INTRODUCTION
The tea plant Camellia sinensis (L.) Kuntze belongs to the family Theaceae. The species has two varieties, namely, C. sinensis var. sinensis (China tea) and C. sinensis var. assamica (Assam tea) [1,2]. The former is grown in China, Japan and Taiwan, while the latter predominates in South and Southeast Asia, including Australia and Africa. Tea is mostly planted in the highlands and rarely in the lowland [2].
Tea var. sinensis is an evergreen, multi-stemmed shrub that grows up to 3 m in height while tea var. assamica can grow up to 10−15 m tall with one main stem [1,3]. Under cultivation, young leaves of C. sinensis are regularly picked and tea plants are pruned and trained to a low profusely branching and spreading bush of 1.0−1.5 m in height. Leaves are alternate and obovate-lanceolate in shape with a short petiole, serrate margin, and pubescent on the lower surface. In var. sinensis, leaves are dark green, leathery, narrower, and marginal veins are indistinct. In var. assamica, leaves are lighter green, thinly leathery, wider, and longer, with distinct marginal veins. Tea flowers are axillary, occurring as single flowers or as clusters of 2−4 flowers and emit a mildly sweet fragrant. Flower petals are white or light pink and stamens bear many yellow anthers (Fig. 1a). Styles are free (var. sinensis) or partly fused (var. assamica) with stigmatic lobes [1,3]. Between varieties of C. sinensis, flowers possess different morphology and fruit yield [4]. Phenotypic traits include pistil length, stamen length, and stigma width.
In China, tea flower buds are produced in May with flowers blooming from September to December [5]. In Sri Lanka, flowering periods of C. sinensis occur from February to April, and from July to November [6]. The tea plant is a facultative outbreeder, i.e., cross-pollination results in a higher fruit set than self-pollination [7]. Most pollen has the ability to germinate on the cross-pollinated stigma [4].
Flies and bees have been observed to be the pollinators of the tea plant and fruiting is from February to May [8]. Another study in Sri Lanka reported that the major flowering season is from September to December and the major fruiting season occurs from April to August [9]. Tea flowers can be classified into four development stages, namely, green or young buds, white or mature buds (Fig. 1b), half-open flowers, and full bloom flowers (Fig. 1a) [10]. The yield of tea flowers varies from 3−12 tons/ha/year [11], with 8.8 tons/ha/year as the average [5]. It has been estimated that China produces 4−12 million tons of tea flowers each year [5,11]. An added advantage of removing the tea flowers is that the yield and quality of tea leaves are enhanced by ~30% the following year [11].
Previously, tea plantations in China were focused on producing tea from the young leaves [5]. Tea flowers were discarded or sprayed with chemicals to induce foliage production and not floral growth. Plant growth regulators such as ethephon, paclobutrazol, and chlormequat have shown to be effective in promoting the abscission of tea flower buds and flowers in tea plantations [12].
In recent years, tea flowers in China have been used to manufacture food, beverage, and cosmetics [5]. Tea flowers are dried (Fig. 1c) and consumed as a tea beverage when steeped in hot water [13]. The drying process involves hot-air drying or in combination with microwave drying [14,15]. Beverage produced from white or mature flower buds is the best, yielding a tea that is bright orange-yellow in color, and has a flowery or chestnut aroma, and a sweet and mellow taste. Black tea produced from tea leaves has been scented with dried tea flowers [16]. A fermentation technology for producing cider from tea flowers has been formulated [17]. Tea flowers can also be used to make a weak alkaline soap with a creamy white color and tea flower fragrance [18]. The tea flower soap has a strong ability in cleaning and protecting the skin. A facial cream from tea flower has been formulated and patented in China [19]. In Japan, tea flowers have been used as preservative for traditional soya products such as miso (fermented soybean paste) and tsukudani (boiled food in sweetened soy sauce) [20]. Honeybees (Apis mellifera) pollinating tea flowers are known to produce quality honey [21]. The honey contains theanine, a very rare amino acid derived from tea flowers. Furthermore, the nectar of tea flower has the highest concentration of caffeine that the activated the brain function of honeybees to produce the honey.
In the past 15 years or so, tea flowers have generated scientific and commercial interest [11]. The importance of this alternative resource has led to the establishment of the International Institute of Tea Flowers in Japan and the International Research and Development Center of Tea Flowers in China. In 2013, the Ministry of Health of China has recognized tea flowers as a new food source.
 | Figure 1. Fresh flowers of C. sinensis bear white petals with a pinkish tinge and produce numerous yellow stamens (a), white or mature flower buds (b), and dried tea flowers (c). [Click here to view] |
Most review articles on the chemical constituents and pharmacological properties of C. sinensis are focused on its young leaves [22–24], with little attention on other parts of the plant. This article is confined to the lesser-known flowers of C. sinensis with some emphasis on their chemical constituents, pharmacological properties, and safety evaluation. Their morphology, reproductive biology, and uses are briefly mentioned.
CHEMICAL CONSTITUENTS
Chemical compounds reported in tea flowers include catechins, polysaccharides, saponins, proteins, alkaloids, spermidine derivatives, flavonol glycosides, and anthocyanins [25,26]. The aqueous extract of tea flowers contains carbohydrates (34%), crude proteins (28%), phenolic compounds (12%), and saponins (2.8%) [26].
In recent years, tea flower polysaccharides (TFPS) have attracted great interest because of their α-glucosidase inhibitory and α-amylase inhibitory activities [11,27]. In general, the molecular weights of TFPS are greater than polysaccharides of tea leaves. Tea flowers contain acid polysaccharides, comprising rhamnose, arabinose, galactose, glucose, xylose, mannose, galacturonic acid, and glucuronic acid [11,27].
Isolated from tea flowers are flavonols (kaempferol, kaempferol glycosides, quercetin, quercetin glycosides, myricetin glycoside, and rutin), and catechins (catechin, epicatechin, gallocatechin, gallocatechin gallate, epigallocatechin, catechin gallate, epicatechin gallate, and epigallocatechin gallate) [11,28−30]. The total concentration of epicatechin gallate and epigallocatechin gallate was 70% of the total concentration of catechins in the ethanol tea flower extract [31]. The contents of total catechins and caffeine ranged from 10 to 38 mg/g and from 3 to 8 mg/g, respectively [32]. The contents of catechins in tea leaves are generally more than 12% higher than those in tea flowers [11].
Caffeine and theobromine are purine alkaloids found in tea flowers with highest contents in the stamens and petals of flower buds [33]. The contents of caffeine are 23.6 and 24.2 kBq/g and the contents of theobromine are 20 and 9.7 kBq/g, respectively [34]. Four spermidine derivatives (tricoumaroyl, triferuoyl, feruoyl dicoumaroyl, and coumaroyl diferuoyl spermidines) have been isolated from tea flowers for the first time [35]. The content of tricoumaroyl spermidine, the major compound, is highest in flower buds (181 μg/g) reducing to 92 μg/g in open flowers. Recently, hydroxycinnamic acid amides (phenolamides) have been reported from tea flowers [36]. All 12 varieties of tea flowers studied possessed p-coumaroyl-spermidine.
Triterpene oligoglycosides or triterpenoid saponins, namely, floratheasaponins (FTS) A−J chakasaponins (CKS) I−VI, and floraasamsaponins (FAS) I−VIII have been isolated from flowers of C. sinensis [10,11,37−41]. FTS A−C and J have been reported from Japan; FTS A−I and CKS I−VI from China; FTS A−F and CKS I−III from Taiwan; and FAS I−VIII from India (Fig. 2). Another group of triterpenoid saponins with highly-substituted oxygen functional groups has been identified as chakasapogenins (CKA) I−III [42]. The contents of saponin in tea flowers range from 9.5 to 79 mg/g [39]. Maximal accumulation of saponins occurs in the green bud stage [10].
The following are some characteristic features of the triterpenoid saponins (Fig. 2). All FTS A−J possess a galactopyranosyl (Gal) component at R6, a H component at R3 and R4 with the exception of FTS I and FTS H that has an acetyl (Ac) component at R3 and R4. Oxyangeloyl (OAng) dominates R1 with the exception of FTS G that has an oxytigloyl (OTig) component instead. FTS C and F have 2 methylbutyryl (2MB) at R2 not found in other FTS.
1. Among CKS I−VI possess a H and Gal component at R4 and R6, respectively. OTig and H dominate R1 and R3 except for CKS IV and CKS VI that have a H and Ac component, respectively.
2. All FAS I−VIII have a H and Rha component at R4 and R7, respectively. Ac dominates R2 except FAS VIII that has a H component instead.
The pink color of tea flowers was attributed to cyanidin-3-O-glucoside, an anthocyanin isolated from the pink petals [41,42]. Earlier studies have reported the presence of cyanidin O-syringic acid, petunidin 3-O-glucoside, and pelargonidin 3-O-β-D-glucoside in pink tea flowers [43,44]. Supercritical carbon dioxide extraction of tea flowers accounted for 86.6% of the essential oil with nonadecane (18.7%) and heneicosane (12.2%) as major volatile components [45].
PHARMACOLOGICAL PROPERTIES
In Table 1, the major pharmacological properties of tea flowers are hypoglycemic (7), anti-cancer (7), antioxidant (4), hypolipidemic (4), modulation of gut health (3), antimicrobial (3), and anti-inflammatory (3) activities. There are two studies each on hepatoprotective and immunoregulatory activities of tea flowers. β-Amyloid aggregation inhibitory, gastroprotective, nephroprotective, anti-obesity, anti-allergic, anti-cholesterol, pancreatic lipase inhibitory, melanin synthesis inhibitory, and non-alcoholic fatty liver disease activities are minor pharmacological properties of tea flowers, represented by one study each.
SAFETY EVALUATION
A study on the safety evaluation of hot water TFE was conducted by Li et al. [75]. Mutagenicity of the TFE was assessed using the Ames test. Results showed that the extract (up to 5.0 mg/plate) had no mutagenic effect towards four tested strains of Salmonella typhimurium. In the acute toxicity study, a single dose of the flower extract (12 g/kg) was administered by gavage, and monitored for 14 days. In the sub-chronic toxicity study, the rats were administered with the extract by gavage at doses of 1, 2, and 4 g/kg daily for 13 weeks [75]. In the acute toxicity study, all animals gained weight, and appeared active and normal with LD50 value >12 g/kg. In the sub-chronic toxicity study, no dose-related effects on survival, growth, hematology, blood chemistry, organ weights, or pathologic lesions were observed. The results of the safety evaluation study showed that the TFE has no mutagenic potential and exhibits an extremely low acute and sub-chronic toxicity to animals.
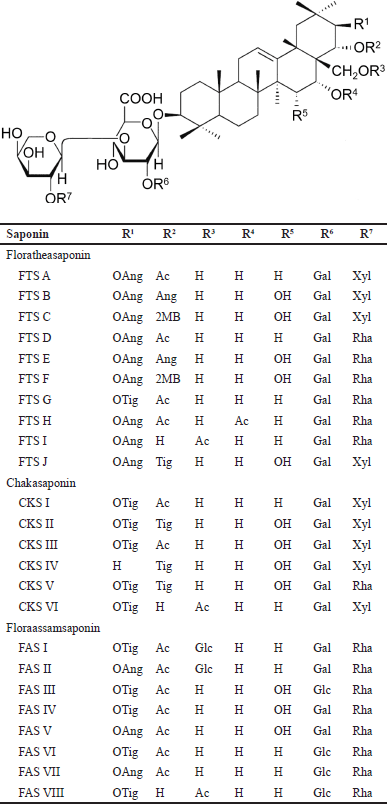 | Figure 2. Types of acylated oleanane-type triterpene oligoglycosides isolated from flowers of C. sinensis. Ac = acetyl, Ang = angeloyl, CKS = chakasaponin, FAS, floraassamsaponin, FTS = floratheasaponin, Gal = galactopyranosyl, Glc = glucopyranosyl, MB = methylbutyryl, Rha = rhamnopyranosyl, Tig = tigloyl, and Xyl = xylopyranosyl. [Click here to view] |
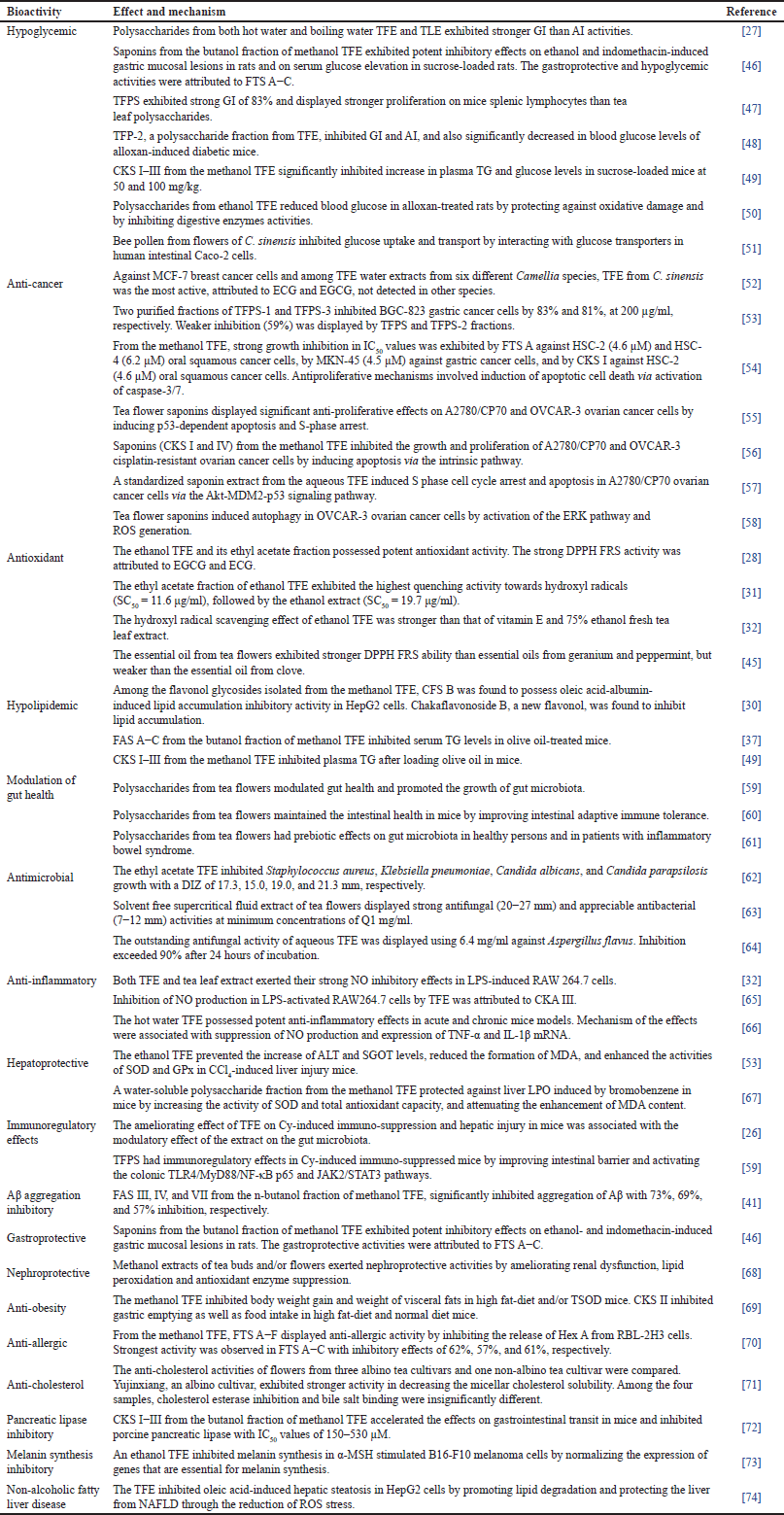 | Table 1. Bioactivities, effects, and mechanisms of extracts and bioactive compounds from flowers of C. sinensis. [Click here to view] |
CONCLUSION
Some of the chemical components of tea flowers, such as flavonols, catechins, caffeine, and theanine, are similar to those of tea leaves, and they share similar health benefits. Much of the previous work on the chemical constituents and pharmacological properties of C. sinensis flowers was conducted by scientists from the Kyoto Pharmaceutical University in Japan. Acylated oleanane-type triterpene oligoglycosides or saponins were isolated and identified from TFE, and their bioactivities described. Bioactivities include anti-hyperlipidemic, anti-hyperglycemic, anti-obesity, and gastroprotective effects, together with anti-allergic, pancreatic lipase inhibitory, and β-amyloid aggregation inhibitory activities. Comparisons were made between the chemical constituents of tea flowers from Japan, China, Taiwan, and India.
Although commercial products such as functional food are being developed from tea flowers, some issues need to be addressed. They include the high cost of harvesting tea flowers that are only available periodically. We envisage that rapid, selective, and mechanized harvesting techniques need to be developed as the flowering season is short, and the picking of white or mature flower buds is preferred over open flowers.
Additionally, the cost of manufacturing tea flower products is high, requiring efficient drying, extraction, and isolation. Post-harvest drying has to be rapid and efficient as fresh flowers containing high moisture content would quickly turn brown due to oxidation of polyphenol oxidases. The development of health supplements from tea flower buds is promising requiring clinical studies to ascertain their effectiveness, dosage, and side-effects. More studies on the safety evaluation of tea flowers are needed, although a preliminary study has shown that TFE has no mutagenic potential, and possesses an extremely low acute and sub-chronic toxicity to animals.
An added advantage of removing the tea flowers is that the yield and quality of tea leaves are enhanced the following year. The recognition of the importance of tea flowers is reflected in the established of the International Institute of Tea Flowers in Japan, and International Research and Development Center of Tea Flowers were established in China. Once considered a waste resource, tea flowers are now recognized as a new food source by the Minister of Health of China in 2013.
AUTHOR CONTRIBUTIONS
The author made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. The author is eligible to be an author as per the International Committee of Medical Journal Editors (ICMJEs) requirements/guidelines.
FINANCIAL SUPPORT
The Lead and Sole Author declares that the funds for publication of this review (Article Processing Charges) in Journal of Applied Pharmaceutical Science (JAPS) are from World’s Top 2% Scientist Research Grant, CERVIE, UCSI University (Grant Code: T2S-2023/004). He is grateful for the financial support provided by UCSI University.
CONFLICTS OF INTEREST
The authors have no conflicts of interest regarding this investigation.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Adiwinata HO, Martosupono M, Schoorel AF. Camellia sinensis. In: Westphal E, Jansen PCM, editors. PROSEA plant resources of Southeast Asia: a selection. Wageningen, The Netherlands: Pudoc Wageningen; 1989. pp 72−9.
2. Chan EWC, Lim YY, Chew YL. Antioxidant activity of Camellia sinensis leaves and tea from a lowland plantation in Malaysia. Food Chem. 2007;102:1214−22. CrossRef
3. Min TL, Bartholomew B. Camellia L. Flora China. 2007;12:367−412.
4. Lo SK, Hu CY, Roan SF, Su TC, Chen IZ. Relationship between flower phenotypic traits and fruit yields in tea (Camellia sinensis L.) varieties. Horticulturae. 2023;9(4):440−56. CrossRef
5. Gao Y, Chen YC. Chapter 17: Tea flowers and their health benefits. In: Yin J, Fu Z, Xu Y, editors. Tea as a food ingredient: properties, processing, and health, Boca Raton, FL: CRC Press/Taylor and Francis Group; 2022. pp 295−324.
6. Ariyarathna HA, Gunasekare MT, Kottawa-Arachchige JD, Paskarathevan R, Ranaweera KK, Ratnayake M, et al. Morpho-physiological and phenological attributes of reproductive biology of tea (Camellia sinensis L., O. Kuntze) in Sri Lanka. Euphytica. 2011;181:203−15. CrossRef
7. Wachira FN, Kamunya SK. Pseudo-self-incompatibility in some tea clones (Camellia sinensis (L.) O. Kuntze). J Hort Sci Biotechnol. 2005;80(6):716−20. CrossRef
8. Wickramaratne MR, Vitarana SI. Insect pollination of tea (Camellia sinensis L.) in Sri Lanka. Trop Agric. 1985;62(3):243−7.
9. Piyasundara JH, Wickramasinghe IP, Gunesekara MT, Wijeratne MA, Perera SA, Ranathunga MA, et al. Reproductive phenology of tea (Camellia sinensis L. O. Kuntze) cultivars in Sri Lanka. Trop Agric Res. 2018;29(3):288−301.
10. Shen X, Shi L, Pan H, Li B, Wu Y, Tu Y. Identification of triterpenoid saponins in flowers of four Camellia sinensis cultivars from Zhejiang province: differences between cultivars, developmental stages, and tissues. Ind Crops Prod. 2017;95:140−7. CrossRef
11. Chen Y, Zhou Y, Zeng L, Dong F, Tu Y, Yang Z. Occurrence of functional molecules in the flowers of tea (Camellia sinensis) plants: evidence for a second resource. Molecules. 2018;23(4):790. CrossRef
12. Tian Y, Chen Z, Jiang Z, Huang X, Zhang L, Zhang Z, et al. Effects of plant growth regulators on flower abscission and growth of tea plant Camellia sinensis (L.) O. Kuntze. J. Plant Growth Regul. 2022;41:1161−73. CrossRef
13. Wu Z, Li X, Xu X, Xing A, Xu Y, Yang X, et al. Quality components identification and formation analysis of tea (Camellia sinensis L.) flower beverages from three cultivars. LWT. 2023;181:114739. CrossRef
14. Shi L, Gu Y, Wu D, Wu X, Grierson D, Tu Y, et al. Hot air drying of tea flowers: effect of experimental temperatures on drying kinetics, bioactive compounds and quality attributes. Int J Food Sci Technol. 2019;54(2):526−35. CrossRef
15. Shi L, Kim E, Yang L, Huang Y, Ren N, Li B, et al. Effect of a combined microwave-assisted drying and air drying on improving active nutraceutical compounds, flavor quality, and antioxidant properties of Camellia sinensis L. (cv. Longjing 43) flowers. Food Qual Saf. 2021;5:fyaa040. CrossRef
16. Shen D, He P, Yu Y, Fan DS, Zhang C, Yu YC. Preliminary study on the technology of scenting black tea with tea flower. Guizhou Sci. 2017;35:92−6.
17. Shan YX, Chen QW, Bai R, Wang X, Wan XC, Jiang J, et al. Study on the fermentation technology of tea-flower cider. Sci Technol Food Ind. 2013;34:207−11.
18. Zhang D, Lu Y, Li B, Yu H, Tu Y. Development and study on properties of tea flower soap. J Zhejiang Univ (Agric Life Sci). 2016;42(3):333−9.
19. Tu YY, Ma ZY, Chen ZC, Gao LD. Development of a tea flower facial cream. CN103251532A. 2013.
20. Harima S, Yoshikawa M, Tokuoka K. Historical consideration of tea trees and tea flowers, especially regarding the use of tea flowers as food. Yakushigaku Zasshi. 2008;43(1):16−32.
21. Saito K, Nagahashi R, Ikeda M, Nakamura Y. Chapter 2. Honeybees (Apis mellifera) produce honey from flowers of tea plants (Camellia sinensis). In: Al-Naggar AMM, editor. Advances and trends in agricultural sciences. India/UK: Book Publisher International; 2019. pp 12−6.
22. Chaudhari S, Gupta SK, Yadav S, Yogi B. A review on phytochemical and pharmacological activity of Camellia sinensis (tea leave). World J Pharm Res. 2020;9:332−48.
23. Rubab S, Rizwani GH, Durrani AI, Liaqat I, Zafar U, Batool F, et al. Phytochemical and pharmacological potential of Camellia sinensis L. Pak J Zool. 2023;55(2):669−78. CrossRef
24. Zhao T, Li C, Wang S, Song X. Green tea (Camellia sinensis): a review of its phytochemistry, pharmacology, and toxicology. Molecules. 2022;27(12):3909. CrossRef
25. Chen D, Chen G, Sun Y, Zeng X, Ye H. Physiological genetics, chemical composition, health benefits and toxicology of tea (Camellia sinensis L.) flower: a review. Food Res Int. 2020;137:109584. CrossRef
26. Chen D, Ding Y, Chen G, Sun Y, Zeng X, Ye H. Components identification and nutritional value exploration of tea (Camellia sinensis L.) flower extract: evidence for functional food. Food Res Int. 2020;132:109100. CrossRef
27. Wang Y, Yang Z, Wei X. Sugar compositions, α-glucosidase inhibitory and amylase inhibitory activities of polysaccharides from leaves and flowers of Camellia sinensis obtained by different extraction methods. Int J Biol Macromol. 2010;47(4):534−9. CrossRef
28. Yang Z, Tu Y, Baldermann S, Dong F, Xu Y, Watanabe N. Isolation and identification of compounds from the ethanolic extract of flowers of the tea (Camellia sinensis) plant and their contribution to the antioxidant capacity. LWT. 2009;42(8):1439−43. CrossRef
29. Morikawa T, Lee IJ, Okugawa S, Miyake S, Miki Y, Ninomiya K, et al. Quantitative analysis of catechin, flavonoid, and saponin constituents in ‘tea flower’, the flower buds of Camellia sinensis, from different regions in Taiwan. Nat Prod Commun. 2013;8(11):1553−7.
30. Morikawa T, Ninomiya K, Miyake S, Miki Y, Okamoto M, Yoshikawa M, et al. Flavonol glycosides with lipid accumulation inhibitory activity and simultaneous quantitative analysis of 15 polyphenols and caffeine in the flower buds of Camellia sinensis from different regions by LCMS. Food Chem. 2013;140(1–2):353−60. CrossRef
31. Yang Z, Xu Y, Jie G, He P, Tu Y. Study on the antioxidant activity of tea flowers (Camellia sinensis). Asia Pac J Clin Nutr. 2007;16(1):148−52.
32. Lin YS, Wu SS, Lin JK. Determination of tea polyphenols and caffeine in tea flowers (Camellia sinensis) and their hydroxyl radical scavenging and nitric oxide suppressing effects. J Agric Food Chem. 2003;51(4):975−80. CrossRef
33. Suzuki T. Purine alkaloids in Camellia sinensis flowers. Agric Biol Chem. 1985;49(9):2803−5.
34. Fujimori N, Ashihara H. Adenine metabolism and the synthesis of purine alkaloids in flowers of Camellia. Phytochemistry. 1990;29(11):3513−6.
35. Yang Z, Dong F, Baldermann S, Murata A, Tu Y, Asai T, et al. Isolation and identification of spermidine derivatives in tea (Camellia sinensis) flowers and their distribution in floral organs. J Sci Food Agric. 2012;92(10):2128−32. CrossRef
36. Liu H, Liu Y, Han H, Lu C, Chen H, Chai Y. Identification and characterization of phenolamides in tea (Camellia sinensis) flowers using ultra-high-performance liquid chromatography/Q-exactive orbitrap mass spectrometry. Food Chem. 2023;424:136402. CrossRef
37. Yoshikawa M, Morikawa T, Yamamoto K, Kato Y, Nagatomo A, Matsuda H. Floratheasaponins A−C, acylated oleanane-type triterpene oligoglycosides with anti-hyperlipidemic activities from flowers of the tea plant (Camellia sinensis). J Nat Prod. 2005;68(9):1360−5.
38. Yoshikawa M, Sugimoto S, Nakamura S, Matsuda H. Medicinal flowers. XXII. Structures of chakasaponins V and VI, chakanoside I, and chakaflavonoside A from flower buds of Chinese tea plant (Camellia sinensis). Chem Pharm Bull. 2008;56(9):1297−303.
39. Morikawa T, Miyake S, Miki Y, Ninomiya K, Yoshikawa M, Muraoka O. Quantitative analysis of acylated oleanane-type triterpene saponins, chakasaponins I–III and floratheasaponins A–F, in the flower buds of Camellia sinensis from different regional origins. J Nat Med. 2012;66:608−13. CrossRef
40. Ohta T, Nakamura S, Nakashima S, Matsumoto T, Ogawa K, Fujimoto K, et al. Acylated oleanane-type triterpene oligoglycosides from the flower buds of Camellia sinensis var. assamica. Tetrahedron. 2015;71(5):846−51. CrossRef
41. Matsuda H, Nakamura S, Morikawa T, Muraoka O, Yoshikawa M. New bio-functional effects of the flower buds of Camellia sinensis and its bioactive acylated oleanane-type triterpene oligoglycosides. J Nat Med. 2016;70(4):689−701. CrossRef
42. Zhang T, Ma X, Zhou Y, Yang H, Wang Y, Chen T, et al. Metabolite profiling of external and internal petals in three different colors of tea flowers (Camellia sinensis) using widely targeted metabolomics. Metabolites. 2023;13(7):784. CrossRef
43. Rothenberg DO, Yang H, Chen M, Zhang W, Zhang L. Metabolome and transcriptome sequencing analysis reveals anthocyanin metabolism in pink flowers of anthocyanin-rich tea (Camellia sinensis). Molecules. 2019;24(6):1064. CrossRef
44. Zhou C, Mei X, Rothenberg DO, Yang Z, Zhang W, Wan S, et al. Metabolome and transcriptome analysis reveals putative genes involved in anthocyanin accumulation and coloration in white and pink tea (Camellia sinensis) flower. Molecules. 2020;25(1):190. CrossRef
45. Chen Z, Mei X, Jin Y, Kim EH, Yang Z, Tu Y. Optimisation of supercritical carbon dioxide extraction of essential oil of flowers of tea (Camellia sinensis L.) plants and its antioxidative activity. J Sci Food Agric. 2014;94(2):316−21. CrossRef
46. Yoshikawa M, Wang T, Sugimoto S, Nakamura S, Nagatomo A, Matsuda H, et al. Functional saponins in tea flower (flower buds of Camellia sinensis): gastroprotective and hypoglycemic effects of floratheasaponins and qualitative and quantitative analysis using HPLC. Yakugaku Zasshi. 2008;128(1):141−51.
47. Wei X, Chen M, Xiao J, Liu Y, Yu L, Zhang H, et al. Composition and bioactivity of tea flower polysaccharides obtained by different methods. Carbohydr Polym. 2010;79(2):418−22. CrossRef
48. Han Q, Yu QY, Shi J, Xiong CY, Ling ZJ, He PM. Molecular characterization and hypoglycemic activity of a novel water-soluble polysaccharide from tea (Camellia sinensis) flower. Carbohydr Polym. 2011;86(2):797−805. CrossRef
49. Matsuda H, Hamao M, Nakamura S, Kon’i H, Murata M, Yoshikawa M. Medicinal flowers. XXXIII. Anti-hyperlipidemic and anti-hyperglycemic effects of chakasaponins I–III and structure of chakasaponin IV from flower buds of Chinese tea plant (Camellia sinensis). Chem Pharm Bull. 2012;60(5):674−80.
50. Wei X, Cai X, Xiong S, Wang Y. Hypoglycemic effect of oral crude tea flower polysaccharides on alloxan modelling Sprague–Dawley rats and the possible mechanism. CyTA J Food. 2012;10(4):325−32. CrossRef
51. Li Q, Ren C, Yan S, Wang K, Hrynets Y, Xiang L, et al. Extract of unifloral Camellia sinensis L. pollen collected by Apis mellifera L. honeybees exerted inhibitory effects on glucose uptake and transport by interacting with glucose transporters in human intestinal cells. J Agric Food Chem. 2021;69(6):1877−87. CrossRef
52. Way TD, Lin HY, Hua KT, Lee JC, Li WH, Lee MR, et al. Beneficial effects of different tea flowers against human breast cancer MCF-7 cells. Food Chem. 2009;114(4):1231−6. CrossRef
53. Xu R, Ye H, Sun Y, Tu Y, Zeng X. Preparation, preliminary characterization, antioxidant, hepatoprotective and antitumor activities of polysaccharides from the flower of tea plant (Camellia sinensis). Food Chem Toxicol. 2012;50(7):2473−80. CrossRef
54. Kitagawa N, Morikawa T, Motai C, Ninomiya K, Okugawa S, Nishida A, et al. The antiproliferative effect of chakasaponins I and II, floratheasaponin A, and epigallocatechin 3-O-gallate isolated from Camellia sinensis on human digestive tract carcinoma cell lines. Int J Mol Sci. 2016;17(12):1979. CrossRef
55. Wang Y, Ren N, Rankin GO, Li B, Rojanasakul Y, Tu Y, et al. Anti-proliferative effect and cell cycle arrest induced by saponins extracted from tea (Camellia sinensis) flower in human ovarian cancer cells. J Funct Foods. 2017;37:310−21. CrossRef
56. Ren N, Chen L, Li B, Rankin GO, Chen YC, Tu Y. Purified tea (Camellia sinensis (L.) Kuntze) flower saponins induce the p53-dependent intrinsic apoptosis of cisplatin-resistant ovarian cancer cells. Int J Mol Sci. 2020;21(12):4324. CrossRef
57. Tu YY, Chen LF, Ren N, Li B, Wu YY, Rankin GO, et al. Standardized saponin extract from Baiye no. 1 tea (Camellia sinensis) flowers induced S phase cell cycle arrest and apoptosis via AKT-MDM2-p53 signaling pathway in ovarian cancer cells. Molecules. 2020;25(15):3515. CrossRef
58. Wang Y, Xia C, Chen L, Chen YC, Tu Y. Saponins extracted from tea (Camellia sinensis) flowers induces autophagy in ovarian cancer cells. Molecules. 2020;25(22):5254. CrossRef
59. Chen D, Chen G, Ding Y, Wan P, Peng Y, Chen C, et al. Polysaccharides from the flowers of tea (Camellia sinensis L.) modulate gut health and ameliorate cyclo-phosphamide-induced immunosuppression. J Funct Foods. 2019;61:103470. CrossRef
60. Chen D, Ding Y, Ye H, Sun Y, Zeng X. Effect of long-term consumption of tea (Camellia sinensis L.) flower polysaccharides on maintaining intestinal health in BALB/c mice. J Food Sci. 2020;85(6):1948−55. CrossRef
61. Chen D, Chen G, Chen C, Zeng X, Ye H. Prebiotics effects in vitro of polysaccharides from tea flowers on gut microbiota of healthy persons and patients with inflammatory bowel disease. Int J Biol Macromol. 2020;158:968−76. CrossRef
62. Eksi S, Ejder NE, Üreyen Ü, Uzunok B, Yazici Z, Beris FA. Screening antiproliferative and antimicrobial effects of ethyl acetate extract driven from Camellia sinensis flowers. J Pharm Drug Res. 2020;3(2):319−28.
63. Sharma R, Kapoor S, Padwad Y, Kumar D. GC-MS based profiling, antimicrobial activity and cytotoxicity studies of Camellia sinensis (L.) O. Kuntze flower extract. J Biol Active Prod Nat. 2022;12(2):137−45. CrossRef
64. Chen F, Chen YP, Wu H, Li Y, Zhang S, Ke J, et al. Characterization of tea (Camellia sinensis L.) flower extract and insights into its antifungal susceptibilities of Aspergillus flavus. BMC Complement Med Ther. 2023;23:286. CrossRef
65. Yoneda T, Nakamura S, Ogawa K, Matsumoto T, Nakashima S, Matsumura K, et al. Oleanane-type triterpenes with highly-substituted oxygen functional groups from the flower buds of Camellia sinensis and their inhibitory effects against no production and HSV-1. Nat Prod Commun. 2018;13(2):131−6.
66. Chen BT, Li WX, He RR, Li YF, Tsoi B, Zhai YJ, et al. Anti-inflammatory effects of a polyphenols-rich extract from tea (Camellia sinensis) flowers in acute and chronic mice models. Oxid Med Cell Longev. 2012;2012:537923.
67. Han Q, Xiong CY, Shi J, Gao Y, Chen YS, Ling ZJ, et al. Isolation, chemical characterization and antioxidant activities of a water-soluble polysaccharide fraction of tea (Camellia sinensis) flower. J Food Biochem. 2012;36(1):46−55. CrossRef
68. Avarave S, Thomas J, Radha V, Altaff K. Synergistic protective effect of Camellia sinensis leaf buds and Camellia sinensis flowers against cisplatin-induced nephrotoxicity in rats and characterization of its bioactive compounds. Nat Prod Res. 2022;36(17):4470–4.
69. Hamao M, Matsuda H, Nakamura S, Nakashima S, Semura S, Maekubo S, et al. Anti-obesity effects of the methanolic extract and chakasaponins from the flower buds of Camellia sinensis in mice. Bioorg Med Chem. 2011;19(20):6033−41. CrossRef
70. Yoshikawa M, Nakamura S, Kato Y, Matsuhira K, Matsuda H. Medicinal flowers. XIV. New acylated oleanane-type triterpene oligoglycosides with antiallergic activity from flower buds of Chinese tea plant (Camellia sinensis). Chem Pharm Bull. 2007;55(4):598−605.
71. Gao, Y, Han Z, Xu YQ, Yin JF. Chemical composition and anti-cholesterol activity of tea (Camellia sinensis) flowers from albino cultivars. Front Nutr. 2023;10:1142971. CrossRef
72. Yoshikawa M, Sugimoto S, Kato Y, Nakamura S, Wang T, Yamashita C, et al. Acylated oleanane-type triterpene saponins with acceleration of gastrointestinal transit and inhibitory effect on pancreatic lipase from flower buds of Chinese tea plant (Camellia sinensis). Chem Biodivers. 2009;6(6):903−15.
73. Dissanayake CY, Moon HH, Yang KM, Lee Y, Han CH. The effects of green tea (Camellia sinensis) flower extract on melanin synthesis in B16-F10 melanoma cells. Korean J Vet Res. 2018;58(2):65−72. CrossRef
74. Zhang X, Gao Y, Xu J, Liu X, Jin F, Li B, et al. Inhibitory effect of tea (Camellia sinensis L. O. Kuntze, Theaceae) flower extracts on oleic acid-induced hepatic steatosis in HepG2 cells. J Food Nutr Res. 2014;2(10):738−43. CrossRef
75. Li B, Jin Y, Xu Y, Wu Y, Xu J, Tu Y. Safety evaluation of tea (Camellia sinensis (L.) O. Kuntze) flower extract:assessment of mutagenicity, and acute and sub-chronic toxicity in rats. J Ethnopharmacol. 2011;133(2):583−90. CrossRef