INTRODUCTION
The Myristicaceae family, also known as Magnoliophyta, is a group of pantropical plants that share traits such as having two homes, trees, axis flowers, meat or hard fruit, red seeds, fragrant leaves, and typically a substance called myristicin. Easily found in tropical Asia, the Pacific Islands, Africa, and tropical America, the Myristicaceae family has 21 genera and 520 species [1]. “Nutmeg” plants are members of the Myristicaceae family, divided into 3 primary genera and 11 species by Chinese taxonomists [2]. The Island of Java is home to roughly 210 species of Myristicaceae, including 100 Myristica species, 70 Horsfieldia species, and 40 Knema species [3,4].
The fruits, leaves, bark, and stems of the Myriticaceae family can all be employed in traditional medicine [5]. Conversely, the fruit is frequently added to dishes as a flavouring [6].
Below is the taxonomy of the Myristicaceae family
Kingdom : Plantae
Division: Magnoliophyta
Class: Maginoliospida
Order: Magnoliales
Family: Myristicaceae
Genus: Myristica/Knema/Horsfieldia
METHODOLOGY
This review cites over 100 published works over the last 25 years. Medicinal Plants Research, Food Chemistry, Natural Product Community, Biodiversity Journal of Biological Diversity, Journal of Medicinal Plants Research, Food Chemistry Toxicology, Phytomedicine, Phytochemistry, Phytochemistry Letters, and so on, were among the online sources and electronic databases from which the articles were sourced. To locate pertinent publications, online databases such as Scopus, Pubmed, and so on, were searched using terms such as Myristicaceae, Myristica, Horsfieldia, and Knema. The writers made an effort to incorporate in Table 1. All publications in which the material was pertinent. Only cytotoxic, antioxidant, antibacterial, anti-inflammatory, and antidiabetic properties are included in this review.
 | Table 1. Previously published review articles focusing on Myristica, Horsfieldia, Knema genus, and their main theme of research. [Click here to view] |
MEDICINAL PROPERTIES
Native populations in tropical and subtropical nations have utilized plants in the Myristicaceae family as medicinal [5,6]. Table 2 lists the therapeutic applications of the Myristicaceae family in indigenous peoples’ traditional medicine in tropical and subtropical regions. Myristicaceae plants are recognized to possess antibacterial, antioxidant, anti-inflammatory, and anti-cancer effects in their seeds and fruits, similar antioxidant and anti-cancer properties are also present in the leaves of various Myristicaceae plants. For instance, A-357, MCF-7, vero, and colon cancer cell lines are all subject to mild cytotoxic activity from M. fragrans [11–13]. This further demonstrates anti-inflammatory properties and a potent inhibitory effect on the RAW264.7 cell line’s ability to produce nitric oxide [14–16]. Specific Myristica fatua plant components are considered to lower obesity and are used to treat diabetes [17–19]. Myristicaceae plants are also used for oral care [20], reducing skin allergies [21], cockroach control [22], and food preservatives [23,24] . According to these applications, these plants could have antibacterial-containing chemicals.
PHYTOCHEMISTRY
Regarding investigations into the components of Myristicaceae’s secondary metabolites, polyketides 1–72, lignans 73–172, terpenoids 173–206, flavonoids 206–270, chalcones, quinones, and alkaloids 271–292 were isolated.
Polyketides
A polyketide, known as beta-polyketone, is a secondary metabolite molecule with alternating carbonyl and methylene groups. Table 3 and Figure 1 present the 72 compound isolated cyclic polyketides that were reported from the Myristicaceae family, within Myristica genus 1–16, 18, 19, 21, 22–24, 42–46, 51, and 60–63 compounds contained in the seeds of Myristica dactyloide from Sri Lanka [25], the fruits of Myristica maingayi [26] and Myristica gigantea [27] from Malaysia, the stem bark and seeds of Myristica malabarica from India [28], the leaves and fruits of Myristica crassa from Malaysia [29], the bark of maxima maxima from Malaysia [30], the barks and seeds of Myristica cinnamomea from Malaysia [31], the seeds of Myristica beddomei from India [32], the leaves and barks of M. fatua from India and Indonesia [17,33], the leaves of Myristica philippensis from the Philippines [34], and all parts of M. fragrans from China, Korea, Africa, India, Indonesia, Malaysia, Korea, and Japan [16,35].
The Horsfiedia genus compounds 17, 20, 21, 25–27, 30-33, 40, 41, 47–50, 52, 53, 56–59, and 64–70, contained in all parts of Horsfieldia macrobotrys from Indonesia [36], the leaves Horsfieldia spicata from Indonesia [37], the leaves and twigs Horsfieldia kingie from Thailand and China [38,39], all parts of Horsfieldia irya from Thailand [40,41], the barks Horsfieldia superba from Malaysia [42], the barks Horsfieldia pandurifolia from China [43], and all parts of Horsfieldia tetratepala from China [44].
In the Knema genus 1, 24–27, 32–37, 43, 52, 53, 62, 69, and 70 compounds contained in leaves and stem bark Knema glauca from Malaysia [45], the twigs Knema furfuracea from China [46], the leaves and twigs Knema elegans from China [47], the roots Knema globularia from Thailand [48], the stem bark Knema hookeriana from Indonesia [49], and the leaves Knema stellate from Philippines [34].
Lignans
Myristicaceae plants are rich in lignan and lignan-derived chemicals. Lignans are a comprehensive class of phenolic compounds defined by two C6–C3 units joined by a bond between the 8 and 8′ or β - β′ positions. The Myristcaceae family has 99 different types of lignan chemicals.(Table 4 and Fig. 2). The Horsfieldia genus contains the compounds 73–76, 108, 109, 126–129, and 167–169 found in the seeds of Horsfieldia iryaghedhi from Sri Lanka [40,50], the leaves and twigs of Horsfieldia glabra from China [50,51], the leaves and twigs of H. kingii from Malaysia [38], and the twigs of H. tetratepala from China [85]. The compounds of Myristica genus 77–88, 90–107, 110, 111, 132-147, and 151–166 contained in the leaves and barks M. fatua from Indonesia [59], all parts of M. fragrans from China, Korea, Africa, India, Indonesia, Malaysia, Korea, and Japan [13,15,19,20,86–88], the barks M. argentea from Indonesia [9,54], the stem barks Myristica dactyloides from Srilanka [89,90], the seeds Myristica otoba from Malaysia [91], and the seeds Myristica schefferi from Indonesia [92]. The compounds of Knema genus 75, 76, 89, 92–94, 108, 112–125, 130–131, 148–150, and 170–172 are contained in roots K. globularia from Thailand [48,65,66], the fruits Knema pachycarpa from Vietnam [67,68], the leaves and stem barks K. glauca from Malaysia [45], the twigs K. furfuracea from Chinese [45,46], and the leaves and twigs K. elegans from China [10,47,74].
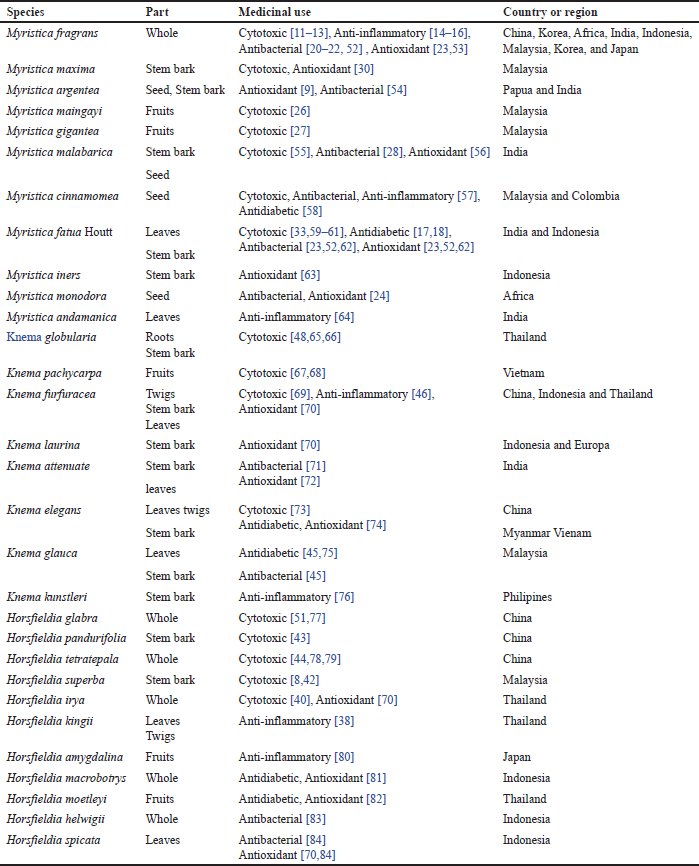 | Table 2. Medicinal uses and some origins of the Myristicaceae family. [Click here to view] |
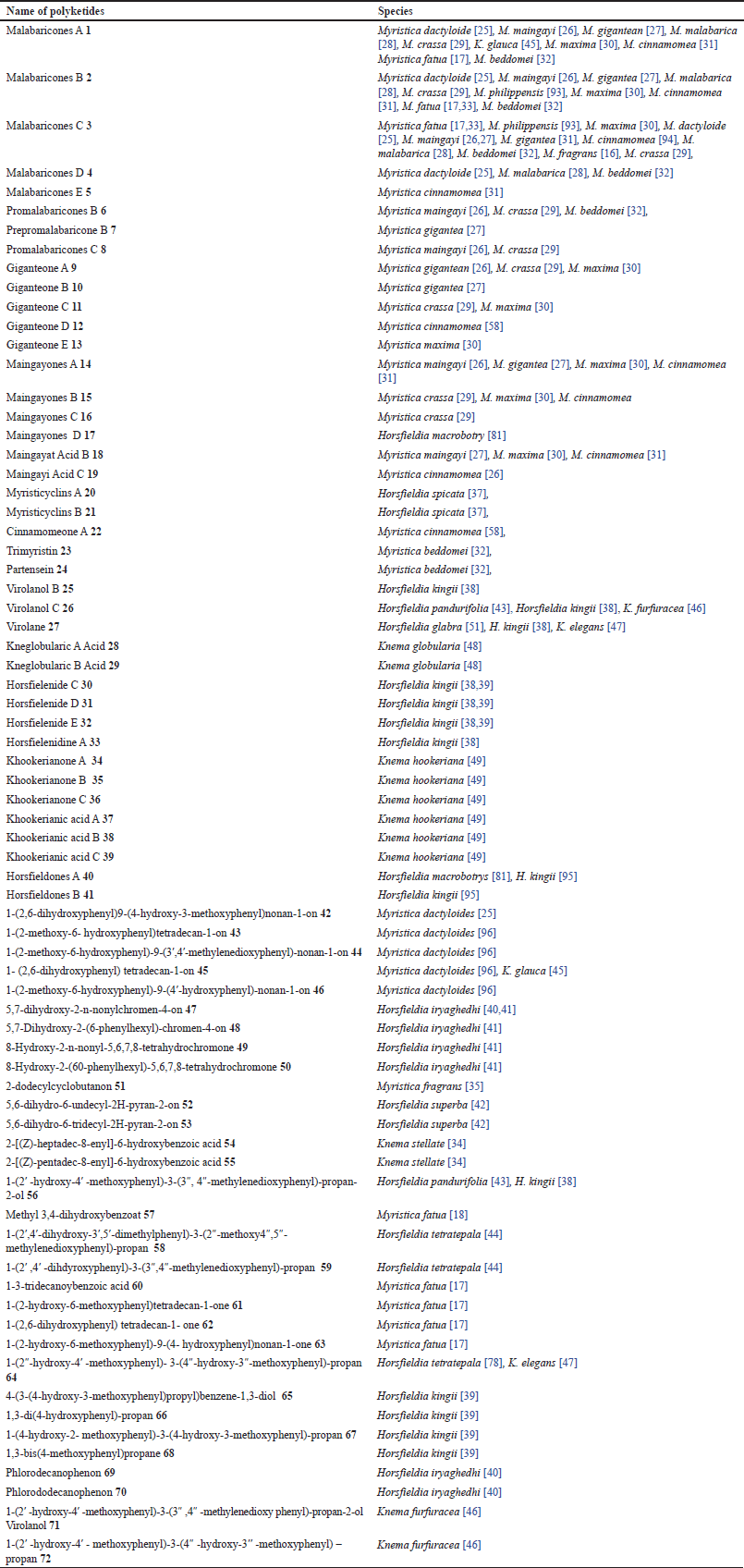 | Table 3. Polyketides isolated from Myristicaceae. [Click here to view] |
Terpenoid
Interestingly, terpenoids have demonstrated encouraging anticancer action, which may lead to more options in cancer treatment [97]. Essential oils (monoterpenes) comprise most terpenoid group constituents in Myristicaceae plants. Horsfieldia, Knema, and Myristica are the genera from which most of the around 33 essential oils (monoterpenes) have been isolated.
The compounds include in the Myristica genus 173, 174, 177–194, 198, and 200 contained in M. fragrans, Myristica monodora, M. schefferi, M. philippensis, M. maxima, and M. monodora. The compound in the Knema genus 195, 198, and 206 contained K. globularia, K. furfuracea, and stem bark Knema patentinervia from Malaysia. The compound in the Horsfieldia genus 173, 17–177, 181–185, 188, 191, 192, 194, 196, 197, 199–205 contained in H. fulva, Horsfieldia hainensis, H. superba, and H. fulva (Table 5 and Fig. 3).
Flavans, isoflavonoids, and flavones
Myristicaceae has about 63 different types of flavonoids. The first flavan compound in Horsfieldia amygdaline was identified as myristinin A 207 in 1992. It was successfully discovered that isomeric compounds of myristinin A were present in the seeds and barks of Myristica cinnamomea (Myristinin B 213, C 214, D 215, E 216, and F 217) (34), the fruits Horsfieldia motley [82] (Myristinin D 215, E 216, and G 228), and Myristinin I 229 from all parts of H. iryaghedhi [40]. The stem bark methanol extract of K. globularia contained the derivative of Kaempferol 235 [66] (Table 6 and Fig. 4).
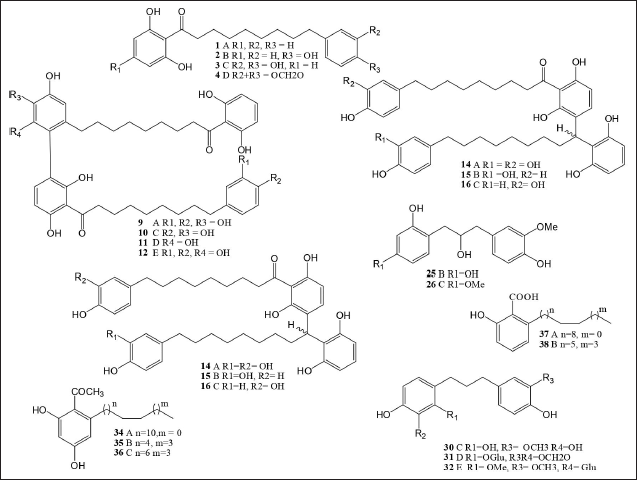 | Figure 1. Polyketides isolated from Myristicaceae species. [Click here to view] |
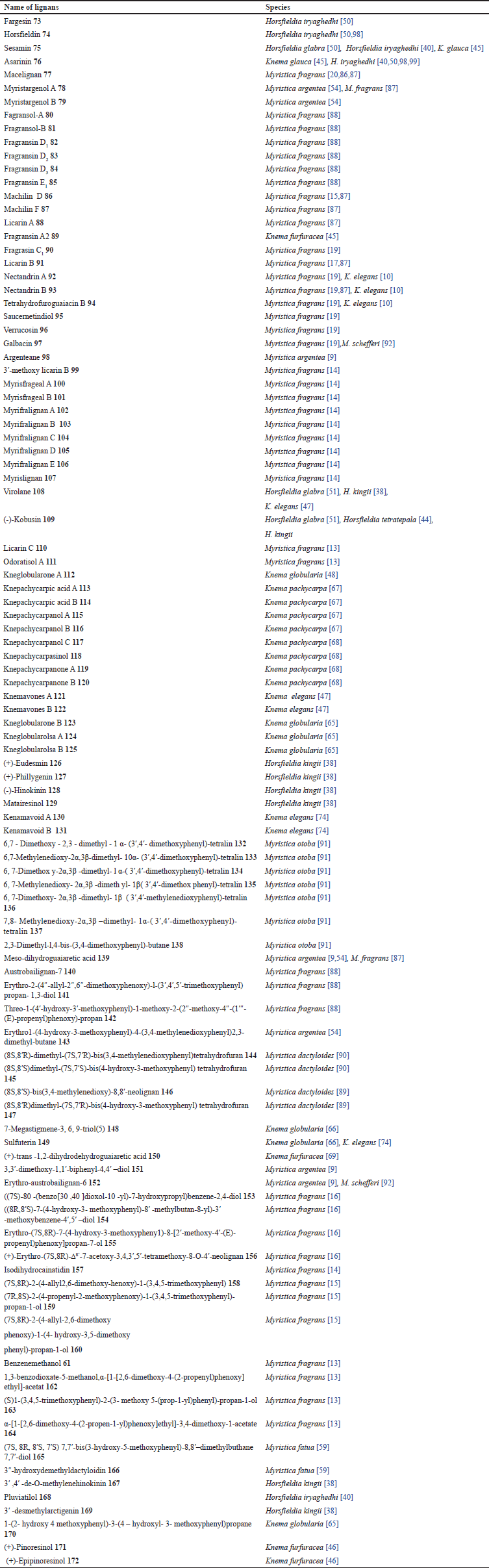 | Table 4. Lignans compounds isolated from Myristicaceae. [Click here to view] |
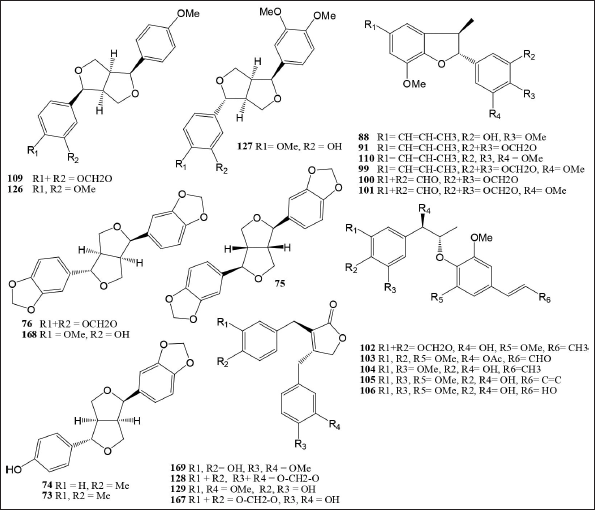 | Figure 2. Lignans compounds isolated from Myristicaceae species. [Click here to view] |
Chalcone, quinone, and alkaloids
The Myristicaceae family contains nine compounds in the chalcone group 271–280, and 290. These compounds are primarily found in the Horfieldia genus and include the trunk methanol extract of H. pandurifolia [43], the stem bark methanol extract of H. superba [42], and the fruits methanol extract of H. glabra [77]. The Quinone compounds are found in the genus Horsfieldia, Horsfiequinone A–F, isolated from the stem extract of H. tetratepala [79]. Compounds derived from napthale 2-methyl-1, 4, 4a, 8a-tetrahydro-endo-1, 4- methanonaphthalene-5,8-dione 281 have been isolated from Myristica argantea seed extract [100]. The Malaysian native tree species H. superba was not known to have alkaloids before. Isolation from leaves including a new alkaloid, Horsfiline1 285, 6-methoxy-2-methyl-1, 2, 3, 4-tetrahydro-β-carboline 286, and 5-mehoxy-N,N-dimethyl-tryptamine 287 Table 7 and their structures are displayed in Figure 5.
PHARMACOLOGY
Studying the effects of medications and other substances on living things is the study of pharmacology, an interesting area. All substances, natural or artificial, that affect a biological system can be considered drugs. Considering all the many ways medications may be utilized to alleviate ailments and enhance people’s quality of life is impressive.
Cytotoxicity
Regarding compounds have been shown to have the ability to inhibit MCF-7 cells. These include compounds from the polyketide groups 1, 2, 3, and 6 that are found in various Myristica genus; more recently, the compound Malabaricones A 1 in the K. glauca species [45], the lignan group 116, 120 that is found in K. pachycarpa [68], and 165, 166 compounds that are found in M. fatua [59]. The terpenoids group 196, 197 are contained in H. superba [42], and the flavones group, Giffithane 240 is contained in K. globularia [65]. In addition to MCF-7 cells, tests were also carried out against HT-29 colon cells 110, and 162 compounds, KB tumor cells test 1, 2, 3, 7, 9, and 10 compounds, PC3 cells 1, 9, 196, and 197 compounds, vero cells 112, 196, 197, and 199–204 compounds, NCI-H187 89, 112, 208, and 240 compounds, Hela cell 119, 120, and 229 compounds, and P388 cells 207, 215 compounds (Table 9).
Anti-inflammatory
Anti-inflammatory effects have been discovered in a wide range of natural substances. In a bioassay, for instance, the methanol extract of Myristica andamanica leaves which contains steroids, carbohydrate alkaloids, and amino acids was used to test the anti-inflammatory properties of the plant’s extracts. Rat wounds treated with this extract have been demonstrated to heal effectively [64] (Table 8). Similarly, the anti-inflammatory activity of M. fragrans performed using the chloroform extract and isolated compounds 154, 155, and 156 from the seeds have been tested on murine monocyte-macrophages [16]. Myristinin 207, 213–217 compounds obtained from the chloroform extract of M. cinnamomea fruit have been found to selectively inhibit the enzyme cyclooxygenase-2 [80]. Other compounds isolated from various plant extracts, such as horsfielenide D 31 and cathechin 212, have also shown anti-inflammatory activity [47]. Acetone extracts of K. furfuracea twigs and leaves 26, 245, and 265 compounds have also been found to be anti-inflammatory active in various compounds. These organic substances provide encouraging possibilities for the creation of novel anti-inflammatory drugs [46] (Table 9).
 | Table 5. Terpenoids compounds isolated from Myristicaceae. [Click here to view] |
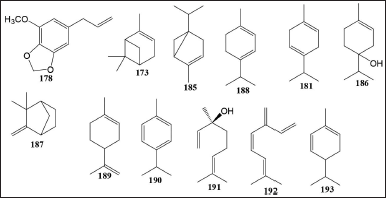 | Figure 3. Terpenoids compound isolated from Myristicaceae species. [Click here to view] |
 | Table 6. Flavan, isoflavonoids, and flavone compounds isolated from Myristicaceae family. [Click here to view] |
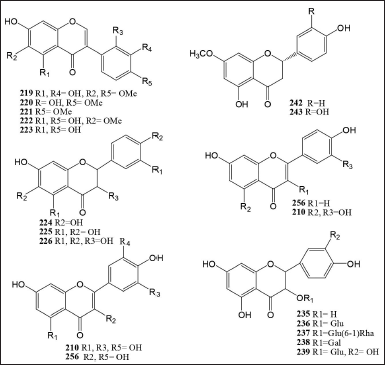 | Figure 4. Flavans, isoflavonoids, and flavone compounds isolated from Myristicaceae family. [Click here to view] |
Antidiabetic activity
Recent research has revealed that K. glauca dichloromethane extracts have antidiabetic qualities [75] (Table 8). In more detail, 2, 3, 12, and 57 compounds from the n-hexane and acetone fractions of M. cinnamomea stem bark, as well as 17 and 40 compounds from the methanol extract of H. macrobotrys fruit, and compounds 130 and 131 from K. elegans twig and leaves extracts, and 215, 216, and 228 compounds from H. motleyi stem bark have all shown an antidiabetic activity against α-amylase and α-glucosidase enzymes [17,18,58,81,82]. Furthermore, it has been discovered that the dichloromethane extract of K. glauca leaves has potent antidiabetic action against α-glucosidase inhibition, IC50 of 4.09 μg/ml [75] (Table 9).
 | Table 7. Chalchone, quinone, and alkaloid compounds isolated from Myristicaceae family. [Click here to view] |
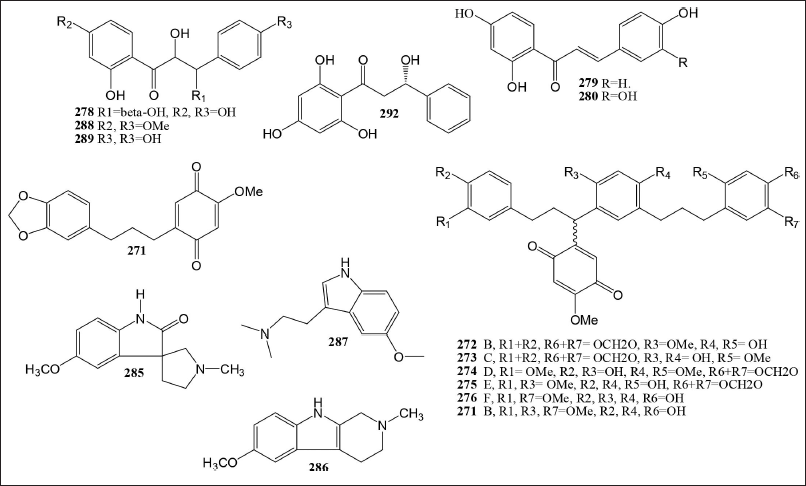 | Figure 5. Chalchone, quinone, and alkaloid compounds isolated from Myristicaceae family. [Click here to view] |
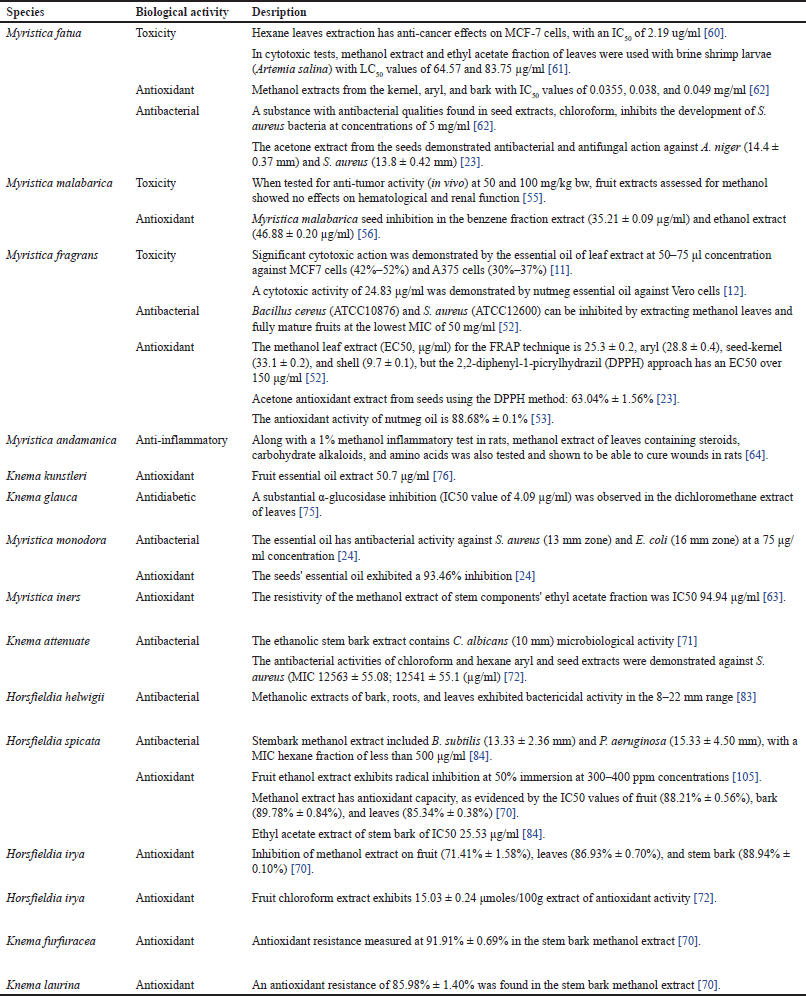 | Table 8. Biological activities extracts of Myristicaceae family. [Click here to view] |
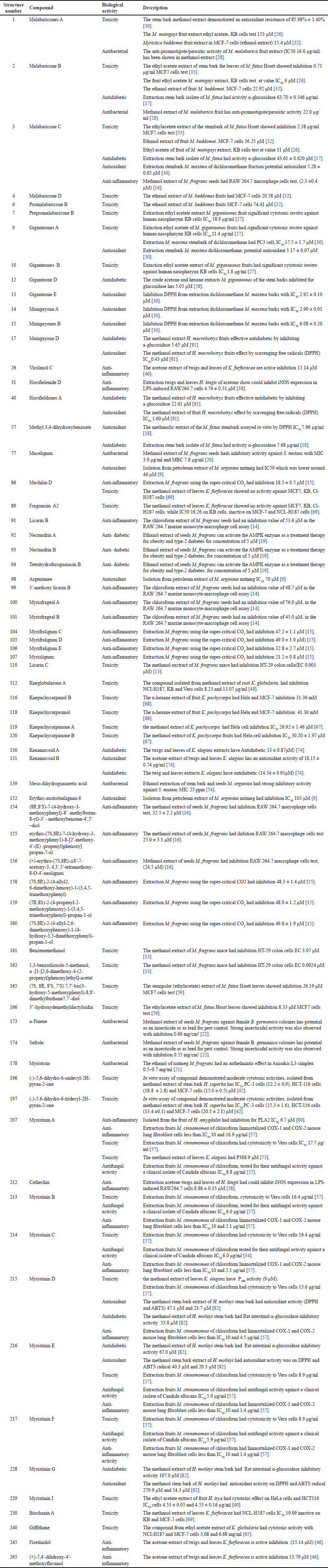 | Table 9. Biological activity of compounds isolated from Myristicacea family. [Click here to view] |
Antibacterial activity
The antibacterial, antifungal, and antiviral properties of several Myristcacae families have been studied. Gram-positive and Gram-negative bacteria were inhibited in the extracts of M. fragrans, Myristica mondora, M. fatua, Knema attenuate, K. glauca, Horsfieldia helwigii, and H. spicata. The chloroform extracts [62] and acetone extracts [23] of M. fatua seeds inhibited Staphylococcus aureus bacteria and Aspergillus niger. MeOH extract leaves and full-ripe fruits M. fragrans inhibit bacteria with the lowest MIC 50 mg/ml against S. aureus and Bacillus cereus [52]. The essential oil of M. mondora has antibacterial Escherichia coli and S. aureus [24]. The ethanolic stem bark extract [71] and chloroform and hexane aryl and seed extracts [72] of K. attenuate microbial activity. Methanol extract of stems H. spicata had microbial activity Bacillus subtilis and Pseudomonas aeruginosa [84] in Table 8.
The antibacterial and antifungal qualities of several substances identified from different sections of the M. fragrans, M. argantea, and M. cinnamomea plants are encouraging. Compounds 77 [20] and 257 [21] from M. fragrans were found to be effective against Streptococcus mutans, while compound 139 from M. argantea displayed strong antibacterial activity against the same target [54]. Myristinin compounds from M. cinnamomea were shown to have antifungal activity. In addition, malabaricone 1 and 2 from M. malabarica demonstrated anti-promastigote/parasitic activity [28]. The results indicate that these substances hold significance in creating novel drugs that combat infections and fight fungal growth (Table 9).
Antioxidant activity
Several species within the Myristicaceae family have been shown to be a source of antioxidants, such as M. fatua, Myristica iners, M. malabarica, M. fragrans, M. monodora, H. spicata, H. irya, K. furfuracea, and Knema laurina. These species were tested using the radical scavenger inhibition DPPH method [81,82] as shown in Table 8. In addition, investigations of compounds 17, 40, 215, 216, and 228 were obtained from methanol extracts of H. macrobotrys and H. motleyi, 131 (K. elegans) [74], 3, 9, 13, 14, and 15 (M. maxima) [30], 139, and 152 (M. argentea) [9], and 57 (M. fatua) [18] demonstrated antioxidant activity as shown in Table 9.
CONCLUSION
This article aims to review the existing knowledge regarding the species of the Myristica, Knema, and Horsfieldia genus (Myristicaceae), which is an important effort to document various reports on the phytochemistry and pharmacology of medicinal plants from this family. Although the multiple benefits and traditional uses of Myristicaceae plants are known, only a few plant species have been investigated for their restorative and food preservative uses based on phytochemical and pharmacological reports, despite more than 520 known species of Myristicaceae. The data provided in this review will likely form the basis of further scientific research regarding this plant family. In addition, understanding the pharmacological studies in this family may be useful for validating their claimed traditional uses. The literature reviewed shows that different Myristica, Knema, and Horsfieldia species are good natural sources for various natural compounds with diverse and interesting chemical structures. The main classes of compounds reported in the literature include lignans and polyketides. A review of the pharmacology of the genus shows that many lignans and polyketides were isolated and exhibited strong, moderate to weak anticancer properties. These two groups of compounds also show a significant effect on antibacterial and anti-inflammatory activity. Pharmacological and phytochemical investigations have established that phytochemicals and crude extracts from various parts of the Myristicaceae family have versatile biological activities. However, modern drugs can be developed only after an intensive investigation of their bioactivity, mechanism of action, toxicity, and proper standardization and clinical trials.
ACKNOWLEDGMENT
The authors would like to thank Dr.rer.nat Gian Primahana and Dr. Sofa Fajriah for great and interesting discussion about natural product and drug discovery.
AUTHOR CONTRIBUTIONS
MM: methodology, investigation, formal analysis, writing original draft, review, and editing.
AD: methodology, investigation, review, and editing. SH : methodology review and editing. All authors have read and agreed to the publisher version of this manuscript.
FINANCIAL SUPPORT
This work was financially support by the Health Research Organization—BRIN for research funding through the drug and vaccine research program for the 2023 fiscal year.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest related to the publication of this article.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Christenhusz MJM, Byng JW. The number of known plants species in the world and its annual increase. Phytotaxa [Internet]. 2016;261(3):201–17. CrossRef
2. Bingtao L, Wilson TK. Myristicaceae. Flora China. 2008;7:99–101.
3. Arrijani A. Phenetic relationship of genus Knema, Horsfieldia, and Myristica in Java based on pollen morphological evidence. Biodivers J Biol Divers. 2003;4(2):83–8. CrossRef
4. Valderrama J. Distribution of flavonoids in the Myristicaceae. Phytochemistry. 2000;55(6):505–11. CrossRef
5. Arrijani A. Biology and conservation of genus Myristica in Indonesia. Biodivers J Biol Divers. 2005;6(2):147–51. CrossRef
6. Pandey R, Mahar R, Hasanain M, Shukla SK, Sarkar J, Rameshkumar KB, et al. Rapid screening and quantitative determination of bioactive compounds from fruit extracts of Myristica species and their in vitro antiproliferative activity. Food Chem [Internet]. 2016;211:483–93. CrossRef
7. Gonzalez MJTG, Deoliveira CJC, Fernandes JO, Kjjoa A. Further alkyl and alkenylphenols of Knema laurina and Knema austrosiamensis. Phytochemistry. 1996;43(6):1333–7. CrossRef
8. Salleh WMNH, Shakri NM, Khamis S, Setzer W, Nadri MH. Chemical composition of three Malaysian Horsfieldia essential oils. Nat Prod Res [Internet]. 2022 Apr 3;36(7):1909–13. CrossRef
9. Calliste C, Kozlowski D, Duroux J, Champavier Y, Chulia A, Trouillas P. A new antioxidant from wild nutmeg. Food Chem [Internet]. 2010;118(3):489–96. CrossRef
10. Salleh WMNHW, Ahmad F. Phytochemistry and biological activities of the genus Knema (Myristicaceae). Pharm Sci [Internet]. 2017;23:249–55. CrossRef
11. Helen M, Vargheese TA, Jeeja K, Abiramy, Sajina, Seree J. Phytochemical analysis and anticancer activity of essential oil from. Int J Curr Pharm Rev Res. 2012;2:188–98.
12. Pillai S, Mahmud R, Lee WC, Perumal S. Anti-parasitic sctivity of Myristica fragrans Houtt. Essential oil against Toxoplasma gondii parasite. SciVerse ScienseDirct [Internet]. 2012;2:92–6. CrossRef
13. Acuna U, Carcache P, Matthew S, Blanco E. New acyclic bis phenylpropanoid and neolignans, from Myristica fragrans Houtt., exhibiting PARP-1 and NF-κB inhibitory effects. Food Chem. 2016;202:269–75. CrossRef
14. Cao GY, Yang XW, Xu W, Li F. New inhibitors of nitric oxide production from the seeds of Myristica fragrans. Food Chem Toxicol [Internet]. 2013;62:167–71. CrossRef
15. Cao GY, Xu W, Yang XW, Gonzalez F, Li F. New neolignans from the seeds of Myristica fragrans that inhibit nitric oxide production. Food Chem [Internet]. 2015;173:231–7. CrossRef
16. Cuong TD, Hung TM, Na M, Ha DT, Kim JC, Lee D, et al. Inhibitory effect on NO production of phenolic compounds from Myristica fragrans. Bioorg Med Chem Lett. 2011;21(22):6884–7. CrossRef
17. Prabha B, Neethu S, Krishnan S, Sherin D, Madhukrishnan M, Ananthakrishnan R, et al. Antidiabetic potential of phytochemicals isolated from the stem bark of Myristica fatua Houtt. var. magnifica (Bedd.) Sinclair. Bioorg Med Chem. 2018;26(12):3461–7. CrossRef
18. Megawati, Darmawan A, Fajriah S, Primahana G, Dewi RT, Minarti, et al. Antioxidant and α-glucosidase activities of benzoic acid derivate from the bark of Myristica fatua Houtt. AIP Conference Proceedings. Indonesia: American Institute of Physics; 2017. p 020027. CrossRef
19. Nguyen PH, Le TVT, Kang HW, Chae J, Kim SK, Kwon KI, et al. AMP-activated protein kinase (AMPK) activators from Myristica fragrans (nutmeg) and their anti-obesity effect. Bioorgan Med Chem Lett [Internet]. 2010;20:4128–31. CrossRef
20. Chung J, Choo J, Lee M, Hwang J. Anticariogenic activity of macelignan isolated from Myristica fragrans (nutmeg) against Streptococcus mutans. Phytomedicine. 2006;13(4):261–6. CrossRef
21. López V, Gerique J, Langa E, Berzosa C, Valero MS, Gómez-Rincón C. Antihelmintic effects of nutmeg (Myristica fragans) on Anisakis simplex L3 larvae obtained from Micromesistius potassou. Res Vet Sci [Internet]. 2015;100:148–52. CrossRef
22. Jung WC, Jang YS, Hieu TT, Lee CK, Ahn YJ. Toxicity of Myristica fragrans seed compounds against Blattella germanica (Dictyoptera: Blattellidae). J Med Entomol. 2007;44(3):524–9. CrossRef
23. Gupta AD, Bansal VK, Babu V, Maithil N. Chemistry, antioxidant and antimicrobial potential of nutmeg (Myristica fragrans Houtt). J Genet Eng Biotechnol [Internet]. 2013;11(1):25–31. CrossRef
24. Okechukwu QN, Ugwuona FU, Ofoedu CE, Juchniewicz S, Okpala COR. Chemical composition, antibacterial efficacy, and antioxidant capacity of essential oil and oleoresin from Monodora myristica and Tetrapleura tetraptera in Southeast Nigeria. Sci Rep [Internet]. 2022;12(1):1–13. CrossRef
25. Cooray N, Jansz E, Wimalasena S, Wijesekera T, Nair B. Acylresorcinols from seed kernels of Myristica dactyloides. Phytochemistry. 1987;26:3369–71. CrossRef
26. Pham VC, Jossang A, Sévenet T, Bodo B. Cytotoxic acylphenols from Myristica maingayi. Tetrahedron. 2000;56(12):1707–13. CrossRef
27. Pham VC, Jossang A, Sévenet T, Bodo B. Novel cytotoxic acylphenol dimers of Myristica gigantea; enzymatic synthesis of giganteones A and B. Tetrahedron. 2002;58(28):5709–14. CrossRef
28. Sen R, Bauri AK, Chattopadhyay SC. Antipromastigote activity of the malabaricones of Myristica malabarica (Rampatri). Phyther Res. 2008;21:592–5. CrossRef
29. Maia A, Schmitz-Afonso I, Martin M, Awang K, Laprévote O, Guéritte F, et al. Acylphenols from Myristica crassa as new acetylcholinesterase inhibitors. Planta Med. 2008;74(12):1457–62. CrossRef
30. Othman MA, Sivasothy Y, Looi CY, Ablat A, Mohamad J, Litaudon M, et al. Acylphenols and dimeric acylphenols from Myristica maxima Warb. Fitoterapia. 2016;111:12–7. CrossRef
31. Wahab SMA, Sivasothy Y, Liew SY, Litaudon M, Mohamad J, Awang K. Natural cholinesterase inhibitors from Myristica cinnamomea King. Bioorg Med Chem Lett [Internet]. 2016;26(15):3785–92. CrossRef
32. Neethu S, Govind MG, Vimalkumar PS, Biji M, Sherin DR, Dan M, et al. Novel flavonoids from the aerial parts of unexplored and endangered wild nutmeg species Myristica beddomei subsp. spherocarpa W. J. de Wilde. Phytochem Lett [Internet]. 2021;45:72–6. CrossRef
33. Megawati, Darmawan A. Resorcinol compounds isolated form the bark of Myristica fatua Houtt. Indones J Pharm. 2017;28(2):82–90. CrossRef
34. Ragasa CY, Torres OB, Mandia EH, Bernardo L, Shen CC. Phenolics from Knema stellata subsp. cryptocaryoides. Chem Nat Compd. 2015;51(6):1169–70. CrossRef
35. Chen S, Tsutsumi T, Takatsuki S, Matsuda R, Kameya H, Nakajima M, et al. Identification of 2-alkylcyclobutanones in nutmeg (Myristica fragrans). Food Chem. 2012;134(1):359–65. CrossRef
36. Ramadhan R, Phuwapraisirisan P. Arylalkanones from Horsfieldia macrobotrys are effective antidiabetic agents achieved by α-glucosidase inhibition and radical scavenging. Nat Prod Commun. 2015;10(2):325–8. CrossRef
37. Lu Z, Van Wagoner RM, Pond CD, Pole AR, Jensen JB, Blankenship D, et al. Myristicyclins A and B: antimalarial procyanidins from Horsfieldia spicata from Papua new guinea. Org Lett. 2014;16(2):346–9. CrossRef
38. Zhan R, Li D, Liu YL, Xie XY, Chen L, Shao LD, et al. Structural elucidation, bio-inspired synthesis, and biological activities of cyclic diarylpropanes from Horsfieldia kingii. Tetrahedron. 2020;76(40):1–8. CrossRef
39. Liu B, Chen YG, Tian XJ, Zhan R. Diarylpropanes from Horsfieldia kingii. Nat Prod Res. 2021 Apr 3;35(7):1127–33. CrossRef
40. Suthiwong J, Sribuhom T, Wongphakham P, Senawong T, Yenjai C. Cytotoxicity of acylphloroglucinol derivatives from the fruits of Horsfieldia irya. Nat Prod Res. 2021 Dec 2;35(23):4930–8. CrossRef
41. Gonzalez MJ, Pinto MMM, Kijjoa A, Kengthong S, Mondanondra I, Silva AMS, et al. 5,7-Dihydroxychromones and 8-hydroxytetrahydrochromones from Horsfieldia irya. Phytochemistry. 2002;61:995–8. CrossRef
42. Al-Mekhlafi NA, Shaari K, Abas F, Jeyaraj EJ, Stanslas J, Khalivulla SI, et al. New flavan and alkyl α,β-lactones from the stem bark of Horsfieldia superb. Nat Prod Commun. 2013;8(4):447–51. CrossRef
43. Ma Q, Liu Y, Zhan R, Chen Y. A new isoflavanone from the trunk of Horsfieldia pandurifolia. Nat Prod Res. 2015;(2):1–9. CrossRef
44. Du SZ, Wang ZC, Liu Y, Zhan R, Chen YG. Diarylpropanes and lignans from Horsfieldia tetratepala. Phytochem Lett. 2017;19:98–100. CrossRef
45. Rangkaew N, Suttisri R, Moriyasu M, Kawanishi K. A new acyclic diterpene acid and bioactive compounds from Knema glauca. Arch Pharm Res. 2009;32(5):685–92. CrossRef
46. Wang CF, Kuang F, Wang WJ, Luo L, Li QX, Liu Y, et al. Phenolic compounds with anti-inflammatory effects from Knema furfuracea. Results Chem [Internet]. 2021;3:100175. CrossRef
47. Lu Z, Wu WC, Wang M, Zhang JQ, Chen YG, Zhan R, et al. Flavonoids from the leaves and twigs of Knema elegans. Biochem Syst Ecol [Internet]. 2020;88:103991. CrossRef
48. Sriphana U, Yenjai C, Koatthada M. Cytotoxicity of chemical constituents from the roots of Knema globularia. Phytochem Lett. 2016;(11):129–33. CrossRef
49. Gény C, Rivière G, Bignon J, Birlirakis N, Guittet E, Awang K, et al. Anacardic acids from Knema hookeriana as modulators of Bcl-xL/Bak and Mcl-1/Bid interactions. J Nat Prod. 2016;79(4):838–44. CrossRef
50. Gunatilaka AL, de Silva AJ, Sotheeswaran S, Tillekeratne L. Horsfieldin, a lignan and other constituents from Horsfieldia iryaghedhi. Phytochemistry. 1982;21(11):2719–23. CrossRef
51. Peng W, Caiqiong Y, Zahn R, Chen Y. Two new flavans from the trunk and leaves of Horsfieldia glabra. Nat Prod Res. 2016;30(20):1–6. CrossRef
52. Sulaiman SF, Ooi KL. Antioxidant and anti food-borne bacterial activities of extracts from leaf and different fruit parts of Myristica fragrans Houtt. Food Control [Internet]. 2012;25(2):533–6. CrossRef
53. Piaru SP, Mahmud R, Majid AMS, Nassar ZD. Antioxidant and antiangiogenic activities of the essential oils of Myristica fragrans and Morinda citrifolia. Asian Pac J Trop Med [Internet]. 2012;5(4):294–8. CrossRef
54. Nakatani N, Ikeda K, Kikuzaki H, Kido M, Yamaguchi Y. Diaryldimethylbutane lignans from Myristica argentea and their antimicrobial action against Streptococcus mutans. Phytochemistry. 1988;27(10):3127–9. CrossRef
55. Bandyopadhyay C, Manna A, De S, Yasmin N, Bauri AK, Chattopadhyay S, et al. Anti-tumor effect of fruit rind of Myristica malabarica in an Ehrlich ascites carcinoma model. Int J Basic Clin Pharmacol. 2019;8(3):383. CrossRef
56. Manjunatha BK, Mankani KL, Mukunda SK, Divakara R, Sridar BK, Paul K. Antioxidant and hepato protective effect of Myristica malabarica seed aril extracts on carbon tetrachloride induced hepatic damage. Glob J Biotechnol Biochem. 2011;6(1):25–30.
57. Sawadjoon S, Kittakoop P, Kirtikara K, Vichai V, Tanticharoen M, Thebtaranonth Y. Atropisomeric myristinins: selective COX-2 inhibitors and antifungal agents from Myristica cinnamomea. J Org Chem. 2002;67(16):5470–5. CrossRef
58. Sivasothy Y, Loo KY, Leong KH, Litaudon M, Awang K. A potent alpha-glucosidase inhibitor from Myristica cinnamomea King. Phytochemistry [Internet]. 2016;122:265–9. CrossRef
59. Fajriah S, Darmawan A, Megawati, Hudiyono S, Kosela S, Hanafi M. New cytotoxic compounds from Myristica fatua Houtt leaves against MCF-7 cell lines. Phytochem Lett. 2017a;20:36–9. CrossRef
60. Fajriah S, Megawati, Hudiyono S, Kosela S, Hanafi M. Chemical constituents and potential cytotoxic activity of n- hexane fraction from Myristica fatua Houtt leaves. AIP Conf Proc. 2017b;1862:030087 CrossRef
61. Fajriah S, Megawati. Phytochemical screening and toxicity assay from Myristica fatua Houtt leaves. Chim Nat Acta. 2015;3(3):116–9. CrossRef
62. Viveka MR, Chandrashekar KR. Antioxidant and antibacterial activities of Myristica fatua var. Magnifica (Beddome) Sinclair. Asian J Pharm Clin Res. 2016;9(4):235–9.
63. Lestari F, Rija’i HRH. DPPH free radical scavenging activity test of ethyl acetate extract of stem penara (Myristica iners Blume.). Proceeding of Mulawarman Pharmaceuticals Conferences [Internet]. Indonesia: Fakultas Farmasi, Universitas Mulawarman; 2021. pp 65–71. CrossRef
64. Arunachalam KD, Subhashini S. Preliminary phytochemical investigation and wound healing activity of Myristica andamanica leaves in Swiss albino mice. J Med Plants Res. 2011;5(7):1095–106.
65. Sriphana U, Yenjai C, Suthiwong J, Poopasit K. A new diarylhexane and two new diarylpropanols from the roots of Knema globularia. Nat Prod Res [Internet]. 2020;36(7):1741–8. CrossRef
66. Wenli M, Wei N, Yan H, Changxiang C. Flavonoids from Knema globularia. Nat Prod Res Dev. 2002;14(5):26–8.
67. Giap TH, Duc PM, Van The N, Popova M, Bankova V, Hue CT, et al. Chemical constituents and biological activities of the fruits of Knema pachycarpa de Wilde. Nat Prod Res [Internet]. 2021 Feb 1;35(3):455–64. CrossRef
68. Giap TH, Thoa HT, Oanh VTK, Hang NTM, Dang NH, Thuc DN, et al. New acetophenone and cardanol derivatives from Knema pachycarpa. Nat Prod Commun. 2019 Jun 16;14(6):1934578X1985004. CrossRef
69. Rangkaew N, Suttisri R, Moriyasu M, Kawanishi K. A new arylnaphthalene lignan from Knema furfuracea. Fitoterapia [Internet]. 2009;80(6):377–9. CrossRef
70. Wulansari D, Chairul. Antioxidant screening activity of several Indonesian medicinal plants using 2,2-difenil 1-1 picrylhidrazil(DPPH). Maj Obat Tradis. 2011;16(1):22–5.
71. Raja S, Suku J. In vitro evaluation of antimicrobial activity of Knema attenuata stem bark extract. Indo Am J Pharm Res. 2017;7(07):242–7.
72. Vinayachandra, Chandrashekar K. Phenolic contents of Knema attenuata fruits and their bioactive potentials. J Herbs Spices Med Plants. 2014;20(2):183–95. CrossRef
73. Deng J, Starck SR, Li S, Hecht SM. (+) Myristinins A and D from Knema elegans, which inhibit DNA polymerase β and cleave DNA. J Nat Prod. 2005;68:1625–8. CrossRef
74. Zhang YX, Lu Z, Wu WC, Chen YG, Zhan R. Bioactive flavonoids from Knema elegans. Phytochem Lett [Internet]. 2021;42:121–4. CrossRef
75. Jaafar FM, Ridhwan MJM, Mustapha NM, Alias A, Ismail N. Antidiabetic effects of Knema glauca leaf extract toward inhibitios of Α- amylase and Α-glucosiase assays. Sci Eng. 2016;3(78):103–8. CrossRef
76. Salleh WMNHW, Anuar MZA, Khamis S, Nafiah MA, Sul’ain MD. Chemical investigation and biological activities of the essential oil of Knema kunstleri Warb. from Malaysia. Nat Prod Res [Internet]. 2019;35(3):1–7. CrossRef
77. Pinto M, Kijjoa A, Tantisewie B, Yoshida M, Gottlieb OR. Arylalkanones from Horsfieldia glabra. Phytochemistry. 1988;27:3988–9. CrossRef
78. Du SZ, Kuang F, Liu Y, Chen YG, Zhan R. A new dimeric diarylpropane from Horsfieldia tetratepala. Nat Prod Res [Internet]. 2018;32(2):162–6. CrossRef
79. Ma Q, Min K, Li HL, Jiang JH, Liu Y, Zhan R, et al. Horsfiequinones A-F, dimeric diarylpropanoids from Horsfieldia tetratepala. Planta Med. 2014;80(8–9):688–94. CrossRef
80. Miyake A, Yamamoto H, Takebayashi Y, Imai H, Honda K. The novel natural product YM-26567-1 [(+)-trans-4-(3-dodecanoyl-2,4,6- trihydroxyphenyl)-7-hydroxy-2-(4-hydroxyphenyl)chroman]a competitive inhibitor of group II phospholipase A2.pdf. J Pharmacol Exp Ther. 1992;263:1302–7. CrossRef
81. Ramadhan R, Phuwapraisirisan P. New arylalkanones from Horsfieldia macrobotrys, effective antidiabetic agents concomitantly inhibiting α-glucosidase and free radicals. Bioorg Med Chem Lett. 2015 Oct 15;25(20):4529–33. CrossRef
82. Ramadhan R, Kusuma IW, Amirta R, Worawalai W, Phuwapraisirisan P. A new 4-arylflavan from the pericarps of Horsfieldia motleyi displaying dual inhibition against α-glucosidase and free radicals. Nat Prod Res. 2018;32(22):2676–82. CrossRef
83. Khan MR, Kihara M, Omoloso AD. Antimicrobial activity of Horsfieldia helwigii and Melia azedarach. Fitoterapia. 2001;72(4):423–7. CrossRef
84. Minarti M, Ariani N, Prastya M, Darmawan A, Megawati M. Antioxidant and antibacterial properties derived from Horsfieldia spicata (Roxb.) J. Sinclair stem bark extract and its active fraction. Proceedings of the 1st International Conference for Health Research—BRIN (ICHR 2022). Dordrecht, Netherlands: Atlantis Press International BV; 2023. pp 327–7. CrossRef
85. Du S, Wang Z, Liu Y, Zhan R, Chen Y. Diarylpropanes and lignans from Horsfieldia tetratepala. Phytochem Lett. 2017;19:98–100. CrossRef
86. Woo WS, Shin KH, Wagner H, Lotter H. The structure of macelignan from Myristica fragrans. Phytochemistry. 1987;26(5):1542–3. CrossRef
87. Lee SU, Ki SS, Shi YR, Yong KM, Seong HK. Machilin A isolated from Myristica fragrans stimulates osteoblast differentiation. Planta Med. 2009;75(2):152–7. CrossRef
88. Hada S, Hattori M, Tezuka Y, Kikuchi T, Namba T. New neolignans and lignans from the aril of Myristica fragrans. Phytochemistry. 1988;27(2):563–8. CrossRef
89. Herath HMTB, Priyadarshini AMA. Lignans from Myristica dactyloides. Phytochemistry. 1997;44(4):699–703. CrossRef
90. Herath HMTB, Priyadarshani AMA. Two lignans and an aryl alkanone from Myristica dactyloides. Phytochemistry. 1996;42(5):1439–42. CrossRef
91. Nemethy EK, Lago R, Hawkins D, Calvin M. Lignans of Myristica otoba. Phytochemistry. 1986;25(4):959–60. CrossRef
92. Wahyuni S, Susilowati M, Bakri A, Bermawie N. Fruit and seed variation of wild nutmeg (Myristica schefferi Warb.) in South Aceh, Indonesia. Indonesia: IOP Publishing Ltd; 2022. p 012067. CrossRef
93. Ragasa CY, Torres OB, Vincent J, Tongco V, Razal RA, Shen CC. Resorcinols from Myristica philippensis Lam. J Chem Pharm Res [Internet]. 2013;(5):614–6. Available from: www.jocpr.com
94. Chong YM, Yin WF, Ho CY, Mustafa MR, Hadi AHA, Awang K, et al. Malabaricone C from Myristica cinnamomea exhibits anti-quorum sensing activity. J Nat Prod. 2011;74(10):2261–4. CrossRef
95. Zhan R, Hu YT, Shao LD, Qin XJ, Kuang F, Du SZ, et al. Horisfieldones A and B, two aromatic ring-contracted dimeric diarylpropanes with human DOPA decarboxylase inhibitory activity from Horsfieldia kingii. Org Lett. 2019;21(10):3678–81. CrossRef
96. Kumar NS, Herath HM, Karunaratne V. Arylalkanones from Myristica dactyloides. Phytochemistry. 1988;27(2):465–8. CrossRef
97. Chopra B, Dhingra A, Dhar KL, Nepali K, Prasad D. Role of terpenoids as anticancer compounds: an insight into prevention and treatment. Key Heterocycl. 2022;1:57–104. CrossRef
98. Wimalasena S, Karunawansha E. Characterization of a new aryl alkanone and other compound presents in Horsfieldia irya seeds. J Natl Sci Found Sri Lanka. 1994;22(3):301–4. CrossRef
99. Tillekeratne L, Jayamanne K, Weerasuria K, Gunatilaka AL. Lignans of Horsfieldia iryaghedhi. Phytochemistry. 1982;21(12):476–8. CrossRef
100. Chatterjee S, AnanthaKumar A, Variyar PS, Sharma A. Identification and estimation of a novel fluorescent compound in nutmeg. J Food Compos Anal. 2008;21(7):577–81. CrossRef
101. Dang JL, Yang XB, Huang YF, Ye F, Luo T, Chen SL, et al. GC-MS analysis on the chemical constituents of essential oil from bark of Horsfieldia hainanensis. Zhong Yao Cai. 2009;32(5):714–6.
102. Taher M, Susanti D, Rezali MF. A new sesquiterpene from Knema patentinervia. Chem Nat Compd. 2013;48(6):985–7. CrossRef
103. Talukdar AC, Jain N, De S, Krishnamurty HG. An isoflavone from Myristica malabarica. Phytochemistry. 2000;53(1):155–7. CrossRef
104. Jossang A, Jossang P, Hadi H, Sevenet T, Bodo B. Horsfiline, an alkaloid from Horsfieldia superba. J Org Chem [Internet]. 1991 Nov 1;56(23):6527–30. CrossRef
105. Susanty, Suryarachma H, Sari AM, Purnawan I, Sari F. Pengaruh Ekstrak Daun Tanaman Pala (Horsfieldia spicata) Terhadap Persentase Nilai Peredaman Radikal Bebas. JurnalUmjAcId/IndexPhp/Semnastek. 2022;(November):1–9.