INTRODUCTION
Macrophages are highly secretory cells that release various mediators, including proinflammatory substances, growth factors, cytotoxic cytokines, hydrolytic enzymes, and nitric oxide (NO). These cells are pivotal in inflammatory pathways [1]. When activated, macrophages express inducible NO synthase (iNOS), an enzyme responsible for the oxidative deamination of L-arginine to produce NO. Excessive NO production by iNOS can lead to detrimental consequences such as septic shock and inflammatory diseases [2–4]. Therefore, measuring NO production induced by lipopolysaccharide (LPS) through iNOS is a valuable way to assess inflammation levels. Changes in NO concentration due to inhibiting iNOS activity provide insights into the impact of external factors on the inflammatory process [5].
In our quest to discover anti-inflammatory agents from natural sources in Vietnam, we identified significant inhibitory activity in vitro in the ethyl acetate (EtOAc) fraction obtained from the root of Polygonum multiflorum Thunb. (Polygonaceae). Polygonum multiflorum is a well-known plant with a rich history of pharmacological effects in traditional oriental medicine. Its versatile uses encompass a wide range of traditional medicines in East Asia [6]. Numerous traditional Chinese medicine books describe its effects in detail, which include improving age-related cognitive dysfunction, nourishing the liver and kidneys, anti-hyperlipidemia, anti-oxidant, anti-cancer, anti-inflammatory, promoting hair darkening, liver protection, cardiovascular protection, and anti-aging effects [7].
Previous studies on the phytochemistry of P. multiflorum have led to the isolation of approximately 70 new compounds, augmenting the total number of known compounds to around 175. These newly identified substances are predominantly dianthrone glycosides and stilbene glycosides. Other chemical constituents in P. multiflorum encompass fatty acids, flavones, quinones, and stilbenes [7,8]. While some investigations have explored the inhibitory effects of water, methanol, and ethyl acetate extracts [9–11], as well as stilbene glycosides (2,3,5,4′ -tetrahydroxystilbene-2-O-β-D-glucopyranoside, TSG) from this plant on NO production [11–17], there has been a notable absence of research on phenolic acids, phenolic glycosides, and especially flavonoids isolated from P. multiflorum and their potential for inhibiting NO production. Driven by the promising pharmaceutical potential of these compounds and the existing research gap, we undertook an extensive investigation into the chemical constituents and their ability to inhibit NO production.
MATERIALS AND METHODS
General experimental procedure
The 1H nuclear magnetic resonance (NMR) (400 MHz) and 13C NMR (100 MHz) spectra were acquired using a Varian Unity Inova 400 MHz spectrometer (Varian, Inc., California, USA), while the 1H NMR (500 MHz) and 13C NMR (125 MHz) spectra were obtained using a Bruker Avance 500 MHz spectrometer (Bruker Daltonics, Ettlingen, Germany). For column chromatography, silica gels (Si 60 F254, 40-63 mesh), YMCGEL (ODS-A, 12 nm S-150 μm), Sephadex LH-20, all solvents, please see Supporting Information.
Plant material
The root of P. multiflorum was collected from Cao Bang province, Vietnam, in January 2021. Botanical identification was expertly conducted by Nguyen Quoc Binh, Ph.D., of the Vietnam National Museum of Nature, Vietnam Academy of Science and Technology (VAST). To ensure traceability, a voucher specimen (PM-R-1502) was meticulously archived at the Natural Product Research and Development Lab, Phenikaa University, Vietnam.
Extraction and isolation
The powdered root of P. multiflorum (10.0 kg) was subjected to sonication extraction using methanol (MeOH) for 1 hour. The resulting extract was then filtered and subsequently evaporated under reduced pressure, yielding a crude MeOH extract. This MeOH extract (330 g) was then dissolved in hot water and partitioned successively with n-hexane, dichloromethane (CH2Cl2), and EtOAc. The resultant fractions were n-hexane (30 g), CH2Cl2 (50 g), EtOAc (105 g), and water (120 g). For detailed extraction and isolation, as well as physicochemical characterization, NMR data, and spectra of isolated compounds (1−10), please see Supporting Information.
Cell culture
RAW264.7 cells were purchased from the American Type Culture Collection (Manssas, VA) and were maintained in Dulbecco’s Modified Essential. These cells were grown at 37°C in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with penicillin (100 units/ml), streptomycin (100 μg/ml), and 10% heat-inactivated fetal bovine serum (FBS, Cambrex, Charles City, IA). The cells were maintained in a humidified 5% CO2 and atmosphere at 37°C. Cells were counted with a hemocytometer, and the number of viable cells was determined by trypan blue dye exclusion.
Cell viability assay
The viability assay was evaluated by MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay [18]. In brief, 100 μl cells (5 × 103 cells per well) in medium (DMEM supplemented with 10% FBS and 1% penicillin-streptomycin) were plated in a 96-well plate and incubated for 24 hours at 37°C in a humidified atmosphere incubator with 5% CO2. The medium was then washed and added with 99 μl new medium and 1 μl of each compound in different concentrations and Dimethyl sulfoxide (DMSO) in triplicate, and then the plate was incubated for 24 hours. Untreated cells served as the control. In brief, 20 μl MTS was added to each well. The plate was incubated in 5% CO2 at 37°C incubator for 4 hours. The absorbance was measured at 490 nm on a microplate reader (Molecular Devices, Emax, Sunnyvale, CA).
Determination of NO production
The level of NO production was determined by measuring the amount of nitrite from the cell culture supernatants as described previously [19–21]. The RAW 264.7 cells were seeded at a density of 1 × 105 cells/well in 24 well plates and incubated for 12 hours at 37°C and 5% CO2. The media of each well were aspirated, and fresh FBS-free DMEM media were replaced. Different concentrations of isolated compounds (1, 3, 10, and 30 μM) were prepared in FBS-free DMEM to give a total volume of 500 μl in each well of a microtiter plate. After 1 hour treatment, cells were stimulated with or without 1 μg/ml of LPS for 24 hours. The quantity of nitrite in the culture medium was measured as an indicator of NO production. Amounts of nitrite, a stable metabolite of NO, were measured using Griess reagent (1% sulfanilamide and 0.1% naphthylethylenediamine dihydrochloride in 2.5% phosphoric acid). In brief, 100 μl of cell culture medium was mixed with 100 μl of Griess reagent. Subsequently, the mixture was incubated at room temperature for 10 minutes, and the absorbance at 540 nm was measured in a microplate reader (Biotek, Winooski, VT). Fresh culture medium was used as a blank in every experiment. The quantity of nitrite was determined from a sodium nitrite (NaNO2) standard curve. For this experiment, N(G)-monomethyl L-arginine (L-NMMA) was used as a positive control.
Statistical analysis
The assay for NO production inhibitory activity was conducted in triplicate. The results are expressed as means ± SEM.
RESULTS AND DISCUSSION
Chemical structure identifications
The methanol (MeOH) extract was fractionated into n-hexane, CH2Cl2, and EtOAc-soluble fractions. From the EtOAc fraction, twelve secondary metabolites (1–10) were isolated using a combination of silica gel and Sephadex LH-20 chromatography (Fig. 1 and Supporting Information).
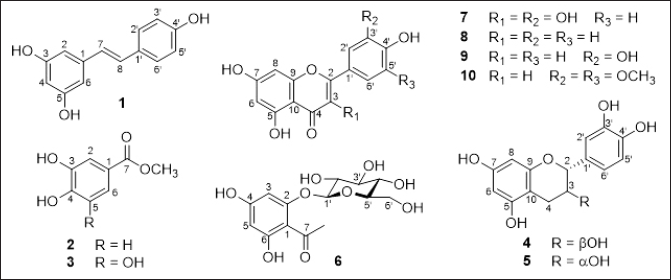 | Figure 1. Chemical structure of isolated compounds (1–10) from P. multiflorum. [Click here to view] |
Compound 1 was obtained as an ivory amorphous powder. Its 1H NMR spectrum exhibited signals indicative of a 1,3,5-trisubstituted benzene ring with an AB2 system [δH 6.43 (2H, d, J = 0.8 Hz, H-2/H-6) and 6.14 (1H, t, J = 0.8 Hz, H-4)], and a 1,4-disubstituted benzene ring with an A2B2 system [δH 7.32 (2H, d, J = 8.4 Hz, H-2′/H-6′) and 6.72 (2H, d, J = 8.0 Hz, H-3′/H-5′)], along with trans-olefinic protons [δH 6.93 (1H, d, J = 16.0 Hz, H-8) and 6.77 (1H, d, J = 16.0 Hz, H-7)] (Fig. 1 and Supporting Information). The 13C-NMR spectrum displayed 14 carbon signals, including 12 aromatic carbons [δC 102.8–159.8], suggesting the presence of two benzene rings and two olefinic carbons [δC 128.9 (C-7) and 127.1 (C-8)]. Compound 1 was unequivocally identified as trans-resveratrol based on this data (Supporting Information) and comparison with published literature [22].
Compounds 2 and 3 were isolated as white powders. Their 1H-NMR spectra displayed characteristic signals for aromatic protons of a benzene ring and methoxy groups, while their 13C-NMR spectra revealed signals of a carboxylic ester (C-7) and a quaternary carbon at C-1 (Fig. 1). Detailed analysis of the 1H- and 13C-NMR spectra (Supporting Information) allowed the identification of compound 2 as methyl protocatechuate [23] and 3 as methyl gallate [24]. This is the first reported identification of methyl protocatechuate (2) and methyl gallate (3) from P. multiflorum.
Compounds 4 and 5 were isolated as colorless solids. Their 1H-NMR spectra displayed five aromatic protons (H-6/H-8/H-2′/H-5′, and H-6′), two oxymethines (H-2 and H-3), and a methylene group (2H-4), while their 13C-NMR spectra revealed signals of two oxymethine carbons (C-2 and C-3) and a methylene carbon (C-4) (Fig. 1). These compounds were identified as catechin [25] for compound 4 and epicatechin for 5 [26] based on this data (Supporting Information) and comparison with published literature.
Compound 6 was isolated as a yellow colorless needle. Its 1H-NMR spectrum showed signals corresponding to a 1,2,4,6-tetrasubstituted benzene ring [δH 6.20 (1H, s, H-3), 5.97 (1H, s, H-5)], a methyl group [δH 2.71 (3H, s, 7-CH3)] attached to an aromatic ketone group, and a glucoside unit [δH 5.05 (1H, d, J = 7.6 Hz, H-1′), 3.94 (1H, br d, J = 11.6 Hz, H-6′a), 3.75 (1H, dd, J = 4.8, 11.6 Hz, H-6′b), 3.37−3.58 (4H, m, H-2′/H-3′/H-4′/H-5′)] attached to C-2 (Fig. 1 and Supporting Information). The 13C-NMR spectrum exhibited 14 carbon signals, including six aromatic carbons belonging to a benzene ring, a ketone carbon [δC 204.9 (C-7)], a methyl group at δC 33.6 (7-CH3), and six carbons of the glucoside unit [δC 102.1 (C-1′), 78.6 (C-5′), 78.4 (C-3′), 74.8 (C-2′), 71.2 (C-4′), 62.5 (C-6′)]. Detailed analysis of the 1H- and 13C-NMR spectra (Supporting Information) allowed the identification of compound 6 as 4,6-dihydroxy-2-O-(β-D-glucopyranosyl)acetophenone [27]. This study marks the first identification of 4,6-dihydroxy-2-O-(β-D-glucopyranosyl)acetophenone from P. multiflorum as well.
Compounds 7–10 displayed characteristic signals for aromatic protons of two benzene rings in their 1H-NMR spectra, along with hydroxyl groups at C-5, C-7, and C-4’. Their 13C-NMR spectra revealed signals of oxygen-substituted carbons at C-2 and C-9, as well as two quaternary carbons at C-10 and C-1′. The presence of an olefinic group [δC 148.9 (C-2) and 137.4 (C-3) for compound 7, and δC 159.0−163.2 (C-2) and 104.5−104.8 (C-3) for compounds 8–10] and a ketone carbon at C-4 in the 1H- and 13C-NMR spectra indicated compound 7 to be a flavonol and compounds 8–10 to be flavones [28], respectively (Fig. 1 and Supporting Information). Detailed analysis of the 1H- and 13C-NMR spectra (Supporting Information) and comparison with published literature, compounds 7 were identified as quercetin [28], 8 as apigenin [28], 9 as luteolin [28], and 10 as tricin [29].
Anti-inflammation activity results
In the initial experiment, a cytotoxicity assay was conducted to determine the safe and non-toxic concentrations of the isolated compounds (1–10) for subsequent assays. The isolated compounds were demonstrated to be non-toxic as they maintained over 90% cell viability, as assessed by the MTS assay [18]. Notably, the isolated compounds exhibited toxicity toward RAW 264.7 cells at a concentration of 100 μM. Therefore, this concentration was excluded from the treatment range, and concentrations ranging from 1 to 30 μM were selected for further investigations (Fig. 2).
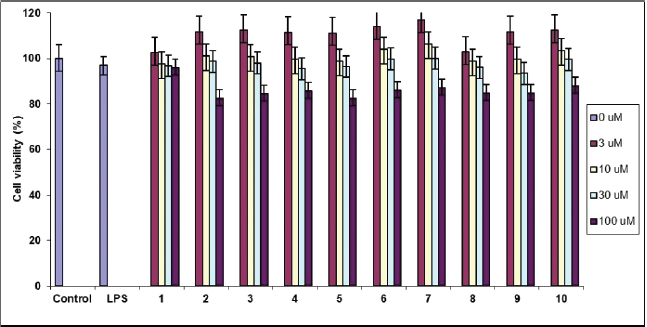 | Figure 2. Effect on cell viability by LPS stimulation in the presence of compounds 1−10. Cell viability was determined by MTS assay and expressed as a percentage of the control without the addition of indicated compounds 1−10. [Click here to view] |
To evaluate the inhibitory activity on NO production, RAW 264.7 cells were exposed to varying concentrations (1–30 μM) of the isolated compounds, and the level of NO production was determined using the Griess reaction [19–21]. As shown in Table 1, compounds 7–9 exhibited the most potent inhibitory activity against LPS-induced NO production, with IC50 values of 12.0, 17.8, and 7.6 μM, respectively. In contrast, compounds 1–4, 6, and 10 displayed IC50 values ranging from 25.5 to 49.3 μM, while compound 5 exhibited no significant activity (IC50 > 50 μM). As a reference, L-NMMA was used as a positive inhibitor, effectively suppressing LPS-induced NO production with an IC50 value of 22.1 μM (Table 1).
It is important to note that the control group, which did not receive either LPS or the samples, did not show any significant cytotoxic effects. Therefore, the observed inhibitory effects of these compounds on NO production were not influenced by any cytotoxic impact. As illustrated in Figure 3, upon stimulation with LPS (1 μg/ml), the control group exhibited an approximately 13-fold increase in NO production after 24 hours. In contrast, compounds 7–9 demonstrated a dose-dependent reduction in NO production 24 hours following LPS stimulation.
Macrophages are known for their pivotal role in inflammatory pathways, releasing various mediators such as proinflammatory cytokines, hydrolytic enzymes, growth factors, cytotoxic cytokines, and NO [1]. Upon activation, macrophages express iNOS, responsible for generating NO from L-arginine. Excessive NO production by iNOS can lead to detrimental effects such as septic shock and inflammatory disorders. Therefore, assessing NO production induced by LPS through iNOS inhibition provides insight into modulating the inflammatory process [2–4].
NO is a molecule that plays a crucial role in the immune response and inflammation. Inflammation is a natural and essential process that helps the body combat infections and heal injuries. However, excessive or prolonged inflammation can lead to tissue damage and chronic diseases. NO is one of the key signaling molecules involved in the inflammatory response, and its production can be regulated to control inflammation. NO is a double-edged sword in inflammation. In moderate amounts, NO serves as a signaling molecule that helps regulate the immune response. It can dilate blood vessels, enhance immune cell function, and facilitate the removal of pathogens. However, in excess, NO can be toxic to cells and tissues. Therefore, to control inflammation, it is sometimes necessary to inhibit NO production. Up to date, several studies on the NO production inhibitions have been published (https://pubmed.ncbi.nlm.nih.gov/?term=Nitrite+Oxide+production+inhibition&sort=date). Remarkably, it is important to note that completely blocking NO production may have negative consequences, as NO also has important roles in the body’s defense mechanisms and vascular regulation. Therefore, the goal is often to modulate NO production rather than eliminate it entirely. Overall, understanding and controlling NO production in inflammation is a complex and important aspect of managing various inflammatory diseases and conditions. The goal is to strike a balance between the beneficial and detrimental effects of NO to promote healing and reduce tissue damage.
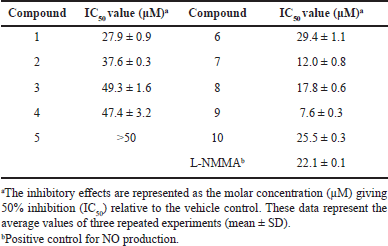 | Table 1. NO production inhibition of isolated compounds (1−10) from P. multiflorum. [Click here to view] |
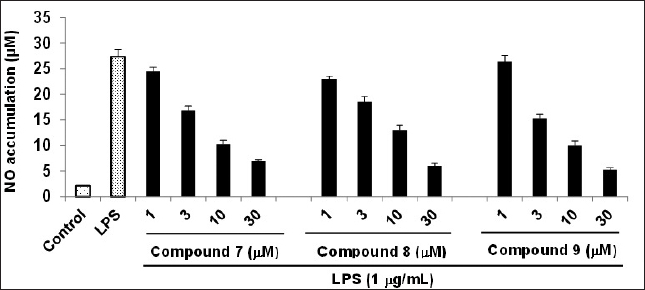 | Figure 3. Inhibitory effect of compounds 7-9 on the LPS-induced NO production in RAW264.7 cells. RAW264.7 cells were pre-treated with various concentrations (1, 3, 10, and 30 μM) of the tested compounds for 1 hour, followed by treatment with LPS (1 μg/ml), and then incubated for 24 hours. Control values were obtained in the absence of both LPS and the compound. The data are presented as the mean ± SD (n = 3). Statistical significance was determined using a two-tailed unpaired Student’s t-test, and p < 0.05 was considered to indicate statistical significance. [Click here to view] |
In our study, the flavonols quercetin (7) and luteolin (9) exhibited the strongest inhibition of NO production, with IC50 values of 12.0 and 7.6 μM, respectively, among the isolated compounds. This inhibitory effect could be attributed to the presence of the conjugated double bond (C-2,3 double bond) and 3,4-hydroxylation(s) of the benzene ring, which provided favorable results (Fig. 1) [19–21]. Compounds 1–3, 8, and 10 showed reduced inhibitory activities (IC50 values ranging from 17.8 to 49.3 μM), likely due to the absence of 3-hydroxylation (compound 8) or the presence of methoxy groups (compounds 1–3 and 10) in the benzene ring [30,31]. Compound 6 displayed further reduced inhibitory activity (IC50 = 29.4 μM) compared to compounds 7 and 9, possibly due to the presence of sugar units (glucose) attached to C-2 in the benzene ring. Compounds 4 and 5 exhibited even greater reduction in inhibitory activities (IC50 value of 47.4 μM) or were inactive (IC50 > 50 μM), respectively, due to the lack of the conjugated double bond (C-2,3 double bond) despite the presence of 3,4-hydroxylation(s) of the benzene ring (Fig. 1) [30,31]. These data suggest that flavonoids bearing the conjugated double bond (C-2,3 double bond) and 3,4-hydroxylation(s) of the benzene ring could serve as promising lead compounds for the development of agents targeting NO production.
This is the first time the evaluation of isolated compounds from Vietnamese P. multiflorum on NO inhibitory activity, and these novel findings will be the foundation for more comprehensive studies in the future. This is also useful information for the production of anti-inflammatory products, through which annual consumption from this species can increase in the future.
CONCLUSION
In conclusion, phytochemical investigation of the root of a Vietnamese P. multiflorum has led to the isolation and structural elucidation of 10 compounds using NMR techniques including trans-resveratrol (1), methyl protocatechuate (2), methyl gallate (3), catechin (4), epicatechin (5), 4,6-dihydroxy-2-O-(β-D-glucopyranosyl)acetophenone (6), quercetin (7), apigenin (8), luteolin (9), and tricin (10). These compounds were evaluated for their anti-inflammatory activity by assessing their ability to inhibit LPS-induced NO production in macrophage RAW264.7 cells. Among the compounds tested, compounds 7−9 demonstrated the most potent inhibitory activity against NO production, with IC50 values of 12.0 ± 0.8, 17.8 ± 0.6, and 7.6 ± 0.3 μM, respectively. Following this, compounds 1–4, 6, and 10 exhibited IC50 values ranging from 25.5 to 49.3 μM, respectively, while compound 5 demonstrated inactivity (IC50 > 50 μM). These findings suggest that P. multiflorum and its natural secondary metabolites (compounds 7−9) may possess anti-inflammatory properties.
ACKNOWLEDGMENTS
The authors thanks to the Vietnam Acedemy of Science and Technology (VAST) for supporting this research (Project code number: UDPTCN 03/21-23).
AUTHORS CONTRIBUTION
All authors have contributed significantly to the work presented here, and these authors contributed equally.
CONFLICTS OF INTEREST
The authors declared that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4(3):281–6. CrossRef
2. Farrell AJ, Blake DR, Palmer RM, Moncada S. Increased concentrations of nitrite in synovial fluid and serum samples suggest increased nitric oxide synthesis in rheumatic diseases. Ann Rheum Dis. 1992;51(11):1219–22. CrossRef
3. Kilbourn RG, Jubran A, Gross SS, Griffith OW, Levi R, Adams J, et al. Reversal of endotoxin-mediated shock by NG-methyl-L-arginine, an inhibitor of nitric oxide synthesis. Biochem Biophys Res Commun. 1990;172(3):1132–8. CrossRef
4. Kröncke KD, Fehsel K, Kolb-Bachofen V. Inducible nitric oxide synthase in human diseases. Clin Exp Immunol. 1998;113(2):147–56. CrossRef
5. Barnes PJ, Liew FY. Nitric oxide and asthmatic inflammation. Immunol Today. 1995;16(3):128–30. CrossRef
6. Moldenke HN. Reynoutria multiflora (Thunb.) Moldenke. Bull Torr Bot Cl. 1941;68:675–6. CrossRef
7. Lin L, Ni B, Lin H, Zhang M, Li X, Yin X, et al. Traditional usages, botany, phytochemistry, pharmacology and toxicology of Polygonum multiflorum Thunb.: a review. J Ethnopharmacol. 2015;159:158–83. CrossRef
8. Teka T, Wang L, Gao J, Mou J, Pan G, Yu H, et al. Polygonum multiflorum: recent updates on newly isolated compounds, potential hepatotoxic compounds and their mechanisms. J Ethnopharmacol. 2021;271:113864. CrossRef
9. Lin CL, Hsieh SL, Wu CC, Huang GC, Leung W, Lee CT, et al. Polygonum multiflorum water extract protects against nitric oxide by LPS-induced in RAW 264.7 macrophages. Biomed Res. 2017;28(6):2854–8.
10. Cha DS, Jeon H. Anti-inflammatory effect of MeOH extracts of the stem of Polygonum multiflorum in LPS-stimulated mouse peritoneal macrophages. Nat Prod Sci. 2009;15(2):83–9.
11. Wang C, Zhang L, Yuan Z, Jin Y, Zhang Z. Blood lipid regulation of ethyl acetate extracting fraction and stilbene glycoside from tuber of Polygonum multiflorum. Chin Tradit Herb Drugs. 2008;39(1):78–83.
12. Li SG, Huang XJ, Li MM, Liu Q, Liu H, Wang Y, et al. Multiflorumisides A-G, dimeric stilbene glucosides with rare coupling patterns from the roots of Polygonum multiflorum. J Nat Prod. 2018;81(2):254–63. CrossRef
13. Li SG, Huang XJ, Zhong YL, Li MM, Li YL, Wang Y, et al. Stilbene glycoside oligomers from the roots of Polygonum multiflorum. Chem Biodivers. 2019;16(6):e1900192. CrossRef
14. Tsai PW, Lee YH, Chen LG, Lee CJ, Wang CC. In vitro and in vivo anti-osteoarthritis effects of 2,3,5,4’-tetrahydroxystilbene-2-O-β-D-glucoside from Polygonum multiflorum. Molecules. 2018;23(3):571. CrossRef
15. Chen LL, Huang XJ, Li MM, Ou GM, Zhao BX, Chen MF, et al. Polygonflavanol A, a novel flavonostilbene glycoside from the roots of Polygonum multiflorum. Phytochem Lett. 2012;5(4):756–60. CrossRef
16. Zhang YZ, Shen JF, Xu JY, Xiao JH, Wang JL. Inhibitory effects of 2,3,5,4’-tetrahydroxystilbene-2-O-β-D-glucoside on experimental inflammation and cyclooxygenase 2 activity. J Asian Nat Prod Res. 2007;9(4):355–63. CrossRef
17. Ye S, Tang L, Xu J, Liu Q, Wang J. Postconditioning’s protection of THSG on cardiac ischemia-reperfusion injury and mechanism. J Huazhong Univ Sci Technol, Med Sci. 2006;26(1):13–6. CrossRef
18. Dewi K, Widyarto B, Erawijantari PP, Widowati W. In vitro study of Myristica fragrans seed (Nutmeg) ethanolic extract and quercetin compound as anti-inflammatory agent. Int J Res Med Sci. 2015;3(9):2303–10. CrossRef
19. Cuong TD, Hung TM, Kim JC, Kim EH, Woo MH, Choi JS, et al. Phenolic compounds from Caesalpinia sappan heartwood and their anti-inflammatory activity. J Nat Prod. 2012;75(12):2069–75. CrossRef
20. Cuong TD, Hung TM, Lee JS, Weon KY, Woo MH, Min BS. Anti-inflammatory activity of phenolic compounds from the whole plant of Scutellaria indica. Bioor Med Chem Lett. 2015;25(5):1129–34. CrossRef
21. Cuong TD, Hung TM, Na MK, Ha DT, Kim JC, Lee D, et al. Inhibitory effect on NO production of phenolic compounds from Myristica fragrans. Bioor Med Chem Lett. 2011;21(22):6884–7. CrossRef
22. Mattivi F, Reniero F, Korhammer S. Isolation, characterization, and evolution in red wine vinification of resveratrol monomers. J Agric Food Chem. 1995;43(7):1820–3. CrossRef
23. Tsuda T, Watanabe M, Ohshima K, Yamamoto A, Kawakishi S, Osawa T. Antioxidative components isolated from the seed of Tamarind (Tamarindus indica L.). J Agric Food Chem. 1994;42(12):2671–4. CrossRef
24. Tan YP, Chan EWC, Lim CSY. Potent quorum sensing inhibition by methyl gallate isolated from leaves of Anacardium occidentale L. (cashew). Chiang Mai J Sci. 2015;42(3):650–6.
25. Meulenbeld GH, Zuilhof H, Van Veldhuizen A, Van Denheuvel RHH, Hartmans S. Enhanced (+)-catechin transglucosylating activity of Streptococcus mutans GS-5 glucosyltransferase-D due to fructose removal. Appl Environ Microbiol. 1999;65(9):4141–7. CrossRef
26. Lv Q, Luo FL, Zhao XY, Liu Y, Hu GB, Sun CD, et al. Identification of proanthocyanidins from Litchi (Litchi chinensis Sonn.) Pulp by LC-ESIQ-TOF-MS and their antioxidant activity. PLoS One. 2015;10(3):e0120480. CrossRef
27. Niumsakul S, Hirunsaree A, Wattanapitayakul S, Junsuwanitch N, Prapanupun K. An antioxidative and cytotoxic substance extracted from Curcuma comosa Roxb. J Thai Trad Alt Med. 2007;5(1):24–9.
28. Agrawal PK. Carbon-13 NMR of flavonoids. 1st edition. Amsterdam, Oxford, New York, Tokyo: Elsevier; 1989. CrossRef
29. Wang Y, Shen JZ, Chan YW, Ho WS. Identification and growth inhibitory activity of the chemical constituents from Imperata cylindrica aerial part ethyl acetate extract. Molecules. 2018;23(7):1807. CrossRef
30. Tanaka K, Ohgo Y, Katayanagi Y, Yasui K, Hiramoto S, Ikemoto H, et al. Anti-inflammatory effects of green soybean extract irradiated with visible light. Sci Rep. 2014;4:4732. CrossRef
31. Nhan NT, Nguyen PH, Bich Hanh DT, Dang Thi TM, Nguyen NPD, Phong TB, et al. Anti-inflammatory activity of ingredients from the heartwood of Vietnamese Dalbergia oliveri Gamble ex Prain. Eur J Inflamm. 2022;20:1–8. doi: https//doi.org/10.1177/1721727X221132273 CrossRef