INTRODUCTION
Among cardiovascular diseases, coronary artery disease (CAD) has led to 16% of the world’s total population mortality in 2019. In Indonesia, about 15 out of 1,000 populations are expected to suffer from CAD. CAD contributed to 14.4% of the total Indonesian population mortality in 2018 [1]. It is a condition caused by atherosclerotic plaque in coronary vessels where its clinical manifestation can either be acute (acute coronary syndrome, ACS) or chronic (chronic coronary syndrome, CCS), depending on the degree of vessel occlusion and phase of the disease. Besides that, CAD is a dynamic disease that can progress from stable to acute, which can result in fatality when modifiable risk factors are not addressed by pharmacological and non-pharmacological interventions to control risk factors such as blood pressure, blood lipids, and glucose levels [2].
Based on the Indonesian national CCS guideline published by PERKI Indonesia, in which evidence is adopted from the European Society of Cardiology 2019 CCS guideline, patients without contraindications to aspirin, beta-blocker, angiotensin converting enzyme inhibitors (ACEi)/angiotensin receptor blockers (ARBs)/angiotensin receptor neprilysin inhibitor (ARNI), and statin should receive the combination of these first-line drugs as secondary prevention of CCS as they are associated with reduction in cardiovascular mortality by 34% [3–5]. Besides that, in patients with moderate or severe ischemia, a conservative pharmacological strategy also appears to be just as beneficial as invasive strategies to address ischemic cardiovascular events or death from any cause, as described by the ISCHEMIA study [6].
In addition to the potential benefits of first-line CCS drugs, there is also growing evidence of the benefits of using second-line CCS drugs and implementation of personalized drugs in CCS patients to control anginal symptoms [7]. In addition, due to the variable healthcare services in Indonesia, which may impact the prescription patterns, and due to the variance of drugs that may be prescribed in CCS patients after adjusting comorbidities, this study aims to observe the prescription patterns in CCS patients together with their appropriateness within the guideline of first-line CCS drugs. Until today, there is no prior study in Indonesia exploring the prescription patterns and their appropriateness within the guidelines of CCS drugs used; therefore, this is the very first study in Indonesia conducted to fill the gap of evidence regarding CCS patients’ treatment in Indonesia.
METHODS
Study design and setting
This is a descriptive cross-sectional study conducted in Bandung, Indonesia, using the data acquired from Dr. Hasan Sadikin Central General Hospital Bandung CAD registry during January–September 2022. Dr. Hasan Sadikin Central General Hospital is one of the two tertiary referral hospitals located in West Java, Indonesia. It is the most crowded province in Indonesia, where about 48 out of the 275 million Indonesians’ population reside (Fig. 1). This is the first and the only registry in Indonesia aiming to register patients diagnosed with CAD to describe their clinical characteristics, management therapy, and clinical outcomes. The registry is completed by interviewing patients and looking into patients’ medical records to acquire associated data and is conducted in several outpatient sites in Dr. Hasan Sadikin Central General Hospital in Bandung: cardiovascular clinics, prevention rehabilitation cardiovascular clinic, and cardiac polyclinic.
Study population
The population of this study includes those diagnosed with CCS as identified retrospectively from the registry during January–September 2022. The targeted population in this study includes all patients diagnosed with CCS in Dr. Hasan Sadikin Central General Hospital; however, the accessible population acquired in this study includes only CCS patients from several sites in the hospital, as described previously. Inclusion criteria include patients diagnosed with CCS from the registry, and the identified population is excluded when associated data required for this study is not present: absence of medication prescription data and data regarding contraindication factors in patients not receiving first-line drugs (aspirin, beta blockers, ACEi/ARB/ARNI, and statin).
In this study, aspirin contraindication factors that may result in non-eligibility to receive this first-line CCS drug include the presence of asthma, chronic obstructive pulmonary disease (COPD), peptic ulcer, gastrointestinal bleeding, hemorrhagic diathesis, and hypersensitivity. However, the use of other antithrombotics, such as clopidogrel, ticagrelor, prasugrel, and warfarin, should also be accounted for as their use could be justified as second-line antithrombotic after aspirin when it is not available or appropriate. Beta blockers contraindication factors included are asthma, COPD, bradycardia (<60 bpm), use of non-dihydropyridine calcium channel blocker (NDHP-CCB), hypotension (<90/60 mmHg), and hypersensitivity. ACEi/ARB/ARNI contraindication factors include pregnancy/lactation, hyperkalemia (>5.5 mmol/l), cough side effects, baseline creatinine level >2.5 mg/dl, and hypersensitivity. Statin contraindication factors include liver dysfunction (serum glutamic-oxaloacetic transaminase [SGOT] >45 μ/l and/or serum glutamic-pyruvic transaminase [SGPT] >35 μ/l), pregnancy/lactation, myopathy side effects, and hypersensitivity.
 | Figure 1. Map of West Java, Indonesia. Dr. Hasan Sadikin Central General Hospital is located in Bandung, West Java, Indonesia, where it is the most populated province in Indonesia as of 2022. Image is acquired from google maps in 2022. [Click here to view] |
Data collection and definitions
A consecutive sampling of population data was identified in the registry during the period January–September 2022. Diagnosis of CCS is defined as patients with stable angina pectoris (condition of angina related to physical and/or emotional stress that is relieved after nitroglycerin use and rest), patients with stabilized symptoms after ACS, and patients post revascularization therapies, either with percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG).
Patients’ clinical characteristics such as gender, physical and blood examination findings [blood pressure, heart rate, low density lipid level (LDL)], risk factors, and medical histories are retrieved from the registry. Blood pressure and heart rate measurements are acquired at the date of the interview, and LDL level is acquired from patients’ medical records where lipid measurement closest to the prior date of the interview is selected. All medical risk factors and histories are acquired through self-reporting of patients.
The main data retrieved in this study is the medication and dosage prescribed to the patients up to the date of the interview. They include all medications, including first-line CCS drugs, other CCS drugs, and drugs used for other comorbidities. In addition, the rate of high-intensity statin used, defined as the use of atorvastatin 40–80 mg or rosuvastatin 20–40 mg, is also described. CCS first-line drug prescriptions are then adjusted for contraindication factors, and appropriateness within the guideline rate is determined based on the percentage of patients receiving optimal pharmacotherapy as described by national guidelines. Optimal pharmacotherapy is defined as the concurrent prescription of four first-line CCS drugs, namely aspirin, beta blockers, ACEi/ARB/ARNI, and statin, unless contraindicated are considered.
Data analysis
Descriptive analysis was performed by using Microsoft Excel and IBM SPSS version 25 to outline the categorical clinical characteristics of CCS patients, varieties and doses of drugs prescribed, contraindication factors in patients not receiving first-line CCS drugs, and appropriateness within guideline rate in CCS patients. They are then described in frequency (n) and percentage (%). Numerical clinical characteristics of patients are calculated in the form of average (x?) with standard deviation (σ) if data are normally distributed based on the Kolmogorov–Smirnov test and with median (interquartile range, IQR) if data are not normally distributed.
Ethical approval and inform consent
The Research Ethics Committee Universitas Padjadjaran Bandung (886/UN6.6.1/TU.00/2022) and Research Ethics Committee RSUP Dr. Hasan Sadikin Bandung (LB.02.01/X.6.5/339/2021) approved this study. Written informed consent was obtained from all individual participants included in this study.
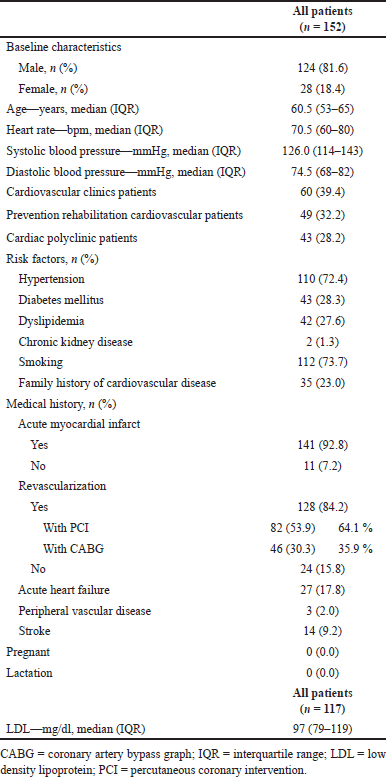 | Table 1. Basic clinical characteristics of CCS patients. [Click here to view] |
RESULTS
A total of 173 patients’ data based on inclusion criteria were initially identified. However, 21 data are excluded due to a lack of information regarding contraindication factors toward first-line CCS drugs. Hence, 152 patients’ data are proceeded for analysis in this study.
The basic clinical characteristics of the included patients are described in Table 1. The majority of the population is male, with a median of 60.5 years old. Included patients have high-risk factors for hypertension and smoking, and the medical history of included patients shows that most of the included patients have prior acute myocardial infarct, and 84.2% of the total population underwent revascularization. All of the patients acquired are outpatients from cardiovascular clinics, followed by prevention rehabilitation cardiovascular clinic and cardiac polyclinic.
Medication prescription patterns, including first-line CCS drugs and other drugs used in this population, are shown in Figure 2 and Table S1. The most frequently prescribed antithrombotic in this study is the first-line CCS antithrombotic aspirin (87.5%), followed by clopidogrel (40.8%), ticagrelor (8.6%), and warfarin (5.3%). First-line anti-ischemic agents are also prescribed at a high rate, where beta blockers and ACEi/ARB/ARNI are prescribed at both 87.5%. The first-line anti-dyslipidemia drug, statin, was prescribed at the highest rate among all first-line CCS drugs; however, the second-line anti-dyslipidemia drugs were only prescribed in one patient.
In this study population, it was shown that the most
antidiabetic medications prescribed are metformin, followed by insulin, Sodium-Glucose Transport Protein 2 (SGLT2) inhibitors, and Dipeptidyl Peptidase 4 (DPP4) inhibitors. It was further shown that nine patients received double antidiabetic drugs, and three patients received triple antidiabetic drugs. Besides that, medications prescribed for other purposes show a high prevalence of the use of proton pump inhibitors such as lansoprazole or omeprazole.
The dosage of each drug prescribed is further delineated in Table S2, in which most prescribed medications are as follows: beta blockers–bisoprolol 5 mg, ACEi–ramipril 2.5 mg, ARB–candesartan 8 mg, statin–atorvastatin 40 mg, aspirin–80 mg, CCB–amlodipine 10 mg. In the statin category, it is also observed that only 66.2% of patients receiving statin received high-intensity statin (Fig. 3).
After retrieving data regarding contraindications of each first-line CCS drug, it was then found that only 1 out of 25 patients not receiving aspirin have a contraindication to it (gastrointestinal bleeding), and 8 out of 19 patients (42.1%) not receiving beta blockers have asthma, COPD, use of NDHP-CCB, and bradycardia each and four others have hypotension. Besides that, in the 19 (5.2%) patients not receiving ACEi/ARB/ARNI, one patient has hypotension. While in the one patient not receiving a statin, there are no contraindication factors discovered.
However, after further analysis in a group of patients not receiving aspirin, it was found that 22 patients used second-line antithrombotic instead of aspirin, 18 patients used clopidogrel alone, two patients used clopidogrel and warfarin, one patient used warfarin alone, and one patient used ticagrelor. When these factors are considered for non-eligibility criteria for aspirin, there is an increase in the percentage of patients receiving appropriate aspirin when contraindications are adjusted (Fig. 4). In total, appropriate prescriptions of aspirin, beta blockers, ACEi/ARB/ARNI, and statin are discovered in 97.7%, 92.4%, 88.1%, and 99.3% of patients, respectively.
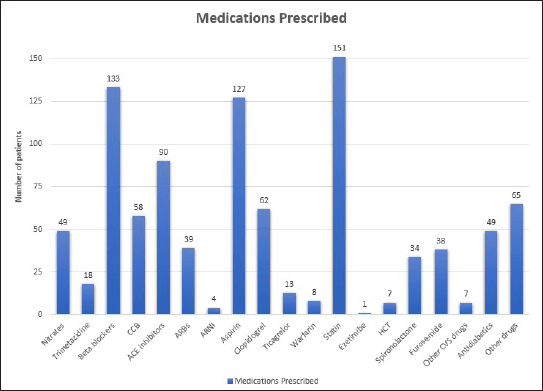 | Figure 2. Medications prescribed in CCS patients. Total population included in the study is 152 patients where the highest rate of prescription is seen statin followed by beta blockers, aspirin, and ACE inhibitors. Other than first line CCS drugs, the highest prescription rate is clopidogrel followed by CCB and nitrates. Mostly prescribed other drugs is proton pump inhibitor mainly lansoprazole. ACE, angiotensin converting enzyme; CCB, calcium channel blocker; CCS, chronic coronary syndrome; CVS, cardiovascular system; HCT, hydrochlorothiazide. [Click here to view] |
All in all, it was found that the appropriateness within the guideline rate where concomitant prescriptions of five first-line CCS drugs were given unless contraindicated in this study population is 82.2%. In the remaining 17.8% of patients not receiving appropriate medications, 85.2% omitted one drug, 11.1% omitted two drugs, and 3.7% omitted three drugs. On the other hand, when the use of second-line antithrombotic is not accounted for aspirin non-eligibility, appropriateness within the guideline rate is only 74.3%.
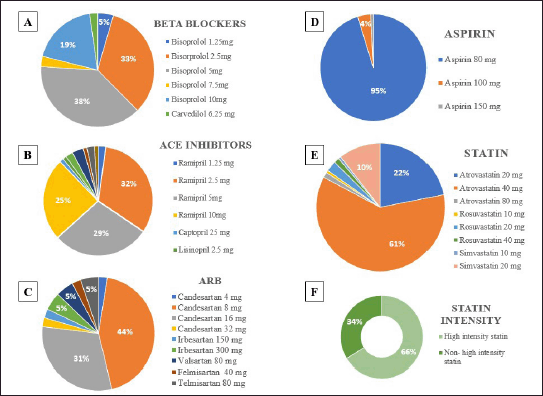 | Figure 3. Dosage of prescribed drugs in CCS patients. Mostly prescribed dosage is bisoprolol 5 mg (A), ramipril 2.5 mg (B), candesartan 8 mg (C), aspirin 80 mg (D), and atorvastatin 40 mg (E). It is observed that high intensity lipid defined as use of atorvastatin 40–80 mg or rosuvastatin 20–40 mg reaches 66.2% (F). CCS, chronic coronary syndrome. [Click here to view] |
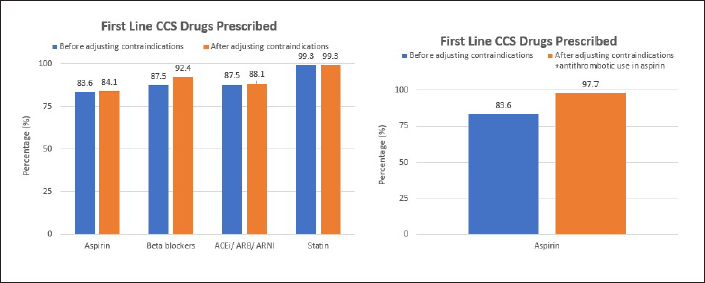 | Figure 4. Appropriateness in first line CCS drugs used before and after contraindication factors adjusted. Significant change in the number of appropriate aspirin use is observed when antithrombotic use is accounted as aspirin non-eligibility (right) compared to only accounting aspirin contraindication factors (left). Antithrombotic used in this study are clopidogrel, ticagrelor, and warfarin. Aspirin contraindications included are asthma, COPD, peptic ulcer, GI bleeding, hemorrhagic diathesis, and aspirin hypersensitivity. CCS, chronic coronary syndrome; COPD, chronic obstructive pulmonary disease; GI, gastrointestinal. [Click here to view] |
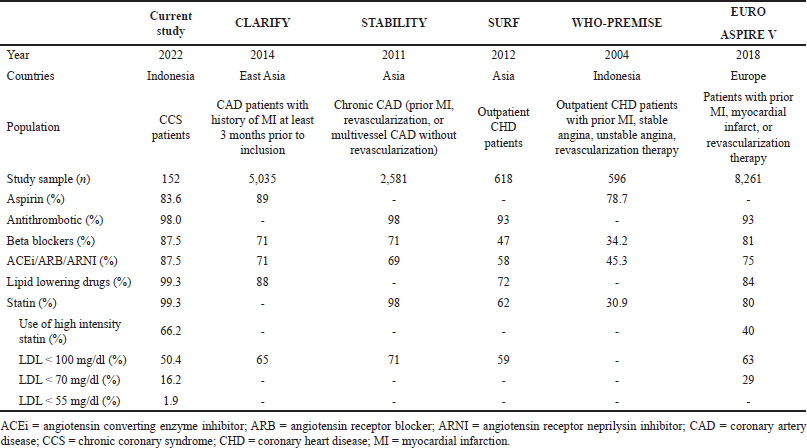 | Table 2. Comparison of first line CCS drugs used in this study and previous studies. [Click here to view] |
DISCUSSION
The basic characteristics of the population in our study are similar to the CLARIFY study conducted in East Asia, where the average age of the population is 62.8, and 76% of the population is male, with 67% having hypertension as comorbid. This study, however, shows a relatively low number of patients having dyslipidemia as a risk factor when compared to previous studies [8], which can be explained by a different method of obtaining patients’ dyslipidemia status. In this study, dyslipidemia status is obtained through patient self-reporting rather than checking the patient’s medical lipid profile; hence, it may result in underreporting. The main reason for not obtaining patients’ dyslipidemia status through medical records in this study is due to a lack of resources regarding patients’ lipid measurements prior to treatment.
In this study, the majority of the population has a substantially higher event of prior myocardial infarct when compared to a previous study [8] (92.2% vs. 50%), and a reasonable cause of it could be due to the different population included in each study. Findings regarding revascularization therapy selected in this study population are also not found to be in line with the previous study, where there is a lower rate of PCI revascularization in this study and a higher rate of CABG revascularization [8]. This finding could be a sign of different population disease severity included in this study compared to the previous study and hence will result in different selections of antithrombotic use depending on the stages of the disease the patients are in.
The finding of the high rate of first-line CCS drugs used in this study is due to the fact that almost a third of patients recruited were from prevention rehabilitation cardiovascular clinics where the use of optimal and appropriate drugs is more common. This level of prescription is also higher than those from the previous study except for a lower aspirin prescription in this study compared to previous studies conducted in Asian countries (CLARIFY [8], STABILITY [9], SURF [10] studies). A similar finding is also observed when it is compared to a study that included the European population (EUROASPIRE V study [11]). In addition, when compared to a study conducted almost 2 decades ago, which included the Indonesian population conducted by WHO-PREMISE [12], it was found that all first-line CCS drugs are prescribed at a higher rate in this study (Table 2). Although the WHO-PREMISE study was done in 2004, data were collected years before and during that time; guidelines regarding dyslipidemia and CAD secondary prevention in Indonesia are also not available; hence, it is not surprising that the use of these agents is uncommon in Indonesia at that time. However, the tremendously growing trend of using first-line CCS drugs in Indonesia in the past 2 decades reflects a better awareness of using first-line drugs as recommended to address morbidities and mortalities of CCS.
However, when exploring the result individually, the main reason for the lower prescription of aspirin in this study when compared to the majority of the previous study is that most of the patients not receiving aspirin in this study that do receive it were found to have already received other antithrombotic such as clopidogrel, ticagrelor, and warfarin, which are in line with the current guidelines where their use could be justified based on patients clinical conditions. Clopidogrel could be used as an alternative to aspirin as secondary prevention of CCS in patients with aspirin intolerance or in patients with a history of ischemic stroke, transient ischemic attack, and peripheral artery disease; ticagrelor can be selected in preference for aspirin after ACS when dual antiplatelet therapy is not preferable; warfarin can be used in the absence of aspirin in patients with previous atrial fibrillation [4]. When considered together, it is observed that the use of antithrombotic in this study reaches 98.0% (149 of 152 patients). In practice, however, several factors should be considered when giving antithrombotics to CCS patients, especially at risk of thrombosis and bleeding; hence, in this study, the use of other antithrombotics in the absence of aspirin could be justified.
In the other hand, when compared to the previous studies, this study shows a higher rate of first-line anti-ischemic drugs used. The high rate of prescription of (ACEi/ARB/ARNI and beta blockers) can be accounted for the high number of hypertension comorbid and medical history of prior MI in this study. This is in line with the national guideline where hypertension is the main indication of ACEi/ARB/ARNI and beta blocker prescription in order to lower blood pressure to a desirable level <130 mmHg when tolerated. Besides, in the condition of prior MI, the use of these two agents could potentially reverse the remodeling process of the cardiac structure; hence, it is not uncommon to be prescribed in this condition. It is further supported by national guidelines where ACEi/ARB/ARNI should be given in patients with high-risk atherosclerotic disease, and long-term beta blockers should be given to patients with prior myocardial infarct [4]. In the other hand, 38.4% of this study population receive the second line of anti-ischemic drugs CCB, which is in line with previous studies [8,9]. Prescription of CCB may be needed to address uncontrollable CCS symptoms as recommended by national guidelines and/or also to treat the highly prevalent comorbidity–hypertension [4].
In this study, almost all of the included patients received statin, and one of them received statin and ezetimibe. This rate of lipid-lowering drugs (including all drugs prescribed to lower lipid levels) prescription is higher compared to previous studies. However, what is concerning is that the amount of patients receiving high-intensity statin as recommended by national guidelines only reaches 66.2%, which may have an impact on an inadequate control of lipid profile in this patient as reflected by the still high level of LDL (median 97 md/dl with IQR 79–119) and only 1.9% (3 out of 117) patients achieving LDL-C level below 55 mg/dl as recommended in the current CCS guideline. When a cutoff of below 70 mg/dl is used, there are still only 16.2% (19 out of 117) patients achieving this higher target. When this result is compared to the EUROASPIRE V study comprising the European population, there is a lower rate of high-intensity statin prescription at 50%. Furthermore, 32% of patients on a statin in the EUROASPIRE V study achieved an LDL target of 70 mg/dl (Table 2). Achieving targeted LDL level is greatly important as all-cause mortality is reduced by 10% per 1.0 mmol/l (18 mg/dl) LDL reduction. Besides that, there is also a reduction in deaths due to coronary heart disease and other cardiac causes [13]. In the current guideline, it is also stated that the lower the LDL level, the better the outcome, remembering that no significant effects are observed in the low LDL concentration level [9]. Hence, the importance of achieving the LDL target cannot be underestimated. In this study, the main reason for the low, high-intensity statin prescription rate may be due to the lack of affordable drugs in Indonesia. Apparently, high-intensity statin drugs are not insured by Indonesian national health insurance, and although generic drugs are present, they are expensive and are only prepared in the lowest prescription dose (rosuvastatin 40 mg), hence requiring patients to consume more drugs when a higher dose is indicated which will affect patient’s compliance. Besides that, physician’s inertia in not prescribing high-intensity statin may also be accounted for this finding and could be made worse by the lack of knowledge and awareness of its use and benefits. As only 1.9% of patients in this population has desirable lipid level, with 66.2% of patients receiving high-intensity statin, it should also be a concern to have a follow-up checking of patients’ LDL level to determine the sufficiency of one lipid-lowering drug. Additional complementary lipid-lowering drugs such as ezetimibe may then have to be added to further lower patients’ LDL levels to the recommended level of below 55 mg/dl.
Besides the prescription pattern of first-line CCS drugs, the use of anti-anginal drugs, namely nitrate, is higher in this study compared to the EUROASPIRE IV study [14] conducted in European populations (32.2% vs. 21.3%). However, second-line anti-anginal drug prescription is found to be low, where only 11.8% use trimetazidine, while none of them use ranolazine and ivabradine. The absence of ranolazine and ivabradine use could be due to the lack of awareness and information exposure regarding their use, hence resulting in a non-familiarity with their use. A lower rate of second-line anti-anginal drugs is also seen in the EUROASPIRE IV study [14], where trimetazidine is used only in 5.8% of patients, ivabradine in 2.3% of patients, and nicorandil in 0.9% patients. CLARIFY study [8] also reported similar findings where ivabradine use is only 2%. The underutilization of second-line anti-anginal drugs in this study is further made worse by the finding that 49 patients (32.2%) still reported having their angina not adequately controlled. The underutilization of these second-line anti-anginal agents may again be explained by the lack of coverage from the national health insurance, resulting in their low utilization rate.
For other drugs, the prescription of furosemide and spironolactone in this study can be elucidated in line with the prevalence of prior heart failure events in this population as they are one of the main drugs prescribed in heart failure therapy and the high prescription rate of proton pump inhibitors drugs in this study population may be explained by its use in addressing aspirin side effects of gastrointestinal discomfort caused by decreased gastric protection. Besides, its usage has also been recommended by current guidelines to prevent complication of therapy [4].
After describing prescription patterns in this study, first-line CCS drugs are adjusted for their contraindication factors in patients not receiving them to identify the appropriateness of prescription within the guideline. It is shown that the majority of patients not receiving aspirin have been prescribed other antithrombotics, hence justifying its omission and leaving three patients in this group to be questionable for not receiving antithrombotics. Besides that, in beta blockers and ACEi/ARB/ARNI group, however, there are still a significant number of patients not being prescribed the drugs with no contraindication factors found. While in the statin group, most patients have already received the drugs. This study, however, does not further explore the main factors regarding the omission of these drugs besides their contraindication factors.
All in all, after adjusting contraindication factors and additional factors as in the aspirin group, a moderately high number of patients (82.2%) adhere to the current national CCS guideline defined as concomitant use of four first-line drugs–aspirin, beta blockers, ACEi/ARB/ARNI, and statin–unless contraindicated. In the remaining 17.8% of this study population, it is found that most of them omitted one drug, mostly ACEi/ARB/ARNI. The less optimal adherence to guidelines could be influenced by doctors’ and patients’ psychological factors in prescribing and receiving four drugs simultaneously for a single disease. Besides that, the patient factor in demanding fewer drugs when symptoms are already controlled also played a significant role, as the patient believes that further pharmacological control in CCS is not required. These factors then would be a challenge for physicians treating CCS patients to encourage patients that first-line CCS drugs should be consumed lifelong unless contraindicated to prevent relapse of disease.
However, this study does have a limitation in that it is conducted mainly in Bandung General Central Hospital, in which patients are treated by perceptive clinicians; hence, there may be an overestimation in the use of first-line CCS drugs. As this is the first study describing the appropriateness of treatment guidelines in CCS patients, it is hoped that future studies will be conducted to further evaluate the trends of guideline appropriateness in CCS patients, especially in a more generalized population in Indonesia. It is also expected that future studies aiming to explore the factors associated with the omission of first-line CCS drug prescriptions are conducted to further elucidate the reasons behind the omissions. Besides that, factors regarding the low prescription rate of high-intensity statin can also be further elucidated by generating further studies to understand this finding. Following longitudinal studies should also be held in order to describe the dynamic nature of CCS patients pharmacological treatment. Finally, studies regarding clinical outcomes of patients who received appropriate drugs should also be sought to further evaluate the benefits of adhering to current guidelines by also considering compliance of patients and other associated factors.
CONCLUSION
This study shows that the most prescribed medications in CCS patients are first-line CCS drugs, and the rate of their use in Indonesia is high compared to previous studies. Besides that, appropriateness within guidelines is also moderately high but could be further improved in the future. The main concern from the findings of this study includes the far-from-target LDL level in patients despite already receiving high-intensity statin.
AUTHOR CONTRIBUTIONS
All authors made a significant contribution to this study, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
FUNDING
The author(s) received no financial support throughout the research process.
CONFLICTS OF INTEREST
The author(s) declared no potential conflict of interest with respect to the research, authorship, and/or publication.
ETHICAL APPROVAL
The Research Ethics Committee Universitas Padjadjaran Bandung (886/UN6.6.1/TU.00/2022) and Research Ethics Committee RSUP Dr. Hasan Sadikin Bandung (LB.02.01/X.6.5/339/2021) approved this study.
INFORMED CONSENT
Written informed consent was obtained from all individual participants included in this study.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Kemenkes RI. Hasil Riset Kesehatan Dasar Tahun 2018. Kementrian Kesehat RI. 2018;53:1689–99.
2. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42:3227–337. CrossRef
3. Du L, Cheng Z, Zhang Y, Li Y, Mei D. The impact of medication adherence on clinical outcomes of coronary artery disease: a meta-analysis. Eur J Prev Cardiol. 2017;24:962–70. CrossRef
4. Neumann FJ, Sechtem U, Banning AP, Bonaros N, Bueno H, Bugiardini R, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–77. CrossRef
5. Perhimpunan Dokter Spesialis Kardiovaskular Indonesia. Panduan Evaluasi dab Tata Laksana Angina Pektoris Stabil.Indonesian Journal of Cardiology. 2019
6. Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Boden WE, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382:1395–407. CrossRef
7. Ferrari R, Camici PG, Crea F, Danchin N, Fox K, Maggioni AP, et al. Expert consensus document: a “diamond” approach to personalized treatment of angina. Nat Rev Cardiol. 2018;15:120–32. CrossRef
8. Ferrari R, Ford I, Greenlaw N, Tardif JC, Tendera M, Abergel H, et al. Geographical variations in the prevalence and management of cardiovascular risk factors in outpatients with CAD: data from the contemporary CLARIFY registry. Eur J Prev Cardiol. 2015;22:1056–65. CrossRef
9. Vedin O, Hagström E, Stewart R, Brown R, Krug-Gourley S, Davies R, et al. Secondary prevention and risk factor target achievement in a global, high-risk population with established coronary heart disease: baseline results from the STABILITY study. Eur J Prev Cardiol. 2013;20:678–85. CrossRef
10. Cooney MT, Reiner Z, Sheu W, Ryden L, Sutter J, De Bacquer D, et al. SURF—SUrvey of Risk Factor management: first report of an international audit. Eur J Prev Cardiol. 2014;21:813–22. CrossRef
11. Kotseva K, De Backer G, De Bacquer D, Rydén L, Hoes A, Grobbee D, et al. Lifestyle and impact on cardiovascular risk factor control in coronary patients across 27 countries: results from the European Society of Cardiology ESC-EORP EUROASPIRE V registry. Eur J Prev Cardiol. 2019;26:824–35. CrossRef
12. Mendis S, Abegunde D, Yusuf S, Ebrahim S, Shaper G, Ghannem H, et al. WHO study on Prevention of REcurrences of Myocardial Infarction And StrokE (WHO-PREMISE). Bull World Health Organ. 2005;83:820–8. CrossRef
13. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010;376:1670–81. CrossRef
14. De Smedt D, De Backer T, Petrovic M, De Backer G, Wood D, Kotseva K, et al. Chronic medication intake in patients with stable coronary heart disease across Europe: evidence from the daily clinical practice. Results from the ESC EORP European Survey of Cardiovascular Disease Prevention and Diabetes (EUROASPIRE IV) Registry. Int J Cardiol. 2020;300:7–13. CrossRef
SUPPLEMENTARY MATERIAL
Supplementary data can be downloaded from the journal’s website