and elastic deformable liposomes and liposomes containing propylene glycol or polysorbate [10–12].
Active ingredients with the therapeutic purpose of accelerating hair growth with the problem of unwanted side effects or environmental impacts are a growing concern, and synthetic medicinal materials are increasingly limited due to their toxicity and health risks. As a result, using active ingredients to accelerate hair growth is increasingly restricted, and this limitation is a severe problem. Therefore, the discovery of medicinal agents that have the potential to accelerate new hair growth of natural origin is an urgent need, and plants can be a good source for this problem. Thus, in this study, researchers are interested in formulating NANO-SERF-OPs: nanoliposomes serum as carriers of peppermint oil. It is a therapy that accelerates hair growth with a controlled small molecule protection system with high sensitivity and low toxicity.
MATERIALS AND METHODS
Materials
The tools used in this study were analytical balance (Mettler Toledo), ultrasonic homogenizer (Model 150VT, BioLogics, Inc., USA), particle size analyzer (PSA) (Horiba Scientific, Nano Particle Analyzer SZ-100), laminar airflow (LAF), autoclave (HICLAVE HVE-50, Japan), microscope (ZEISS SteREO Discovery. V8, Germany), refrigerator, magnetic stirrer, micropipette (BRAND TransferpetteÒ, Germany), Petri dish, pH meter (Horiba LAQUAact D71, Japan), viscometer (Brookfield DV-I Prime, USA), transmission electron microscope (JEM 1400, JEOL), homogenizer (IKA T25 digital Ultra Turrax), rotary evaporator (Heidolph Instruments GmbH & Co. KG, Germany), vortex (Heidolph, Germany), hot plate stirrer (Scilogex MS7-H550-S), and a set of glassware (Iwaki, Indonesia). The materials used were peppermint essential oil obtained from Young Living Essential Oils, a vital oil company based in Lehi, Utah, USA, Lipoid Phospolipon® 80H, and Lipoid Phytosolve® (Lipoid GMBH) purchased from Germany. Chloroform, Polysorbate 20, methanol Pa, (Merck), carbopol 940, methyl paraben, propyl paraben, distilled water, TEA (triethanolamine), 3-ml tube solution in (SUPELCO) (Sigma-Aldrich), and PBS (phosphate-buffered saline) (Oxoid) pH 7.4 were purchased from ThermoFisher Scientific (United States).
Preparation of NANO-SERF-OPs
Liposomes are produced by a thin-layer hydration method with modified phospholipids and Polysorbate 20 in a matrix that allows control over the permeability of the liposome membrane (Fig. 1).
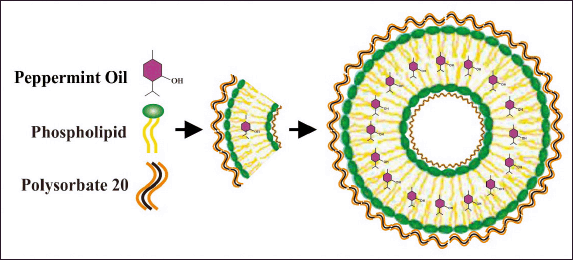 | Figure 1. NANO-SERF-OPs design prepared in this study. [Click here to view] |
The preparation of NANO-SERF-OPs is based on the formulation in Table 1, which includes a flow chart of the research procedure in Figure 2. Phase 1 ingredients consisting of Lipoid Phospolipon® 80H and Lipoid Phytosolve® were mixed in chloroform and methanol (1:1 v/v) and homogenized using a vortex for 1–3 minutes. The mixture was then evaporated using a rotary evaporator with a vacuum pressure of 200 bar and a temperature of 50°C at 125 rpm for ±5 minutes. After the sample was seen to form a thin layer on the wall of the round bottom flask, the sample was incubated at room temperature to remove all remaining solvent for 30 minutes, after which the sample was added to peppermint oil and then back in the rotary evaporator for 30 minutes with the exact mechanism but without being conditioned using vacuum. Finally, the ingredients in phase 2, consisting of PBS pH 7.4 and Polysorbate 20, were added and returned to the rotary evaporator for 30 minutes with the exact mechanism without being conditioned using a vacuum. After the liposome preparation is formed, the preparation is put into the refrigerator at a temperature of 2°C–8°C for 10–15 minutes.
 | Table 1. Formulation of NANO-SERF-OPs. [Click here to view] |
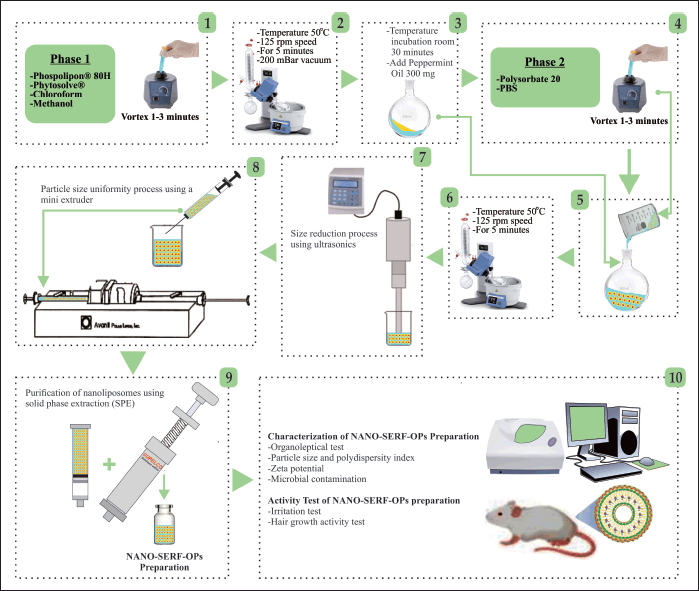 | Figure 2. Liposomes prepared using the thin-layer hydration method are reduced and homogenized into nanoliposomes by sonication, followed by an extrusion process using a mini extruder [7]. [Click here to view] |
The formed liposome suspension was reduced in size using ultrasonics (130 W, 20 kHz, USA), power 50%, pleasure 70%, and three cycles (one cycle consists of 5 minutes, of which 2 minutes of ultrasonic treatment and 3 minutes of break to allow cooling of the sample). Furthermore, the nanoliposome suspension was sized using a mini extruder for three cycles. One milliliter of nanoliposome suspension was passed through a 0.2-μm polycarbonate membrane filter at 40°C over a water bath. A water bath was used to maintain the desired liposome temperature. The nanoliposome preparation formed was purified using solid phase extraction (SPE). Samples taken as much as 3 ml were put into the solution tube inside and then flowed through the membrane with the help of an SPE pusher, and further characterization was carried out [7,13].
Preparation of NANO-SERF-OPs Serum Gel
NANO-SERF-OPs serum gel preparation was made based on the formulation in Table 2; 60 ml of distilled water and 10 ml of propylene glycol were heated simultaneously on an electric stove using different containers. After that, 20 mg of methylparaben and 20 mg of propyl paraben were added to the propylene glycol container until dissolved. Carbopol 940 was then developed in heated distilled water and homogenized using an ultraturrax homogenizer. The propylene glycol mixture was added gradually, and 10 ml of distilled water was added until a serum gel was formed. Next, 20 ml of peppermint essential oil nanoliposome preparation was added and homogenized again until peppermint essential oil nanoliposome gel was formed [14].
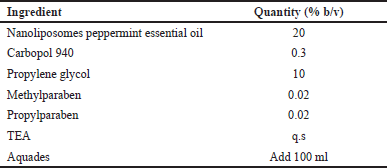 | Table 2. Formulation of NANO-SERF-OPs serum gel [Click here to view] |
Evaluation of physical characteristics of NANO-SERF-OPs
Particle size, polydispersity index (PdI) value, and potential zeta value were observed using Horiba Scientific’s PSA, Nano Particle Analyzer SZ-100. One milliliter of the sample was put into a cuvette, inserted into the PSA holder, and observed. As for the zeta potential value, the sample was put into a special zeta cuvette, inserted in the PSA holder, and followed. Morphological nanoliposome observation was done using transmission electron microscopy (TEM) JEOLJEM-1010. Five hundred microliters of sample solution were placed on a grid of electrical mesh, absorbed using filter paper with the help of a vacuum, and then observed [7,14].
Evaluation of physical characteristics of NANO-SERF-OPs serum gel
An organoleptic test was conducted to visually observe the sample with color, shape, and odor parameters of the nanoliposome gel. A homogeneity test was conducted by applying 1 mg of nanoliposome gel on an objective glass plate. It was then covered in another aim glass and observed whether it showed the presence or absence of coarse grains and inhomogeneous texture. The pH test was conducted using a semi-solid pH meter (Horiba Laqua) previously calibrated using pH four, seven, and ten buffers. Adhesion testing was done by weighing 0.5 g of nanoliposome gel placed between two glass plates and given a load weighing 500 g for 5 minutes and then tested by removing the glass plate using a load weighing 80 g and determining the release time using a stopwatch. The spreadability test was carried out by weighing 0.5 g of the preparation, placing it between two glass plates, giving a load of 50–2,000 g, and measuring the diameter of the spread of each load. Viscosity testing was conducted using a Brookfield DV-I Prime Viscometer with an S63 spindle speed of 30 rpm. Furthermore, the spindle was dipped into 100 g of nanoliposome gel in a glass beaker, and the centipoise value obtained was recorded [14].
NANO-SERF-OPs gel serum microbial contamination test
Testing begins by sterilizing test tubes, Erlenmeyers, and Petri dishes using an autoclave at 121°C for 15 minutes. Agar media is prepared by dissolving 22.5 g of plate count agar into 1 l of distilled water. The solution is cooked until boiling and fully dissolved and then sterilized again using an autoclave at 121°C for 15 minutes. Afterward, the agar medium is poured into Petri dish containers. To prevent contamination, materials and dilutions are handled in an LAF cabinet. Next, 10 g of nanoliposome gel is placed into a sterile bag, and 90 ml of peptone water is added, followed by homogenization for approximately 1 minute using a homogenizer.
One milliliter of the sample is taken from the sterile bag, and a 10-1 dilution is prepared by transferring it into 10 ml of peptone water. This process is repeated to prepare serial dilutions of 10-², 10-³, 10-4, and 10-4. Then, 1000 μl of each diluted sample is transferred onto agar media in Petri dishes. The samples are spread evenly using a sterilized spreader, allowed to solidify, and wrapped in parchment paper before being incubated at 35°C–37°C for 24 hours. Finally, the number of colonies that grow is counted [15].
Test animal preparation
Rats were acclimatized before testing to get used to living in a new environment, and treatment was given during the study. Previously, this study received ethical committee approval for using test animals as research subjects, with No. 159/KEP-PKU/X/2023. Acclimatization was carried out for ±7 days at laboratory room temperature ±20°C to 25°C, humidity 30%–70%, 12-hour light cycle, and 12-hour dark cycle. Rats were placed in a plastic box, 60 × 40 × 20 cm, with a wire lid and a base of rice husk. Rats were fed twice daily and drank ad libitum, replacing the husks every three days. After the rat is ready, its hair is shaved in the area it wants to be treated, either through irritation testing or hair growth activity. Before shaving, rats were anesthetized using a combination injection of ketamine 0.2 ml and xylazine 0.02 ml taken using a syringe and injected into the thigh of the rat and waited until fainting. Shearing was performed on the rat’s back with an area of 3 × 3 cm. After that, each rat’s back was shaved using scissors, and fine hair was cleaned until clean [16].
NANO-SERF-OPs gel serum irritation test
The irritation test was conducted with 15 Sprague Dawley male white rats as research subjects. The research subjects were treated by applying 0.5 g of each preparation and then covered with sterile gauze and plaster, after which the symptoms were observed after 24 hours. The data obtained were analyzed to determine the primary irritation index (PII) and then continued by scoring erythema and edema based on skin severity [17].
NANO-SERF-OPs gel serum hair growth activity test
The hair growth activity test was conducted with 30 male Sprague Dawley white rats as research subjects. Treatment of the research subjects was performed by applying 0.5 g of each preparation with a frequency of twice daily every 12 hours. Then, the hair was plucked every week for 1 month. The hair that grew was plucked on different sides ± ten strands. Then, the length, length, and thickness of the hair that has been plucked are observed on the thickest side. This observation was done using an electron microscope [18].
RESULTS AND DISCUSSION
Results of physical characteristics of NANO-SERF-OPs
Organoleptic tests of the resulting NANO-SERF-OPs showed a milky yellow color, which is likely due to the dispersion of the nanoparticles and the nature of the active ingredients and excipients, such as carrier oils or surfactants, which affect the system’s refractive index. The characteristic peppermint odor indicates the aroma stability of peppermint essential oil as an active ingredient. The NANO-SERF-OPs preparation also showed a slight fat content and a slightly viscous viscosity. This can be attributed to the lipid or phospholipid composition used in the nanoliposome formulation, which physicochemically tends to form a stable bilayer. The slightly viscous viscosity indicates thickening excipients or interactions between surfactants, phospholipids, and the liquid phase, which provide physical stability to the preparation [19]. After storage for 24 hours at 2°C–8°C, the preparation showed no phase separation or uneven color. This indicates that the formulation has good physical stability. This is most likely due to the ability of surfactants and phospholipids to form homogeneous nano-systems, prevent particle coalescence, and support the formulation’s thermodynamic stability—Figure 3A [20].
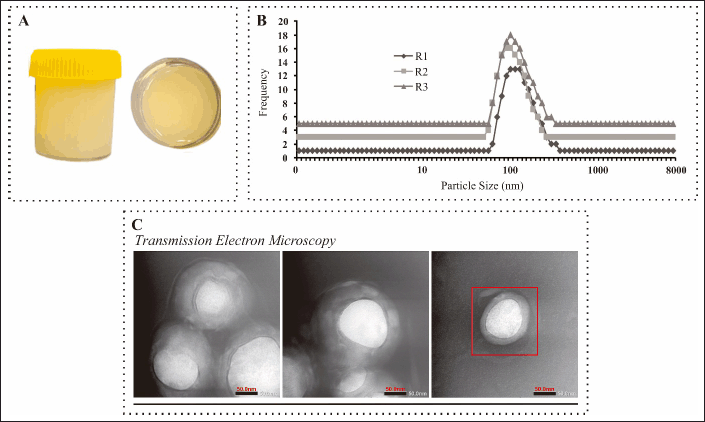 | Figure 3. Evaluation results of physical characteristics of NANO-SERF-OPs. A: Image of NANO-SERF-OPs serum gel preparation. B: Particle size distribution graph with three readings. C: morphology test preparation observed using TEM. [Click here to view] |
NANO-SERF-OPs measurements were analyzed using a PSA to determine the size and particle size distribution of the nanoliposomes formed. The measurement data of NANO-SERF-OPs can be seen in Table 3. The particle size test results have shown promising results and are by the nanoliposome size range of 10–200 nm [21]. NANO-SERF-OPs are formed based on a bottom-up manipulation technique, which manipulates the formation of phospholipids into a series of nanoliposome matrices [22]. This explains the formation of phospholipid particles from the synthesized form assembled into a nanoliposome matrix of nanometer size as a protective base and carrier encapsulating peppermint essential oil [7]. This size range reflects the efficiency of the manufacturing process, such as high-pressure homogenization or sonication, which is supported by the physicochemical properties of the excipients in the formulation. Phospholipids, as the main component of the nanoliposome bilayer, have amphiphilic properties that enable the formation of nano-sized vesicles through a self-assembly mechanism. Surfactants in the formulation stabilize the lipid–water interface, prevent particle aggregation, and help produce uniform particle size [20]. In addition, particle sizes in this nano range provide additional advantages, such as increasing the surface area for interaction with the target tissue, facilitating penetration through biological membranes, and improving the formulation’s physical stability. Thus, these results indicate that the excipients used have been appropriately selected to support the formation of stable and effective nanoliposomes [23].
 | Table 3. Test results for particle size, PdI value, and zeta potential value. [Click here to view] |
The particle size distribution of NANO-SERF-OPs can be seen in Figure 3B, illustrating that the resulting NANO-SERF-OPs preparation has a uniform particle size and shows an even distribution of particles. This result is confirmed by the polydispersity index value of 0.4 ± 0.02 PdI Table 3, indicating that the preparation is polydisperse, with nonuniform particle size but relatively even distribution. The polydispersity index represents the particle size distribution, and the polydispersity index values are between 0 (for samples with very uniform particle size) and 1 (for highly polydispersed samples with a large particle size population), among others [7,24,25]. This polydispersity index value describes the size distribution uniformity in the nanoparticle dosage system. The smaller the polydispersity index value, the better particle size and distribution uniformity [7]. The uniformity of the measured particle size distribution is strongly influenced by the physicochemical properties of the excipients, such as phospholipids and surfactants. Phospholipids, as a bilayer-forming component of nanoliposomes, have amphiphilic properties that allow the formation of vesicles of uniform size [26]. In addition, manufacturing processes, such as sonication or high-pressure homogenization, produce a more uniform particle size by evenly distributing energy in the system. Tins, the PdI value obtained reflects the success of the combination of excipients and manufacturing methods in producing a stable nanoliposome system [26,27].
The zeta potential value of peppermint essential oil nanoliposomes obtained was −12.6 ± 0.5 mV. A negative zeta potential value indicates that the interaction between peppermint essential oil and liposome-forming components creates a negative surface charge. The zeta potential values obtained suggest that the nanoliposomes have sufficient negative charge to provide some stability but may not be high enough to prevent accumulation completely. Typically, potential zeta values outside the range of −30 to +30 mV indicate good stability in suspension [7,24]. The zeta potential value of −12.6 mV indicates that the nanoliposomes have sufficient stability, although it is in a range close to the limit of moderate stability. This is likely due to the polarity of the phospholipid combination, which can affect the charge of the produced nanoliposomes [28]. The physicochemical properties of the excipients, such as phospholipids and surfactants, can also influence it. Phospholipids in the nanoliposome bilayer can produce a negative surface charge, especially if they contain negatively charged polar head groups such as Lipoid Phospolipon® 80H and Phytosolve®, which are highly purified forms of phospholipids, consisting mainly of phosphatidylcholine (>80%) [26]. The phosphatidylcholine head group is neutral, as there is a balance between the negatively charged phosphate group (-PO4²-) and the positively charged choline group (N+=(CH3)3). Overall, the charge on the polar head group of phosphatidylcholine is neutral because the positive charge on the choline group neutralizes the negative charge on the phosphate group [29]. Although phosphatidylcholine is neutral overall, the phosphate group on the polar head has a negative charge that is more exposed on the lipid bilayer surface than the positively charged choline group. As a result, the surface of nanoliposomes tends to show a negative charge. This is consistent with the NANO-SERF-OPs preparation produced with a combination of Phospolipon® 80H and Phytosolve® lipoid bilayers.
TEM testing was performed to observe nanoliposome morphology and distribution. Based on Figure 3C, the nanoliposomes have a spherical shape without particle aggregation. These results confirm that the nanoliposomes formed are of the small unilamellar vesicle (SUV) type with a particle size range of 2–200 nm. The obtained particle size of 96.7 ± 1.9 nm shows a uniform size distribution by the characteristics of a stable nanoparticle system [30]. These morphological properties are strongly influenced by the physicochemical properties of the excipients, especially phospholipids such as Lipoid Phospholipon® 80H and Lipoid Phytosolve®, which have an essential role in forming the lipid bilayer. Phosphatidylcholine, the main component of such excipients, has amphiphilic properties with polar head groups and nonpolar fatty acid chains, allowing spherical vesicles to form with a single layer [23,31].
The homogeneity test results showed that the resulting NANO-SERF-OPs serum gel was homogeneous, with no lumps or unevenness in the preparation. This indicates that the formulation created a stable and uniform system, which is crucial to ensure even distribution of the NANO-SERF-OPs serum gel on the skin during application. This stability can be influenced by the physicochemical properties of the excipients used, such as phospholipids in the NANO-SERF-OPs serum gel, which function to maintain the uniformity of the system by forming stable and well-dispersed vesicles in the gel matrix [32].
The pH value obtained was 5.78 ± 0.01, within the acceptable pH range of the human scalp (pH 4.5–6.5) [14]. This appropriate pH is vital to ensure comfortable use and prevent irritation. Excipients, such as phospholipids in NANO-SERF-OPs serum gel, gelling agents, and buffers, play a role in stabilizing the formula’s pH. The phospholipids used, such as Lipoid Phospholipon® 80H, have amphiphilic properties that help maintain pH stability in aqueous systems, keeping the serum gel formulation [33].
Meanwhile, the viscosity value obtained was 3,904 ± 45.49 cP. A suitable viscosity value of a serum gel-based preparation is in the range of 2,000–4,000 cP [34]. The viscosity of NANO-SERF-OPs serum gel preparation is strongly influenced by the concentration of gelling agents and other ingredients, such as triethanolamine, which is used to increase the viscosity of the gel. Triethanolamine is an alkali that helps the gel formation by increasing viscosity through interaction with the gelling agent polymer. With an increase in the concentration of gelling agents, the viscosity of the preparation will increase, resulting in a thicker texture and easier to apply without dripping or flowing quickly. These results indicate that the NANO-SERF-OPs serum gel formula has achieved optimal viscosity for comfortable and practical topical application [35].
The results of the spreadability test in Table 4 show that the spreadability obtained ranges from 5 to 12 cm. The greater the spreadability given, the ability of the active substance to spread and make contact with the skin to provide maximum effect [36]. When applied to the skin surface, it can affect the absorption and release of active substances and can ensure user comfort [37]. The physicochemical properties of excipients, especially the gelling agents of NANO-SERF-OPs serum and emulsifiers in the formulation, are essential in affecting spreadability. Excipients, such as gelling agents and solvents, affect the viscosity of the preparation. A preparation that is too thick may reduce spreadability, while a preparation that is too liquid may not provide sufficient contact with the skin. Therefore, a balance between gelling agent and solvent concentration is required to achieve optimal spreadability [38]. In addition, using nanoliposomes in formulations can improve the penetration and permeability of active substances into the deeper layers of the skin. When a formulation contains nanoliposomes, for example, the presence of phospholipids in the liposomes can help facilitate the absorption of the active substance through the lipid layer of the skin, increasing the effectiveness of releasing the active substance and ensuring the convenience of use [27]. In other words, good spreadability also contributes to the comfort of use, as a preparation that spreads quickly on the skin will not leave a sticky or uncomfortable feeling.
 | Table 4. Results of scatter ability testing of NANO-SERF-OPs serum gel. [Click here to view] |
The adhesion test results obtained results of 1.75 ± 0.01 seconds, showing that the NANO-SERF-OPs serum gel meets the requirements of more than 1 second. These results indicate that the NANO-SERF-OPs serum gel can adhere well to the skin. Adhesion is directly proportional to viscosity; the higher the viscosity, the higher the ability to adhere. Adhesion that is too strong will block the skin’s pores, while adhesion that is too weak will reduce the effectiveness of the active substance [35]. The adhesion test results showed a value of 1.75 ± 0.01 seconds, which met the requirement of more than 1 second. This indicates that the NANO-SERF-OPs serum gel can adhere well to the skin, providing sufficient contact time to release active substances effectively. Good adhesion is essential in ensuring that the preparation remains on the desired skin area, thus allowing enough time for absorption of the active substances [35]. Adhesion is directly proportional to the preparation’s viscosity; the higher the viscosity, the greater the ability to adhere to the skin surface. Viscosity is influenced by excipients such as gelling agents and other thickening agents, which increase the formulation’s viscosity. The higher the concentration of gelling agent used, the thicker the gel, which allows the gel to adhere to the skin for longer without flowing or drying too quickly [39]. However, it is essential to note that an adhesion that is too strong may cause skin irritation or clog pores, interfering with the skin’s breathing process and reducing user comfort. On the other hand, adhesions that are too weak will cause the formulation to be quickly erased or lost from the skin, reducing the effectiveness of releasing active substances. Therefore, the NANO-SERF-OPs serum gel formulation has achieved the right balance in viscosity and adhesion to ensure use comfort while maintaining the active substances’ effectiveness [40].
Results of NANO-SERF-OPs gel serum microbial contamination test
The results of the microbial contamination test obtained the calculation of the number of colonies as in Table 5. The number of colonies obtained was 1–29, so it did not meet the calculation rules. Total plate numbers can be calculated and analyzed when the number of colonies ranges from 30 to 300 colonies. This is because the agar media with the number of colonies >300 colonies is not valid to count. Hence, the possibility of miscalculation is enormous, whereas if the number of colonies produced <30, it results in invalidation when statistically calculated [15]. Therefore, the resulting preparation is free from contamination because it already contains formulated antimicrobials.
 | Table 5. Microbial contamination test results total plate count. [Click here to view] |
Results of NANO-SERF-OPs serum gel irritation test
Tests using rat test animals have met previous ethical clearance with registration number 159/KEP-PKU/X/2023. The results of the NANO-SERF-OPs serum gel irritation test can be seen in Figure 4. The PII results can be seen in Table 6. Based on these results, neither the control nor treatment groups did not cause irritation characterized by the absence of erythema and edema on the rats’ skin, so it was safe to use. This may be due to the pH of the nanoliposome gel preparation made by the skin’s acidity so that its use does not irritate. The nanoliposome gel formulation’s ingredients are also within the appropriate range in the Handbook of Pharmaceutical Excipients guidelines [18].
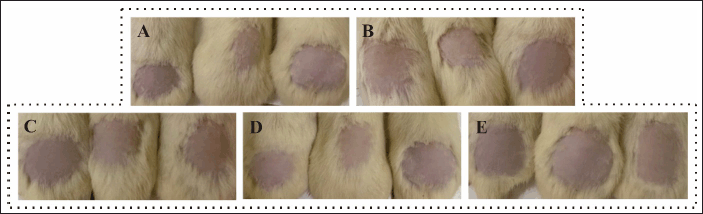 | Figure 4. Observation results of irritation test. A: Normal control (no treatment); B: Negative control (gel base); C: Positive control (Natur Hair Tonic GinsengTM); D: Treatment one (Peppermint essential oil gel); E: Treatment two (NANO-SERF-OPs serum gel). [Click here to view] |
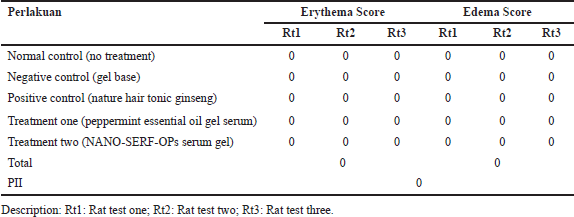 | Table 6. Irritation test calculation result. [Click here to view] |
Results of NANO-SERF-OPs gel serum hair growth activity test
The hair growth activity test results show the rat hair graph obtained in Figure 5A. The first week showed no significant hair length growth in all groups compared to the standard group. This is due to the adaptation period of rat skin, where in the early stages, rat skin may take time to adapt to the new formula. NANO-SERF-OPs serum gel may take time to penetrate the skin layer and reach the hair follicles. Samples of observation results of measuring the length and width of rat hair using an in vivo microscope for rat hair growth can be seen in Figure 6.
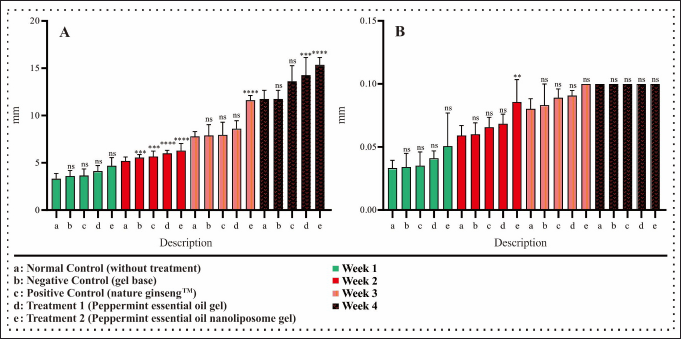 | Figure 5. Graph of mouse hair growth activity test. A: Rat hair length growth chart. B: Rat hair width growth graph. Samples were analyzed using one-way ANOVA with 95% confidence and a post hoc test to determine significant differences in each group. n: 5; ns: not significant, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. [Click here to view] |
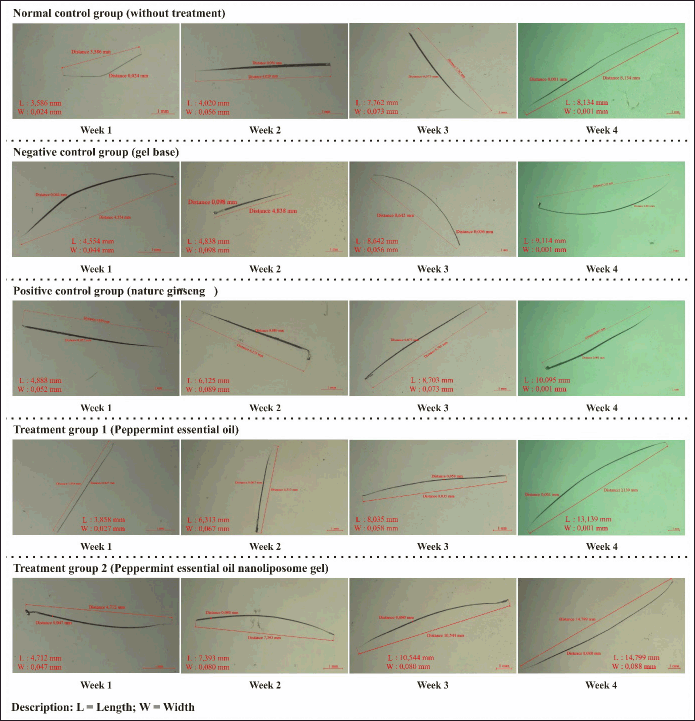 | Figure 6. Observation samples measuring the length and width of rat hair using a microscope. [Click here to view] |
Hair growth measurements may be more sensitive in the second week, so small changes in the first week may become more visible and measurable in the second week, giving the impression of a significant improvement [1]. The observation results in the second week showed a significant difference between the standard group with the negative control group and the positive control group at p < 0.001 in Figure 5A. The negative control group was treated using a gel base, which does not have ingredients that can stimulate hair growth. Therefore, the group only given the gel base should not have experienced significant changes in mouse hair growth. However, in this case, it may be because carbopol, when used as a gel base, can increase the water content in the skin. Hydrated skin can provide a better environment for hair growth, as hydration increases skin moisture in rats and reduces dryness, supporting hair follicle health. Carbopol also provides a cooling sensation that can cause vasodilation (dilation of blood vessels), which increases the supply of oxygen and nutrients to the hair follicles, thus promoting hair growth [42]. The positive control group was treated with Nature Hair Tonic GinsengTM, which is a preparation with active ingredients that have activity to accelerate hair growth on the market. These results showed a significant difference from the standard control group in hair length growth evaluated at p < 0.001. Treatment groups one and two showed a significant difference compared to the standard, negative, and positive control groups at p < 0.0001. Treatment group one was given peppermint essential oil gel serum. This shows that peppermint essential oil has active compound agents that function to accelerate hair growth. This is confirmed by Oh et al. [5], who state that peppermint contains menthol, the most effective penetration-enhancing compound, and limonene, the prototype for using terpenes as penetration enhancers. This suggests that peppermint facilitates hair growth by promoting the conservation of hair skin papilla vascularization and alkaline phosphatase activity, which improves blood circulation by relaxing vascular smooth muscle, which may contribute to the induction of the early anagen stage in the hair growth cycle [5,43]. Treatment group two, given NANO-SERF-OPs serum gel, showed a significant difference. This is because the nanoliposome gel delivery system is proven to accelerate hair growth. The small size of nanoliposomes can increase the surface area, which will increase solubility and allow good penetration into the scalp so that peppermint essential oil can reach its target better than conventional formulations [7].
The rat hair length growth results in the third and fourth weeks showed no significant difference between the standard and negative and positive control groups. As for treatment one, the third week also showed no significant difference, but the fourth week showed a significant difference of p < 0.001 in Figure 5A. This may be because, after the first 2 weeks, the hair and scalp may enter a phase of adaptation and stabilization to the treatment. Significant changes may not be seen during this period even though the treatment is still being applied [1]. The results of hair length growth of rats in treatment group two compared to other groups in the third and fourth weeks showed a very significant difference of p < 0.0001. This indicates that the physical form of nanoliposomes has excellent penetration ability. Therefore, there is a positive relationship between the delivery techniques using a nanoliposome base, which results in faster growth than conventional delivery. These results indicate that nanoparticle encapsulation of drug preparation can indirectly affect the penetration speed of the drug preparation to penetrate the membrane barrier [44]. These results can occur because nanoliposomes are useful as carriers of peppermint essential oil. They have flexible properties that control and deliver drugs to penetrate the membrane barrier, especially the challenge of passing through the stratum corneum into the dermis to the circulatory system or through the route into the hair follicles [7].
Similar to the growth of rat hair length in the first week, the growth of rat hair width also did not show a significant difference in the first week of all groups compared to the standard control group in Figure 5B.
The growth of rat hair width in the second week showed no significant difference in rat hair width growth compared to the standard control group between the negative, positive, and treatment groups in Figure 5B, while treatment group two showed a significant difference of p < 0.01. By the second week, the NANO-SERF-OPs serum gel may have reached the hair follicles and started working, while the gel base may not have shown the same effect due to slower absorption. Nanoliposomes can function as enhanced absorption because of their flexible nature and minimal structure, enabling them to penetrate the skin layers more effectively in gel-based formulations [45].
The growth of rat hair width at weeks 3 and 4 of each group did not show significant differences between the standard control group without treatment compared to the negative control group, positive control group, and treatments 1 and 2 in Figure 5B. This may be because the growth of rat hair width does not last forever. After reaching a particular stage, hair growth will stop, and the hairs will get the maximum length and width according to genetics and other factors such as hair growth cycle, environmental factors, nutritional health, and age of the rats [7,46]. The stimulating effect of the treatment from each treatment has reached its maximum point, and the growth rate of hair width begins to decrease or stabilize in the fourth week [47].
Thus, it can be concluded that NANO-SERF-OPs serum gel affects accelerating rat hair growth, where in the second week, significant hair growth was seen until the fourth week. This has been stated in Oh et al. [5] research, proving that peppermint essential oil topically can accelerate hair growth and induce rapid anagen hair growth in rat skin. In addition, it can also stimulate dermal activity that can improve blood circulation by relaxing smooth muscles in blood vessels to encourage blood flow and accelerate hair growth. When modified in nanoliposome gel serum, it accelerates hair growth further [5]. The small size of nanoliposomes can increase their surface area, which will increase solubility and allow good penetration into the scalp, allowing the drug to reach its target better than conventional formulations [7].
CONCLUSION
The resulting peppermint essential oil nanoliposome gel preparation is included in the SUV category, with a size of 96.7 ± 1.9 nm. It has an even particle size distribution and morphology with a sphere shape. Several other evaluations and characterizations, such as the pH test, viscosity test, spreadability test, and adhesion test, have met the requirements for the acceptability of the preparation. Meanwhile, the microbial contamination test showed that the preparation made was sterile. The peppermint essential oil nanoliposome gel preparation showed no signs of erythema and edema on the rat skin, so the preparation was safe for topical use. The results of graphical data on the effectiveness of hair growth of peppermint essential oil nanoliposome gel showed that the preparation increased the development of the length and width of rat hair, which correlated with each week and was superior to the other groups.
ACKNOWLEDGMENTS
The authors would like to thank the Nanopharmaceutical Research Center, Pharmaceutical Science and Technology Laboratory, Undergraduate Pharmacy Study Program, Universitas Islam Indonesia, and the Pharmaceutical Technology Research Center, Undergraduate Pharmacy Study Program, Sultan Agung Islamic University, as well as those who have supported the publication of this paper.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Ethical approvals details are given in the ‘Material and Method section’.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Natarelli N, Gahoonia N, Sivamani RK. Integrative and mechanistic approach to the hair growth cycle and hair loss. JCM. 2023;12(3):893. doi: CrossRef
2. Alanazi AS, Alsalhi WA, Alghuyaythat WK, Almutairi AN, Almazrou MA, Alabdulminaim JA, et al. Stress-related hair loss among the general population in Al Majma’ah, Saudi Arabia: a cross-sectional study. Cureus. 2023;15(10):e46517. doi: CrossRef
3. Lin CS, Chan LY, Wang JH, Chang CH. Diagnosis and treatment of female alopecia: focusing on the iron deficiency-related alopecia. Tzu Chi Med J. 2023;35(4):322–8. doi: CrossRef
4. Sattur SS, Sattur IS. Pharmacological management of pattern hair loss. Indian J Plast Surg. 2021;54(04):422–34. doi: CrossRef
5. Oh JY, Park MA, Kim YC. Peppermint oil promotes hair growth without toxic signs. Toxicol Res. 2014;30(4):297–304. doi: CrossRef
6. Souto EB, Fangueiro JF, Fernandes AR, Cano A, Sanchez-Lopez E, Garcia ML, et al. Physicochemical and biopharmaceutical aspects influence skin permeation and SLN and NLC’s role in skin drug delivery. Heliyon. 2022;8(2):e08938. doi: CrossRef
7. Suryani A, Laksitorini MD, Sulaiman TNS. Ferrous fumarate nanoliposomes: formulation, characterization, and diffusion profiles. J Appl Pharm Sci. 2024;14(5):157–65. doi: CrossRef
8. Chabib L, Suryani A, Pangestu MI, Hidayat AMUJ, Trianloka AMB. The development of Origanum vulgare L. into nanoparticles in dosage forms. Pharm Educ. 2021;21(2):205–9. doi: CrossRef
9. Abbasi H, Kouchak M, Mirveis Z, Hajipour F, Khodarahmi M, Rahbar N, et al. What we need to know about liposomes as drug nanocarriers: an updated review. Adv Pharm Bull. 2022:13(1):7–23. doi: CrossRef
10. Guillot AJ, Martínez-Navarrete M, Garrigues TM, Melero A. Skin drug delivery using lipid vesicles: a starting guideline for their development. J Control Release. 2023;355:624–54. doi: CrossRef
11. Chabib L, Hidayat AMUJ, Trianloka AMB, Pangestu MI, Suryani A, Yulianto. The therapeutic potential of Cymbopogon schoenanthus (L.) developed into nanoparticle technology. Pharm Educ. 2021;21(2):210–4. doi: CrossRef
12. Chabib L, Suryani A, Hakim SNP, Rizki MI, Firmansyah F, Yulianto, et al. Stevia rebaudiana is a nutraceutical for COVID-19 patients on a no-sugar diet during recovery and its nanoparticle application. Pharm Educ. 2022;22(2):174–9. doi: CrossRef
13. Ong S, Chitneni M, Lee K, Ming L, Yuen K. Evaluation of extrusion technique for nanosizing liposomes. Pharmaceutics. 2016;8(4):36. doi: CrossRef
14. Chabib L, Suryani A, Dewi LS, Noviani H, Maharani WHP, Indraswari AA. Pineapple fruit extract (Ananas comosus L. Merr) is an antioxidant and anti-acne agent made with the nano-emulsion gel delivery system. Pharm Educ. 2023;23(2):126–132. doi: CrossRef
15. Sundari S. Uji angka lempeng total (ALT) pada sediaan kosmetik lotion X di BBPOM medan. Jurnal Biologica Samudra. 2019;1(1):25–33.
16. Apriani EF, Ahmadi A, Noviani V. Formulation and evaluation of water fraction hair tonic containing flavonoids from ethanolic extract of green tea leaves (Camellia sinensis L.). TradMedJ. 2021;26(2):77. doi: CrossRef
17. Verawaty V, Halim A, Febriyenti F. Efektivitas sistem penghantaran liposom pada katekin sebagai antioksidan. JSainsFarKlin. 2016;2(2):176. doi: CrossRef
18. Kuncari ES, Iskandarsyah I, Praptiwi P. Uji iritasi dan aktivitas pertumbuhan rambut tikus putih: efek sediaan gel apigenin dan perasan herba seledri (Apium graveolens L.). Media Litbangkes. 2015;25(1):15–22. doi: CrossRef
19. Kuznetcova DV, Linder M, Jeandel C, Paris C, Desor F, Baranenko DA, et al. Nanoliposomes and nanoemulsions based on chia seed lipids: preparation and characterization. Int J Mol Sci. 2020;21(23):9079. doi: CrossRef
20. Lombardo D, Kiselev MA. Methods of liposomes preparation: formation and control factors of versatile nanocarriers for biomedical and nanomedicine application. Pharmaceutics. 2022;14(3):543. doi: CrossRef
21. Bochicchio S, Dalmoro A, Lamberti G, Barba AA. Advances in nanoliposomes production for ferrous sulfate delivery. Pharmaceutics. 2020;12(5):445. doi: CrossRef
22. Trevisan JE, Cavalcanti LP, Oliveira CL, de La Torre LG, Santana MH. Technological aspects of scalable processes for the production of functional liposomes for gene therapy. In: Yuan X, editor. Non-viral gene therapy. London, UK: InTech; 2011. doi: CrossRef
23. Majumdar S, Mahanti B, Kar AK, Parya H, Ghosh A, Kar B. Nanoliposome: as a smart nanocarrier in transdermal drug delivery system. Intelligent Pharm. 2024;2(6):768–76. doi: CrossRef
24. Chabib L, Suryani A, Indraswari AA, Fitria A, Laksitorini MD. Nanosilver synthesis of bromelain isolate from pineapple extract: a green approach for antibacterial applications. J Chem Health Risks. 2024;14(1):134–40.
25. Chabib L, Ar Rodli FH, Nugroho BH, Suryani A, Firmansyah F. Development of nanoliposome formulation of beta-carotene using high-speed homogenizer method. Pharm Educ. 2024;24(2):1–8. doi: CrossRef
26. Danaei M, Kalantari M, Raji M, Samareh Fekri H, Saber R, Asnani GP, et al. Probing nanoliposomes using single particle analytical techniques: effect of excipients, solvents, phase transition, and zeta potential. Heliyon. 2018;4(12):e01088. doi: CrossRef
27. Aguilar-Pérez KM, Avilés-Castrillo JI, Medina DI, Parra-Saldivar R, Iqbal HMN. Insight into nanoliposomes as smart nanocarriers for greening the twenty-first century biomedical settings. Front Bioeng Biotechnol. 2020;8:579536. doi: CrossRef
28. Rihhadatulaisy S, Sriwidodo S, Putriana NA. Stabilisasi liposom dalam sistem penghantaran Obat. Maj Farmasetika. 2020;5(5):257. doi: CrossRef
29. Hariharan VN, Nakamura T, Shin M, Tang Q, Sontakke V, Caiazzi J, et al. Phosphatidylcholine head group chemistry alters the extrahepatic accumulation of lipid-conjugated siRNA. Mol Ther Nucleic Acids. 2024;35(2):102230. doi: CrossRef
30. Barba AA, Bochicchio S, Dalmoro A, Lamberti G. Lipid delivery systems for nucleic-acid-based-drugs: from production to clinical applications. Pharmaceutics. 2019;11(8):360. doi: CrossRef
31. Nsairat H, Khater D, Sayed U, Odeh F, Al Bawab A, Alshaer W. Liposomes: structure, composition, types, and clinical applications. Heliyon. 2022;8(5):e09394. doi: CrossRef
32. Pasarin D, Ghizdareanu AI, Enascuta CE, Matei CB, Bilbie C, Paraschiv-Palada L, et al. Coating materials to increase the stability of liposomes. Polymers. 2023;15(3):782. doi: CrossRef
33. Arana-Linares AC, Barrera-Ocampo A, Salamanca CH. Determination of the critical aggregation concentration of phospholipids widely used in nanoliposomal development from different experimental methodologies. J Mol Liq. 2025;417:126677. doi: CrossRef
34. Nurwaini S, Fatimah MN. Formulation and characterization of gels of telang flower extract (Clitoria Ternatea L) with variations of carbopol concentration and antioxidant activity test using DPPH methods. ISSN. 2024;21(1).
35. Apriani EF, Kornelia N, Amriani A. Optimizing gel formulations using carbopol 940 and sodium alginate containing andrographis paniculata extract for burn-wound healing. JFIKI. 2023;10(3):300–11. doi: CrossRef
36. Putri Rahmani SI, Zulkarnain AK. Optimization of HPMC and Na-CMC as gelling agents on physical properties and stability in sunflower seed oil gel formulation. J Food Pharm Sci. 2023;11(2):812–9. doi: CrossRef
37. Irianto IDK, Purwanto P, Mardan MT. Aktivitas antibakteri dan uji sifat fisik sediaan gel dekokta sirih hijau (Piper betle L.) sebagai alternatif pengobatan mastitis sapi. Majalah Farmaseutik. 2020;16(2):202. doi: CrossRef
38. Milutinov J, Krstonoši? V, ?irin D, Pavlovi? N. Emulgels: promising carrier systems for food ingredients and drugs. Polymers. 2023;15(10):2302. doi: CrossRef
39. Ar N. Review on pharmaceutical gelling agents. PSBJ. 2024;8(1):1–11. doi: CrossRef
40. Sadia A, Muhammad Z, S Sadia N, Zahra F, HM Fayzan S, Rehan ZA. Ultrasound hydrogel: a review on materials and method. J Mod Polym Chem Mater. 2022;1(1):1–24. doi: CrossRef
41. .Safitri FI, Nawangsari D, Febrina D. Overview: application of carbopol 940 in gel: In: Proceedings of the International Conference on Health and Medical Sciences (AHMS 2020). Atlantis Press; 2021. doi: CrossRef
42. Agarwal P, Sebghatollahi Z, Kamal M, Dhyani A, Shrivastava A, Singh KK, et al. Citrus essential oils in aromatherapy: therapeutic effects and mechanisms. Antioxidants. 2022;11(12):2374. doi: CrossRef
43. Andra VVSNL, Pammi SVN, Bhatraju LVKP, Ruddaraju LK. A comprehensive review on novel liposomal methodologies, commercial formulations, clinical trials and patents. BioNanoSci. 2022;12(1):274–91. doi: CrossRef
44. Akombaetwa N, Ilangala AB, Thom L, Memvanga PB, Witika BA, Buya AB. Current advances in lipid nanosystems intended for topical and transdermal drug delivery applications. Pharmaceutics. 2023;15(2):656. doi: CrossRef
45. Orasan MS, Roman II, Coneac A, Muresan A, Orasan RI. Hair loss and regeneration performed on animal models. Med Pharm Rep. 2016;89(3):327–34. doi: CrossRef
46. Hwang D, Lee H, Lee J, Lee M, Cho S, Kim T, et al. Micro-current stimulation has potential effects of hair growth-promotion on human hair follicle-derived papilla cells and animal model. Int J Mol Sci. 2021;22(9):4361. doi: CrossRef