INTRODUCTION
Alzheimer’s disease (AD) is the most common neurodegenerative disorder, accounting for more than 80% of dementia cases among the elderly around the world. By 2050, one new case of AD is expected to develop every 33 seconds, or nearly a million new cases per year [1]. Pathogenesis of AD begins with the accumulation of insoluble Amyloid β (Aβ) oligomers because of altered cleavage of Amyloid precursor protein (APP). These aggregated plaques and tangles promote the activation of microglia and astrocytes, increase mitochondrial oxidative stress, decrease energy metabolism, and cause degeneration of hippocampal pyramidal neurons [2]. Diabetes and Hyperinsulinemia occur as a result of higher body mass index (BMI) by increasing insulin resistance. Thus, it would be expected that a higher BMI would be associated with a higher risk of AD through diabetes and hyperinsulinemia-related mechanisms; some studies have suggested an association between hyperlipidemia, or high cholesterol and a higher risk of AD, particularly in middle age [3]. Brain cholesterol is a primary component of the brain membrane, and it plays a role in maintaining the plasticity of neurons. The presence of the ?4 allele of ApoE, a cholesterol carrier, represents the major genetic risk factor for the late-onset form of the disease. Another potential mechanism is cerebrovascular disease, although hypertension is a much more important risk factor for AD. The use of lipid-lowering medications could also reduce the risk of AD with widely available medication. Statins are inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA reductase) that represent the rate-limiting enzyme in cholesterol biosynthesis. These drugs are used among elderly patients to treat vascular death, stroke, and myocardial infarction.
Cholesterol levels in the brain are increased during AD condition. These regions are known as lipid rafts, and they contain β and γ secretases. These β secretases are capable of cleaving APP into sAPPβ and a membrane-bound APP-CTF (C99). The γ-secretase later cleaves the C99 into Aβ and the APP intracellular domain, which eventually results in the worsening of AD. Hence, Simvastatin, a cholesterol synthesis inhibitor, was evaluated in this study.
Acetyl-CoA is the precursor for the synthesis of cholesterol. HMG CoA reductase is inhibited by statins, which is the rate-limiting step for cholesterol synthesis. Inhibits isoprenylation, which is required for the processing of APP and inflammation [4,5]. Doxycycline is endowed with anti-amyloidogenic properties and better crosses the blood-brain barrier. AD progression is mainly due to the accumulation of Aβ, and Doxycycline inhibits the aggregation of Aβ42 Amyloid fibrils and disassembles mature Amyloid fibrils. A hopeful repositioned drug is counteracting crucial neuropathological AD targets. The present study aims to evaluate the effect of a combination of Simvastatin and Doxycycline on Aβ clearance in an Amyloid beta-induced AD mice model.
MATERIALS AND METHODS
Animals
C57BL/6 female mice of body weight 25–30 g were acquired with an IAEC approval No: JSSAHER/CPT/ IAEC/081/2021. Animals were divided into groups and held in polypropylene cages in an aerated environment, retaining temperature at 23°C ± 3°C and humidity levels at 40%–70%. The rooms followed the 12 hours L and 12 hours D cycle and minimal sound (?80 decibels). Animals were fed with quality rodent feed and pure water. Animals were allowed to familiarize themselves with the new environment for 7 days before starting the experimental work. All the animal investigational procedures were approved and supervised by IAEC- JSSCPM.
*Alzheimer’s proteins rise sharply in response to stress in female mice [6].
Treatment drugs
The treatment drugs Simvastatin and Doxycycline and the standard drug Donepezil are procured from JSS Hospital in Mysuru, Karnataka, India.
Grouping of animals for induction of AD by mouse model
The animals were divided into groups, with 56 animals equally divided into groups (n = 7). The dose given to animals and the treatment period were mentioned in Table 1.
Induction of Alzheimer’s by Aβ1-42
Aβ monomer solution (1 μM) was prepared using dimethylsulphoxide and diluting it 10 times with phosphate buffer saline (PBS). Incubation of monomer solution at 37°C for 5 days under humid conditions results in oligomer formation [7]. Next, Aβ1-42 was injected by i.c.v with the help of a stereotaxic apparatus (Steolting, USA) consisting of a 28-gauge stainless-steel needle of 3.0 mm length (Hamilton). An intraperitoneal (i.p) injection of xylazine (20 mg/kg) and Ketamine (80 mg/kg) cocktail was used for inducing anesthesia in mice, after which they were placed on a stereotaxic frame. 5 μl of Aβ1-42 was injected slowly into the right lateral ventricle using the following coordinates from bregma: anteroposterior = −0.9 mm, mediolateral = 1.3 mm, and dorsoventral = −2.0 mm. Sham-treated mice received PBS (5 μl) in the same coordinates. Special care was taken to prevent temperature falls in the mice. The rapid dispersion of the peptide throughout the brain is a real advantage of this i.c.v. mode of administration [8].
Behavioral parameters
Buried pellet
We used a buried pellet test to examine the mice’s odor detection, as previously described. Individually housed mice were fed a meal containing 90% of their body weight for two days previous to the test and for the duration of the experiment.
1–2 pieces of pellets were given to each mouse to get adjusted with the pellet throughout the test and during food restriction. This phase is crucial because the mice must become used to the pellet odor. In the test cage, a portion of the pellet was buried 0.5 cm below the bedding. The mouse was placed in the center of the cage at the start of each trial. The time required to discover the pellet was noted [9].
Morris water maze (MWM)
MWM is used to measure spatial learning and memory performance. This metallic pool was filled with water, and the temperature was maintained stable at 23°C. The light intensity, external cue, and opacity of water (with black food coloring) were made reproducible. The entire pool was segmented into four quadrants with an escape platform (diameter: 4.5 cm) kept at the southwest quadrant. 5 days of acquisition were performed, with 2 trials being performed daily for 2 days and 1 trial per day for the rest 3 days. The final 6th day was the test day. The mice were kept at random in the water, in the opposite quadrant to the one where the platform was placed. Mice were allowed to swim for around the 60 seconds until the platform was discovered by animals. Once it reached the platform, it was made to stay for 15 seconds, and the escape latency within the 60 seconds was noted, after which the parameters such as path length and the time the mice voluntarily stayed on the platform for 15 seconds were recorded. The mice were returned to their respective cages, and on the final day, memory preservation was performed using 60 seconds probe trials. The platform was then removed, and the mice were placed in the water facing the wall opposite to the platform, where they were allowed to move in the surroundings for 60 seconds. A digital camera was used to record the movement of each mouse, which was placed atop the midpoint of the maze. It was connected to a computer, and video was recorded [10].
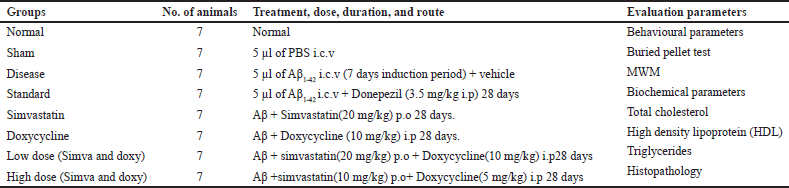 | Table 1. Grouping of animal, dosing, and duration of study. [Click here to view] |
Biochemical parameters
Histopathology
The brains were removed from the animals, and the tissues were stored in 10% formalin. The brains were removed and postfixed in the same fixative overnight at 48°C. The brains were embedded in paraffin and stained with Hematoxylin-Eosin. The hippocampus lesions were assessed microscopically at 400× magnifications.
Statistical analysis
For in vivo, the values are expressed as mean ± SEM of samples. All data were analyzed by one-way ANOVA followed by Tukey’s post-hoc test using Graph pad Prism version 5.0 software. The difference between the control and treated groups was expressed using a p-value, and considered significant if p < 0.05.
RESULTS
Behavioral parameters
Effect of Simvastatin and Doxycycline on olfactory function after Aβ induction in mice
Sham control did not show any significant changes in the time taken to unbury the buried pellet from day 0 to 35 (8 ± 1.046 vs. 8.57 ± 0.57). The disease group showed increased time in identifying the pellet from day 0 to 35 (8.42 ± 0.94 vs. 28.28 ± 1.26) when compared to normal. Simvastatin- and Doxycycline-treated groups showed an increase in time taken to unbury the buried pellet on day 35 when compared to the standard group (18.28 ± 0.99 vs. 16.42 ± 1.19 and 18.85 ± 1.28 vs. 16.42 ± 1.19). A low-dose combination of (Simvastatin and Doxycycline) has shown an increase in the time taken to unbury the buried pellet on day 35 when compared to standard (17.42 ± 0.84 and 16.42 ± 1.19). A high-dose combination of (Simvastatin and Doxycycline) has a significant decrease in the time taken to unbury the buried pellet on day 35 when compared to the standard group (16.71 ± 1.16 vs. 16.42 ± 1.19). The effect of Simvastatin and Doxycycline on buried pellet test are reported in Figure 1 and Table 2.
Effect of Simvastatin and Doxycycline on memory function in mice after Aβ induction in mice
Sham control did not show any significant changes in the time spent in the target quadrant from day 0 to 28 (15.21 ± 2.05 vs. 15.65 ± 1.00). The disease group showed a decrease in the time spent in the target quadrant from day 0 to 28 (17.32 ± 0.51 vs. 10.61 ± 1.62) when compared to normal. Simvastatin- and Doxycycline-treated groups have not shown any significant difference in time spent in the target quadrant on day 28 when compared to the standard group (14.66 ± 0.73 vs. 13.77 ± 0.9 and 13.61 ± 0.92 vs. 13.77 ± 0.97). A low-dose combination of (Simvastatin and Doxycycline) has shown a significant difference in time spent in the target quadrant on day 28 when compared to the standard group (15.24 ± 0.80 vs. 13.77 ± 0.9 and 13.61 ± 0.92 vs. 13.77 ± 0.97). A high-dose combination of Simvastatin and Doxycycline has a slight difference in a variation on day 35 when compared to the standard group (16.71 ± 1.16 vs. 16.42 ± 1.19). The effect of Simvastatin and Doxycycline on memory enhancement is reported in Figure 2 and Table 3.
Biochemical parameters
Effect of Simvastatin and Doxycycline on total cholesterol after Aβ induction in mice
The sham group did not show any significant increase in total cholesterol when compared to normal (38.92 ± 2.37 vs. 40.31 ± 2.73). The disease group has shown an increase in the amount of total cholesterol when compared to normal (59.89 ± 0.95 vs. 40.31 ± 2.73). Simvastatin-treated group has shown a decrease in the amount of total cholesterol when compared to the disease group (37.68 ± 2.48 vs. 59.89 ± 0.95). Doxycycline-treated group has shown a less significant decrease in the amount of total cholesterol when compared to the disease group (52.39 ± 0.85 vs. 59.89 ± 0.95). In a low-dose combination of Simvastatin and Doxycycline and a high-dose combination of Simvastatin and Doxycycline, a treated group has shown a decrease in the amount of total cholesterol when compared to disease (49.09 ± 0.94 vs. 59.89 ± 0.95 and 41.07 ± 1.91 vs. 59.89 ± 0.95). The effect of Simvastatin and Doxycycline on cholesterol levels is reported in Figure 3 and Table 4.
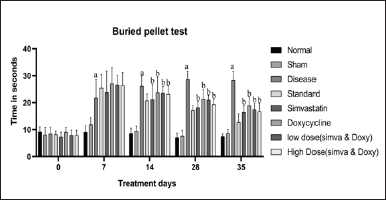 | Figure 1. Effect of Simvastatin and Doxycycline on buried pellet test after Aβ induction in mice. All values are expressed in the form of Mean ± SEM, n = 7; data were analyzed by employing two-way ANOVA and “a” represents p-value <0.05 when compared to normal versus disease, and “b” represents p-value <0.05 when compared to disease versus treatment groups. [Click here to view] |
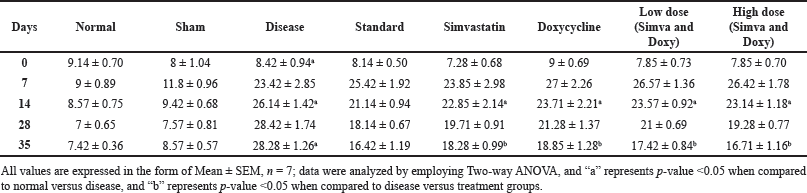 | Table 2. Effect of Simvastatin and Doxycycline on buried pellet test after Aβ induction in mice. [Click here to view] |
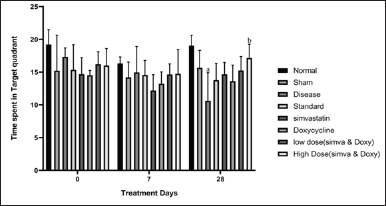 | Figure 2. Effect of Simvastatin and Doxycycline on time spent in target zone after Aβ induction in mice. All values are expressed in the form of Mean ± SEM, n = 7; data were analyzed by employing two-way ANOVA, and “a” represents p-value <0.05 when compared to normal versus disease and “b” represents p-value < 0.05 when compared to disease versus treatment groups. [Click here to view] |
All values are expressed in the form of Mean ± SEM, n = 7, Statistical analysis was executed employing one way ANOVA, and ‘a’ represents p-value < 0.05 when compared to disease vs. treatment groups and ‘b’ represents p-value < 0.05 when compared to Standard vs. treatment groups.
Effect of Simvastatin and Doxycycline on triglycerides after Aβ induction in mice
The sham group did not show any significant increase in triglycerides when compared to normal (48.73 ± 2.30 vs. 50.91 ± 1.99). The disease group has shown an increase in the number of triglycerides when compared to normal (64.10 ± 2.33 vs. 50.91 ± 1.99). Simvastatin-treated group has shown a decrease in the amount of total cholesterol when compared to the disease (47.25 ± 1.62 vs. 64.10 ± 2.33). Doxycycline-treated group has shown a less significant decrease in the amount of total cholesterol when compared to the disease (52.39 ± 0.85 vs. 50.91 ± 1.99). A low-dose combination of Simvastatin and Doxycycline and a high-dose combination of Simvastatin- and Doxycycline-treated groups have shown a decrease in the amount of total cholesterol when compared to disease (51.91 ± 1.99 vs. 64.10 ± 2.33 and 49.33 ± 2.80 vs. 64.10 ± 2.33). The effect of Simvastatin and Doxycycline on triglyceride levels are reported in Figure 4 and Table 4.
Effect of Simvastatin and Doxycycline on HDL after Aβ induction in mice
The sham group did not show any significant increase in the HDL when compared to normal (28.80 ± 3.48 vs. 27.81 ± 2.66). The disease group has shown an increase in the amount of HDL when compared to normal (41.40 ± 1.06 vs. 27.81 ± 2.66). Simvastatin-treated group has shown a decrease in the amount of HDL when compared to the disease (39.59 ± 0.96 vs. 41.40 ± 1.06). Doxycycline-treated group has shown an increase in the amount of HDL when compared to the disease (43.93 ± 1.93 vs. 41.40 ± 1.06). A low-dose combination of Simvastatin and Doxycycline has not shown any significant decrease in the amount of HDL when compared to the disease group (41.44 ± 1.00 vs. 41.40 ± 1.06). A high-dose combination of Simvastatin and Doxycycline has shown a decrease in the amount of HDL when compared to the disease group (33.52 ± 1.98 vs. 41.40 ± 1.06). The effect of Simvastatin and Doxycycline on HDL levels are reported in Figure 5 and Table 4.
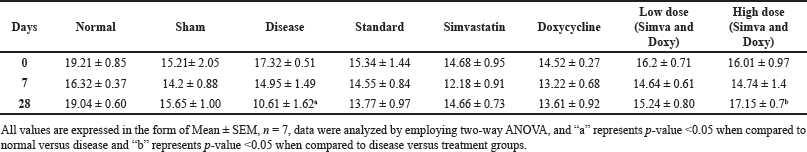 | Table 3. Effect of Simvastatin and Doxycycline on time spent in target zone after Aβ induction in mice. [Click here to view] |
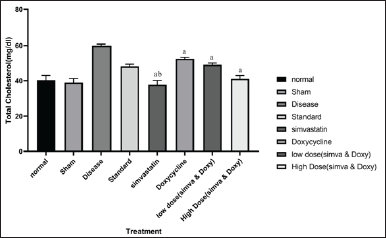 | Figure 3. Effect of Simvastatin and Doxycycline on total cholesterol after Aβ induction in mice. All values are expressed in the form of Mean ± SEM, n = 7; statistical analysis was executed employing one-way ANOVA, and “a” represents p-value <0.05 when compared to disease versus treatment groups and “b” represents p-value <0.05 when compared to standard versus treatment. [Click here to view] |
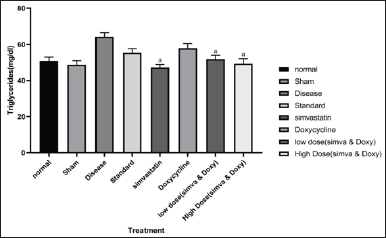 | Figure 4. Effect of Simvastatin and Doxycycline on triglycerides after Aβ induction in mice. All values are expressed in the form of Mean ± SEM, n = 7; statistical analysis was executed employing one-way ANOVA, and “a” represents p-value <0.05 when compared to disease versus treatment. [Click here to view] |
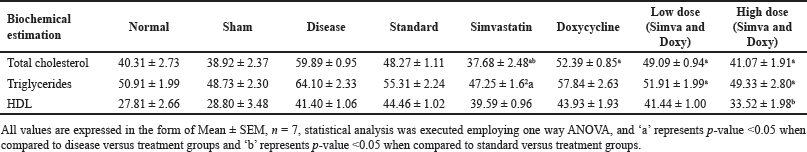 | Table 4. Effect of Simvastatin and Doxycycline on various biochemical parameters. [Click here to view] |
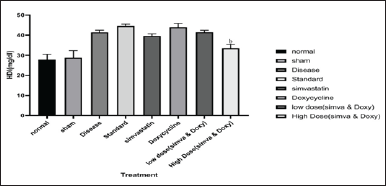 | Figure 5. Effect of Simvastatin and Doxycycline on HDL after Aβ induction in mice. All values are expressed in the form of Mean ± SEM, n = 7; statistical analysis was executed by employing one-way ANOVA, and “b” represents p-value <0.05 when compared to standard versus treatment. [Click here to view] |
Histopathology
The histopathological data is shown in Figure 6. The data revealed that the disease group showed degeneration of pyramidal neurons and the presence of macrophages, whereas the Simvastatin, Doxycycline group, and combination (high dose) group showed significant recovery from the damage caused by Aβ1-42 induction
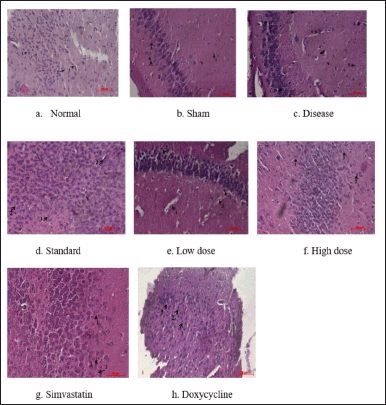 | Figure 6. Photomicrographs showing the histological architecture of the brain hippocampus region. 1 – nerve cell; 2 – glial cell; 3 – blood Vessel; 4 – infiltration with the presence of macrophages; and 5 – degeneration of pyramidal neurons. [Click here to view] |
DISCUSSION
AD is a complicated illness caused by a combination of hereditary and environmental factors. Despite recent promising developments in the study of AD, the link between cholesterol and APP processing remains poorly understood. During AD conditions, the cholesterol levels in the brain increase, and these regions are called lipid rafts. These lipid rafts are hosts for β and γ secretases. As a result, mono fibrils develop, which leads to Aβ1-42 oligomerization and, eventually, Aβ1-42 accumulation. Cholesterol inhibits α-secretase and non-Amyloid pathways as well. Simvastatin reduces cholesterol levels; thereby, the amount of Aβ peptides decreases. A decrease in the amount of Aβ peptides decreases the progression of AD. Doxycycline is endowed with anti-amyloidogenic properties and better crosses the blood–brain barrier. The present study showed that administration of Aβ1-42 by single unilaterally after i.c.v surgery to the female C57BL/6 mice caused AD symptoms such as olfactory damage and cognitive impairment. The olfactory damage was assessed by the time taken to unbury the pellet. The cognitive impairment was assessed by the time spent in the target quadrant and the latency to reach the target quadrant. Olfactory function was assessed by performing the buried pellet test. In this study, after the administration of Aβ1-42, it was observed that there was a decrease in the olfactory function in the disease when compared to the sham control and normal group. The standard group has shown a significant increase in olfaction ability when compared to the disease. The treatment groups have shown a significant increase in olfactory function when compared to the disease after the completion of treatment.
The cognitive impairment was assessed by the MWM. The time spent in the quadrant zone was decreased in disease when compared to the sham control and control group. The standard drug has shown a significant increase in time spent in the target quadrant. The treatment groups such as Simvastatin and Doxycycline and low dose combination (Simva and doxy) have shown less significance when compared to the standard. A high combination dose of (Simva and doxy) has shown an increase in time spent in the target quadrant. Similarly, the latency to reach the target quadrant was also assessed by the MWM. The latency to reach the target quadrant in the disease was increased when compared to the normal and sham controls. The standard group has shown a significant decrease in latency to reach the target quadrant. The treatment groups such as Simvastatin and Doxycycline and low dose combination (Simva and doxy) have shown less significance when compared to the standard. A high combination dose of (Simva and doxy) has shown decreased latency to reach the target quadrant.
Total cholesterol, triglycerides, and HDL were estimated in serum and were measured by enzymatic assay from serum by using commercial RANDOX KITS. The amount of total cholesterol, triglycerides, and HDL was increased in the disease when compared to the normal. The standard drug does not have any significant effect on total cholesterol, triglycerides, and HDL. The treatment group Simvastatin and low dose combination (simva and doxy) and high dose combination (simva and doxy) has a significant decrease in the amount of total cholesterol and triglycerides and HDL. These results show that a high-dose combination of (simva and doxy) exhibits cholesterol-lowering properties by blocking the HMG CoA reductase pathway, thereby regulating cholesterol. Hence, a high dose combination of (simva and doxy) has an anti-Alzheimer’s effect by regulating the cholesterol and clearance of Aβ1-42 aggregation by blocking the HMG CoA reductase pathway. AD is a complicated illness caused by a combination of hereditary and environmental factors. Despite recent promising developments in the study of AD, the link between cholesterol and APP processing remains poorly understood. During AD conditions, the cholesterol levels in the brain increase, and these regions are called lipid rafts. These lipid rafts are hosts for β and γ secretases. As a result, mono fibrils develop, which leads to Aβ1-42 oligomerization and, eventually, Aβ1-42 accumulation. Cholesterol inhibits α-secretase and non-Amyloid pathways as well.
CONCLUSION
These results show that Aβ1-42 administration through i.c.v induces Alzheimer’s, and upregulation of cholesterol is seen in disease-induced animals. Administration of Simvastatin and Doxycycline in various doses attenuates the olfactory function and cognitive impairment. High-dose administration of (Simvastatin and Doxycycline) reduces the total cholesterol, triglycerides, and HDL levels. Hence, the study shows that the administration of a high dose administration of (Simvastatin and Doxycycline) produces an anti-Alzheimer’s effect in Aβ1-42-induced Alzheimer’s model.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by the IAEC of JSSCPM (Approval no: JSSAHER/CPT/IAEC/081/2021).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2016 Apr 1;12(4):459–509. CrossRef
2. Sharma P, Srivastava P, Seth A, Tripathi PN, Banerjee AG, Shrivastava SK. Comprehensive review of mechanisms of pathogenesis involved in Alzheimer’s disease and potential therapeutic strategies. Prog Neurobiol. 2019;174:53–89. CrossRef
3. Kivipelto M, Laakso MP, Tuomilehto J, Nissinen A, Soininen H. Hypertension and hypercholesterolaemia as risk factors for Alzheimer’s disease: potential for pharmacological intervention. CNS Drugs. 2002;16:435–44. CrossRef
4. Hamilton Health Sciences Corporation. Effects of treatment with Doxycycline and Rifampicin on biomarkers of Alzheimer’s disease in the cerebrospinal fluid. clinicaltrials.gov; 2018 Mar [cited 2023 Aug 29]. Report No.: NCT00439166. Available from: https://clinicaltrials.gov/study/NCT00439166
5. Diomede L, Cassata G, Fiordaliso F, Salio M, Ami D, Natalello A, et al. Tetracycline and its analogues protect Caenorhabditis elegans from β amyloid-induced toxicity by targeting oligomers. Neurobiol Dis. 2010;40:424–31. CrossRef
6. Bhandari T. Stress increases Alzheimer’s risk in female mice but not males. St. Louis, MO: Washington University School of Medicine. Available from: https://medicine.wustl.edu/news/stress-increases-alzheimers-risk-in-female-mice-but-not-males/
7. Kim HY, Lee DK, Chung BR, Kim HV, Kim Y. Intracerebroventricular injection of amyloid-# peptides in normal mice to acutely induce Alzheimer-like cognitive deficits. J Vis Exp. 2016;2016:1–6. CrossRef
8. Souza LC, Jesse CR, Antunes MS, Ruff JR, de Oliveira Espinosa D, Gomes NS, et al. Indoleamine-2,3-dioxygenase mediates neurobehavioral alterations induced by an intracerebroventricular injection of amyloid-β1-42 peptide in mice. Brain Behav Immun. 2016;56:363–77. CrossRef
9. Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. Curr Protoc Neurosci. 2009;48:8.24.1–12. CrossRef
10. Pereira IT, Burwell RD. Using the spatial learning index to evaluate performance on the water maze. Behav Neurosci. 2015;129:533–9. CrossRef
11. Meiattini F, Prencipe L, Bardelli F, Giannini G, Tarli P. The 4-hydroxybenzoate/4-aminophenazone chromogenic system used in the enzymic determination of serum cholesterol. Clin Chem. 1978 Dec 1;24(12):2161–5. CrossRef
12. Young DS. Effects of drugs on clinical laboratory tests. Washington, DC: AACC Press; 1995. Available from: https://lib.ugent.be/en/catalog/rug01:000423281.
13. Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973 May 1;19(5):476–82. CrossRef
14. Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982 Oct 1;28(10):2077–80. CrossRef
15. Fassbender K, Simons M, Bergmann C, Stroick M, Lü Tjohann D, Keller P, et al. Simvastatin strongly reduces levels of Alzheimer’s disease-amyloid peptides A42 and A40 in vitro and in vivo. Proc Nat Acad Sci. 2001;98(10):5856–61. CrossRef
16. Cleeman JI, Lenfant C. The national cholesterol education program: progress and prospects. JAMA. 1998;280:2099–104. CrossRef