INTRODUCTION
Doxorubicin is one of the potent chemotherapy drugs that has been used in the treatment of a wide variety of cancers for several decades [1]. The drug works by intercalating between the base pairs of the DNA molecule, which disrupts the replication and transcription of DNA, leading to cell death [2]. In addition, doxorubicin generates free radicals that damage cellular components, including the DNA. Due to its broad mechanism of action, it has been effective against various malignancies, including breast cancer, lymphomas, and sarcomas, among others [3,4]. However, while doxorubicin is a valuable tool in oncology, its use is not without challenges. Doxorubicin has been reported to cause cardiotoxicity, indicated by apparent damage to cardiac muscle cells [5]. These deleterious effects could be both acute and chronic [6].
Among various mechanisms underlying cardiotoxicity, doxorubicin action to deactivate protein, namely sirtuin 1 (SIRT1), in the cardiomyocytes is obvious. A study has shown that doxorubicin administration could lead to a substantial reduction in SIRT1 expression and activity in the heart muscle cells [7]. The downregulation of SIRT1 compromises its protective roles, such as the regulation of oxidative stress, prevention of cellular hypertrophy, and promotion of cellular survival pathways [8]. The reduced SIRT1 activity in the presence of doxorubicin may exacerbate oxidative damage, mitochondrial dysfunction, and cellular apoptosis in cardiomyocytes, contributing to the overall cardiotoxicity of the drug [9]. In another way, doxorubicin also has been observed to negatively impact the adenosine monophosphate-activated protein kinase (AMPK) signaling pathway in cardiomyocytes [10], which plays a crucial role in cellular energy homeostasis. Under normal conditions, AMPK acts as an energy sensor and is activated during energy-deprived states, subsequently promoting catabolic pathways to generate Adenosine Triphosphate (ATP) while inhibiting anabolic processes [11]. However, exposure to doxorubicin disrupts this finely tuned-mechanism [12]. Mechanistically, doxorubicin-induced reactive oxygen species (ROS) production and the subsequent oxidative stress in cardiomyocytes can impair the activity of major upstream kinase responsible for AMPK activation [13]. In addition, doxorubicin may directly interfere with AMPK’s phosphorylation status [14]. The impairment of AMPK activity due to doxorubicin not only disrupts cellular energy balance but also attenuates the cardioprotective effects of AMPK, including enhancement of mitochondrial function, inhibition of pathological hypertrophy, and reduction of apoptosis [15]. Hence, considering the detrimental implication of doxorubicin-induced reduction in SIRT1 and AMPK activity in cardiomyocytes, it could be crucial to explore potent natural compounds including from the plants that are capable of activating SIRT1 and AMPK as a strategy to counteract cardiotoxicity.
Among the diverse tropical plants rich in phytochemical compounds, Vitis gracilis (Family: Vitaceae) is one of the species shown to have protective effects on various organs [16,17]. Our previous research indicated that a leaf decoction of V. gracilis can protect testicular tissue in a hyperglycemic animal model [18]. A nano herbal preparation derived from V. gracilis leaf extract also has been shown to effectively protect lung cells against apoptosis in rats [19]. In addition, V. gracilis has demonstrated the ability to prevent apoptosis in rodent models [20]. Furthermore, phytochemical screening using ultra-performance liquid chromatography revealed that V. gracilis leaves are rich in bioactive compounds belonging to the groups of phenols, fatty acids, terpenoids, steroids, and glycosides [18]. Although several studies have highlighted the health benefits of V. gracilis, the protective effects of its compounds on cardiomyocytes remain unexplored. In particular, the molecular interactions of the bioactive compounds from V. gracilis with SIRT1 and AMPK in addressing doxorubicin-induced cardiotoxicity are largely unknown. Moreover, whether bioactive compounds of V. gracilis exert equal activation on SIRT1 and AMPK remains to be elucidated. Since in vitro and in vivo investigations can be both time-consuming and expensive, the use of in silico approaches has been validated as a dependable method to ascertain the potential actions of compounds, including those derived from plants, in treating various diseases [21].
Among the myriad tropical plants abundant in phytochemical compounds, V. gracilis (Family: Vitaceae), locally known as “gagatan harimau” in Indonesia, stands out as a species known for its protective effects on various organs [16,17]. Previous study has demonstrated that a leaf decoction of V. gracilis offers protection to testicular tissue in hyperglycemic animal models [18]. Moreover, a nano-herbal preparation derived from V. gracilis leaf extract has been found to shield lung cells against apoptosis in rats [19]. In addition, V. gracilis has proven its capability to prevent apoptosis in rodent models [20]. Phytochemical analyses, utilizing ultra-performance liquid chromatography, have unveiled that the leaves of V. gracilis are laden with bioactive compounds, notably phenols, fatty acids, terpenoids, steroids, and glycosides [18]. While various studies have emphasized the health merits of V. gracilis, the potential protective impact of its compounds on cardiomyocytes has yet to be explored. Specifically, the molecular interplay between the bioactive compounds from V. gracilis and proteins such as SIRT1 and AMPK, especially in the context of doxorubicin-induced cardiotoxicity, remains a mystery. Furthermore, it is still undetermined whether the bioactive compounds from V. gracilis offer equivalent activation of both SIRT1 and AMPK.
Considering that in vitro and in vivo research can be resource-intensive, in silico methodologies have emerged as reliable tools for determining the potential therapeutic roles of various compounds, including plant-derived ones, in addressing diverse ailments [21]. Given the promising nature of phytochemical compounds in V. gracilis, it is important to undertake an exploratory study using an in-silico approach. This will facilitate a deeper understanding of the mechanisms that could potentially mitigate cardiotoxicity induced by doxorubicin. Therefore, this present research seeks to shed light on the bioactivity of compounds from V. gracilis leaves, particularly focusing on their interactions with SIRT1 and AMPK, to counteract the cardiotoxic effects of doxorubicin.
MATERIALS AND METHODS
Bioactive compounds from V. gracilis leaf extract
A total of 13 selected compounds from the leaf extract of V. gracilis were obtained from our previous study [18]. The selection criteria were based on the molecular weight of the compounds (≤500 g/mol). Subsequently, the structures of the compounds were retrieved from the database of PubChem [https://pubchem.ncbi.nlm.nih.gov/]. The compound names and the respective PubChem CID were as follows: valproic acid (PubChem CID: 3121), phenyl 2-hydroxybenzoate, phenyl salicylate (PubChem CID: 8361), ribonic acid, 2,3,4,5-tetrahydroxypentanoic acid (PubChem CID: 5460677), ethylparaben (PubChem CID: 8434), propyl 4-hydroxybenzoate (PubChem CID: 7175), 3’,4’-dimethoxy-alpha-naphthoflavone (PubChem CID: 276138), 6,6,9-trimethyl-3-(3-methyloctan-2-yl)-7,8,9,10-tetrahydrobenzo[c]chromen-1-ol (PubChem CID: 36276), dipropyleneglycol methyl ether acetate (PubChem CID: 9815489), arbutin (PubChem CID: 440936), 5-[6-hydroxy-5-(3-methylbut-2-enyl)-1-benzofuran-2-yl]benzene-1,3-diol (PubChem CID: 641376), lauric acid (PubChem CID: 3893), 4,4-dimethyl-5alpha-cholesta-8,14,24-trien-3beta-ol (PubChem CID: 443212), and norethindrone acetate (PubChem CID: 5832). The 2-D structures of the compounds are presented in Figure 1.
Screening of the biological activities of the compounds using the PASS online test
The screening on the biological activities of the compounds from V. gracilis leaf extract was performed using a Prediction of Activity Spectra of Substances (PASSs) online test through the Way2drug server (http://way2drug.com/PassOnline/). Briefly, the compound name was first submitted to the PubChem server (http://pubchem.ncbi.nlm.nih.gov) to retain the SMILES structure. Thereafter, the structure of the compound was submitted to the Way2drug server to define its predictive biological activities. The criteria of the PASS online test’s result (Pa value) were as follows: (i) Pa > 0.7 indicates a high probable activity of the compound, (ii) 0.5 < Pa < 0.7 indicates a lower biological activity, and (iii) Pa < 0.5 indicates a very low activity [22].
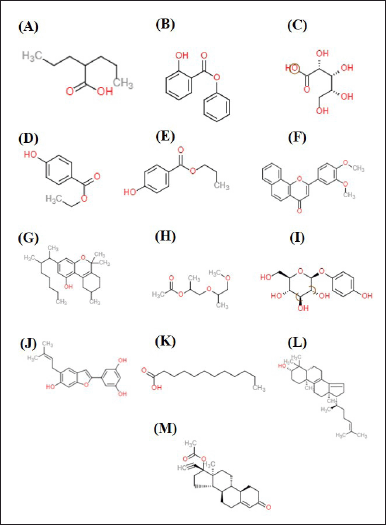 | Figure 1. 2D structures of the compounds from V. gracilis leaf extract. (A) valproic acid, (B) phenyl 2-hydroxybenzoate, phenyl salicylate, (C) ribonic acid, 2,3,4,5-tetrahydroxypentanoic acid, (D) ethylparaben, (E) propyl 4-hydroxybenzoate, (F) 3’,4’-dimethoxy-alpha-naphthoflavone, (G) 6,6,9-trimethyl-3-(3-methyloctan-2-yl)-7,8,9,10-tetrahydrobenzo[c]chromen-1-ol, (H) dipropyleneglycol methyl ether acetate, (I) arbutin, (J) 5-[6-hydroxy-5-(3-methylbut-2-enyl)-1-benzofuran-2-yl]benzene-1,3-diol, (K) lauric acid, (L) 4,4-dimethyl-5alpha-cholesta-8,14,24-trien-3beta-ol, and (M) norethindrone acetate. [Click here to view] |
Prediction of the absorption, distribution, metabolism, excretion, and toxicity (ADMET) of the compounds
To determine the ADMET, the 2-D structure of the compound in .sdf format and the SMILES structure were first downloaded from PubChem Database [http://pubchem.ncbi.nlm.nih.gov]. Furthermore, the ADME was determined using the SwissADME server (http://www.swissadme.ch/index.php). Thereafter, the prediction of the compound to cross the blood–brain barrier (BBB) was conducted via the pkcSM web server ((http://biosig.lab.uq.edu.au/pkcsm/prediction). Furthermore, the prediction of compound toxicity was carried out by using the ProTox-II server (http://tox-new.charite.de/protox_II) with the criteria of toxicity as per the standard of the Organization for Economic Co-operation and Development [22].
Prediction of drug-likeness of the compounds using Lipinski’s rule of five test
The drug-likenesses of the compounds were evaluated based on the criteria of Lipinski’s rule of five. The canonical SMILE molecular structures of the compounds were submitted to the Molinspiration Cheminformatics server (https://www.molinspiration.com/cgi/properties) to define their compliance with Lipinski’s rules [23].
Molecular docking
Preparation of ligands
Ligand preparations were carried out using BIOVIA Discovery Studio Software (https://discover.3ds.com/discovery-studio-visualizer-download). The ligand structures were downloaded from the PubChem database [http://pubchem.ncbi.nlm.nih.gov] and saved in the Standard Data Format (SDF) format for the further step of the analysis. The ligands were also subjected to energy minimalized by using Open Babel software integrated with Pyrx v.0.8. In addition to phytochemical compounds from V. gracilis, the known native ligands and standard drugs suitable for SIRT1 and AMPK were also prepared. The native ligand for SIRT1 was ATQ ((3S)-1,3-dimethyl-N-[3-(1,3-oxazol-5-yl)phenyl]-6-[3 (trifluoromethyl)phenyl]-2,3-dihydropyrido[2,3-b]pyrazine-4(1H)-carboxamide)) and Native ligand for AMPK was C1V (native ligand of AMPK) (3-[4-(2-hydroxyphenyl)phenyl]-4-oxidanyl-6-oxidanylidene-7H-thieno[2,3-b]pyridine-5-carbonitrile). A standard commercial drug, namely resveratrol, was chosen as a potent ligand to activate SIRT1 and AMPK [24]. Resveratrol is known as a potent activator for SIRT1 and AMPK.
Preparation of targeted proteins
The 3-D structures of targeted proteins, namely SIRT1 (PDB ID: 4ZZI) and AMPK (PDB ID: 4CFF), were retrieved from a protein data bank (PDB; https://www.rcsb.org/) and subsequently validated by X-ray diffraction method with resolution of 2.73 and 3.92 Å and R-value free score of 0.235 and 0.264 for SIRT1 and AMPK, respectively. Thereafter, the water molecules were eliminated from the proteins by using PYMOL software.
Docking and visualization of the results
Molecular docking was conducted by using Autodock Vina software integrated in Pyrx v. 08. It used targeted docking with exhaustiveness 8 to obtain the best pose of binding conformation for the docked complex of protein ligand. The grid box dimension for SIRT1 was adjusted as X = 20.7739, Y = 20.8390, and Z = 20.7404, and the center grid box was adjusted as X = 8.3946, Y = 35.4962, and Z = −3.9606. Moreover, the grid box dimension for AMPK was adjusted as X = 17.0822, Y = 13.6879, and Z = 18.8745, while the center grid box was adjusted as X = 26.1826, Y = −11.0162, and Z = 207.6170. The results of docking were presented as binding energy values and the interactions between the ligands (compounds of V. gracilis leaf extract) and targeted proteins (SIRT1 and AMPK) were visualized by using BIOVIA Discovery Studio 2019 software [25].
RESULTS AND DISCUSSION
Predicted bioactivity of the compounds from V. gracilis based on PASS online test
The results of virtual screening on the bioactivity by using the PASS online test (Table 1) suggests that arbutin is the compound that could exert the most diverse potent bioactivities related to the cardiovascular system including as cardio protectant, lipid peroxidase inhibitor, antioxidant, and free radical scavenger. Other compounds namely valproic acid, ribonic acid, 2,3,4,5-tetrahydroxypentanoic acid, and ethylparaben might exert high bioactivity as Nicotinamide Adenine Dinucleotide Phosphate (NADPH) peroxidase inhibitors. Moreover, some compounds might exert an immunomodulatory activity (anti-inflammatory, immunostimulant, and immunosuppressant) including phenyl 2-hydroxybenzoate, phenyl salicylate, arbutin, lauric acid, and norethindrone acetate. In addition, some compounds are also suggested to have high bioactivity to regulate lipid metabolism (lipid metabolism regulator and anti-hypercholesterolemic) such as ribonic acid, 2,3,4,5-tetrahydroxypentanoic acid, ethylparaben, and propyl 4-hydroxybenzoate.
The virtual screening results from the PASS online test highlight the potential therapeutic benefits of the studied compounds [26]. Arbutin emerges as a significant player, showcasing diverse bioactivities beneficial for the cardiovascular system. It suggests its utility as a cardio-protectant and an agent against oxidative stress, which is crucial in heart-related disorders. Several compounds, including valproic acid and ribonic acid, have been identified as NADPH peroxidase inhibitors. Given that NADPH peroxidase is instrumental in producing ROS, its inhibition might offer therapeutic advantages in conditions marked by oxidative stress [27]. Immunomodulatory properties of compounds such as phenyl 2-hydroxybenzoate indicate potential applications in managing inflammatory and related diseases. Meanwhile, the ability of certain compounds to regulate lipid metabolism could address the widespread issues of dyslipidemia and its cardiovascular implications [28]. In essence, while these compounds showcase promising bioactivities based on virtual screening, it is imperative to validate their actual therapeutic potential and safety through comprehensive in vitro and in vivo studies.
Predicted pharmacokinetics of the compounds from V. gracilis
Absorption of the compounds
The prediction on the absorption of the compounds from V. graclicis (Table 2) revealed that the majority of the compounds (11 of the total of 13 compounds) could be absorbed highly through the human intestine. Only two compounds (ribonic acid, 2,3,4,5-tetrahydroxypentanoic acid, and arbutin) have a low level of absorption in the human intestine. In addition, the analysis on Caco-2 permeability as an indicator of absorption rate for orally given drugs also confirmed that the majority of the compounds (11 of 13) could be easily absorbed, while two compounds namely ribonic acid, 2,3,4,5-tetrahydroxypentanoic acid and arbutin are limitedly absorbed.
Distribution of the compounds
To define the distribution of the compounds from V. gracilis, the human oral bioavailability (the presence of the compound in the circulatory upon oral administration) and BBB permeant (the possibility of the compounds reaching the central nervous system) were analyzed. As shown in Table 2, commonly the compounds of V. gracilis have lower human oral bioavailability (9 of 13 compounds). However, three other compounds (valproic acid, phenyl 2-hydroxybenzoate, phenyl salicylate, and 3’,4’-dimethoxy-alpha-naphthoflavone) are predicted to have higher oral bioavailability. Moreover, eight compounds could cross the BBB, thereby, reaching the brain tissue.
Metabolism of the compounds
To predict the metabolism of the compounds, their availability as a substrate of the metabolic enzyme commonly found in the liver namely cytochrome P450 2D6 (CYP2D6) was assessed. As depicted in Table 2, 11 of 13 compounds from V. gracilis do not act as a substrate for CYP2D6 enzyme, while the other two (6,6,9-trimethyl-3-(3-methyloctan-2-yl)-7,8,9,10-tetrahydrobenzo[c]chromen-1-ol and 5-[6-hydroxy-5-(3-methylbut-2-enyl)-1-benzofuran-2-yl]benzene-1,3-diol) could be metabolized by the enzyme. In addition, none of the compounds acts as a CYP2D6 inhibitor, suggesting that all of the selected compounds from V. gracilis will not alter the concentration of other drugs that are dependent on the CYP2D6 enzyme for its activation of elimination.
Excretion of the compounds
To assess the excretion of the compounds from V. gracilis, their possibility to act as inhibitors of organic cation transporter 2 (OCT2) protein in the kidney was examined. The results in Table 2 indicate that none of the 13 compounds from V. gracilis leaf extract functions as an OCT2 inhibitor. This finding suggests that all of the compounds would not affect the excretion of other drugs or compounds in the kidney. It also means that none of the compounds would affect the concentration of other drugs or compounds in the circulatory system while combined.
Water solubility of the compounds
As depicted in Table 2, the majority of the compounds of V. gracilis leaf are water-soluble (ranging from moderate to high solubility). However, two compounds are poorly soluble in water namely 6,6,9-trimethyl-3-(3-methyloctan-2-yl)-7,8,9,10-tetrahydrobenzo[c]chromen-1-ol and 4,4-dimethyl-5alpha-cholesta-8,14,24-trien-3beta-ol.
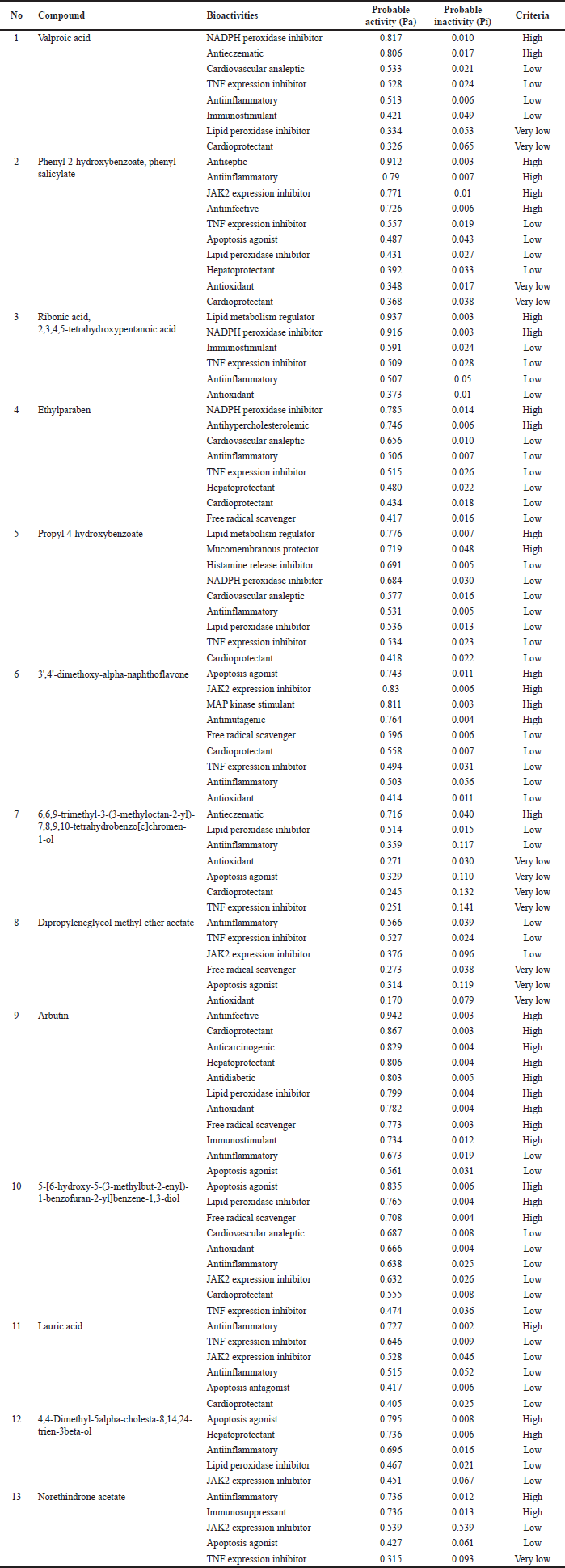 | Tabel 1. Predicted biological activity spectrum of the compounds from V. gracilis leaf based on PASS online test. [Click here to view] |
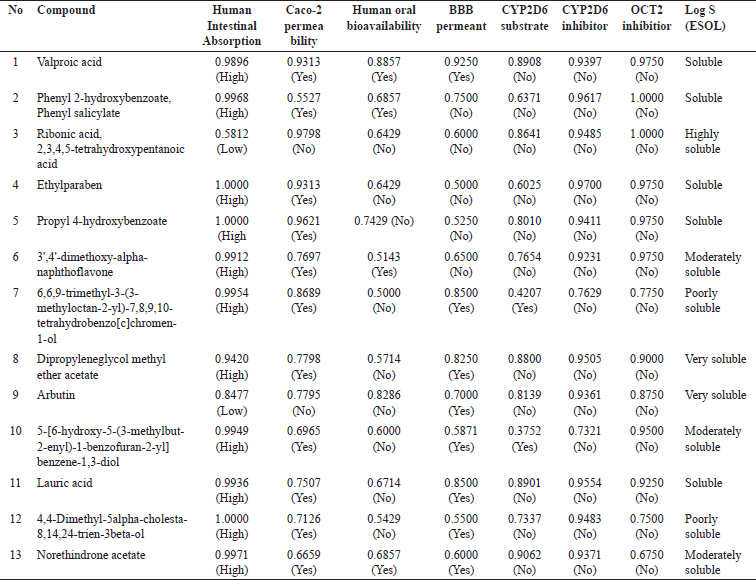 | Table 2. Pharmacokinetics properties of compounds from V. gracilis leaf extract. [Click here to view] |
The absorption, distribution, metabolism, excretion, and solubility (often referred to as ADME properties) of pharmacological compounds play a pivotal role in determining their therapeutic effectiveness and potential side effects [26]. The present analysis offers valuable insights into the ADME properties of compounds derived from V. gracilis. The predominant absorption of most compounds from V. gracilis through the human intestine suggests a high potential for oral administration drugs. However, two compounds, specifically ribonic acid, 2,3,4,5-tetrahydroxypentanoic acid, and arbutin, demonstrate reduced absorption. This divergence underscores the necessity for alternative delivery mechanisms or modifications to enhance their bioavailability if they possess therapeutic potential. Interestingly, while a majority of the compounds have lower oral bioavailability, a select few, such as valproic acid and phenyl 2-hydroxybenzoate, show higher potential. Moreover, the ability of eight compounds to cross the BBB is particularly notable, suggesting the distribution of the compounds to the central nervous system [29]. From a metabolic perspective, the majority of V. gracilis compounds not acting as substrates for CYP2D6 is promising. It suggests a reduced risk of drug–drug interactions, especially with medications metabolized by this particular enzyme [30]. The compounds’ inability to inhibit OCT2 further strengthens their safety profile, indicating a lower likelihood of interfering with the renal excretion of other concurrently administered drugs [31]. Such a trait can be critical when considering drug combinations or treatments for patients on multiple medications. Water solubility plays a crucial role in a drug’s pharmacokinetics and pharmacodynamics [32]. The high water solubility of most V. gracilis compounds could result in better absorption and distribution. However, the poor solubility of the two mentioned compounds might necessitate formulation strategies to enhance their solubility and, by extension, their bioavailability. In summary, the compounds from V. gracilis present a broad spectrum of ADME properties, with several showcasing attributes favorable for therapeutic application. Furthermore, in vivo studies will be instrumental in translating these in silico findings to real-world applications, with an emphasis on the compounds’ efficacy, safety, and potential therapeutic niches.
Predicted toxicity of the compounds
The prediction of toxicity levels (Table 3) showed that the toxicity classes of the compounds of V. gracilis leaf ranged from harmful (class 4) to nontoxic (toxicity class 6). Specifically, there are two compounds belonging to nontoxic class, namely ribonic acid, 2,3,4,5-tetrahydroxypentanoic acid (LD50: 7,800 mg/kg BW), and dipropyleneglycol methyl ether acetate (LD50: 9,456 mg/kg BW). Meanwhile, there are three compounds categorized as harmful (toxicity class 4) such as valproic acid (LD50: 670 mg/kg BW), 6,6,9-trimethyl-3-(3-methyloctan-2-yl)-7,8,9,10-tetrahydrobenzo[c]chromen-1-ol (LD50: 500 mg/kg BW), and lauric acid (LD50: 900 mg/kg BW). Nine other compounds are possibly hazardous (toxicity class 5). The results of toxicity prediction (Table 4) showed that of 13 selected compounds in V. gracilis leaf extract, 6 compounds are without any potential toxicity, while 7 compounds are predicted to cause toxicity. Two compounds are predicted to cause hepatoxicity (valproic acid and phenyl 2-hydroxybenzoate, phenyl salicylate), two compounds could exert carcinogenicity (dipropyleneglycol methyl ether acetate and norethindrone acetate), four compounds are predicted to cause immunotoxicity (3’,4’-dimethoxy-alpha-naphthoflavone, 6,6,9-trimethyl-3-(3-methyloctan-2-yl)-7,8,9,10-tetrahydrobenzo[c]chromen-1-ol, 4,4-dimethyl-5alpha-cholesta-8,14,24-trien-3beta-ol, and norethindrone acetate). Moreover, no compound is indicated to cause mutagenicity and cytotoxicity.
The assessment of toxicity levels of the compounds extracted from V. gracilis leaves reveals a spectrum of potential risks and safety profiles. With toxicity classes ranging from harmful to nontoxic, it underscores the heterogeneity in the pharmacological properties of these compounds, emphasizing the necessity for discerning selection in therapeutic applications [26]. Interestingly, two compounds, namely ribonic acid, 2,3,4,5-tetrahydroxypentanoic acid, and dipropyleneglycol methyl ether acetate, were identified with notably high LD50 values, placing them in the nontoxic class. Such compounds might offer therapeutic advantages given their apparent safety in the examined doses. Conversely, compounds such as valproic acid, 6,6,9-trimethyl-3-(3-methyloctan-2-yl)-7,8,9,10-tetrahydrobenzo[c]chromen-1-ol, and lauric acid were categorized as harmful, indicating that their application, if any, would necessitate caution, monitoring, and possibly dose adjustments to ensure patient safety [33]. Furthermore, the differentiated toxicity concerns, spanning hepatoxicity, carcinogenicity, and immunotoxicity among seven of the compounds, provide a detailed roadmap for future investigations. Specifically, the potential carcinogenicity of two compounds warrants rigorous in-depth studies before any clinical consideration [34]. It is equally noteworthy that none of the examined compounds exhibited mutagenic or cytotoxic properties, a positive sign for their broader safety profile. The absence of mutagenicity and cytotoxicity across all the tested compounds is a positive indication, suggesting that these compounds, even those deemed harmful or potentially hazardous based on LD50 values, might not necessarily pose genetic or cell damage risks [35]. Hence, while the compounds from V. gracilis leaf present a diverse range of toxicity profiles, each compound’s specific risk factors must be taken into account in potential therapeutic or pharmacological applications. Furthermore, in vivo studies are imperative to corroborate these in silico findings and provide a more comprehensive understanding of the safety and efficacy of these compounds.
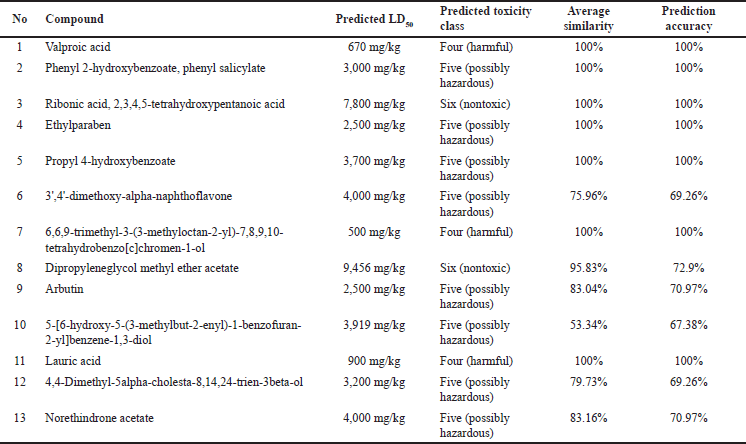 | Table 3. Predicted toxicity levels of the compounds from V. gracilis leaf extract. [Click here to view] |
Drug-likeness properties of the bioactive compounds from V. gracilis
Analysis of the physicochemical properties of the compounds from V. gracilis and their compliance with Lipinski’s rule (Table 5) revealed that 12 of the total 13 selected compounds meet the criteria as drug-like compounds. However, one compound, namely ribonic acid, 2, 3, 4, 5-tetrahydroxypentanolic acid fails to fully meet the drug-likeness criteria due to its higher H-bond receptor (>5) and lower molecular refractory (<40). The evaluation of the physicochemical properties of compounds from V. gracilis in relation to Lipinski’s rule offers valuable insights into their potential as drug candidates. A striking majority (12 out of 13) of the analyzed compounds meet the criteria for drug-likeness, suggesting their suitability for therapeutic applications, given that compounds aligning with Lipinski’s rule often exhibit good ADME profiles [36]. However, the exception of ribonic acid, 2, 3, 4, 5-tetrahydroxypentanolic acid due to its noncompliance, specifically its higher H-bond receptor and lower molecular refractory, implies potential challenges in its bioavailability or interaction profiles [37]. This divergence underlines the importance of further studies on this compound to determine its pharmacokinetic behavior and potential modifications to enhance its drug-like properties.
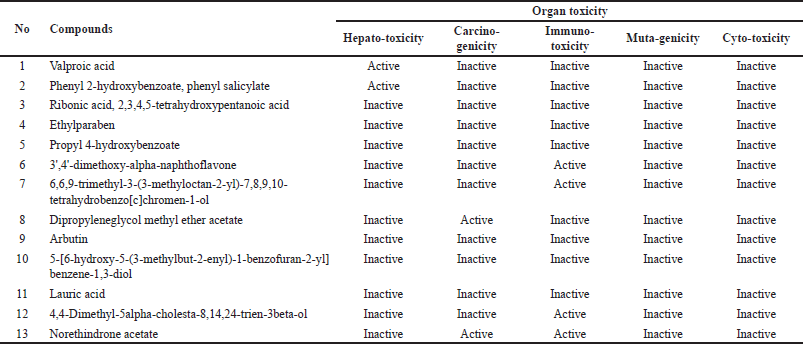 | Table 4. Predicted organ toxicity of the compounds from V. gracilis extract. [Click here to view] |
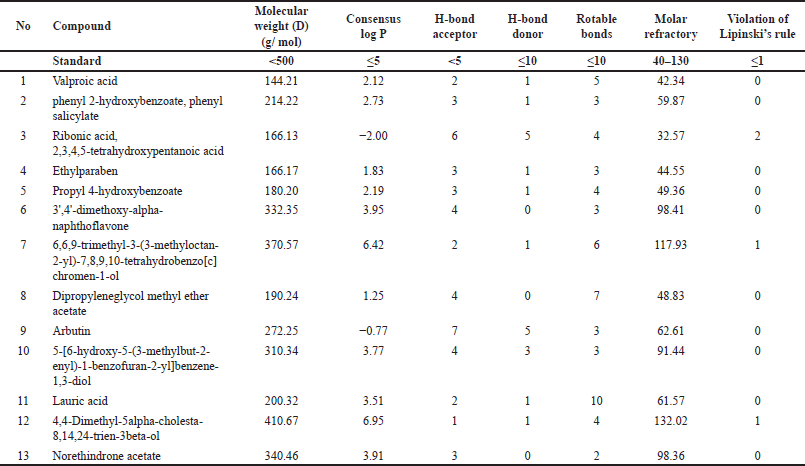 | Table 5. Physicochemical properties of compounds from V. gracilis leaf extract based on Lipinski’s rule of five test. [Click here to view] |
Molecular interactions of bioactive compounds from V. gracilis with SIRT1 and AMPK
The interactions of the compounds with the SIRT1 and AMPK proteins were simulated by molecular docking. As shown in Table 6, the compounds have substantial variations in binding energy values toward SIRT1 and AMPK. There are 7 of 13 compounds of V. gracilis that have high binding affinity to SIRT1 protein (with binding energies ranging from −7.6 to −10.7 kcal/mol) (Figs. 2 and 3). Interestingly, six of them have higher binding affinity as compared with a potent SIRT1 activator namely resveratrol (standard drug; −8.3 kcal/mol). Analysis of the interactions formed between amino acid residues of SIRT1 and selected bioactive compounds of V. gracilis (Table 7) indicated that the compounds interact via hydrogen bonds and hydrophobics. However, none of the compounds interact via the van der Wals bond. Furthermore, it was found that compounds from V. gracilis exhibit lesser binding affinity to AMPK protein than to SIRT1 protein. In addition, none of the compounds has binding energy higher than −7.0 kcal/mol (a standard value for a stable bond between two different compounds). However, given the resveratrol as a standard ligand with −4.5 kcal/mol of binding energy, there are four compounds that exhibited higher binding affinity to AMPK (with binding energies ranging from −4.7 to −5.0 kcal/mol) (Figs. 4 and 5). The compounds also interact with AMPK via hydrogen and hydrophobic bonds (Table 8).
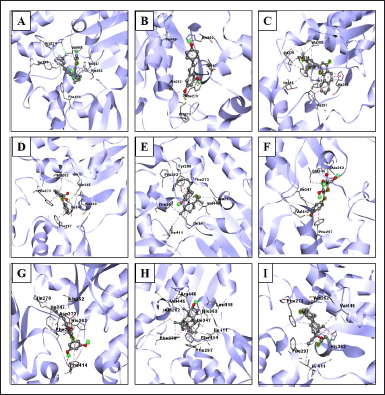 | Figure 2. Visualization of interactions between ligands and SIRT1 protein. (A) SIRT1/native ligand, (B) SIRT1/resveratrol, (C) SIRT1/phenyl 2-hydroxybenzoate, phenyl salicylate, (D) SIRT1/3',4'-dimethoxy-alpha-naphthoflavone, (E) SIRT1/6,6,9-trimethyl-3-(3-methyloctan-2-yl)-7,8,9,10-tetrahydrobenzo[c]chromen-1-ol, (F) SIRT1/arbutin, (G) SIRT1/5-[6-hydroxy-5-(3-methylbut-2-enyl)-1-benzofuran-2-yl]benzene-1,3-diol, (H) SIRT1/4,4-dimethyl-5alpha-cholesta-8,14,24-trien-3beta-ol, and (I) SIRT1/norethindrone acetate. [Click here to view] |
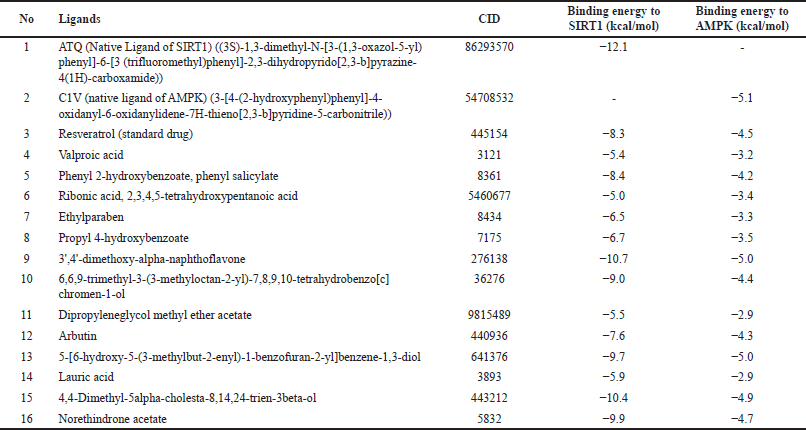 | Table 6. Binding affinity of the native ligand, standard drug and compounds from V. gracilis leaf extract with SIRT1 and AMPK proteins. [Click here to view] |
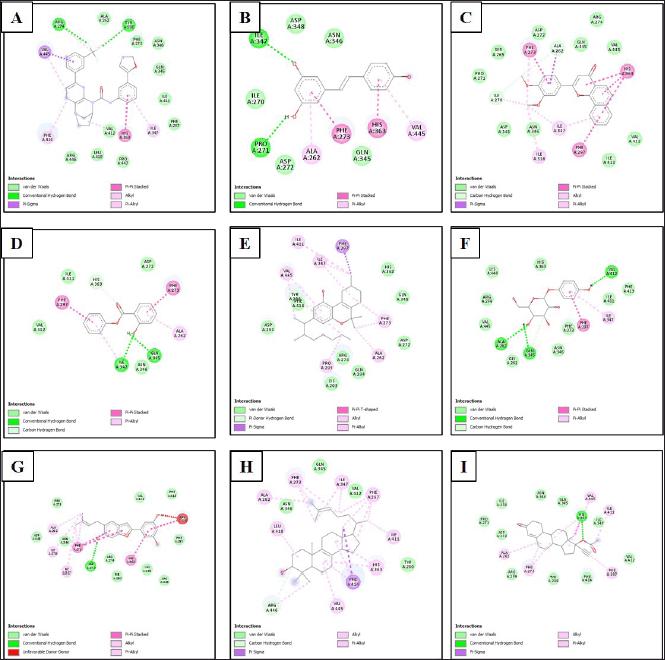 | Figure 3. Type of bonds formed between ligands and amino acid residues of SIRT1 protein. (A) SIRT1/native ligand, (B) SIRT1/resveratrol, (C) SIRT1/phenyl 2-hydroxybenzoate, phenyl salicylate, (D) SIRT1/3',4'-dimethoxy-alpha-naphthoflavone, (E) SIRT1/6,6,9-trimethyl-3-(3-methyloctan-2-yl)-7,8,9,10-tetrahydrobenzo[c]chromen-1-ol, (F) SIRT1/arbutin, (G) SIRT1/5-[6-hydroxy-5-(3-methylbut-2-enyl)-1-benzofuran-2-yl]benzene-1,3-diol, (H) SIRT1/4,4-dimethyl-5alpha-cholesta-8,14,24-trien-3beta-ol, and (I) SIRT1/norethindrone acetate. [Click here to view] |
Overall, four compounds show a consistency as high affinitive ligands to both SIRT1 and AMPK such as 3’,4’-dimethoxy-alpha-naphthoflavone, 5-[6-hydroxy-5-(3-methylbut-2-enyl)-1-benzofuran-2-yl]benzene-1,3-diol, 4,4-dimethyl-5alpha-cholesta-8,14,24-trien-3beta-ol, and norethindrone acetate. Besides the standard drug (resveratrol), the molecular docking performed in this study also included native ligands for SIRT1 and AMPK. It was found that the ATQ (a native ligand for SIRT1) has the highest binding affinity to SIRT (with binding energy −12.1 kcal/mol) among all tested ligands. Likewise, the C1V (a native ligand for AMPK also has the highest binding affinity to AMPK (−5.1 kcal/mol) among all tested ligands.
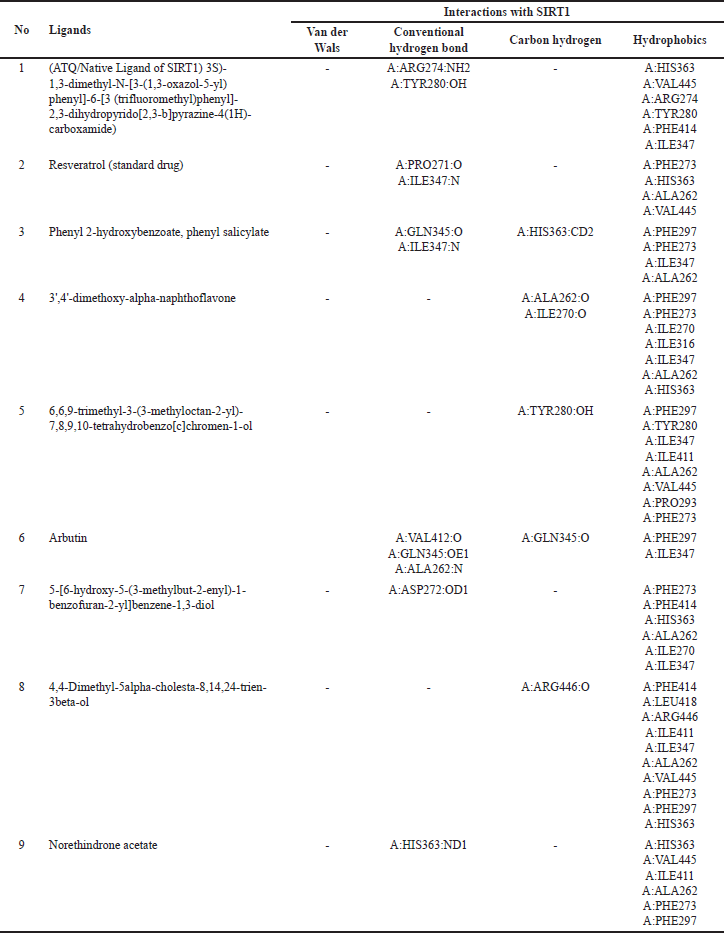 | Table 7. Interactions of amino acid residues of SIRT1 protein with selective potent compounds from V. gracilis leaf extract. [Click here to view] |
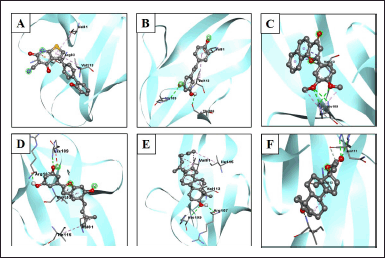 | Figure 4. Visualization of interactions between ligands and AMPK protein. (A) AMPK/native ligand, (B) AMK/resveratrol, (C) AMPK/3',4'-dimethoxy-alpha-naphthoflavone, (D) AMPK/5-[6-hydroxy-5-(3-methylbut-2-enyl)-1-benzofuran-2-yl]benzene-1,3-diol, (E) AMPK/4,4-dimethyl-5alpha-cholesta-8,14,24-trien-3beta-ol, and (F) AMPK/norethindrone acetate. [Click here to view] |
The molecular docking of V. gracilis compounds with the SIRT1 and AMPK proteins presents intriguing results that expand our understanding of the potential therapeutic applications of these compounds. A notable majority of the analyzed compounds demonstrated a high binding affinity toward the SIRT1 protein, surpassing the binding energy of the recognized SIRT1 activator, resveratrol. This suggests the possibility of these compounds being more potent activators of SIRT1 than the current standard drug (resveratrol) [24]. Such potency could translate into therapeutic advantages in clinical contexts where SIRT1 activation is desired, including in the way to minimize doxorubicin-induced cardiotoxicity [38]. However, when it comes to AMPK, the compounds from V. gracilis seem to exhibit a diminished binding affinity compared to their interaction with SIRT1. The fact that none surpassed the standard bonding energy of −7.0 kcal/mol indicates potential limitations in their therapeutic role in AMPK-related pathways [39]. When benchmarked against resveratrol, four compounds still managed to outperform, suggesting some promise in their applicability in AMPK-centric treatments. Importantly, it has been reported that the SIRT1 activation could also subsequently induce AMPK activation [24], suggesting that in the real physiological context, bioactive compounds of V. gracilis may also potently activate AMPK via SIRT1 activation to preclude doxorubicin-induced cardiotoxicity. It is also worth highlighting the consistency of four compounds that show strong binding affinities to both SIRT1 and AMPK. These dual-affinity compounds may be of particular interest in multi-target therapeutic strategies [40], warranting further investigations. Overall, this study indicates the potential of V. gracilis compounds as therapeutic agents, especially in the context of SIRT1 modulation to counteract doxorubicin-induced cardiotoxicity, while also shedding light on potential candidates for AMPK activation. Furthermore, in vivo and clinical studies are needed to validate these in silico findings and to explore the broader implications of these interactions in managing doxorubicin-induced cardiotoxicity.
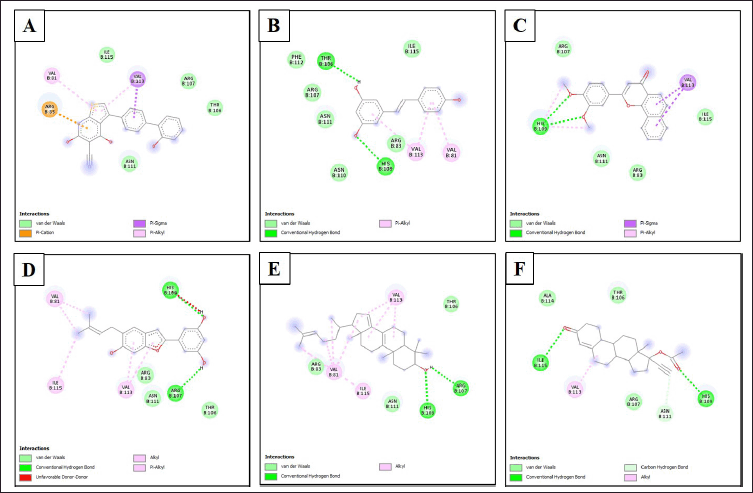 | Figure 5. Type of bonds formed between ligands and amino acid residues of AMPK. (A) AMPK/native ligand, (B) AMK/resveratrol, (C) AMPK/3',4'-dimethoxy-alpha-naphthoflavone, (D) AMPK/5-[6-hydroxy-5-(3-methylbut-2-enyl)-1-benzofuran-2-yl]benzene-1,3-diol, (E) AMPK/4,4-dimethyl-5alpha-cholesta-8,14,24-trien-3beta-ol, and (F) AMPK/norethindrone acetate. [Click here to view] |
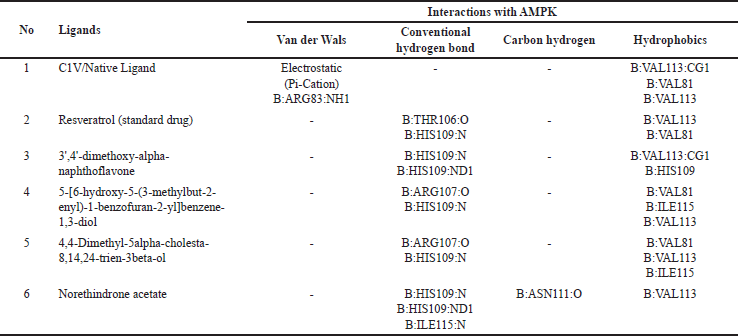 | Table 8. Interactions of amino acid residues of AMPK protein with selective compounds from V. gracilis leaf extract. [Click here to view] |
The study has several limitations that should be considered when interpreting its findings. First, the study relies heavily on computational approaches, particularly virtual screening and molecular docking, which may not accurately predict the actual biological activities of bioactive compounds from V. gracilis leaves and interactions of the compounds in a real biological context to SIRT1 and AMPK. The predicted bioactivity, binding affinities, and interactions should be confirmed through rigorous experimental studies, such as in vitro assays and animal models. Second, the study mainly focuses on in silico predictions and lacks in vitro and in vivo validation of selected compounds from V. gracilis. While the predicted toxicity and ADME properties provide valuable insights, they need to be corroborated with real-world experimental data. Toxicity assessments, such as LD50 values, should be confirmed through animal studies to establish a more accurate understanding of potential risks and safety profiles. Third, the study uses standard drug-like criteria, such as Lipinski’s rule, to assess the compounds’ potential as drug candidates. However, drug development involves complex considerations beyond these rules, including target specificity, off-target effects, and pharmacokinetic profiles. Therefore, meeting Lipinski’s rule does not guarantee successful drug development. Fourth, the study assumes that the compounds’ predicted interactions with SIRT1 and AMPK correlate directly with therapeutic benefits particularly against doxorubicin-induced cardiotoxicity, disregarding the complex network of cellular pathways and potential downstream effects. Experimental validation of these interactions and their functional consequences is essential. Finally, the study predominantly focuses on individual compound interactions with specific proteins. It overlooks potential synergistic or antagonistic effects when compounds are combined, which is a common approach in herbal medicine. Evaluating compound interactions in combination could yield different outcomes than individual assessments.
While the present study offers valuable insights into the potential bioactivities, ADME properties, toxicity, and interactions of compounds from V. gracilis, its findings are largely based on computational predictions. The study’s limitations highlight the need for comprehensive experimental validation, including in vitro assays, animal models, and potential clinical trials, to confirm the compounds’ therapeutic potential, safety profiles, and interactions before considering them for drug candidates.
CONCLUSION
This study indicated that 11 out of 13 selected compounds from V. gracilis leaf extract can be easily absorbed by the human intestine. However, generally, the compounds have lower human oral bioavailability, with only a few exceptions. Notably, eight of the compounds can cross the BBB. Metabolically, the majority of these compounds are not substrates for the liver enzyme CYP2D6, and none of the compounds act as inhibitors of the OCT2 protein in the kidneys. Most of the V. gracilis compounds are water-soluble, barring two exceptions. In terms of toxicity, the compounds range from nontoxic to harmful, with some posing risks of hepatoxicity, carcinogenicity, and immunotoxicity. Moreover, 12 out of 13 compounds meet drug-likeness criteria. Finally, several compounds exhibit high binding affinities to the proteins SIRT1 and AMPK, with some even outperforming the standard drug, resveratrol such as 3’,4’-dimethoxy-alpha-naphthoflavone, 5-[6-hydroxy-5-(3-methylbut-2-enyl)-1-benzofuran-2-yl]benzene-1,3-diol, 4,4-dimethyl-5alpha-cholesta-8,14,24-trien-3beta-ol, and norethindrone acetate. Therefore, bioactive compounds from V. gracilis could be considered potent drug candidates to overcome doxorubicin-induced cardiotoxicity via SIRT1 and AMPK activation.
ACKNOWLEDGMENT
Andalas University, Universitas Sumatra Utara, and Universitas Indonesia through the Indonesian Collaborative Research Program (RKI) provided the funds for this study (Contract No. 29/UN16.19/PT.01.03/KO-RKI Skema A (Mitra)/2023 for Andalas University; Contract No. 1/UNS.2.3.1/PPM/KPRKI/2023 For Universitas Sumatra Utara; and contract No. NKB-1065/UN2.RST/HKP.05.00/2023 for Universitas Indonesia).
AUTHOR CONTRIBUTIONS
PS, SI, and YHM conceptualized the study, PS, SI, and DFB performed phytochemical screening, PS, DFB, and RM performed the in-silico study, PS, SI, and YHM analyzed the data and prepared the manuscript. All authors evaluated the manuscript content and approved its publication.
CONFLICTS OF INTEREST
The authors do not have any conflict of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
The journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Peter S, Alven S, Maseko RB, Aderibigbe BA. Doxorubicin-based hybrid compounds as potential anticancer agents: a review. Molecules. 2022;27(14):4478–98. CrossRef
2. Van der Zanden SY, Qiao X, Neefjes J. New insights into the activities and toxicities of the old anticancer drug doxorubicin. FEBS J. 2021;288:6095–111. CrossRef
3. Pilco-Ferreto N, Calaf GM. Influence of doxorubicin on apoptosis and oxidative stress in breast cancer cell lines. Int J Oncol. 2016;49:753–62. CrossRef
4. Uceda-Castro R, Margarido AS, Cornet L, Vegna S, Hahn K, Song JY, et al. Re-purposing the pro-senescence properties of doxorubicin to introduce immunotherapy in breast cancer brain metastasis. Cell Rep Med. 2022;3(11):100821–48. CrossRef
5. Songbo M, Lang H, Xinyong C, Bin X, Ping Z, Liang S. Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol Lett. 2019;307:41–8. CrossRef
6. Syahputra RA, Harahap U, Dalimunthe A, Nasution MP, Satria D. The role of flavonoids as a cardioprotective strategy against doxorubicin-induced cardiotoxicity: a review. Molecules. 2022;27(4):1320–39. CrossRef
7. Ahmad N, Ullah A, Chu P, Tian W, Tang Z, Sun Z. Doxorubicin induced cardio toxicity through sirtuins mediated mitochondrial disruption. Chem Biol Interact. 2022;365:110028. CrossRef
8. He L, Liu F, Li J. Mitochondrial sirtuins and doxorubicin-induced cardiotoxicity. Cardiovasc Toxicol. 2021;21(3):179–91. CrossRef
9. Packer M. Cardioprotective effects of sirtuin-1 and its downstream effectors: potential role in mediating the heart failure benefits of SGLT2 (sodium-glucose cotransporter 2) inhibitors. Circ Heart Fail. 2020;13(9):e007197. CrossRef
10. Timm KN, Tyler DJ. The role of AMPK activation for cardioprotection in doxorubicin-induced cardiotoxicity. Cardiovasc Drugs Ther. 2020;34(2):255–69. CrossRef
11. Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121–35. CrossRef
12. Wu R, Wang HL, Yu HL, Cui XH, Xu MT, Xu X, et al. Doxorubicin toxicity changes myocardial energy metabolism in rats. Chem Biol Interact. 2016;244:149–58. CrossRef
13. Chen H, Zhu J, Le Y, Pan J, Liu Y, Liu Z, et al. Salidroside inhibits doxorubicin-induced cardiomyopathy by modulating a ferroptosis-dependent pathway. Phytomedicine. 2022;99:153964. CrossRef
14. Jin C, Chai Y, Hu Z, Tian W, Ling W, Li J, et al. Higenamine attenuates doxorubicin-induced cardiac remodeling and myocyte apoptosis by suppressing AMPK activation. Front Cell Dev Biol. 2022;10:809996. CrossRef
15. Tokarska-Schlattner M, Kay L, Perret P, Isola R, Attia S, Lamarche F, et al. Role of cardiac AMP-activated protein kinase in a non-pathological setting: evidence from cardiomyocyte-specific, inducible AMP-activated protein kinase α1α2-knockout mice. Front Cell Dev Biol. 2021;9:731015. CrossRef
16. Aththorick TA, Berutu L. Ethnobotanical study and phytochemical screening of medicinal plants on Karonese people from North Sumatra, Indonesia. J Phys Conf Ser. 2018;116(5):1–13. CrossRef
17. Wasnis NZ, Ilyas S, Hutahaean S, Silaban R, Situmorang PC. Analysis of apoptotic cells and lung inflammation after given by Vitis gracilis. Pak J Biol Sci. 2022;25(11):1033–9. CrossRef
18. Santoso P, Ilyas S, Midoen Y, Yuniarti A. Protective effect of Vitis gracilis Wall (Vitaceae) leaf decoction on sexual vitality and testis of alloxan-induced diabetic mice. Trad Integr Med. 2023;8(3): 256–68. CrossRef
19. Santoso P, Ilyas S, Midoen YH, Situmorang PC. Effect of Vitis gracilis Wall. administration on maximal swimming exercise apoptosis via cytochrome C in rat lung cells. J Pharm Pharmacogn Res. 2023;11(3):381–90. CrossRef
20. Midoen YH, Ilyas S, Santoso P, Situmorang PC. Effect of maximal physical exercise on apoptosis via cytochrome C in hippocampus cells after administration of Vitis gracilis Wall. J Pharm Pharmacogn Res. 2023;11(2):297–307. CrossRef
21. Pinzi L, Rastelli G. Molecular docking: shifting paradigms in drug discovery. Int J Mol Sci. 2019;20(18):4331–54. CrossRef
22. Alam N, Banu N, Aziz MA, Barua N, Ruman U, Jahan I, et al. Chemical profiling, pharmacological insights and in silico studies of methanol seed extract of Sterculia foetida. Plants (Basel). 2021;10(6):1135–56. CrossRef
23. Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, et al. PubChem 2019 update: improved access to chemical data. Nucleic Acids Res. 2019;47(D1):D1102–9. CrossRef
24. Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15(5):675–90. CrossRef
25. Jadhav PB, Jadhav SB, Zehravi M, Mubarak MS, Islam F, Jeandet P, et al. Virtual screening, synthesis, and biological evaluation of some carbohydrazide derivatives as potential DPP-IV inhibitors. Molecules. 2022;28(1):149–53. CrossRef
26. Shinde MG, Modi SJ, Kulkarni VM. Synthesis, pharmacological evaluation, molecular docking and in silico ADMET prediction of nitric oxide releasing biphenyls as anti-inflammatory agents. J App Pharm Sci. 2017;7(10) 37–47. CrossRef
27. Reis J, Massari M, Marchese S, Ceccon M, Aalbers FS, Corana F, et al. A closer look into NADPH oxidase inhibitors: validation and insight into their mechanism of action. Redox Biol. 2020;32:101466. CrossRef
28. Trautwein EA, McKay S. The role of specific components of a plant-based diet in management of dyslipidemia and the impact on cardiovascular risk. Nutrients. 2020;12(9):2671. CrossRef
29. Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32(11):1959–72. CrossRef
30. Taylor C, Crosby I, Yip V, Maguire P, Pirmohamed M, Turner RM. A review of the important role of CYP2D6 in pharmacogenomics. Genes (Basel). 2020;11(11):1295. CrossRef
31. Bi Y, Wang X, Ding H, He F, Han L, Zhang Y. Transporter-mediated natural product-drug interactions. Planta Med. 2023;89(2):119–33. CrossRef
32. Liu X, Zhao L, Wu B, Chen F. Improving solubility of poorly water-soluble drugs by protein-based strategy: a review. Int J Pharm. 2023;634:122704. CrossRef
33. Pratama MRF, Poerwono H, Siswodiharjo S. ADMET properties of novel 5-O-benzoylpinostrobin derivatives. J Basic Clin Physiol Pharmacol. 2019;30(6):251–62. CrossRef
34. Suarez-Torres JD, Orozco CA, Ciangherotti CE. The 2-year rodent bioassay in drug and chemical carcinogenicity testing: performance, utility, and configuration for cancer hazard identification. J Pharmacol Toxicol Methods. 2021;110:107070. CrossRef
35. Traesel GK, Castro LH, Silva PV, Muzzi RM, Kassuya CA, Arena AC, et al. Assessment of the cytotoxic, genotoxic, and mutagenic potential of Acrocomia aculeata in rats. Genet Mol Res. 2015;14(1):585–96. CrossRef
36. Chagas CM, Moss S, Alisaraie L. Drug metabolites and their effects on the development of adverse reactions: revisiting Lipinski’s rule of five. Int J Pharm. 2018 5;549(1–2):133–49. CrossRef
37. Chen D, Oezguen N, Urvil P, Ferguson C, Dann SM, Savidge TC. Regulation of protein-ligand binding affinity by hydrogen bond pairing. Sci Adv. 2016;2(3):e1501240. CrossRef
38. Kuno A, Hosoda R, Tsukamoto M, Sato T, Sakuragi H, Ajima N, et al. SIRT1 in the cardiomyocyte counteracts doxorubicin-induced cardiotoxicity via regulating histone H2AX. Cardiovasc Res. 2023;118(17):3360–73. CrossRef
39. Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31(2):455–61. CrossRef
40. Xin T, Lu C. SirT3 Activates AMPK-related mitochondrial biogenesis and ameliorates sepsis-induced myocardial injury. Aging (Albany NY). 2020;12(16):16224–37. CrossRef